Abstract
The study of the telomeric complex in oogenesis and early development is important for understanding the mechanisms which maintain genome integrity. Telomeric transcripts are the key components of the telomeric complex and are essential for regulation of telomere function. We study the biogenesis of transcripts generated by the major Drosophila telomere repeat HeT-A in oogenesis and early development with disrupted telomeric repeat silencing. In wild type ovaries, HeT-A expression is downregulated by the Piwi-interacting RNAs (piRNAs). By repressing piRNA pathway, we show that overexpressed HeT-A transcripts interact with their product, RNA-binding protein Gag-HeT-A, forming ribonucleoprotein particles (RNPs) during oogenesis and early embryonic development. Moreover, during early stages of oogenesis, in the nuclei of dividing cystoblasts, HeT-A RNP form spherical structures, which supposedly represent the retrotransposition complexes participating in telomere elongation. During the later stages of oogenesis, abundant HeT-A RNP are detected in the cytoplasm and nuclei of the nurse cells, as well as in the cytoplasm of the oocyte. Further on, we demonstrate that HeT-A products co-localize with the transporter protein Egalitarian (Egl) both in wild type ovaries and upon piRNA loss. This finding suggests a role of Egl in the transportation of the HeT-A RNP to the oocyte using a dynein motor. Following germline piRNA depletion, abundant maternal HeT-A RNP interacts with Egl resulting in ectopic accumulation of Egl close to the centrosomes during the syncytial stage of embryogenesis. Given the essential role of Egl in the proper localization of numerous patterning mRNAs, we suggest that its abnormal localization likely leads to impaired embryonic axis specification typical for piRNA pathway mutants.
Introduction
Telomeres are DNA-protein complexes that protect the ends of linear eukaryotic chromosomes. In most species telomeric repeats are synthesized by telomerase consisting of reverse transcriptase and an RNA template. Telomere-associated proteins form the telomere protection shelterin complex [1]. Recently it was found that telomeric repeats are transcribed and give rise to long non-coding RNAs, TERRA [2]. TERRA molecules interact with shelterin proteins, telomerase, chromatin remodeling factors and localize near telomeres suggesting a structural role in telomere architecture [3]. However, TERRA may also bind and regulate different genic targets indicating its role as an epigenetic factor in gene expression [4]. Moreover, the large network of TERRA interacting proteins suggests that TERRA plays a significant role not only in telomere regulation but also in various cellular pathways [4, 5]. However, the functional significance of most interactions between TERRA and its partners remains poorly understood.
Using Drosophila we conducted a systematic study of the biogenesis and function of telomeric transcripts and their associated proteins in the female germline and in early development. Telomerase was not found in the Drosophila genome [6]. A unique feature of Drosophila telomeres is that they are composed of LINE (long interspersed nuclear element) retrotransposons; namely, HeT-A, TART and TAHRE, among which HeT-A is the most abundant [7]. Despite the different nature of telomeric repeats between Drosophila and species encoding telomerase, the basic mechanisms of telomere maintenance are similar [8]. Drosophila telomeric protein complex is structurally different from human shelterin, but is functionally analogous and protects the chromosome ends from degradation and fusion [9, 10]. Transcription of telomeric repeats is a conserved feature described in all studied species. Telomeric transcripts in Drosophila were described a decade earlier than human TERRA [11, 12]. The Drosophila telomeric transcriptome in the germline consists of both long retrotransposon transcripts and small RNAs [13, 14]. HeT-A and TART produce multiple sense and antisense transcripts, the latter containing multiple introns [14–16]. Unusual regulatory regions of HeT-A and TART retrotransposons drive the bidirectional transcription [16, 17]. HeT-A sense transcripts may be considered as functional analogues of both the telomerase RNA template and TERRA RNA. Clearly, the expression of telomeric elements must be strictly controlled to ensure regulation of telomere length. In Drosophila ovaries, such control is mediated by a distinct mechanism of RNA interference, the piRNA (PIWI interacting RNA) pathway. In the germline, antisense transcripts of telomeric retrotransposons serve as piRNA precursors, regulating the abundance of sense coding transcripts [13, 14]. In the presence of piRNAs, the expression of telomeric repeats is repressed, while mutations in piRNA pathway genes lead to a drastic accumulation of telomeric transcripts and an increased rate of heritable terminal retrotranspositions [13]. piRNA pathway disruption strongly affects telomeric chromatin state and HeT-A expression in the germline leading to severe developmental defects [18–21].
Telomeres have certain features of heterochromatin, therefore, their transcriptional activity in a normal cell is repressed. Increased level of telomere transcription in response to telomere damage may be a part of telomere signaling affecting various cellular processes [22]. An important question in Drosophila telomere biology is whether the level of HeT-A RNA could play a role in cellular response to the state of telomeres. To address this question, it is critical to examine the HeT-A RNA and HeT-A Gag biogenesis in normal development and with telomere dysfunctions.
Previously, we have shown that the mechanisms of chromosome end protection are closely related to the silencing of telomeric repeats: upon depletion of the piRNA pathway components and other factors suppressing HeT-A expression, abundant long telomeric RNAs accumulate in the germline, then transported to the oocyte and form aggregates at the mitotic spindles during the syncytial stage of embryogenesis [20]. This phenotype is accompanied by severe mitotic defects, chromosome missegregation and leads to early embryonic lethality [20, 21]. However, the functional link between accumulation of telomeric transcripts upon telomere dysfunction and developmental defects remained unclear. To advance the understanding of the interplay between dysfunctional telomeres and various cellular pathways, we explored the localization and functional interactions of abundant HeT-A RNA and Gag protein in the germline and early development upon piRNA pathway disruption.
RNA-binding proteins are involved in RNA metabolism at different stages of its life cycle, from transcription to degradation. Transcripts and proteins encoded by HeT-A and TART were detected at different stages of germline development in wild type Drosophila strains as well as upon depletion of telomere silencing components [20, 23, 24]. Despite being crucial for telomere targeting and lengthening, the biogenesis of Drosophila telomeric RNP in the germline and in early development is still poorly understood since most studies are focused on either HeT-A and TART RNA or their protein products separately.
Due to robust piRNA-mediated silencing in the germline, telomeric retrotransposon products are present at very low levels in wild type ovaries. Even in the Gaiano strain which is characterized by increased HeT-A and TART copy numbers [25], HeT-A Gag protein is barely detected in germ cells [23], which can be explained by enhanced production of HeT-A piRNAs in Gaiano ovaries [26]. Valuable data on Drosophila telomeric RNP were recently obtained from studies of somatic tissues. HeT-A spherical particles consisting of HeT-A Gag and HeT-A RNA were discovered in proliferating neuroblasts where they associate with telomeres undergoing DNA replication and are considered a prerequisite for somatic HeT-A transposition [27].
Here, we show that upon piRNA loss overexpressed HeT-A transcripts interact with the RNA-binding protein Gag-HeT-A, which they encode, and that they form RNP particles during most of their life cycle in oogenesis and early embryogenesis. In germarium, HeT-A RNP form spherical structures in the nuclei of dividing cystoblasts and seem to be the intermediates of telomere elongation in the germline. It has been shown that interaction of HeT-A RNPs with the main carrier protein Egalitarian (Egl) [28] is required for their transport into the oocyte. Here, we describe for the first time the life cycle of maternal HeT-A RNP in early development. Retention of Egl at HeT-A RNP causes its ectopic localization at syncytial stage of embryogenesis which likely impairs embryonic development in the piRNA pathway mutants.
Materials and methods
Drosophila strains and transgenic constructs
Full-length HeT-A element encoding Gag protein tagged with HA (hemagglutinin) and FLAG epitopes was cloned into pUASp-attB as described previously [29]. To generate pUAST-attB-HeTA-HA-FLAG_ms2, 8 MS2 hairpins were introduced in the end of HeT-A 3`UTR. The construct pUAST-attB-HeTA-HA-FLAG-mut_ms2 was created on the basis of pUAST-attB-HeTA-HA-FLAG-ms2 by cloning of the mutated HeT-A hairpin (S6 Fig). Constructs were integrated in the attP docking site on chromosome 3 (strain #24862, BDSC). GLKD (Germline Knockdown) flies were F1 progeny of the genetic cross of a strain bearing a construct with short hairpin (sh) RNA (spnE_sh, #103913, VDRC; piwi_sh, #101658, VDRC; egl-sh, #21779, VDRC) and a driver strain #25751 (P{UAS-Dcr-2.D}. v1, w1118, P{GAL4-nos.NGT}40, Bloomington Stock Center). The transgenic strain expressing fused protein Egl-GFP was kindly provided by S. Bullock [28]. The Gaiano III (GIII) is a strain carrying a third chromosome with Tel locus derived from natural Gaiano strain characterized by extremely long telomeres [25].
RNA immunoprecipitation (RIP)
RIP was performed according to [30] with modifications. Briefly, ovaries from 3-day old females or 0-2-hour old embryos were homogenized in the Dounce homogenizer (Sigma) in 5 volumes (V) of cold lysis buffer (10 mM HEPES, pH 7.0, 100 mM KCl, 5 mM MgCl2, 0.5% NP-40, Complete mini protease inhibitor cocktail (Roche), 20 mM NaF, 0.2 mM NaVO4, 1 U/μl RiboLock RNase Inhibitor (ThermoScientific)). For RIP with anti-HA beads, lysis buffer was supplemented with 1% Triton X-100, 0.1% SDS and 10% glycerol. The extracts were cleared by centrifugation at 16,000 g at 4° C for 10 min and diluted with 9 volumes of NT2 buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.05% NP-40, 0,2 mM NaVO4, 20 mM NaF, RiboLock 1U/μl). The lysates were incubated with anti-HA magnetic beads (Pierce) or with antibody-coated (5 μg of the primary antibodies per sample) Dynabeads Protein A (Invitrogen) for 2 h at 4°C on a rotator. After 3 washes in NT2 buffer, 1/10 of beads were saved for Western blot analysis. RNA was isolated from the remaining beads using Trizol reagent (Life Technologies). Reverse transcription was performed with a random hexanucleotide primer and SuperScriptIII reverse transcriptase (Life Technologies) according to the manufacturer’s instructions. qPCR was performed on a LightCycler96 (Roche). Primers are listed in S1 Table. Antibodies used for RIP: rabbit anti-EGL [31]; rabbit anti-GFP (Abcam), rabbit anti-HA (Cell signaling technology), normal rabbit IgG (Santa Cruz).
RNA FISH and immunostaining
RNA FISH combined with immunostaining was carried out according to the previously described procedure [20] with modifications. After fixation, ovaries or dechorionized embryos were incubated in PBS containing 50 ug/ml proteinase K for 6 or 2 min, respectively. Proteinase K treatment was omitted in case of further staining for cytoplasmic proteins in the ovaries. Digoxigenin (DIG)-labeled antisense HeT-A riboprobe containing a fragment of the ORF (nucleotides 4330–4690 of GenBank sequence DMU06920) was used. The specificity of this probe was previously confirmed by Northern blotting and in situ hybridization with polytene chromosomes of salivary glands [19, 26]. To amplify hybridization signal, we incubated the sample with anti-DIG- fluorescein (FITC) antibodies (Roche), followed by incubation with anti-FITC Alexa Fluor 488 antibodies (Life Technologies). Blocking solution was supplemented with 3% BSA before immunostaining. Samples were mounted in Vectashield Antifade Mounting Medium with DAPI (Vector Laboratories). Images were captured using Zeiss LSM 510 Meta or Olympus FV10i confocal microscopes and analyzed using ImageJ and Adobe Photoshop.
Co-immunoprecipitation (co-IP)
To prepare embryonic extracts, 0-2-hour old embryos were dechorionated, frozen and stored at -80°C. Ovaries (400 pairs) or embryos (the volume of dechorionized embryos ~70 μl) were homogenized in a Dounce homogenizer in 9 volumes of cold IP Buffer (20 mM HEPES pH 7.0, 150 mM NaCl, 2,5 mM MgCl2, 0.1% Triton X-100, Complete Mini protease inhibitor cocktail (Roche), 0,2 mM NaVO4, 20 mM NaF, RiboLock 1U/μl (ThermoScientific)). The extracts were cleared by centrifugation at 16,000 g at 4°C for 10 min. Supernatants were incubated with anti-HA magnetic beads (20 μl, Pierce) for 30 min at room temperature on a rotator. After 3 washes in IP buffer the bound proteins were eluted from the beads by boiling in 100 μl of Laemmli protein loading buffer (31,25 mM Tris-HCl, pH 6.8, 12,5% glycerol, 1% SDS, 0.005% Bromophenol Blue, 2,5% β-mercaptoethanol) for 5 min. Beads were pelleted and the supernatant was saved for Western blot analysis. Samples were resolved on 8% SDS-PAGE gel and transferred onto Immobilon-P PVDF membrane (Millipore). Blots were developed using the Immun-Star AP detection system (Bio-Rad Laboratories), in accordance with the manufacturer’s recommendations.
Antibodies
The following primary antibodies were used: rabbit anti-Egl [31] (kindly provided by R. Lehmann); mouse anti-Dhc (Developmental Studies Hybridoma Bank; donor J. Scholey); mouse anti-BicD (Developmental Studies Hybridoma Bank, donor R. Steward); rat anti-Vasa (Developmental Studies Hybridoma Bank; donors A.C. Spradling/D/Williams); rabbit anti-gamma-tubulin (Sigma); mouse anti-GFP (Abcam); mouse anti-HA (Cell signaling technology); rabbit anti-HA (Cell signaling technology); rabbit anti-HOAP, guinea pig anti-HipHop and anti-Gag HeT-A (kindly provided by Y. Rong); rabbit anti-Gag HeT-A (kindly provided by E. Casacuberta (23) Alexa fluor conjugated secondary antibodies (Life Technologies) were diluted 1:500.
Results
HeT-A RNA and HeT-A Gag are co-localized in ovaries and form HeT-A RNPs at different stages of oogenesis
RNA biogenesis is orchestrated by RNA-binding proteins that determine the localization, lifetime and the specificity of transcript interactions. Therefore, in order to understand the role of telomeric coding RNAs, it is first necessary to identify their protein partners. Direct recognition of the retrotransposon transcript by the protein it encodes was discovered for human retrotransposon LINE-1 (L1) and is referred to as cis-preference [32, 33]. We suggested that the Drosophila telomeric protein Gag, encoded by HeT-A, interacts with HeT-A RNA forming RNP in the germline. Recombinant engineering of epitope tags and inducible tissue-specific expression of transgene containing full-length HeT-A have made detection and purification of HeT-A Gag complexes routine [29]. Due to the activity of the piRNA pathway and other telomere silencing mechanisms, expression of HeT-A is strongly repressed in the germline [13, 20]. Indeed, HeT-A Gag is not detectable by Western blotting in the ovaries of GIII and transgenic strains expressing HeT-A-HA in contrast to somatic tissues (S1 Fig). However, upon piRNA loss, caused by depletion of the RNA helicase Spindle-E (SpnE) [34], abundant HeT-A Gag accumulates in the ovaries (Part A in S1 Fig). To get an insight into the biogenesis and pathological significance of abundant telomeric RNAs and proteins generated upon telomere dysfunction, we characterized their localization during oogenesis and first hours of development after germline knockdown (GLKD) of spnE.
To determine whether HeT-A Gag associates with HeT-A RNA, a RIP experiment followed by RT-qPCR of co-precipitated RNA was performed. Ovarian lysates expressing HeT-A Gag-HA upon spnE_GLKD were immunoprecipitated with a/HA antibodies. Ovaries of spnE_GLKD lacking the UAS-HeT-A-HA transgene were used as controls (Fig 1A). HeT-A transcripts are significantly enriched in the HeT-A Gag-HA precipitate (Fig 1A). Interestingly, endogenous TART RNA was also present in this complex suggesting that HeT-A RNPs are multicomponent complexes.
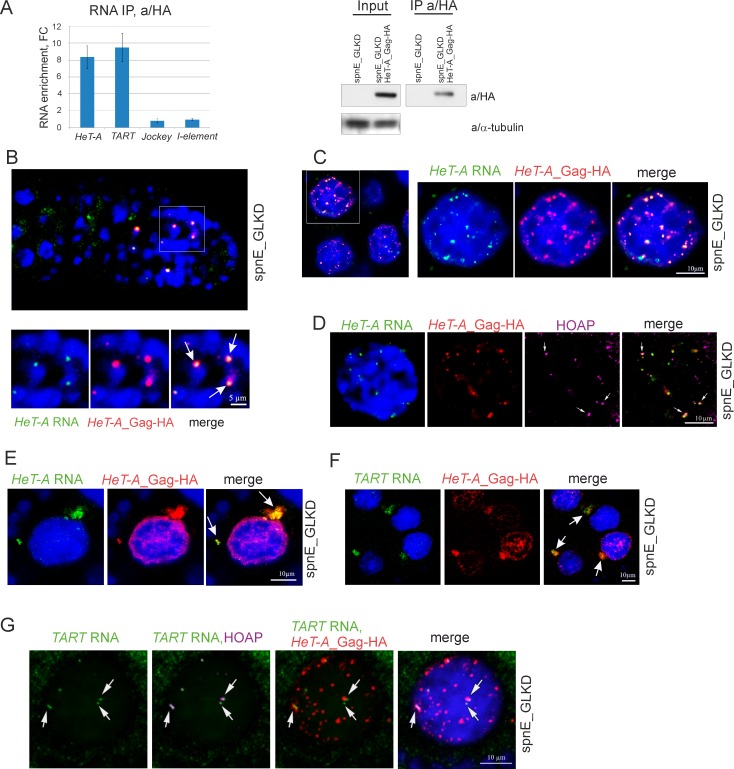
Fig 1. HeT-A RNA and HeT-A Gag protein form RNP in ovaries.
(A) RT-qPCR analysis of RNA immunoprecipitated with anti-HA from ovary lysates of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies and control nosGal4; UAS-spnE_sh flies. Fold changes (FC) for RNA enrichments in RNA IP from ovaries of HeT-A-HA transgenic strain versus control are shown. rp49 was used for normalization. The error bars represent standard deviations (SD) of 2 biological replicas. Western blot analysis of immunoprecipitated HeT-A Gag-HA is shown to the right. The antibodies used for Western blotting are indicated to the right. (B-G) RNA FISH combined with immunostaining was performed on ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. (B) HeT-A RNA (green) colocalizes with HeT-A Gag-HA (red) in germarium. Enlarged region (white rectangle) is shown. HeT-A spheres (arrows) are observed in cystoblasts. (C) HeT-A RNA (green) colocalizes with HeT-A Gag-HA (red) in nurse cell nuclei. A fragment of a stage 7 egg chamber is shown to the left. An enlarged nucleus of a germline nurse cell (white rectangle) is shown. (D) HeT-A RNA FISH (green) combined with HeT-A Gag-HA (red) and HOAP (magenta) immunostaining in a nurse cell nucleus from a stage 7 egg chamber is shown. Telomeric localization of HeT-A RNP is shown by arrows. (E) HeT-A RNA (green) and HeT-A Gag-HA (red) form large aggregates (arrows) in the cytoplasm of nurse cells. A fragment of a stage 5 egg chamber is shown. For an entire egg chamber see Part A in S2 Fig. (F) TART transcripts (green) are co-localized with HeT-A Gag-HA (red) in the nurse cell cytoplasm (arrows). A fragment of a stage 7 egg chamber is shown. (G) TART RNA (green) colocalization with telomere-specific protein HOAP (magenta) is shown (arrows). HeT-A Gag-HA (red) partially colocalizes with TART RNA in nurse cell nuclei. Image of an individual nurse cell nucleus from a stage 6 egg chamber.
Next, we analyzed the co-localization of HeT-A RNA and Gag protein at different stages of oogenesis upon spnE_GLKD using RNA fluorescence in situ hybridization (FISH) combined with immunostaining. In the germarium region 2A, HeT-A transcripts co-localize with Gag-positive particles forming spherical RNPs of 1–2 microns in size (Fig 1B) resembling those discovered in brain cells [27]. Numerous HeT-A spheres observed in the nuclei of dividing cystoblasts are presumably intermediates of the telomere elongation complex, in agreement with the high rate of telomeric attachments in spnE mutants [13]. We failed to detect spherical HeT-A RNPs in the female germ cells of GIII strain (Parts B and C in S1 Fig). Taking into account the extremely low rate of spontaneous terminal transpositions [35], assembly of HeT-A spheres appears to be a rare event under normal conditions.
During the later stages of oogenesis, HeT-A RNPs are detected in the nurse cells and oocyte upon piRNA loss. In the oocyte, HeT-A Gag and HeT-A RNA staining are evenly distributed and extensively overlapped (Part A in S2 Fig). Most of the nuclear HeT-A RNP in the nurse cells have a spherical shape (Fig 1C), however, only some of them co-localize with telomeres stained for telomere-specific proteins HOAP or HipHop [36, 37] (Fig 1D and Part B in S2 Fig). We observed similar patterns of HeT-A RNP localization both for endogenous and transgenic HeT-A, which argues against nonspecific effects caused by transgene overexpression (Part C in S2 Fig). HeT-A RNA FISH combined with HA-immunostaining on wild type yw ovaries served as a negative control (Part D in S2 Fig). In the nurse cell cytoplasm, some of the HeT-A RNP foci form large aggregates of irregular shape differed from nuclear spherical particles (Fig 1E). HeT-A Gag-HA staining was also revealed in the perinuclear ribonucleoprotein structure—the nuage. However, immunostaining of HeT-A Gag and a nuage component Vasa shows only partial colocalization of these proteins (Part A in S3 Fig). HeT-A Gag staining is more diffuse and found not only around the nucleus but also in the nucleus close to the envelope. Most likely, nuclear import of abundant HeT-A Gag is accompanied by its sequestration in the nuage.
In accordance with RIP, TART sense transcripts co-localize with aggregates of HeT-A RNP in the nurse cell cytoplasm (Fig 1F). In nurse cell nuclei, TART transcripts are revealed nearby telomeres but not in the HeT-A spheres (Fig 1G).
All these data taken together suggest that HeT-A RNA and HeT-A Gag form RNPs of different structures at different stages of oogenesis upon loss of piRNA silencing.
In order to demonstrate direct interaction between the HeT-A Gag protein and its RNA template (cis-preference rule) we took advantage of another transgenic strain containing a HeT-A copy, tagged by the MS2 bacteriophage hairpins, and encoding for Gag-HA. Combined MS2 RNA FISH and HA-immunostaining shows colocalization of transgenic RNA and protein in the nuclei and cytoplasm of germ cells (Fig 2). Distribution patterns of transgenic HeT-A RNPs are similar to that of endogenous HeT-As. However, due to the lower sensitivity of the MS2 probe, we used HeT-A ORF probe in further experiments. To rule out artifacts due to nonspecific MS2 RNA probe hybridization we performed control experiment. The MS2 probe does not recognize nontagged HeT-A-HA RNA in transgenic ovaries expressing HeT-A Gag-HA upon spnE_GLKD (Fig 2C).
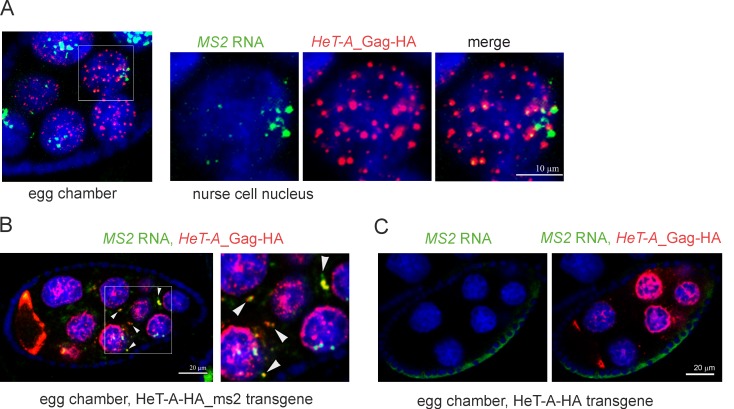
Fig 2. HeT-A Gag demonstrates cis-preferential activity to the template HeT-A mRNA.
(A) MS2 RNA FISH combined with anti-HA immunostaining was performed on ovaries of a transgenic strain expressing HeT-A-HA-ms2 upon spnE_GLKD. Transgenic HeT-A-HA_ms2 RNA (green) colocalizes with HeT-A Gag-HA (red) in nurse cell nuclei. A stage 6 egg chamber and enlarged nurse cell nucleus (rectangle) are shown. Intensive HeT-A signals in the nurse cell nuclei most likely correspond to the actively transcribed HeT-A transgenes. (B) MS2 RNA (green) and HeT-A Gag-HA (red) form large aggregates (arrowheads) in the cytoplasm of nurse cells of transgenic strain expressing HeT-A-HA-ms2 upon spnE_GLKD. A stage 7 egg chamber and its enlarged fragment (rectangle) is shown. (C) MS2 RNA FISH (green) and HA immunostaining (red) on ovaries of transgenic strain expressing HeT-A-HA upon spnE_GLKD serves as a negative control and confirms specificity of the MS2 probe.
We conclude that HeT-A RNA and Gag form RNP according to the cis-preference rule.
HeT-A RNPs interact with Egl in ovaries and form granules in the cytoplasm of nurse cells and oocyte
Upon piRNA pathway disruption, HeT-A products accumulate in the oocyte from the early stages of oogenesis, suggesting that some mechanism provides their relocation from the nurse cells to oocyte [13, 24] (Part A in S2 Fig). Egl, a protein that interacts with the dynein motor complex, is a major carrier which provides the transportation and localization of maternal RNAs at different stages of oogenesis [28, 31, 38]. Egl is essential for oocyte determination since in Egl mutants all 16 germ cells of the egg chamber become nurse cells [39, 40]. We therefore suggested that Egl might be involved in HeT-A RNA localization. In ovaries with double piwi, egl GLKD, HeT-A specific localization is not observed (Fig 3A). Both HeT-A RNA and dynein accumulated in the cytoplasm of nurse cells. HeT-A delocalization is likely caused by the severe disorganization of microtubules caused by Egl depletion [39, 40]. Based on this observation, we focused on examining the role of Egl in HeT-A RNP transport.
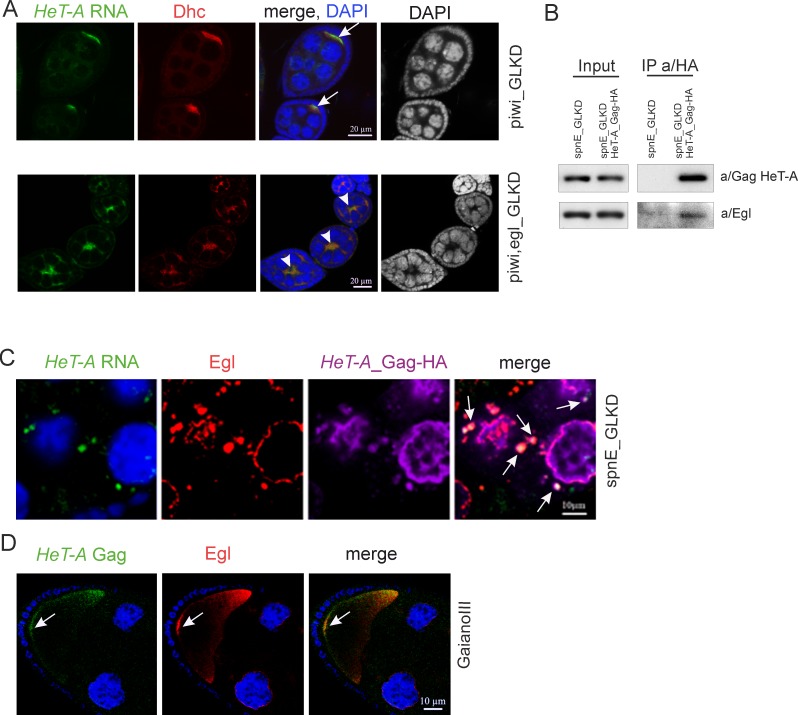
Fig 3. Egl participates in the transport of HeT-A RNPs in ovaries.
(A) Egl germline knockdown results in mis-localization of HeT-A transcripts. Egg chambers at stage 4–5 of oogenesis from piwi_GLKD (top panel) and double piwi-egl_GLKD (bottom panel) are stained with a/Dynein heavy chain (Dhc) (red). HeT-A RNA (green) is localized in the oocyte upon piwi_GLKD (arrows), but is mis-localized upon piwi,egl double GLKD (arrowheads). Ectopic Dhc is observed at this stage according to published data [40]. DAPI is shown separately in a grey. (B) Co-IP of HeT-A Gag. Western blot analysis of proteins immunoprecipitated with anti-HA from ovaries of spnE-GLKD flies (control) and nosGal4; UAS-HeT-A-HA; UAS-spnE_sh. Anti-HA immunoprecipitates HeT-A Gag-HA and Egl proteins. The antibodies used for Western blotting are indicated to the right and the antibodies used for co-IP are indicated above the IP lanes. (C) HeT-A RNA (green), HeT-A Gag-HA (magenta) and Egl (red) form aggregates (arrows) in the cytoplasm of the nurse cells in ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. The fragment of a stage 9 egg chamber is shown. For an entire egg chamber see Part A in S5 Fig. (D) HeT-A Gag (red) and Egl (green) co-localize at the posterior pole of the oocyte (arrows) at stage 10 in ovaries of GIII strain. A fragment of a stage 10 egg chamber including oocyte is shown. DNA is stained with DAPI (blue).
To determine whether Egl directly associates with HeT-A RNAs, we performed RIP followed by RT-qPCR. RIP was performed on ovaries with a HeT-A overexpression or wild type background. Lysates of spnE GLKD ovaries expressing HeT-A Gag-HA were immunoprecipitated with a/Egl or normal rabbit serum. Alternatively, Egl-GFP was immunoprecipitated using rabbit a/GFP or normal rabbit IgG from ovary lysate of Egl-GFP strain at a wild type background. As expected, nanos (nos), oskar (osk) and gurken (grk) mRNAs are associated with Egl-BicD complex in wild type ovaries (S4 Fig) [38]. It is worth noting, that association of nos, osk and grk transcripts with Egl is not affected by spnE_GLKD (S4 Fig). However, HeT-A RNA enrichment was insignificant in both cases, and HeT-A Gag is undetectable in Egl-BicD complex (S4 Fig). Of note, the majority of HeT-A Gag is present in an insoluble pellet after tissue lysis indicating its strong tendency to aggregate.
We suggested that if Egl does not associate directly with HeT-A RNA, it probably interacts with HeT-A Gag, and that this interaction is sensitive to the stringent purification conditions used in RIP (see Materials and methods). Co-IP experiments on spnE_GLKD ovaries using an HA antibody show that Egl is co-purified with HeT-A Gag-HA (Fig 3B) confirming that Egl indeed associates with transgenic HeT-A Gag. HeT-A RNA FISH combined with immunostaining confirmed the co-localization of Egl and HeT-A RNPs in the cytoplasm of the nurse cells and oocyte (Fig 3C and Part A in S5 Fig). In the nurse cells, Egl and HeT-A RNP form large aggregates of irregular shape in the cytoplasm and are partially colocalized in the nuage (Figs 3C and S3). Immunostaining of Egl and endogenous HeT-A Gag in ovaries of spnE_GLKD lacking the UAS-HeT-A-HA transgene revealed numerous Egl granules co-localized with HeT-A Gag indicating that their formation is not a side effect of transgenic Gag-HA aggregation (Part B in S5 Fig). In agreement with this observation, Co-IP experiments on spnE_GLKD ovaries lacking the HeT-A transgene show that Egl is co-purified with endogenous HeT-A Gag (Part C in S5 Fig).
We proposed that in the wild type ovaries, HeT-A RNP also interact with Egl and therefore performed Egl and HeT-A Gag immunostaining combined with HeT-A RNA FISH on ovaries of the GIII strain, which is characterized by an increased HeT-A copy number [25]. Egl was previously shown to be localized at the posterior pole of oocytes at stages 9–10 of oogenesis [31, 38]. Despite the low level of HeT-A Gag in GIII ovaries, we detected its enrichment at the posterior pole and co-localization with Egl (Fig 3D). This result confirms Egl association with endogenous HeT-A Gag in wild type ovaries.
Taken together, our data indicate that Egl carrier function is required for translocation and localization of HeT-A RNP in the oocyte upon HeT-A overexpression caused by piRNA loss. The experiment using the GIII strain indicates that probably the same mechanism operates in a wild type background.
Association of abundant HeT-A RNP with Egl causes ectopic accumulation of Egl near to centrosomes in early embryos upon piRNA loss
Previously, we reported, that maternal HeT-A transcripts and transgenic HeT-A Gag-HA form aggregates around centrosomes during blastoderm formation in spnE_GLKD background [20, 29]. Specific localization of HeT-A RNA is dependent on microtubules since disruption of microtubules caused delocalization of HeT-A transcripts [29]. Similarly, endogenous HeT-A RNA and HeT-A Gag form granules and accumulate around centrosomes in 0-2-hour old embryos collected from spnE_GLKD females (Fig 4A and 4B). At this stage, HeT-A transcripts do not co-localize with the telomeres of mitotic chromosomes suggesting a non-telomeric role for HeT-A RNA.
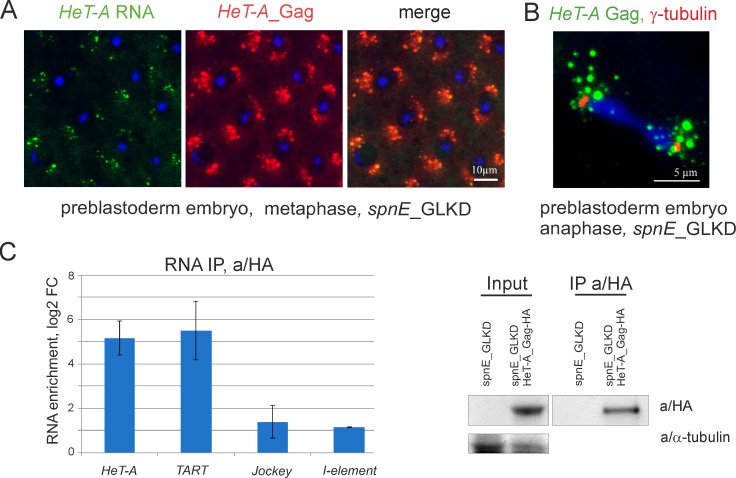
Fig 4. HeT-A RNA and HeT-A Gag form RNPs in early embryos.
(A) Endogenous HeT-A RNA FISH (green) and anti-HeT-A Gag immunostaining (red) was performed on 0-2-hour old embryos after spnE_GLKD. Syncytial metaphase stage is shown. (B) HeT-A Gag (green) and gamma-tubulin (red) immunostaining was performed on 0-2-hour old embryos upon spnE_GLKD. Syncytial anaphase is shown. (C) RT-qPCR analysis of RNA precipitated with anti-HA from lysates of 0-2-hour old embryos laid by nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies relative to control nosGal4; UAS-spnE_sh embryos. log2 of fold changes (log2_FC) for RNA enrichments in RNA IP from embryos of HeT-A-HA transgenic strain versus control are shown. rp49 was used for normalization. The error bars represent SD of 2 biological replicas. Western blot analysis of immunoprecipitated HeT-A Gag-HA is shown to the right. The antibodies used for Western blotting are indicated to the right.
First, we addressed a question whether HeT-A RNA and HeT-A Gag form RNP in early embryos. A RIP experiment was performed on lysates of 0-2-hour old embryos of a transgenic strain expressing UAS-HeT-A-HA in the germline upon spnE_GLKD. As a control, embryo lysates from non-transgenic spnE_GLKD flies were used (Fig 4C). HeT-A transcripts are significantly enriched in HeT-A Gag-HA precipitate from 0-2-hour embryos. Similar to RIP data on ovaries, TART RNA is also present in HeT-A RNPs of early embryos.
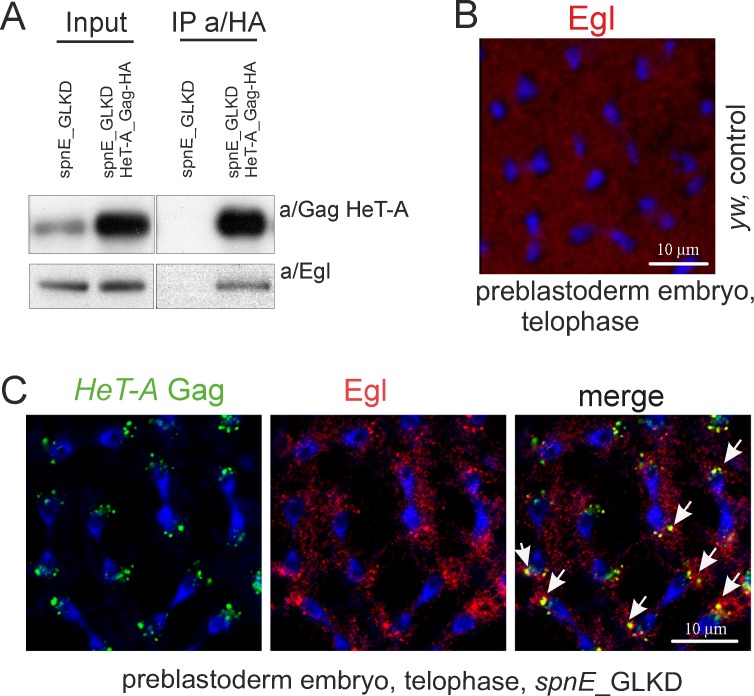
Next, we asked if Egl associates with HeT-A Gag during early embryogenesis. Co-IP using the lysate of 0-2-hour old embryos from spnE_GLKD flies expressing HeT-A Gag-HA shows that Egl co-purifies with transgenic HeT-A Gag-HA (Fig 5A). Immunostaining reveals co-localization of endogenous HeT-A Gag and Egl near the spindle poles in 0-2-hour old embryos upon spnE_GLKD while in wild type, Egl is evenly distributed in the syncytial embryo interior (Fig 5B and 5C). These data suggest that interaction with HeT-A Gag may cause Egl retention and its ectopic localization in early embryos with spnE_GLKD background.
Fig 5. HeT-A RNPs associate with Egl in early embryos.
(A) Western blot analysis of proteins immunoprecipitated with anti-HA from 0-2-hour old embryos laid by spnE_GLKD flies with and lacking the UAS-HeT-A-HA transgene. Anti-HA immunoprecipitates HeT-A Gag-HA and Egl proteins. The antibodies used for Western blotting are indicated to the right and the antibodies used for co-IP are indicated above the IP lanes. (B) 0-2-hour old embryos of y1 w67c23 strain were immunostained with anti-Egl (red). Telophase stage of syncytial preblastoderm embryo is shown. (C) Immunostaining of 0-2-hour old embryos collected from spnE_GLKD flies was performed with anti/Gag HeT-A (green) and anti/Egl (red) antibodies. DNA is stained with DAPI (blue). Telophase is shown. Endogenous HeT-A Gag colocalizes with Egl around spindle poles (arrows).
It is known that mRNA stem-loop elements are important for recognition by protein carriers [41–43]. We suggested that the hairpin structure detected in the HeT-A 3'UTR (untranslated region) is presumably involved in the HeT-A RNA localization, and therefore, generated а transgenic strain, encoding HeT-A transcripts with impaired hairpin structure (S6 Fig). The mutated HeT-A construct contains sequences of bacteriophage MS2 hairpins which allowed us to specifically detect transgenic transcripts using an MS2 probe. Transgenic strains containing HeTA-HA-mut_ms2 construct were used to decipher the role of the identified structural motif in the transport and localization of HeT-A mRNA. In spnE GLKD ovaries, HeT-A-HA-mut_ms2 transcripts colocalize with HeT-A Gag-HA and Egl and accumulate in the oocyte just like endogenous HeT-A RNA (Part C in S6 Fig). In early embryos, HeT-A-HA-mut_ms2 RNAs localize near to the centrosomes stained with gamma-tubulin (Part D in S6 Fig). Similar to endogenous HeT-A Gag, Gag-HA encoded by HeT-A-HA-mut_ms2 transgenic RNA co-localizes with Egl close to spindle poles (Part E in S6 Fig.). These data show that this particular hairpin structure is dispensable for Egl-mediated transportation and localization of HeT-A RNA in ovaries and embryos.
Discussion
In this study, we describe the life cycle of maternal telomeric RNPs in Drosophila. To study the localization of telomeric RNA in oogenesis and early development, we focused on the major telomere repeat HeT-A which is overexpressed upon piRNA pathway disruption. It was shown that in the ovaries and embryos HeT-A transcripts interact with the RNA-binding protein Gag-HeT-A that they encode forming RNP complexes. Using tagged transgenic HeT-A, we confirmed that this interaction occurs according to the cis-preference rule discovered previously for the human L1 retrotransposon [32, 33].
HeT-A RNPs form structures with different characteristics during oogenesis upon piRNA loss. In the early stages of oogenesis, during cystoblast cell divisions, nuclear HeT-A RNPs form spherical particles which are seemingly participate in telomere elongation. HeT-A spheres were revealed in germarium germ cells with spnE depletion, but not in wild type ovaries. This fact is in agreement with extremely low levels of HeT-A expression and therefore, low transposition rate to chromosome termini in wild type flies [13, 35]. Accumulation of HeT-A spheres in cystoblast nuclei is in accordance with the observation that identical telomeric attachments to the terminally truncated chromosomes have been identified in several descendants from the same female bearing a heterozygous spnE mutation, suggesting that terminal elongation occurs at premeiotic stages of oogenesis [13]. The germarium region where we have seen HeT-A spheres, and where expression of HeT-A-lacZ transgene was previously detected [19], corresponds to the so-called “Piwi-less pocket”, a small developmental window when transposons escape host control and can transpose [44]. Interestingly, telomeres appear to be elongated at the same stage suggesting that telomere length control and transposon regulation are coupled.
During later stages of oogenesis in spnE deficient ovaries, numerous spherical HeT-A RNPs are also detected in the nuclei of terminally differentiated nurse cells, with only a few of them associated with telomeres. We speculate that HeT-A RNPs could act here independently of telomere elongation and be involved in dysfunctional telomere signaling. Indeed, mammalian telomeric factors are able to localize outside telomeres, where they can regulate the transcription of various genes [22].
In the cytoplasm of nurse cells, HeT-A products form irregularly shaped aggregates which appear to be the transportation cargoes. Thus, only a portion of HeT-A RNPs generated in the nurse cells are translocated into nucleus while others are transported into the oocyte. It is noteworthy, that compositionally and functionally distinct L1 RNPs were also revealed in human cells [45]. Taking into account the strong tendency of HeT-A Gag to aggregate, one may suggest that formation of regular spherical particles revealed in the nuclei is compartment-specific and requires additional factors.
HeT-A Gag co-IP in combination with RNA FISH and immunostaining suggests that the Egl-dynein carrier complex provides migration of cytoplasmic HeT-A RNPs in the germline. In piRNA pathway mutant, cytoplasmic aggregates consisting of dynein, Egl and BicD proteins, as well as transcripts of bicoid, grk and retrotransposon I-element were detected in both nurse cells and the oocyte [46]. We also show that cytoplasmic HeT-A RNPs co-localize with Egl. Seemingly, HeT-A RNPs can catalyze the aggregation of their interactors. If this is the case, interaction between Egl-BicD complex and HeT-A RNP particles can promote the formation of Egl-positive granules upon HeT-A overexpression caused by the piRNA loss.
Telomere targeting is an essential but not the only function of HeT-A RNPs. Maternal telomeric RNPs transported to the oocyte have a specific destination during early development. HeT-A RNPs accumulate near to the centrosomes in 0-2-hour old syncytial embryos suggesting some interplay between telomeric and mitotic machinery. Indeed, HeT-A overexpression in the germline is followed by the severe mitotic defects during early development that leads to the embryonic lethality [20]. Physical interaction between HeT-A RNPs and transportation pathway components may be in part responsible for the developmental defects that accompany HeT-A overexpression. Indeed, Egl and BicD associate with dynein motors and mediate transport of RNAs essential for the initial oocyte specification and subsequent embryonic patterning [31, 38, 39, 41]. Egl retention at centrosomes in complex with HeT-A RNP may lead to mis-localization of its typical cargoes, nos and osk mRNAs, observed in early embryos upon piRNA pathway disruption [47, 48]. Indeed, we show that upon spnE_GLKD nos, osk and grk transcripts are still loaded into Egl-BicD complex. However, Egl aggregation and its ectopic localization caused by interaction with abundant HeT-A Gag could affect the localization pattern of developmental mRNAs leading to embryonic axis defects observed upon piRNA loss [47, 49, 50]. We speculate that the abundant telomeric RNPs generated upon telomere dysfunction could cause developmental arrest of such embryos, thus protecting genome integrity.
The exact nature of the signal responsible for HeT-A RNP enrichment in the oocyte remains elusive. The stem-loop structure in the 3’ UTR of maternal mRNAs mediates transport of these RNAs from the nurse cells into the oocyte and their specific localization in early embryos [28]. Egl despite lacking a canonical RNA-binding domain is an RNA-binding protein capable of direct recognition of RNA localization signals within transcripts of grk, hairy, K10, and the I-element retrotransposon [28]. However, our data show weak if any HeT-A RNA binding to Egl. In addition, mutation of a hairpin in the 3`UTR of HeT-A, that is similar to Egl- bound hairpins of certain mRNAs, did not affect HeT-A localization. The interaction of HeT-A Gag with Egl-BicD-microtubule transport components was instead revealed which supports the idea that protein-protein interactions are essential for HeT-A RNP transportation by the Egl-BicD complex.
Further dissection of the biochemical composition of HeT-A RNPs during oogenesis and early development will increase understanding of the telomeric retrotransposition mechanism as well as non-telomeric functions of HeT-A products. Other telomeric retroelements represented by a few copies per genome, TART and TAHRE, encode reverse transcriptases which are likely critical for HeT-A retrotranspositions. It is known that transiently expressed HeT-A and TART Gag proteins are able to form telomere-associated structures in cultured cells; TART Gag moves to telomeres only when co-expressed with HeT-A Gag [51]. Moreover, co-localization of HeT-A Gag and TART Pol proteins was observed by immunostaining in neuroblast nuclei [23]. According to our data, TART transcripts along with HeT-A RNA are bound to HeT-A Gag supporting the idea that HeT-A RNP is a core of the multicomponent telomeric RNP complex. The role of proteins encoded by TART and TAHRE in telomere targeting and retrotransposition remains to be determined.
Conclusions
We show that upon piRNA depletion transcripts of telomeric retrotransposon HeT-A interact with the HeT-A Gag protein, that they encode, according to the cis-preference rule, to form RNP particles during oogenesis and early embryogenesis. Our data suggest that HeT-A RNPs are multicomponent complexes which are present both in the nuclei and cytoplasm of germ cells, and in preblastoderm embryos. Spherical HeT-A RNPs found in the germ cells of germarium supposedly perform telomere elongation. Cytoplasmic HeT-A aggregates interact with carrier protein Egl which mediates the transportation of the HeT-A RNPs to the oocyte. HeT-A Gag-Egl complex is detected in early embryos near to centrosomes after HeT-A overexpression caused by piRNA pathway disruption. Interaction between HeT-A RNP and Egl carrier protein which is essential for embryonic axis specification likely impairs embryonic development in piRNA pathway mutants.
Supporting information
(A) Detection of HeT-A Gag in ovaries and carcasses (imago after ovary dissection) by Western blotting. Extracts were prepared from the GIII strain (first lane) and transgenic strains expressing UAS-HeT-A-HA in the germline on a wild type background (second lane) or upon spnE_GLKD (third lane). Antibodies are indicated to the left. In ovaries, HeT-A Gag is detected only upon piRNA pathway disruption (spnE_GLKD). (B, C) HeT-A RNA FISH (green) combined with endogenous HeT-A Gag (red) immunostaining on ovaries of GIII strain. HeT-A RNPs are not reveled in the germ cells but detected in somatic cells of germarium (B, arrows) in accordance to previously published observation (23). Intensive HeT-A staining observed in the nurse cell nuclei appear to be correspond to the transcribed telomeres (arrows) (C).
(TIF)
(A) HeT-A RNA (green) and HeT-A Gag-HA (red) form HeT-A RNPs (arrows) in the cytoplasm of nurse cells in the ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. Egg chamber at stage 7 of oogenesis is shown. (B) Colocalization of HeT-A RNA (green), HeT-A Gag-HA (red) and telomeric protein HipHop (magenta) is indicated by arrows. Nurse cell nucleus of a stage 6 is shown. (C) HeT-A RNA FISH (green) combined with endogenous HeT-A Gag (red) immunostaining on ovaries of non-transgenic spnE_GLKD strain. A fragment of a stage 7 egg chamber is shown. (D) HeT-A RNA FISH (green) combined with HeT-A Gag (red) immunostaining was performed on ovaries of yw wild type strain. An egg chamber at stage 7 of oogenesis is shown. DNA is stained with DAPI (blue).
(TIF)
Immunostaining of a nuage component Vasa (red) and HeT-A Gag-HA (green) (A) or Egl (green) (B) is shown. Stage 6 egg chambers of transgenic strains expressing UAS-HeT-A-Gag-HA in the germline in wild type background (bottom panels) or upon spnE_GLKD (top panels). Arrows indicate nuage. Egl colocalizes with Vasa in nuage, while HeT-A Gag-HA staining is more diffuse and only partially overlapped with Vasa. Magnification is 63x.
(TIF)
(A) RT-qPCR analysis of RNA precipitated with anti-Egl relative to negative control (normal rabbit IgG) from ovary lysates of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. rp49 was used for normalization. Western blot analysis of co-immunoprecipitated proteins is shown to the right. The antibodies used for Western blotting are indicated to the right. The antibodies used for co-IP are indicated above the IP lanes. Lane designation: input (total lysate), pellet (insoluble fraction), IP (precipitates). Anti-Egl immunoprecipitates both Egl and BicD proteins but not HeT-A Gag which is enriched in insoluble fraction. (B) RT-qPCR analysis of RNA precipitated with anti-GFP relative to negative control (normal rabbit IgG) from ovary lysates of w; tub-Egl-GFP flies. Western blot analysis of co-immunoprecipitated proteins is shown to the right; the indications are as in (A). Anti-GFP immunoprecipitates both Egl-GFP and Egl as well as BicD indicating that Egl-BicD is an oligomeric complex. For RIP panels, the error bars represent SEM of 2 biological replicas.
(TIF)
(A) HeT-A RNA (green), HeT-A Gag-HA (magenta) and Egl (red) form granules (arrows) in the cytoplasm of nurse cells in ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. Egg chamber at stage 7 of oogenesis is shown. (B) Egl (red) and endogenous HeT-A Gag (green) form granules (arrows) in ovaries of spnE_GLKD not carrying UAS-HeT-A-HA transgene. DNA is stained with DAPI (blue). (C) Co-IP of HeT-A Gag. Western blot analysis of proteins immunoprecipitated with anti-Gag HeT-A from ovaries of spnE_GLKD flies. Anti-Gag HeT-A immunoprecipitates Egl protein. The antibodies used for Western blotting are indicated to the right and the antibodies used for co-IP are indicated above the IP lanes.
(TIF)
(A) A putative HLS1 (HeT-A localization signal 1) is revealed in the 3’UTR of HeT-A copies (start corresponds to 5798 position of canonical HeT-A, DM06920). Folding of HLS1 is shown. (B) Sequence of mutated HeT-A hairpin is shown. (C) Colocalization of MS2 RNA (green), HeT-A Gag-HA (magenta) and Egl (red) in the cytoplasm of nurse cells (arrowheads) and in the oocyte (arrows) in ovaries of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. Two egg chambers at different stages of oogenesis are shown. (D) MS2 RNA FISH (green) combined with immunostaining of gamma-tubulin (red) was performed on 0-2-hour old embryos of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. DNA is stained with DAPI (blue). Syncytial metaphase is shown. (E) HeT-A Gag-HA (red) and Egl (green) immunostaining were performed on 0-2-hour old embryos of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. DNA is stained with DAPI (blue).
(TIF)
(XLSX)
Acknowledgments
We thank R. Lehmann for a/Egl antibodies, Y. Rong for a/HOAP, a/HipHop and a/Gag HeT-A antibodies, E. Casacuberta for a/Gag HeT-A antibodies, S. Bullock for Egl-GFP strains. We thank the Bloomington Stock Center and Vienna Drosophila RNAi Center for fly strains and the Developmental Studies Hybridoma bank for antibodies. We thank Dr. M. Semenova and User Facilities Centre of M.V. Lomonosov Moscow State University as well as User Centre of the Institute of Molecular Genetics, RAS, for the provided opportunity to use microscopy equipment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Russian Science Foundation (16-14-10167) to A.K. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol. 2013;14(2):69–82. Epub 2013/01/10. doi: nrm3505 [pii] 10.1038/nrm3505 ; PubMed Central PMCID: PMC3805138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801. Epub 2007/10/06. doi: 1147182 [pii] 10.1126/science.1147182 . [DOI] [PubMed] [Google Scholar]
- 3.Azzalin CM, Lingner J. Telomere functions grounding on TERRA firma. Trends Cell Biol. 2015;25(1):29–36. Epub 2014/09/27. doi: S0962-8924(14)00144-5 [pii] 10.1016/j.tcb.2014.08.007 . [DOI] [PubMed] [Google Scholar]
- 4.Chu HP, Cifuentes-Rojas C, Kesner B, Aeby E, Lee HG, Wei C, et al. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell. 2017;170(1):86–101 e16. 10.1016/j.cell.2017.06.017 ; PubMed Central PMCID: PMCPMC5552367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheibe M, Arnoult N, Kappei D, Buchholz F, Decottignies A, Butter F, et al. Quantitative interaction screen of telomeric repeat-containing RNA reveals novel TERRA regulators. Genome Res. 2013;23(12):2149–57. Epub 2013/08/08. doi: gr.151878.112 [pii] 10.1101/gr.151878.112 ; PubMed Central PMCID: PMC3847783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garavis M, Gonzalez C, Villasante A. On the origin of the eukaryotic chromosome: the role of noncanonical DNA structures in telomere evolution. Genome Biol Evol. 2013;5(6):1142–50. 10.1093/gbe/evt079 ; PubMed Central PMCID: PMCPMC3698924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardue ML, DeBaryshe PG. Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annu Rev Genet. 2003;37:485–511. 10.1146/annurev.genet.38.072902.093115 . [DOI] [PubMed] [Google Scholar]
- 8.Kordyukova MY, Olovnikov I, Kalmykova A. Transposon control mechanisms in telomere biology. Curr Opin Genet Dev. 2018;49:56–62. 10.1016/j.gde.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Raffa GD, Cenci G, Ciapponi L, Gatti M. Organization and Evolution of Drosophila Terminin: Similarities and Differences between Drosophila and Human Telomeres. Front Oncol. 2013;3:112 Epub 2013/05/16. 10.3389/fonc.2013.00112 ; PubMed Central PMCID: PMC3650302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang L, Tang X, Bhardwaj SR, Ji J, Rong YS. MTV, an ssDNA Protecting Complex Essential for Transposon-Based Telomere Maintenance in Drosophila. PLoS Genet. 2016;12(11):e1006435 10.1371/journal.pgen.1006435 ; PubMed Central PMCID: PMCPMC5105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danilevskaya ON, Arkhipova IR, Traverse KL, Pardue ML. Promoting in tandem: the promoter for telomere transposon HeT-A and implications for the evolution of retroviral LTRs. Cell. 1997;88(5):647–55. . [DOI] [PubMed] [Google Scholar]
- 12.Danilevskaya ON, Tan C, Wong J, Alibhai M, Pardue ML. Unusual features of the Drosophila melanogaster telomere transposable element HeT-A are conserved in Drosophila yakuba telomere elements. Proc Natl Acad Sci U S A. 1998;95(7):3770–5. Epub 1998/05/09. ; PubMed Central PMCID: PMC19912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 2006;20(3):345–54. 10.1101/gad.370206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shpiz S, Kwon D, Rozovsky Y, Kalmykova A. rasiRNA pathway controls antisense expression of Drosophila telomeric retrotransposons in the nucleus. Nucleic Acids Res. 2009;37(1):268–78. Epub 2008/11/28. doi: gkn960 [pii] 10.1093/nar/gkn960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danilevskaya ON, Traverse KL, Hogan NC, DeBaryshe PG, Pardue ML. The two Drosophila telomeric transposable elements have very different patterns of transcription. Mol Cell Biol. 1999;19(1):873–81. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell PH, Belote JM, Levis RW. Identification of multiple transcription initiation, polyadenylation, and splice sites in the Drosophila melanogaster TART family of telomeric retrotransposons. Nucleic Acids Res. 2006;34(19):5498–507. 10.1093/nar/gkl709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radion E, Ryazansky S, Akulenko N, Rozovsky Y, Kwon D, Morgunova V, et al. Telomeric Retrotransposon HeT-A Contains a Bidirectional Promoter that Initiates Divergent Transcription of piRNA Precursors in Drosophila Germline. J Mol Biol. 2017;429(21):3280–9. 10.1016/j.jmb.2016.12.002 . [DOI] [PubMed] [Google Scholar]
- 18.Rozhkov NV, Hammell M, Hannon GJ. Multiple roles for Piwi in silencing Drosophila transposons. Genes Dev. 2013;27(4):400–12. Epub 2013/02/09. doi: gad.209767.112 [pii] 10.1101/gad.209767.112 ; PubMed Central PMCID: PMC3589557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shpiz S, Olovnikov I, Sergeeva A, Lavrov S, Abramov Y, Savitsky M, et al. Mechanism of the piRNA-mediated silencing of Drosophila telomeric retrotransposons. Nucleic Acids Res. 2011;39(20):8703–11. Epub 2011/07/19. doi: gkr552 [pii] 10.1093/nar/gkr552 ; PubMed Central PMCID: PMC3203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgunova V, Akulenko N, Radion E, Olovnikov I, Abramov Y, Olenina LV, et al. Telomeric repeat silencing in germ cells is essential for early development in Drosophila. Nucleic Acids Res. 2015;43(18):8762–73. Epub 2015/08/05. doi: gkv775 [pii] 10.1093/nar/gkv775 ; PubMed Central PMCID: PMC4605298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana JS, Xu J, Weng Z, Theurkauf WE. Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet. 2010;6(12):e1001246 Epub 2010/12/24. 10.1371/journal.pgen.1001246 ; PubMed Central PMCID: PMC3003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye J, Renault VM, Jamet K, Gilson E. Transcriptional outcome of telomere signalling. Nat Rev Genet. 2014;15(7):491–503. 10.1038/nrg3743 . [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Panades E, Gavis ER, Casacuberta E. Specific Localization of the Drosophila Telomere Transposon Proteins and RNAs, Give Insight in Their Behavior, Control and Telomere Biology in This Organism. PLoS ONE. 2015;10(6):e0128573 Epub 2015/06/13. 10.1371/journal.pone.0128573 [pii]. ; PubMed Central PMCID: PMC4467039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vagin VV, Klenov MS, Kalmykova AI, Stolyarenko AD, Kotelnikov RN, Gvozdev VA. The RNA interference proteins and vasa locus are involved in the silencing of retrotransposons in the female germline of Drosophila melanogaster. RNA Biol. 2004;1(1):54–8. . [PubMed] [Google Scholar]
- 25.Siriaco GM, Cenci G, Haoudi A, Champion LE, Zhou C, Gatti M, et al. Telomere elongation (Tel), a new mutation in Drosophila melanogaster that produces long telomeres. Genetics. 2002;160(1):235–45. Epub 2002/01/24. ; PubMed Central PMCID: PMC1461955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryazansky S, Radion E, Mironova A, Akulenko N, Abramov Y, Morgunova V, et al. Natural variation of piRNA expression affects immunity to transposable elements. PLoS Genet. 2017;13(4):e1006731 Epub 2017/04/28. 10.1371/journal.pgen.1006731 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Beaucher M, Cheng Y, Rong YS. Coordination of transposon expression with DNA replication in the targeting of telomeric retrotransposons in Drosophila. Embo J. 2014;33(10):1148–58. Epub 2014/04/16. doi: embj.201386940 [pii] 10.1002/embj.201386940 ; PubMed Central PMCID: PMC4193921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dienstbier M, Boehl F, Li X, Bullock SL. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 2009;23(13):1546–58. 10.1101/gad.531009 ; PubMed Central PMCID: PMCPMC2704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olovnikov IA, Morgunova VV, Mironova AA, Kordyukova MY, Radion EI, Olenkina OM, et al. Interaction of Telomeric Retroelement HeT-A Transcripts and Their Protein Product Gag in Early Embryogenesis of Drosophila. Biochemistry (Mosc). 2016;81(9):1023–30. 10.1134/S000629791609011X . [DOI] [PubMed] [Google Scholar]
- 30.Jain R, Devine T, George AD, Chittur SV, Baroni TE, Penalva LO, et al. RIP-Chip analysis: RNA-Binding Protein Immunoprecipitation-Microarray (Chip) Profiling. Methods Mol Biol. 2011;703:247–63. 10.1007/978-1-59745-248-9_17 . [DOI] [PubMed] [Google Scholar]
- 31.Mach JM, Lehmann R. An Egalitarian-BicaudalD complex is essential for oocyte specification and axis determination in Drosophila. Genes Dev. 1997;11(4):423–35. Epub 1997/02/15. . [DOI] [PubMed] [Google Scholar]
- 32.Kulpa DA, Moran JV. Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol. 2006;13(7):655–60. 10.1038/nsmb1107 . [DOI] [PubMed] [Google Scholar]
- 33.Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, et al. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol. 2001;21(4):1429–39. Epub 2001/02/07. 10.1128/MCB.21.4.1429-1439.2001 ; PubMed Central PMCID: PMC99594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malone CD, Brennecke J, Dus M, Stark A, McCombie WR, Sachidanandam R, et al. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137(3):522–35. Epub 2009/04/28. doi: S0092-8674(09)00377-8 [pii] 10.1016/j.cell.2009.03.040 ; PubMed Central PMCID: PMC2882632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn T, Savitsky M, Georgiev P. Attachment of HeT-A sequences to chromosomal termini in Drosophila melanogaster may occur by different mechanisms. Mol Cell Biol. 2000;20(20):7634–42. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao G, Walser JC, Beaucher ML, Morciano P, Wesolowska N, Chen J, et al. HipHop interacts with HOAP and HP1 to protect Drosophila telomeres in a sequence-independent manner. Embo J. 2010;29(4):819–29. Epub 2010/01/09. doi: emboj2009394 [pii] 10.1038/emboj.2009.394 ; PubMed Central PMCID: PMC2829166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cenci G, Siriaco G, Raffa GD, Kellum R, Gatti M. The Drosophila HOAP protein is required for telomere capping. Nat Cell Biol. 2003;5(1):82–4. 10.1038/ncb902 . [DOI] [PubMed] [Google Scholar]
- 38.Sanghavi P, Liu G, Veeranan-Karmegam R, Navarro C, Gonsalvez GB. Multiple Roles for Egalitarian in Polarization of the Drosophila Egg Chamber. Genetics. 2016;203(1):415–32. 10.1534/genetics.115.184622 ; PubMed Central PMCID: PMCPMC4858789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro C, Puthalakath H, Adams JM, Strasser A, Lehmann R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat Cell Biol. 2004;6(5):427–35. 10.1038/ncb1122 . [DOI] [PubMed] [Google Scholar]
- 40.Theurkauf WE, Alberts BM, Jan YN, Jongens TA. A central role for microtubules in the differentiation of Drosophila oocytes. Development. 1993;118(4):1169–80. . [DOI] [PubMed] [Google Scholar]
- 41.Bullock SL, Ish-Horowicz D. Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature. 2001;414(6864):611–6. 10.1038/414611a . [DOI] [PubMed] [Google Scholar]
- 42.Cohen RS, Zhang S, Dollar GL. The positional, structural, and sequence requirements of the Drosophila TLS RNA localization element. RNA. 2005;11(7):1017–29. 10.1261/rna.7218905 ; PubMed Central PMCID: PMCPMC1370787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van De Bor V, Hartswood E, Jones C, Finnegan D, Davis I. gurken and the I factor retrotransposon RNAs share common localization signals and machinery. Dev Cell. 2005;9(1):51–62. 10.1016/j.devcel.2005.04.012 . [DOI] [PubMed] [Google Scholar]
- 44.Dufourt J, Dennis C, Boivin A, Gueguen N, Theron E, Goriaux C, et al. Spatio-temporal requirements for transposable element piRNA-mediated silencing during Drosophila oogenesis. Nucleic Acids Res. 2014;42(4):2512–24. 10.1093/nar/gkt1184 ; PubMed Central PMCID: PMCPMC3936749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor MS, LaCava J, Mita P, Molloy KR, Huang CR, Li D, et al. Affinity proteomics reveals human host factors implicated in discrete stages of LINE-1 retrotransposition. Cell. 2013;155(5):1034–48. 10.1016/j.cell.2013.10.021 ; PubMed Central PMCID: PMCPMC3904357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarro C, Bullock S, Lehmann R. Altered dynein-dependent transport in piRNA pathway mutants. Proc Natl Acad Sci U S A. 2009;106(24):9691–6. 10.1073/pnas.0903837106 ; PubMed Central PMCID: PMCPMC2688001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Megosh HB, Cox DN, Campbell C, Lin H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr Biol. 2006;16(19):1884–94. 10.1016/j.cub.2006.08.051 . [DOI] [PubMed] [Google Scholar]
- 48.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467(7319):1128–32. 10.1038/nature09465 ; PubMed Central PMCID: PMCPMC4505748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell. 2007;12(1):45–55. Epub 2007/01/03. doi: S1534-5807(06)00559-4 [pii] 10.1016/j.devcel.2006.12.001 . [DOI] [PubMed] [Google Scholar]
- 50.Pane A, Wehr K, Schupbach T. zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev Cell. 2007;12(6):851–62. Epub 2007/06/05. doi: S1534-5807(07)00147-5 [pii] 10.1016/j.devcel.2007.03.022 ; PubMed Central PMCID: PMC1945814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashkova S, Karam SE, Kellum R, Pardue ML. Gag proteins of the two Drosophila telomeric retrotransposons are targeted to chromosome ends. J Cell Biol. 2002;159(3):397–402. 10.1083/jcb.200205039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Detection of HeT-A Gag in ovaries and carcasses (imago after ovary dissection) by Western blotting. Extracts were prepared from the GIII strain (first lane) and transgenic strains expressing UAS-HeT-A-HA in the germline on a wild type background (second lane) or upon spnE_GLKD (third lane). Antibodies are indicated to the left. In ovaries, HeT-A Gag is detected only upon piRNA pathway disruption (spnE_GLKD). (B, C) HeT-A RNA FISH (green) combined with endogenous HeT-A Gag (red) immunostaining on ovaries of GIII strain. HeT-A RNPs are not reveled in the germ cells but detected in somatic cells of germarium (B, arrows) in accordance to previously published observation (23). Intensive HeT-A staining observed in the nurse cell nuclei appear to be correspond to the transcribed telomeres (arrows) (C).
(TIF)
(A) HeT-A RNA (green) and HeT-A Gag-HA (red) form HeT-A RNPs (arrows) in the cytoplasm of nurse cells in the ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. Egg chamber at stage 7 of oogenesis is shown. (B) Colocalization of HeT-A RNA (green), HeT-A Gag-HA (red) and telomeric protein HipHop (magenta) is indicated by arrows. Nurse cell nucleus of a stage 6 is shown. (C) HeT-A RNA FISH (green) combined with endogenous HeT-A Gag (red) immunostaining on ovaries of non-transgenic spnE_GLKD strain. A fragment of a stage 7 egg chamber is shown. (D) HeT-A RNA FISH (green) combined with HeT-A Gag (red) immunostaining was performed on ovaries of yw wild type strain. An egg chamber at stage 7 of oogenesis is shown. DNA is stained with DAPI (blue).
(TIF)
Immunostaining of a nuage component Vasa (red) and HeT-A Gag-HA (green) (A) or Egl (green) (B) is shown. Stage 6 egg chambers of transgenic strains expressing UAS-HeT-A-Gag-HA in the germline in wild type background (bottom panels) or upon spnE_GLKD (top panels). Arrows indicate nuage. Egl colocalizes with Vasa in nuage, while HeT-A Gag-HA staining is more diffuse and only partially overlapped with Vasa. Magnification is 63x.
(TIF)
(A) RT-qPCR analysis of RNA precipitated with anti-Egl relative to negative control (normal rabbit IgG) from ovary lysates of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. rp49 was used for normalization. Western blot analysis of co-immunoprecipitated proteins is shown to the right. The antibodies used for Western blotting are indicated to the right. The antibodies used for co-IP are indicated above the IP lanes. Lane designation: input (total lysate), pellet (insoluble fraction), IP (precipitates). Anti-Egl immunoprecipitates both Egl and BicD proteins but not HeT-A Gag which is enriched in insoluble fraction. (B) RT-qPCR analysis of RNA precipitated with anti-GFP relative to negative control (normal rabbit IgG) from ovary lysates of w; tub-Egl-GFP flies. Western blot analysis of co-immunoprecipitated proteins is shown to the right; the indications are as in (A). Anti-GFP immunoprecipitates both Egl-GFP and Egl as well as BicD indicating that Egl-BicD is an oligomeric complex. For RIP panels, the error bars represent SEM of 2 biological replicas.
(TIF)
(A) HeT-A RNA (green), HeT-A Gag-HA (magenta) and Egl (red) form granules (arrows) in the cytoplasm of nurse cells in ovaries of nosGal4; UAS-HeT-A-HA; UAS-spnE_sh flies. Egg chamber at stage 7 of oogenesis is shown. (B) Egl (red) and endogenous HeT-A Gag (green) form granules (arrows) in ovaries of spnE_GLKD not carrying UAS-HeT-A-HA transgene. DNA is stained with DAPI (blue). (C) Co-IP of HeT-A Gag. Western blot analysis of proteins immunoprecipitated with anti-Gag HeT-A from ovaries of spnE_GLKD flies. Anti-Gag HeT-A immunoprecipitates Egl protein. The antibodies used for Western blotting are indicated to the right and the antibodies used for co-IP are indicated above the IP lanes.
(TIF)
(A) A putative HLS1 (HeT-A localization signal 1) is revealed in the 3’UTR of HeT-A copies (start corresponds to 5798 position of canonical HeT-A, DM06920). Folding of HLS1 is shown. (B) Sequence of mutated HeT-A hairpin is shown. (C) Colocalization of MS2 RNA (green), HeT-A Gag-HA (magenta) and Egl (red) in the cytoplasm of nurse cells (arrowheads) and in the oocyte (arrows) in ovaries of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. Two egg chambers at different stages of oogenesis are shown. (D) MS2 RNA FISH (green) combined with immunostaining of gamma-tubulin (red) was performed on 0-2-hour old embryos of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. DNA is stained with DAPI (blue). Syncytial metaphase is shown. (E) HeT-A Gag-HA (red) and Egl (green) immunostaining were performed on 0-2-hour old embryos of nosGal4; UAS-HeT-A-HA-ms2_mut flies upon spnE_GLKD. DNA is stained with DAPI (blue).
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.