Abstract
Epithelial-to-mesenchymal transition (EMT) is a complex process involving multiple genes, steps and stages. It refers to the disruption of tight intercellular junctions among epithelial cells under specific conditions, resulting in loss of the original polarity, order and consistency of the cells. Following EMT, the cells show interstitial cell characteristics with the capacity for adhesion and migration, while apoptosis is inhibited. This process is critically involved in embryogenesis, wound-healing, tumor invasion and metastasis. The tumor microenvironment is composed of infiltrating inflammatory cells, stromal cells and the active medium secreted by interstitial cells. Most patients with hepatocellular carcinoma (HCC) have a history of hepatitis virus infection. In such cases, major components of the tumor microenvironment include inflammatory cells, inflammatory factors and virus-encoded protein are major components. Here, we review the relationship between EMT and the inflammatory tumor microenvironment in the context of HCC. We also further elaborate the significant influence of infiltrating inflammatory cells and inflammatory mediators as well as the products expressed by the infecting virus in the tumor microenvironment on the EMT process.
Keywords: Hepatocellular carcinoma, Epithelial-to-mesenchymal transition, Inflammatory microenvironment, Tumor-related macrophages, Expressed products of infected virus
Background
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide and is a typical inflammatory-related tumor that is associated with early metastasis and poor prognosis. From a global perspective, between 75 and 80% of patients with liver cancer have a history of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections [1, 2]. Sub-Saharan Africa and East Asia are regions with a high incidence of hepatocellular carcinoma, and the HCC patients in China account for 50% of the total number of patients around the world, a fact that is inseparable from the huge numbers of people with hepatitis infections in this region [3]. In addition, the accumulation of toxic compounds (such as alcohol and aflatoxins) as well as metabolic liver injury are also important causative factors in the development of liver cancer. These infection-associated and non-infection-associated factors can lead to a state of chronic inflammation of the liver [4]. Over time, the chronic inflammatory microenvironment may gradually and imperceptibly promote the development of liver fibrosis and early liver cancer, as well as the development, invasion and metastasis of tumor cells.
Biological behaviors, such as early invasion, metastasis and recurrence, are challenges to the clinical treatment of liver cancer. Specifically, EMT is considered to be a key step for tumor invasion and metastasis [5]. Tumor cells develop powerful invasive and metastatic ability through the EMT process, which allows the migration of tumor cells to different sites via the circulatory system [6]. In HCC, the long-term chronic inflammatory microenvironment is undoubtedly the decisive factor in the development of the tumor. Inflammatory cell aggregation, inflammatory cell infiltration and inflammatory mediator-induced activation of related pathways are critically involved in tumor invasion and metastasis. However, the association between the occurrence of EMT and the inflammatory microenvironment in the tumor is not yet clear. Here, we review the current knowledge regarding this issue.
The inflammatory microenvironment of hepatocellular carcinoma
HCC is a typical inflammation-related tumor. The process of tumor growth and infiltration is always accompanied by apoptosis or necrosis, which causes the release of numerous inflammatory mediators. Tumor cells and inflammatory cells also produce chemokines, cytokines, and growth factors, which induce angiogenesis and further inflammation [7]. These inflammatory mediators, inflammatory cells and tumor cells interact to form an inflammatory cascade reaction. Moreover, the persistent inflammatory microenvironment not only promotes tumor induction, but also accelerates tumor progression and promotes the formation of new blood vessels [8], activation of cancer-associated fibroblasts (CAF) [9] and remodeling of the extracellular matrix (ECM) [10]. These conditions also enhance tumor cell survival and proliferation, which play a significant role in the occurrence, development and metastasis of tumors.
Epithelial-to-mesenchymal transition
Recent studies have shown that EMT is a key step in tumor invasion and metastasis [5, 11, 12]. Normal epithelial cells are highly ordered and have close intercellular connections. These cells also exhibit significant polarity of the free and basal surfaces, with relatively stable morphology. In contrast, interstitial cells, which assist the parenchymal cells to perform organ functions, have different forms and a loose arrangement [13]. They typically lack polarity and have greater migration and invasive capacity. EMT refers to the disruption of tight intercellular junctions among epithelial cells under specific conditions, resulting in loss of the original polarity, order and consistency. Under these circumstances, epithelial cells tend to show interstitial cell characteristics and develop the capacity for migration and apoptosis is inhibited [14].
The molecular mechanism of epithelial-to-mesenchymal transition
The most significant feature of the surface of a cell following EMT is the decrease in E-cadherin expression and the increase in N-cadherin expression [15]. E-cadherin is a connecting structure among epithelial cells and has strong and stable adhesion properties. N-cadherin, which can be defined as the connecting structure among mesenchymal cells, shows weaker adhesion ability, a characteristic that is one of the causes underlying the increase in cell migration and invasion following EMT [16]. The dynamic properties of the intermediate filament protein vimentin are very important for cell flexibility and increased expression of vimentin is an important sign of EMT in tumor invasion and metastasis [17].
The common transcription factors Snail, Slug, Twist, ZEB1, ZEB2, FOXC1, and FOXC2 participate in the induction of the EMT process [18] by reducing E-cadherin expression via intracellular signaling pathways, such as the JAK/STAT3, MAPK/ERK, and PI3K/AKT [19–21]. In addition, many growth factors, such as epidermal growth factor (EGF), transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF), also play a role in the intracellular conduction pathway [17, 22, 23].
The relationship between the inflammatory microenvironment of hepatocellular carcinoma and the epithelial-to-mesenchymal transition process
The occurrence and development of HCC are accompanied by a persistent inflammatory reaction. Inflammatory cells, inflammatory mediators, and the products of the infecting virus have a great influence on the process of EMT in hepatocellular carcinoma.
Inflammatory cells in the inflammatory microenvironment of hepatocellular carcinoma
Similar to other tumor microenvironments, inflammatory cells in the HCC microenvironment include mainly macrophages, neutrophils, lymphocytes, mast cells, dendritic cells, and eosinophils. Among these tumor-related macrophages, infiltrating lymphocytes and neutrophils are the three most common leukocytes [24].
Tumor-associated macrophages (TAMs) and EMT in hepatocellular carcinoma
Tumor-related macrophages are the primary inflammatory cells infiltrating in the tumor microenvironment [25]. These cells, which have a high degree of heterogeneity and plasticity and are derived from circulating monocytes and Kupffer cells, are recruited into tumor tissues by chemokines, vascular endothelial growth factor (VEGF) and macrophage colony-stimulating factor (MC-SF). Under the influence of cytokines and microbial products, TAMs show specific features of specialization and polarization [24, 26].
According to the characteristics of polarization, macrophages can be divided into M1 and M2 subtypes. In the tumor microenvironment, the M2 phenotype tends to predominate, which promotes tumor invasion and metastasis [27]. TAMs are not intrinsically malignant. Nevertheless, their interactions with tumor cells can directly promote tumor growth, invasion and metastasis, and their association with EMT can also be mediated by secreting inflammatory factors, cytokines and related proteases.
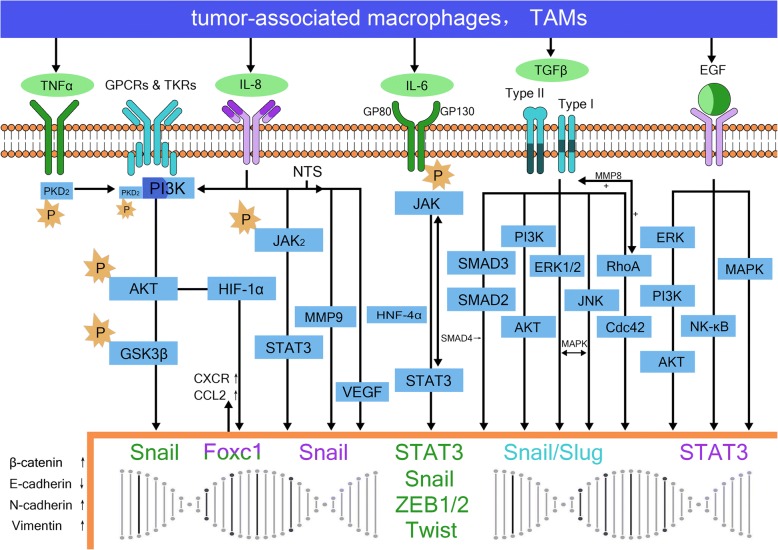
In HCC, TAMs are the major cell type promoting tumor invasion and metastasis [7, 25, 28] and their secreted inflammatory cytokines as well as other cytokines and proteases are the main mediators that promote EMT. TAMs induce EMT of tumor cells by secreting factors such as interleukelin-6 (IL-6), interleukelin-8 (IL-8), tumor necrosis factorα (TNFα), TGFβ, EGF, VEGF, matrix metalloproteinase-2 (MMP-2) and MMP-9. Additionally, these factors act synergistically to stimulate neovascularization, degrade the matrix and promote local invasion and distant metastasis of tumor cells (Fig. 1).
Fig. 1.
Tumor-associated macrophages and epithelial-to-mesenchymal transition in hepatocellular carcinoma. TNFα binds to the receptor TNFR (mainly TNFR1) to phosphorylate PKD2, which then forms a complex with PI3K. This complex stabilizes the high expression of β-catenin via the PI3K/AKT/GSK-3β pathway, upregulates Snail and Twist transcription, and participates in the process of epithelial-to-mesenchymal transition (EMT) to promote tumor invasion and metastasis. IL-8 secreted by TAMs participates in the EMT via the JAK2/STAT3/Snail pathway. It also activates FOXC1 via PI3K/AKT HIF-1α, leading to transactivation of CXC chemokine receptor (CXCR) and CC chemokine ligand 2 (CCL2), otherwise, neurotensin (NTS) and IL-8 are also activated abnormally, leading to upregulated expression of VEGF and MMP9 via the NTS/IL-8 pathway. IL-6 induces EMT by binding to the IL-6R receptor to induce STAT3 phosphorylation via the JAK/STAT3 pathway, leading to downregulated E-cadherin expression and upregulated vimentin expression. This interaction can also induce upregulation of the expression of Snail, ZEB1, ZEB2, Twist and other transcription factors to promote tumor metastasis. TGFβ secreted by TAMs modulates the expression of EMT-related genes at the epigenetic level via the classic TGF-β/TGF-β R/Smad signaling pathway. It also acts on Snail, Slug and other transcription factors via the RhoA/Cdc42, JKN/p38, Erk1/2 and PI3K/Akt pathways. EGF binds to hepatoma cell epidermal growth factor receptor (EGFR), activating downstream ERK/PI3K/AKT, ras/raf/MEK/MAPK, NF-κB and other pathways
IL-6 secreted by TAMs is an important factor involved in the occurrence and development of tumors [29, 30]. It is relatively clear that IL-6 mediates EMT mainly via the IL-6/STAT3 pathway. In this process, IL-6 binds to its receptor IL-6R, which consists of two polypeptide chains; a ligand-binding chain (GP80) and a signal conduction chain (GP130). The latter is phosphorylated following interaction with Janus kinase resulting in activation of STAT3 to form two homologous polymers that enter the nucleus to regulate transcription and promote EMT, a process that is observed in liver cancer [31]. Studies in both human samples and human HCC cell lines in vitro have shown that the IL-6/STAT3 axis comprises a variety of “circuits”, including microRNAs such as miR-24, miR-629, and miR-124, and hepatocyte nuclear factor 4α (HNF4α). In this circuit, IL-6/STAT3 activates the transcription of miRNAs, such as miR-24 and miR-629, which inhibit the activity of HNF4α. HNF4α is an important factor in the maintenance of the growth and normal biological functions of hepatocytes. When its activity is inhibited, hepatocytes enter the inflammatory state, which is exacerbated via a positive feedback mechanism resulting in a severe inflammatory microenvironment that promotes tumor invasion and metastasis (Fig. 2). That is quite similar to the “snowball effect”, with miR-124 representing the key factor in this circuit. In HepG2 and SNU-449 cells, it has been shown that miR-124 suppresses STAT3 activation, restores the function of HNF4α and terminates the further development of the inflammatory environment. Furthermore, miR-124 was shown to inhibit the tumor invasion and metastasis in a mouse model [32]. The effectiveness of this approach has also been confirmed in studies of lung adenocarcinoma, breast cancer and head and neck tumors [33]. In addition to activating the JAK/STAT3 pathway via phosphorylation of STAT3, the IL-6/IL-6R interaction leads to low E-cadherin expression and high vimentin expression as well as upregulated expression of Snail, ZEB1, ZEB2, Twist and other transcription factors that promote tumor metastasis [34–36].
Fig. 2.
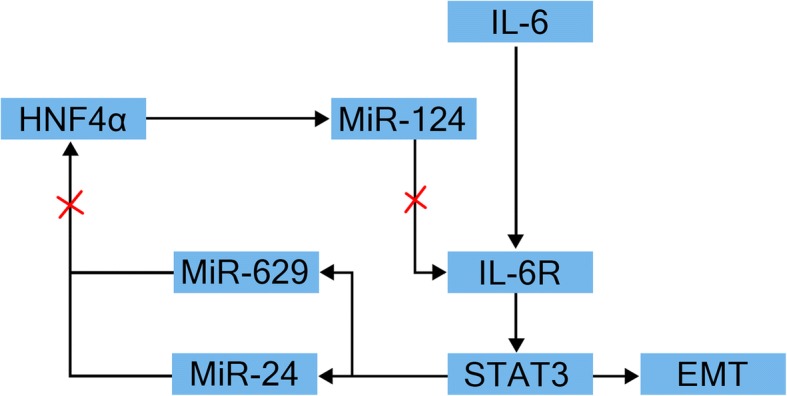
HNF4α feedback circuit in hepatocellular oncogenesis. IL-6/STAT3 activates the transcription of miRNAs, such as miR-24 and miR-629, which inhibit the activity of HNF4α. HNF4a directly regulates miR-124 expression. MiR-124 can suppresses STAT3 activation, restores the function of HNF4α. When IL-6/STAT3 pathway is activated, HNF4α activity is inhibited, miR-124 levels is reduced, hepatocytes enter the inflammatory state, which is exacerbated via a positive feedback mechanism resulting in a severe inflammatory microenvironment that promotes tumor invasion and metastasis
IL-8 is another important inflammatory factor secreted by TAMs in HCC. Its expression is associated with tumor growth and survival, as well as increased tumor invasion, migration and angiogenesis. Studies in MHCC97H and HepG2 cell lines have shown that the IL-8 secreted by TAMs participates in EMT via the JAK2/STAT3/Snail pathway [37]. Studies in both human HCC cell lines and mouse models have shown that IL-8 also activates FOXC1 via PI3K/AKT HIF-1α to promote the invasion and metastasis of HCC by trans-activation of CXC chemokine receptor (CXCR) and CC chemokine ligand 2 (CCL2) [38]. The increased IL-8 levels also lead to a higher incidence of portal vein invasion [39]. During the development of HCC, the neurotensin (NTS)/IL-8 signaling pathway is also activated abnormally, leading to increased expression of VEGF and MMP9. These factors co-mediate the process of tumor EMT to promote tumor invasion and metastasis, which has an adverse effect on prognosis [40]. Furthermore, the role of IL-8 in EMT has also been confirmed in pancreatic, breast, prostate and ovarian cancer [41–43].
TNFα is another important inflammatory factor secreted by TAMs in HCC [44]. Studies in human HCC cell lines and mouse models showed that the expression of TNFα and protein kinase D2 (PKD2) in the metastatic liver cancer tissues are significantly higher than that in normal tissues [45]. Moreover, binding of TNFα to the receptor TNFR (mainly TNFR1) on the cell membrane surface induces phosphorylation of intracellular PKD2, which then forms a complex with PI3K to stabilize the high expression of β-catenin via the PI3K/AKT/GSK-3β pathway and participate in EMT to promote tumor invasion and metastasis. The role of TNFα has also been confirmed in other tissues, including malignant tumors, such as tongue cancer, laryngeal carcinoma, cholangiocarcinoma, thyroid cancer and colorectal cancer. Nonetheless, the mechanisms underlying the influence of TNFα are varied, and include the promotion of stromal cell-derived factor-1 (SDF1) secretion in the tongue cancer, high expression of the Snail gene in cholangiocarcinoma and colorectal cancer, and upregulation of Twist transcription in laryngeal carcinoma [46–51].
The TGFβ secreted by TAMs changes the expression of EMT-related genes at the epigenetic level via the classic TGF-β/TGF-βR/Smad signaling pathway [52, 54]. In a study of HCC, Reichl et al. [53] showed that TGFβ-overexpression inhibited the Smad pathway but not the EMT process. TGFβ can also act on Snail, Slug and other transcription factors via the RhoA/Cdc42, JKN/p38, Erk1/2 and PI3K/Akt pathways to downregulate E-cadherin expression and upregulate vimentin expression and mediate EMT in tumor cells [54–56].
TAMs can also produce EGF, which binds to hepatoma cell epidermal growth factor receptor (EGFR) to activate downstream signaling pathways, including the ERK/PI3K/AKT, ras/raf/MEK/MAPK, and NF-κB pathways. As a result, EGF downregulates E-cadherin and upregulates vimentin to induce EMT by activation of STAT3 [57–59]. Similarly, it has also been confirmed that VEGF induces EMT in the highly metastatic hepatoma cell line MHCC97H [60]. Finally, members of the MMP family, including MMP-1, MMP-2, MMP-7 and MMP-14, also play an important role in the EMT process in liver cancer [61–63]. In addition, MMP-8 also mediates positive feedback regulation of TGFβ, and participates in the process of EMT via the downstream PI3K/Akt/Rac1 pathway [64].
In conclusion, TAMs are one of the most important inflammatory cell types in the inflammatory microenvironment of HCC. These cells secrete numerous inflammatory factors, which are significant in the EMT process in HCC.
Tumor-associated neutrophils (TANs) and HCC EMT
In the occurrence and development of HCC, tumor-associated neutrophils (TANs) also play an important role. Similar to TAMs, TANs also differentiate into two phenotypes; N1 and N2. The N1 phenotype inhibits tumor growth, while N2 promotes tumor growth and metastasis [65]. Specifically, N2 type TANs secrete a variety of cytokines, such as CCL2, neutrophil elastase (NE), hepatocyte growth factor (HGF), MMP9, and VEGF, which affect the growth, angiogenesis, invasion and metastasis of the tumor [66–69] (Fig. 3).
Fig. 3.
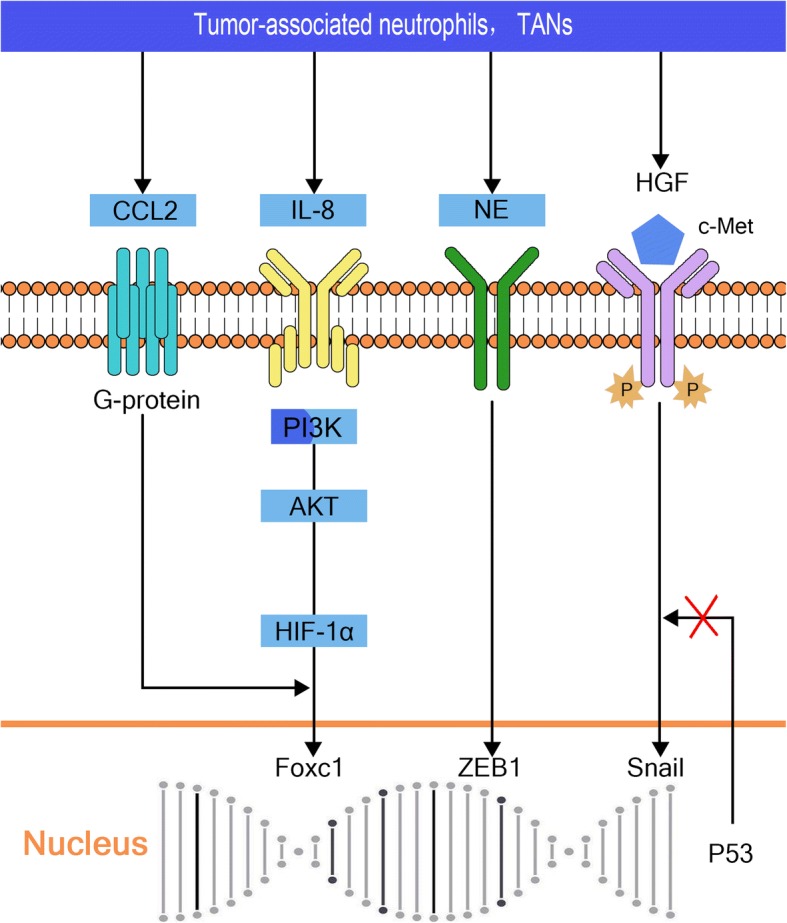
Tumor-associated neutrophils (TANs) and epithelial-to-mesenchymal transition in hepatocellular carcinoma. CCL2 secreted by TANs associates with IL-8 to promote epithelial-to-mesenchymal transition (EMT) via the PI3K/AKT HIF-1α pathway. TANs also upregulate the downstream ZEB1 transcription factors by secreting NE. In addition, HGF promotes EMT of tumor cells and increases hematogenous dissemination by binding to its receptor c-Met. In the absence of p53 gene expression, HGF/Met also mediates EMT of hepatocellular carcinoma by upregulation of Snail and other transcription factors
In a study of human HCC cell lines and mouse models, Huang et al. [38], found that TANs secrete large amounts of CCL2, which interacted with IL-8 to participate in EMT, and reduce the therapeutic effect of sorafenib [70]. Zhou et al. [71] verified these findings in a study of tissue specimens from 452 patients. CCL2, which is a member of the chemotactic factor family, is a low molecular weight protein responsible for leukocyte migration to sites of infection. Moreover, CCL2 interacts with Snail factors in EMT of pancreatic cancer cell lines, melanoma cells and colon cancer cell lines in vitro [72]. CCL2/CCR2 also cooperates with IL-6 to activate the STAT3-Twist pathway in EMT of non-small cell lung cancer [34].
NE, which is another important inflammatory mediator secreted by TANs, participates in the invasion and development of lung, ovarian and pancreatic cancer as well as the EMT [73–75]. A study of the Huh7 HCC cell line and 115 patient HCC tissue samples indicated that, during tumor progression, TANs upregulate the downstream ZEB1 transcription factors by secreting NE. It also reduces the expression of cytokeratin and E-cadherin and increases the expression of beta-catenin to mediate the EMT of hepatoma cells [75].
HGF is also one of the cytokines secreted by TANs [76]. By constructing the circulating tumor cell model of liver cancer in mice, Olorunseun et al. [77] showed that HGF promotes EMT of tumor cells and increases hematogenous dissemination by binding to its receptor c-Met. Liu et al. [78] also confirmed that in the absence of the p53 gene, HGF/Met mediates EMT of HCC by upregulation of Snail and other transcription factors. The role of HGF in promoting EMT has also been confirmed in non-small cell lung cancer, prostate cancer and others [79, 80].
The role of MMPs and VEGF in EMT has been described previously. In different tissues, these two factors are secreted by different cells, although their functions are similar.
In conclusion, the essential role of TANs in EMT of HCC is mediated by cytokines, such as NE, HGF and CCL2.
Tumor-infiltrating lymphocytes and EMT of HCC
Tumor-infiltrating lymphocytes (TILs) were first discovered and reported by the Rosenberg group in 1986 [81–83]. The level of infiltration is closely related to the prognosis of HCC. Among the TILs, Treg cells (CD4+ CD25+ FoxP3+) are most closely related to the occurrence and development of tumors. Treg cells weaken the function of CD8 + T cells and inhibit the effects of cytotoxic CD8 + T cells on malignant tumor cells, thus, promoting the development of HCC. In HCC patients, high levels of Treg cells in pre-operative circulating blood are closely related to high mortality and low survival rates. Therefore, the imbalance between Treg cells and cytotoxic T cells can be used as a prognostic factor for HCC patients [84, 85].
There are few reports of the role of Treg cells in EMT in HCC. However, in study of the breast cancer cell lines BT474 and MCF-7 [86], Treg cells were shown to activate the downstream Smad signaling pathway via the TGFβ pathway, which promoted EMT of breast cancer cells, increased the local frequency of cancer stem cell (CSC)-like cells, and enhanced their invasion and migration ability.
Inflammatory mediators in the microenvironment of HCC
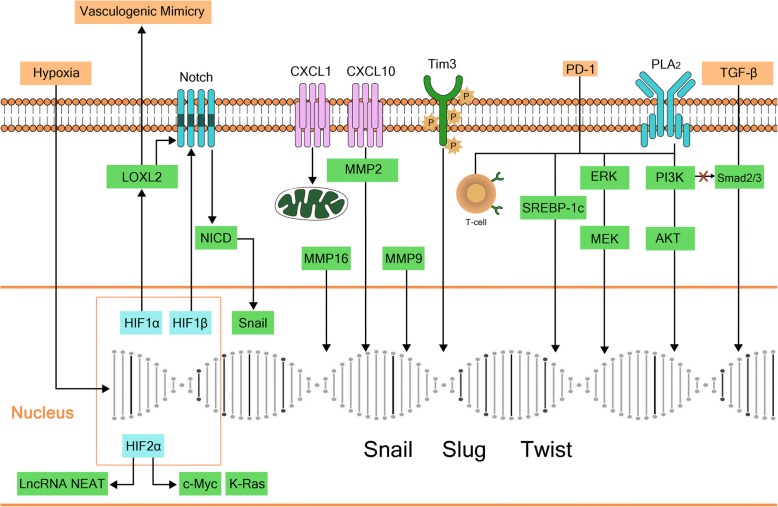
In addition to the influence of inflammatory cells on the EMT of HCC, various kinds of inflammatory factors also participate in the EMT process of HCC, either directly or indirectly (Fig. 4).
Fig. 4.
Inflammatory mediators in the microenvironment of hepatocellular carcinoma. HIF-1α promotes vasculogenic mimicry (VM) and epithelial-to-mesenchymal transition (EMT) by upregulation of LOXL2. HIF-1β downregulates E-cadherin expression via the Notch signaling pathway, and interacts with numerous oncogene-encoded proteins including epidermal growth factor receptor (EGFR), c-Myc, K-Ras, even some lncRNAs, such as NEAT1, to promote EMT. CXCL1 participates in tumor promotion by stimulating mitochondrial metabolism and activating EMT. CXCL10 upregulates MMP-2 expression to participate in EMT; MMP-16 and MMP-9 are also key factors. cPLA2 plays an opposing role in TGF-β-induced signaling pathways by inhibiting Smad2/3 phosphorylation and promoting activation of PI3K/AKT/ERK signaling pathways to mediate EMT. PD-1/PD-L1 induces EMT via the PI3K/AKT and ERK/MEK signaling pathways, and upregulation of SREBP-1c
Hypoxia inducible factor (HIFs), which are also involved in tumor inflammation, enhance the metabolic activity of the tissue by causing infiltration of inflammatory cells and inflammatory reactions. The resulting increase in inflammation and the associated inflammatory reaction leads to the increased demand for oxygen. The inflammatory factors also cause vasoconstriction, which further reduces oxygen levels in the inflammatory environment. As a result, high levels of HIFs are generated in the hypoxic microenvironment [87].
HIF-1 (HIF-1α and HIF-1β) is the most common HIF expressed during the development of HCC, which is associated with long-term chronic inflammation. Studies have shown that HIF-1α in the inflammatory microenvironment of HCC promotes vasculogenic mimicry (VM) and the occurrence of EMT by upregulation of LOXL2 [88]. HIF-1β is involved in the EMT process by downregulation of E-cadherin expression via the Notch signaling pathway [89]. HIF-2α interacts with many oncogene-encoded proteins, including EGFR, c-Myc and K-Ras, which participate in the tumor development. HIF-2α also promotes EMT in HCC through upregulation of the lncRNA NEAT1 [90, 91].
Similar to the previously mentioned CC chemokine family, the CXC chemokine family, especially CXCL1 and CXCL10, also plays an important role EMT in HCC. CXCL1 promotes tumorigenesis by stimulating mitochondrial metabolism and activating the EMT process [92]. CXCL10 is involved in EMT by upregulating MMP-2 expression [93], and similarly, MMP-16 and other MMPs are also key factors in EMT [94].
T cell immunoglobulin mucin-3 (Tim3) is a specific target for activating T cells in inflammatory responses [95]. In the SMMC-7721 cell line, Tim-3 overexpression upregulated the expression of Snail, Slug, Twist 1, MMP-9 and other transcription factors and enhanced the EMT process compared with that observed in the control group [96].
cPLA2 is one a member of the phospholipase family, the main physiological function of which is to reconstruct the phospholipid structure and to promote the autogenous removal of necrotic tissue. Inflammation can be mediated by COX-1 (cyclooxygenase − 1), releases arachidonic acid through oxidation and peroxide, and leads to the biosynthesis of prostaglandins, especially prostacyclin, which induce inflammation and pain [97]. Using a xenograft tumor transplant model, Fu et al. [98] showed that cPLA2 can play an opposing role in TGF-β-induced signaling pathways by inhibiting Smad2/3 phosphorylation and promoting the activation of PI3K/AKT/ERK pathways to mediate EMT of HCC.
Programmed cell death receptor-1 (PD-L1) is a transmembrane receptor present on T cells. It was first identified in an apoptotic T cell hybridoma and named based on its involvement in apoptosis [99]. Although PD-1/PD-L1 is not an inflammatory factor, it is widely expressed in the liver tissues of patients with chronic HBV infection and even liver cancer [100, 101]. Additionally, in patients with a more aggressive HCC and shorter survival, Critella et al. [102] found a markedly immunosuppressed microenvironment (as shown by the local upregulation of both PD-1 and PD-L1) against a background of higher systemic inflammation, with a distinct switch toward EMT and extremely poor differentiation at the histological level compared with the conditions detected in patients with a less aggressive disease and longer survival. However, the specific mechanisms were not investigated. In other studies of the relationship between PD-1/PD-L1 and EMT, Alsuliman et al. [103] showed that PD-L1 induced EMT in tumor cell lines via the PI3K/AKT and ERK/MEK pathways in breast cancer and that the involvement of the PI3K/AKT pathway was more important in this process. Wang et al. [104] found that PD-L1 induced EMT and enhanced RCC cell cancer stemness through upregulation of SREBP-1c in the renal cell carcinoma (RCC) cancer cell lines, 769P and ACHN. Furthermore, the relationship between PD-L1 and EMT has also been recently demonstrated in head and neck squamous cell carcinoma, esophageal cancer, and pulmonary adenocarcinoma [105–107]. PD-1/PD-L1 also plays important roles in regulating T cell proliferation and differentiation and maintaining autoimmune tolerance, as well as the development of tumor immune escape and chronic infection [108].
Virus-related products in the inflammatory tumor microenvironment
The occurrence and development of liver cancer is closely related to hepatitis virus infection, especially HBV and HCV. The products of viral expression are important factors that affect the development, invasion and metastasis of liver cancer (Fig. 5).
Fig. 5.
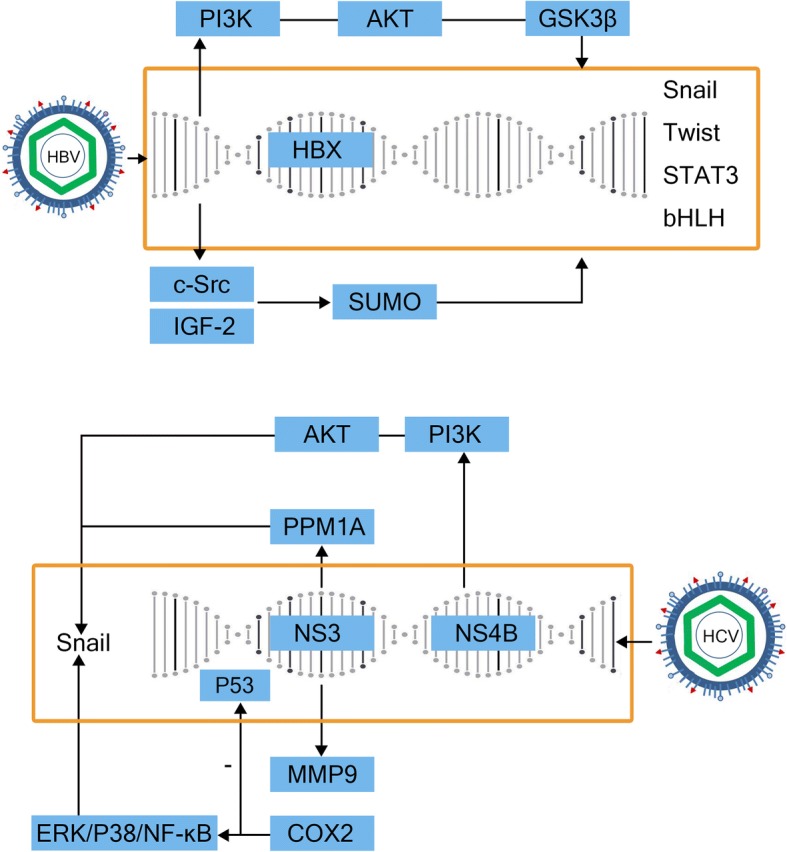
Virus-related products in the inflammatory tumor microenvironment. HBx stabilizes the transcription of Snail in the PI3K/AKT/GSK-3b pathway to mediate epithelial-to-mesenchymal transition (EMT). It also participates in the EMT process by inducing upregulation of Twist expression and activation of STAT3 transcription. In addition, HBx activates c-Src and mediates the expression of IGF2 in the SUMO pathway, or directly upregulates the expression of bHLH transcription factor E12/E47, which inhibits E-cadherin expression and induce EMT. NS3 promotes EMT by downregulating PPM1A through ubiquitination. It also enhances cancer cell invasion by activating matrix metalloproteinase-9 (MMP-9) and cyclooxygenase-2 (COX-2) via the ERK/p38/NF-κB signaling cascade, and interacts with p53 to inhibit p53-dependent transcription. NS4B upregulates the Snail transcription factor via the PI3K/AKT signaling pathway and induce EMT
In China and Africa, most patients with HCC are infected with HBV. The HBV genome mainly includes four overlapping open reading frames (ORFs): S, C, P and X. The S ORF is divided into PreS1, PreS2 and S, which are translated predominantly into virus surface antigen or virus envelope protein. The C ORF contains two intra-frame codons, encoding HBV core protein (HBc) and HBV e antigen (HBe), respectively. The P ORF encodes a DNA polymerase protein, which also has reverse transcriptase activity and is responsible for replication of the HBV genomic DNA. The X ORF encodes the X protein, which is considered to be the key factor in the occurrence and progression of liver cancer. It has a wide range of non-specific transactivation effects and functions. In the nucleus, HBx cannot directly bind the double-stranded DNA, but it can combine with the transcription factors through protein-protein interactions. HBx also mediates the formation of the transcriptional initiation complex and participates in the EMT process of HCC [109]. In studies of the Huh-7 and SMMC7721 cell lines, Liu and Lu et al. [110, 111] showed that HBx stabilizes the transcription of Snail, including its superfamily member Snail1, to mediate EMT via the PI3K/AKT/GSK-3β signaling pathway. Teng et al. [112] also showed that HBx is involved in the upregulation of Twist expression and activation of STAT3 transcription leading to EMT in the MHCC97H and HL-7702 cell lines. In addition, HBx has been shown to activate c-Src (a non-receptor tyrosine kinase) to induce the expression of insulin-like growth factor 2 (IGF2), and reduce E-cadherin expression via the small ubiquitin like small ubiquitin-related modifier (SUMO) pathway to induce EMT in the SMMC-7721 hepatoma cell line [113, 114]. A study of the HepG2 and HUH-7 cell lines also suggested that HBx directly upregulates expression of the bHLH transcription factor E12/E47, inhibits E-cadherin expression, and induces the process of EMT [115, 116]. In contrast, Wang et al. [117] demonstrated that EMT was suppressed in the HepG2.2.15 cell line in the presence of high levels of HBV virus replication. However, the underlying mechanism is still unclear, and moreover, the effect of virus on EMT in liver cancer may not be dependent only on HBx levels, an issue which requires further investigation.
In Europe, America and Japan, HCV infection is the main cause of hepatitis infection. The HCV genome includes 5′-untranslated region, an ORF encoding 3011 amino acids and a 3′-untranslated region. The ORF encodes a large precursor protein, which can be processed to form 10 proteins (structural proteins, core, E1, E2 and P7, and non-structural protein, NS2–5) [118]. Accumulating experimental evidence suggests that HCV contributes to HCC by directly modulating signaling pathways that promote the malignant transformation of hepatocytes [119]. Among the HCV-encoded proteins, the core proteins NS3, NS4B, and NS5A have received much attention, since all of them possess cell-transforming potential by interacting with a number of host factors and signaling pathways when expressed in cell culture or transgenic animal models [120]. Zhou et al. [121] found that NS3 promotes EMT in the Huh-7 and Huh-7.5.1 cell lines by inducing the decomposition and downregulation of protein phosphatase 1A (PPM1A) through ubiquitination. In addition, Lu et al. [122] suggested that in the HepG2 and Huh7.5.1 cell lines, NS3 also enhances cancer cell invasion by activating MMP-9 and COX-2 via the ERK/p38/NF-κB signal cascade, and interacts with p53 to inhibit p53-dependent transcription [123]. In the same way, NS4B also increases expression of the Snail transcription factor via the PI3K/AKT pathway and induces EMT in liver cancer [124].
Conclusions
The importance of EMT in the invasion and metastasis of HCC has gradually been clarified. There is no doubt that the inflammatory microenvironment formed by the inflammation associated with hepatitis virus infection is an important factor affecting the invasion and metastasis of liver cancer. The virus not only participates in the process of liver inflammation, but also directly promotes the development of the tumor by combining with the host genome and encoding proteins. However, numerous transcriptional factors are involved in EMT, and many pathways are activated by inflammatory factors. The cytokines involved in the inflammatory microenvironment are also complex. Although knockout or overexpression of relevant genes has been shown to block tumor invasiveness metastasis in cells and small animal models, this strategy is still far from clinical application. On the one hand, despite blocking an individual pathway or inhibiting a single gene, the upstream factors still have many other mechanisms through which to continue to promote tumor progression. On the other hand, the level of gene suppression that can be achieved in cells and small animal models is difficult to apply to large animals or even in the clinic. Furthermore, the cost and prolonged timeframe of research on targeted inhibitory drugs for the identified gene will delay confirmation of the actual clinical effect of the gene modification.
Therefore, the future direction of research will involve investigation of potential commonality among different inflammatory factors in promoting the EMT of HCC, as well as strategies to modulate the tumor microenvironment or block the expression of inflammatory factors and signaling pathways to inhibit EMT. We anticipate that control of hepatitis will play a decisive role in the treatment of inflammatory-related tumors like HCC. Similar to TNF, TGF, EGF, IL-8, PLA2 and other inflammatory factors, the virus-encoded proteins HBx and NS3, NS4B also signal via classic conduction pathways such as PI3K/AKT/GSK3β and ERK/NF-κB. This may account for the interaction of viral gene-coding proteins and inflammatory factors produced in the microenvironment. Elimination of viral infection and control of inflammatory responses may be an important approach to inhibiting tumor progression, invasion and metastasis in the future.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- CCL2
CC chemokine ligand 2
- CSCs
Cancer stem cells
- CXCR
CXC chemokine receptor
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-to-mesenchymal transition
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- HIFs
Hypoxia inducible factor
- HNF4α
Hepatocyte nuclear factor 4α
- IL-6
Interleukelin-6
- IL-8
Interleukelin-8
- MET
Mesenchymal-epithelial transition
- MMP-2
Matrix metalloproteinase-2
- MMP-9
Matrix metalloproteinase-9
- NTS
Neurotensin
- PDGF
Platelet-derived growth factor
- PKD2
Protein kinase D2
- SDF1
Stromal cell-derived factor-1
- TAMs
Tumor related macrophages
- TANs
Tumor Related Neutrophils
- TGF-β
Transforming growth factor factor
- Tim3
T cell immunoglobulin mucin-3
- TNFα
Tumor necrosis factorα
- VEGF
Vascular endothelial growth factor
- VM
Vasculogenic mimicry
- COX-1
(cyclooxygenase − 1)
- PD-1
Programmed cell death receptor-1
- HBc
HBV core protein
- HBe
HBV e antigen
- IGF2
Insulin-like growth factor 2
- SUMO
Small ubiquitin-related modifier
- ORF
Open reading frame
- PPM1A
Protein phosphatase 1A
- MMP-9
Matrix metalloproteinase-9
- COX-2
Cyclooxygenase-2
Authors’ contributions
LY searched the literature and drafted and revised the manuscript. FX and CD revised and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Long Yan, Phone: 15382088876, Email: snihyaami@sina.com.
Feng Xu, Phone: 18940255203, Email: xufengsjh@126.com.
Chao-liu Dai, Phone: 18940251697, Email: 384267542@qq.com.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma? J Gastroenterol Hepatol. 2011;26(2):221–227. doi: 10.1111/j.1440-1746.2010.06576.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Liang HW, Wang N, Wang Y, Wang F, Fu Z, Yan X, Zhu H, Diao W, Ding Y, Chen X, et al. Hepatitis B virus-human chimeric transcript HBx-LINE1 promotes hepatic injury via sequestering cellular microRNA-122. J Hepatol. 2016;64(2):278–291. doi: 10.1016/j.jhep.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Beach JR, Hussey GS, Miller TE, Chaudhury A, Patel P, Monslow J, Zheng Q, Keri RA, Reizes O, Bresnick AR, et al. Myosin II isoform switching mediates invasiveness after TGF-beta-induced epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(44):17991–17996. doi: 10.1073/pnas.1106499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9(12):1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 7.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35(4):467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89(1):31–39. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 10.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1–2):195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 11.Xiang X, Zhuang X, Ju S, Zhang S, Jiang H, Mu J, Zhang L, Miller D, Grizzle W, Zhang HG. miR-155 promotes macroscopic tumor formation yet inhibits tumor dissemination from mammary fat pads to the lung by preventing EMT. ONCOGENE. 2011;30(31):3440–3453. doi: 10.1038/onc.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352(2):160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. CELL. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 15.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125(Pt 19):4417–4422. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- 17.David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, Iacobuzio-Donahue CA, Massague J. TGF-beta tumor suppression through a lethal EMT. Cell. 2016;164(5):1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, Zhang J, Liu Q, Tan G, Feng D, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363(2):137–145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT. JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 20.Bao RF, Shu YJ, Hu YP, Wang XA, Zhang F, Liang HB, Ye YY, Li HF, Xiang SS, Weng H, et al. miR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and Smad pathways in gallbladder carcinoma. Oncotarget. 2016;7(16):22339–22354. doi: 10.18632/oncotarget.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SC, Chai DS, Chen CB, Wang ZY, Wang L. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/AKT signaling pathway. Biomed Pharmacother. 2015;75:33–39. doi: 10.1016/j.biopha.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, Chen DY, Fang R, Liu H, Cai SH, et al. Snail regulated by PKC/GSK-3beta pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358(2):491–502. doi: 10.1007/s00441-014-1953-2. [DOI] [PubMed] [Google Scholar]
- 23.Bastiaans J, van Meurs JC, van Holten-Neelen C, Nagtzaam NM, van Hagen PM, Chambers RC, Hooijkaas H, Dik WA. Thrombin induces epithelial-mesenchymal transition and collagen production by retinal pigment epithelial cells via autocrine PDGF-receptor signaling. Invest Ophthalmol Vis Sci. 2013;54(13):8306–8314. doi: 10.1167/iovs.13-12383. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 27.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 28.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W, Zhang L. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2015;34:116. doi: 10.1186/s13046-015-0229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, Karin M, Struhl K, Hadzopoulou-Cladaras M, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147(6):1233–1247. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Cheng X, Wang Y, Han R, Li L, Xiang T, He L, Long H, Zhu B, He Y. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS One. 2014;9(4):e95884. doi: 10.1371/journal.pone.0095884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Gao Q, Han S, Pan F, Fan W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-twist signaling. Tumour Biol. 2015;36(2):973–981. doi: 10.1007/s13277-014-2717-z. [DOI] [PubMed] [Google Scholar]
- 35.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9(12):1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/snail pathway. Int J Oncol. 2015;46(2):587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, Wu K, Xia L. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149(4):1053–1067. doi: 10.1053/j.gastro.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 39.Akiba J, Yano H, Ogasawara S, Higaki K, Kojiro M. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int J Oncol. 2001;18(2):257–264. doi: 10.3892/ijo.18.2.257. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Ren X, Chen Y, Liu P, Wei X, Li H, Ying G, Chen K, Winkler H, Hao X. Dysfunctional activation of neurotensin/IL-8 pathway in hepatocellular carcinoma is associated with increased inflammatory response in microenvironment, more epithelial mesenchymal transition in cancer and worse prognosis in patients. PLoS One. 2013;8(2):e56069. doi: 10.1371/journal.pone.0056069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, Dang W, Tang H, Huang Y, Wei L, et al. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16(8):1220–1230. doi: 10.1080/15384047.2015.1056409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71(15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J, Zeng F, Wu N, Kang K, Yang Z, Yang H. Interleukin-8 promotes human ovarian cancer cell migration by epithelial-mesenchymal transition induction in vitro. Clin Transl Oncol. 2015;17(5):365–370. doi: 10.1007/s12094-014-1240-4. [DOI] [PubMed] [Google Scholar]
- 44.Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015;6:e1942. doi: 10.1038/cddis.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Cheng Y, Guo Y, Chen J, Chen F, Luo R, Li A. Protein kinase D2 contributes to TNF-alpha-induced epithelial mesenchymal transition and invasion via the PI3K/GSK-3beta/beta-catenin pathway in hepatocellular carcinoma. Oncotarget. 2016;7(5):5327–5341. doi: 10.18632/oncotarget.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou B, Zhuang XM, Wang YY, Lin ZY, Zhang DM, Fan S, Li JS, Chen WL. Tumor necrosis factor alpha induces myofibroblast differentiation in human tongue cancer and promotes invasiveness and angiogenesis via secretion of stromal cell-derived factor-1. Oral Oncol. 2015;51(12):1095–1102. doi: 10.1016/j.oraloncology.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Techasen A, Namwat N, Loilome W, Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H, Yongvanit P. Tumor necrosis factor-alpha (TNF-alpha) stimulates the epithelial-mesenchymal transition regulator snail in cholangiocarcinoma. Med Oncol. 2012;29(5):3083–3091. doi: 10.1007/s12032-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 48.Lv N, Gao Y, Guan H, Wu D, Ding S, Teng W, Shan Z. Inflammatory mediators, tumor necrosis factor-alpha and interferon-gamma, induce EMT in human PTC cell lines. Oncol Lett. 2015;10(4):2591–2597. doi: 10.3892/ol.2015.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L, Mu Y, Sa N, Wang H, Xu W. Tumor necrosis factor alpha induces epithelial-mesenchymal transition and promotes metastasis via NF-kappaB signaling pathway-mediated TWIST expression in hypopharyngeal cancer. Oncol Rep. 2014;31(1):321–327. doi: 10.3892/or.2013.2841. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8(2):e56664. doi: 10.1371/journal.pone.0056664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. CELL. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 52.Papageorgis P, Lambert AW, Ozturk S, Gao F, Pan H, Manne U, Alekseyev YO, Thiagalingam A, Abdolmaleky HM, Lenburg M, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010;70(3):968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichl P, Haider C, Grubinger M, Mikulits W. TGF-beta in epithelial to mesenchymal transition and metastasis of liver carcinoma. Curr Pharm Des. 2012;18(27):4135–4147. doi: 10.2174/138161212802430477. [DOI] [PubMed] [Google Scholar]
- 54.Steinway SN, Zanudo JG, Ding W, Rountree CB, Feith DJ, Loughran TJ, Albert R. Network modeling of TGFbeta signaling in hepatocellular carcinoma epithelial-to-mesenchymal transition reveals joint sonic hedgehog and Wnt pathway activation. Cancer Res. 2014;74(21):5963–5977. doi: 10.1158/0008-5472.CAN-14-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, Factor V, Heuchel R, Mercurio F, Thorgeirsson SS, et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22(3):412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- 56.Moon H, Ju HL, Chung SI, Cho KJ, Eun JW, Nam SW, Han KH, Calvisi DF, Ro SW. Transforming growth factor-beta promotes liver tumorigenesis in mice via up-regulation of snail. Gastroenterology. 2017;153(5):1378–1391. doi: 10.1053/j.gastro.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Lin CY, Lin CJ, Chen KH, Wu JC, Huang SH, Wang SM. Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett. 2006;580(13):3042–3050. doi: 10.1016/j.febslet.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 58.Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2014;18(2):218–230. doi: 10.1111/jcmm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang CH, Guo FL, Xu GL, Jia WD, Ge YS. STAT3 activation mediates epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. 2014;61(132):1082–9. [PubMed]
- 60.Ai JH, Zheng SG, Zhang LD, Jiang P, Dong JH. Vascular endothelial growth factor receptor-1 activation induces epithelial to mesenchmal transition in hepatocellular carcinoma cell line MHCC97-H. Zhonghua Gan Zang Bing Za Zhi. 2009;17(2):112–116. [PubMed] [Google Scholar]
- 61.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatogastroenterology. 2009;50(5):1464–74. [DOI] [PubMed]
- 62.Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong XY, Gu Q, Yao Z, Sun BC. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med. 2011;15(3):691–700. doi: 10.1111/j.1582-4934.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90(6):1265–1273. doi: 10.1038/sj.bjc.6601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin G, Luo M, Chen J, Dang Y, Chen G, Li L, Zeng J, Lu Y, Yang J. Reciprocal activation between MMP-8 and TGF-beta1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 2016;374(1):85–95. doi: 10.1016/j.canlet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Allavena P, Sica A, Garlanda C, Mantovani A. The yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 66.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9(6):e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, Antoine M, Soler P, Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63(6):1405–1412. [PubMed] [Google Scholar]
- 68.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu J, Lin H, Li G, Sun Y, Shi L, Ma W, Chen J, Cai X, Chang C. Sorafenib with ASC-J9® synergistically suppresses the HCC progression via altering the pSTAT3-CCL2/Bcl2 signals. Int J Cancer. 2017;140(3):705–717. doi: 10.1002/ijc.30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to Sorafenib. Gastroenterology. 2016;150(7):1646–1658. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 72.Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis. 2013;30(4):393–405. doi: 10.1007/s10585-012-9545-6. [DOI] [PubMed] [Google Scholar]
- 73.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayer C, Darb-Esfahani S, Meyer AS, Hubner K, Rom J, Sohn C, Braicu I, Sehouli J, Hansch GM, Gaida MM. Neutrophil granulocytes in ovarian Cancer - induction of epithelial-to-mesenchymal-transition and tumor cell migration. J Cancer. 2016;7(5):546–554. doi: 10.7150/jca.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hansch GM, Gaida MM. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012;2012:720768. doi: 10.1155/2012/720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hurt B, Schulick R, Edil B, El KK, Barnett CJ. Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surg. 2017;214(5):938–944. doi: 10.1016/j.amjsurg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Ogunwobi OO, Puszyk W, Dong HJ, Liu C. Epigenetic upregulation of HGF and c-met drives metastasis in hepatocellular carcinoma. PLoS One. 2013;8(5):e63765. doi: 10.1371/journal.pone.0063765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu WT, Jing YY, Yu GF, Chen H, Han ZP, Yu DD, Fan QM, Ye F, Li R, Gao L, et al. Hepatic stellate cell promoted hepatoma cell invasion via the HGF/c-met signaling pathway regulated by p53. Cell Cycle. 2016;15(7):886–894. doi: 10.1080/15384101.2016.1152428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu F, Song S, Yi Z, Zhang M, Li J, Yang F, Yin H, Yu X, Guan C, Liu Y, et al. HGF induces EMT in non-small-cell lung cancer through the hBVR pathway. Eur J Pharmacol. 2017;811:180–190. doi: 10.1016/j.ejphar.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 80.Zhou D, Zhang Y, Xu L, Zhou Z, Huang J, Chen M. A monocyte/granulocyte to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Sci Rep. 2015;5:15263. doi: 10.1038/srep15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu HJ, Lin XL, Liu MH, Fan XJ, Zou WW. Curcumin mediates reversion of HGF-induced epithelial-mesenchymal transition via inhibition of c-met expression in DU145 cells. Oncol Lett. 2016;11(2):1499–1505. doi: 10.3892/ol.2015.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 84.Kayadibi H, Sertoglu E, Uyanik M, Tapan S. Neutrophil-lymphocyte ratio is useful for the prognosis of patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20(28):9631–9632. doi: 10.3748/wjg.v20.i28.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, Gao YT, DU Z. Tumor-infiltrating FoxP3+ Tregs are associated with CD34 expression and prognosis of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2012;20(1):25–29. doi: 10.3760/cma.j.issn.1007-3418.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Yu X, Li H, Ren X. Interaction between regulatory T cells and cancer stem cells. Int J Cancer. 2012;131(7):1491–1498. doi: 10.1002/ijc.27634. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Liu Y, Yan X, Xu Y, Luo F, Ye J, Yan H, Yang X, Huang X, Zhang J, et al. HIFs enhance the migratory and neoplastic capacities of hepatocellular carcinoma cells by promoting EMT. Tumour Biol. 2014;35(8):8103–8114. doi: 10.1007/s13277-014-2056-0. [DOI] [PubMed] [Google Scholar]
- 88.Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, Zhang Y, Dong X, Sun B. HIF-1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60. doi: 10.1186/s13046-017-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu JN, Jiang L, Jiang JH, Yang X, Li XY, Zeng JX, Shi RY, Shi Y, Pan XR, Han ZP, et al. Hepatocyte nuclear factor-1beta enhances the stemness of hepatocellular carcinoma cells through activation of the notch pathway. Sci Rep. 2017;7(1):4793. doi: 10.1038/s41598-017-04116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Dong J, Jia L, Zhao T, Lang M, Li Z, Lan C, Li X, Hao J, Wang H, et al. HIF-2-dependent expression of stem cell factor promotes metastasis in hepatocellular carcinoma. Cancer Lett. 2017;393:113–124. doi: 10.1016/j.canlet.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 91.Zheng X, Zhang Y, Liu Y, Fang L, Li L, Sun J, Pan Z, Xin W, Huang P. HIF-2alpha activated lncRNA NEAT1 promotes hepatocellular carcinoma cell invasion and metastasis by affecting the epithelial-mesenchymal transition. J Cell Biochem. 2017; [DOI] [PubMed]
- 92.Ren T, Zhu L, Cheng M. CXCL10 accelerates EMT and metastasis by MMP-2 in hepatocellular carcinoma. Am J Transl Res. 2017;9(6):2824–2837. [PMC free article] [PubMed] [Google Scholar]
- 93.Cui X, Li Z, Gao J, Gao PJ, Ni YB, Zhu JY. Elevated CXCL1 increases hepatocellular carcinoma aggressiveness and is inhibited by miRNA-200a. Oncotarget. 2016;7(40):65052–65066. doi: 10.18632/oncotarget.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng X, Zhang Y, Liu Y, Fang L, Li L, Sun J, Pan Z, Xin W, Huang P. HIF-2alpha activated lncRNA NEAT1 promotes hepatocellular carcinoma cell invasion and metastasis by affecting the epithelialmesenchymaltransition. J Cell Biochem. 2017;119:3247-3256. [DOI] [PubMed]
- 95.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2(5):393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 96.Lin H, Yang B, Teng M. T-cell immunoglobulin mucin-3 as a potential inducer of the epithelial-mesenchymal transition in hepatocellular carcinoma. Oncol Lett. 2017;14(5):5899–5905. doi: 10.3892/ol.2017.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnwal B, Kini RM. Characterization of inflamin, the first member of a new family of snake venom proteins that induces inflammation. Biochem J. 2013;455(2):239–250. doi: 10.1042/BJ20130599. [DOI] [PubMed] [Google Scholar]
- 98.Fu H, He Y, Qi L, Chen L, Luo Y, Chen L, Li Y, Zhang N, Guo H. cPLA2alpha activates PI3K/AKT and inhibits Smad2/3 during epithelial-mesenchymal transition of hepatocellular carcinoma cells. Cancer Lett. 2017;403:260–270. doi: 10.1016/j.canlet.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 99.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci U S A. 2013;110(37):15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han Y, Li J, Jiang L, Xu Q, Liu B, Jin K, Liu Y, Huang Z. Regulation of B7-H1 expression on peripheral monocytes and IFN-gamma secretion in T lymphocytes by HBeAg. Cell Immunol. 2013;283(1–2):25–30. doi: 10.1016/j.cellimm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Critelli R, Milosa F, Faillaci F, Condello R, Turola E, Marzi L, Lei B, Dituri F, Andreani S, Sighinolfi P, et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 2017;8(8):e3017. doi: 10.1038/cddis.2017.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, Al-Alwan M, Ghebeh H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Wang H, Zhao Q, Xia Y, Hu X, Guo J. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol. 2015;32(8):212. doi: 10.1007/s12032-015-0655-2. [DOI] [PubMed] [Google Scholar]
- 105.Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim JH, Jeon YK, Lee JS, Kwon SK, Hah JH, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. ONCOTARGET. 2016;7(13):15901–15914. doi: 10.18632/oncotarget.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L, Xiong Y, Li J, Zheng X, Zhou Q, Turner A, Wu C, Lu B, Jiang J. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal Cancer. Cell Physiol Biochem. 2017;42(6):2267–2280. doi: 10.1159/000480000. [DOI] [PubMed] [Google Scholar]
- 107.Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, Jeon YK, Chung DH. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016;58:7–14. doi: 10.1016/j.humpath.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Bu X, Mahoney KM, Freeman GJ. Learning from PD-1 resistance: new combination strategies. Trends Mol Med. 2016;22(6):448–451. doi: 10.1016/j.molmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 110.Liu H, Xu L, He H, Zhu Y, Liu J, Wang S, Chen L, Wu Q, Xu J, Gu J. Hepatitis B virus X protein promotes hepatoma cell invasion and metastasis by stabilizing snail protein. Cancer Sci. 2012;103(12):2072–2081. doi: 10.1111/cas.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu Q, Yan J, Xiong Y, Cai L, Li XW, Dong JH. Hepatitis B virus X protein upregulates the transcription activity of zinc finger transcription factor SNAI1. Zhonghua Yi Xue Za Zhi. 2009;89(14):990–993. [PubMed] [Google Scholar]
- 112.Teng J, Wang X, Xu Z, Tang N. HBx-dependent activation of twist mediates STAT3 control of epithelium-mesenchymal transition of liver cells. J Cell Biochem. 2013;114(5):1097–1104. doi: 10.1002/jcb.24450. [DOI] [PubMed] [Google Scholar]
- 113.Yang SZ, Zhang LD, Zhang Y, Xiong Y, Zhang YJ, Li HL, Li XW, Dong JH. HBx protein induces EMT through c-Src activation in SMMC-7721 hepatoma cell line. Biochem Biophys Res Commun. 2009;382(3):555–560. doi: 10.1016/j.bbrc.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 114.Ha HL, Kwon T, Bak IS, Erikson RL, Kim BY, Yu DY. IGF-II induced by hepatitis B virus X protein regulates EMT via SUMO mediated loss of E-cadherin in mice. Oncotarget. 2016;7(35):56944–56957. doi: 10.18632/oncotarget.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shin KS, Yeom S, Kwak J, Ahn HJ, Lib JK. Hepatitis B virus X protein induces epithelial-mesenchymal transition by repressing E-cadherin expression via upregulation of E12/E47. J Gen Virol. 2016;97(1):134–143. doi: 10.1099/jgv.0.000324. [DOI] [PubMed] [Google Scholar]
- 116.Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276(29):27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- 117.Wang T, Jin Y, Zhao R, Wu Y, Zhang Y, Wu D, Kong D, Jin X, Zhang F. High load hepatitis B virus replication inhibits hepatocellular carcinoma cell metastasis through regulation of epithelial-mesenchymal transition. Int J Infect Dis. 2014;20:37–41. doi: 10.1016/j.ijid.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 118.Kang SM, Choi SH, Park CY, Kim MH, Kim TK, Park JM, Koh MS, Kang HJ, Hwang SB. Monoclonal antibody recognizing N-terminal epitope of hepatitis C virus nonstructural 5B inhibits viral RNA replication. J Viral Hepat. 2008;15(4):305–313. doi: 10.1111/j.1365-2893.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 119.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25(27):3834–3847. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- 120.Lai VC, Zhong W, Skelton A, Ingravallo P, Vassilev V, Donis RO, Hong Z, Lau JY. Generation and characterization of a hepatitis C virus NS3 protease-dependent bovine viral diarrhea virus. J Virol. 2000;74(14):6339–6347. doi: 10.1128/JVI.74.14.6339-6347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y, Zhao Y, Gao Y, Hu W, Qu Y, Lou N, Zhu Y, Zhang X, Yang H. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J Exp Clin Cancer Res. 2017;36(1):42. doi: 10.1186/s13046-017-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu L, Zhang Q, Wu K, Chen X, Zheng Y, Zhu C, Wu J. Hepatitis C virus NS3 protein enhances cancer cell invasion by activating matrix metalloproteinase-9 and cyclooxygenase-2 through ERK/p38/NF-kappaB signal cascade. Cancer Lett. 2015;356(2 Pt B):470–478. doi: 10.1016/j.canlet.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 123.Hu B, Xie S, Hu Y, Chen W, Chen X, Zheng Y, Wu X. Hepatitis C virus NS4B protein induces epithelial-mesenchymal transition by upregulation of snail. Virol J. 2017;14(1):83. doi: 10.1186/s12985-017-0737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deng L, Nagano-Fujii M, Tanaka M, Nomura-Takigawa Y, Ikeda M, Kato N, Sada K, Hotta H. NS3 protein of hepatitis C virus associates with the tumour suppressor p53 and inhibits its function in an NS3 sequence-dependent manner. J Gen Virol. 2006;87(Pt 6):1703–1713. doi: 10.1099/vir.0.81735-0. [DOI] [PubMed] [Google Scholar]