Abstract
Background
Although harness racing is of high economic importance to the global equine industry, significant genomic resources have yet to be applied to mapping harness racing success. To identify genomic regions associated with harness racing success, the current study performs genome-wide association analyses with three racing performance traits in the Norwegian-Swedish Coldblooded Trotter using the 670 K Axiom Equine Genotyping Array.
Results
Following quality control, 613 horses and 359,635 SNPs were retained for further analysis. After strict Bonferroni correction, nine genome-wide significant SNPs were identified for career earnings. No genome-wide significant SNPs were identified for number of gallops or best km time. However, four suggestive genome-wide significant SNPs were identified for number of gallops, while 19 were identified for best km time. Multiple genes related to intelligence, energy metabolism, and immune function were identified as potential candidate genes for harness racing success.
Conclusions
Apart from the physiological requirements needed for a harness racing horse to be successful, the results of the current study also advocate learning ability and memory as important elements for harness racing success. Further exploration into the mental capacity required for a horse to achieve racing success is likely warranted.
Electronic supplementary material
The online version of this article (10.1186/s12863-018-0670-3) contains supplementary material, which is available to authorized users.
Keywords: Genetic, Horse, Performance, Racehorse
Background
Regardless of horseracing discipline, speed, or perhaps more appropriately, unparalleled speed, is the “holy grail” of almost every horse owner, trainer, and breeder. However, speed alone does not necessarily equate to success on the racecourse. The manner in which a horse demonstrates speed is critical to its racing success [1–3]. For example, while the ability to gallop fast may result in a champion Thoroughbred (TB) or Quarter Horse (QH), the same ability in a Standardbred (SB) or Coldblooded Trotter (CT) is of little value. In SB and CT racing, horses undoubtedly require speed, but galloping, a four-beat gait, results in disqualification [1–3]. Thus, speed in these breeds must be demonstrated at trot, a contralateral two-beat gait, or pace, an ipsilateral two-beat gait [1–4]. Consequently, racing success in SBs, CTs, and other harness racing breeds depends not on an individual’s capacity for speed, but on an individual’s capacity for speed in a specific gait.
To date, significant genomic resources have been applied in studies attempting to map speed and racing success in TBs and QHs [4–18]. While these studies have proven to be of great value for gallop racing breeds, their applicability to harness racing breeds has been limited [19–21]. In fact, a genomic study exploring locomotion pattern in Icelandic horses is arguably the most influential study to date when it comes to racing success in harness racing breeds [22]. The study discovered that a premature stop codon in the doublesex and mab-3 related transcription factor 3 (DMRT3) gene had a major effect on the pattern of locomotion in the horse, resulting in some horses possessing the ability to trot or pace at high speed without transitioning into gallop [22]. Follow-up studies were then able to demonstrate that for many harness racing breeds the DMRT3 mutation was associated with racing success (e.g. increased earned prize money) [20, 23–26]. However, the mutation appears to only account for between 0 and 6.3% of the phenotypic variation in traits widely used to evaluate racing success [24, 26]. Considering that heritability estimates for some of these traits are as high as 0.38, the likelihood of other genes playing a significant role in a harness racing horse’s success is high [20, 23, 27–38]. Nevertheless, genome-wide association (GWA) studies with harness racing performance are lacking [23].
Despite the fact that harness racing success is of high economic importance to the global equine industry, genome scans for performance appear to predominantly target Thoroughbreds and sport horses (e.g. Warmbloods) [4–6, 8–14, 39–41]. Most studies involving harness racing breeds tend to focus on detecting genes underlying certain conditions and diseases [42–47]. While disease studies are undoubtedly important for improved animal well-being, a greater awareness of the genes, and by extension the underlying biological mechanisms, involved in racing success are also likely to prove highly valuable. A deeper understanding of the biology underpinning success in a racehorse ultimately can lead to more targeted medical treatments as well as a heightened awareness of the physical limitations (e.g. lack of speed at trot) some horses will inevitably possess.
Motivated by these facts, we conducted a GWA study to identify genomic regions and genes associated with harness racing success using the Norwegian-Swedish Coldblooded Trotter (NSCT). Norwegian-Swedish Coldblooded Trotters are ideally suited for GWA analyses of harness racing performance as their small population is not only likely to correspond with low within breed genetic variation, but the limited region in which NSCTs are eligible to race is also likely to reduce environmental variation. Thus, a more accurate assessment of the relationship between genomic regions and harness racing performance is achievable.
Methods
Data collection
Pedigree information and performance data on all raced and unraced NSCTs born between 1 January 2000 and 31 December 2009 were provided by the trotting associations in both Norway and Sweden (Norsk Rikstoto and Svensk Travsport). Pedigree information included horse name, horse id, date of birth, country of birth, sex, breeder, sire id, and dam id. Performance data, as of 8 February 2017, was presented per race per horse (i.e. data included individual race records with each record corresponding to a given horse’s specific performance in the race). This data included non-competitive premie and qualification races, with each record containing information on horse id, race date, race track, race type, race distance, trainer, owner, driver, finish position, prize money earned, gallop status, and average km time [48, 49].
Sample acquisition
In order to reflect the raced population as accurately as possible, a list of raced individuals was randomly generated from the data described above using the statistical software R [50]. In addition to having raced, two requirements were set for inclusion in the study: 1. Hair and/or blood samples had to be readily accessible from the pedigree registration authorities in either Norway (Department of Basic Sciences and Aquatic Medicine, Norwegian University of Life Sciences) or Sweden (Animal Genetics Laboratory, Swedish University of Agricultural Sciences) 2. Sufficient sample material had to be available to ensure DNA quality standards would likely be achieved. The first 661 horses on the list that met these criteria were included in the study. Average relatedness within the selected group, estimated using the genetic software Contribution, Inbreeding, Coancestry, was 0.16 (interquartile range [IQR] 0.11–0.18) [51].
DNA isolation
Deoxyribonucleic acid was prepared from the hair roots using a standard hair-preparation procedure. Briefly, 186 μL Chelex 100 Resin (Bio-Rad Laboratories, Hercules, CA) and 14 μL of proteinase K (20 mg/mL; Merck KgaA, Darmstadt, Germany) were added to the sample. The mix was incubated at 56 °C for 2 h and the proteinase K was inactivated for 10 min at 95 °C. For DNA preparation from blood samples, 350 μL of blood was used and isolated on the Qiasymphony instrument using the Qiasymphony DSP DNA mini kit (Qiagen, Hilden, Germany). A summary of the final horses selected for genotyping is shown in Table 1.
Table 1.
Summary information for the genotyped and the final sample of horses
| Genotyped Horses | Final GWA Horses | |
|---|---|---|
| N | N | |
| Sex | ||
| Intact males | 70 | 62 |
| Females | 271 | 247 |
| Geldings | 320 | 304 |
| Country of birth | ||
| Norway | 360 | 312 |
| Sweden | 301 | 301 |
| Year of birth | ||
| 2000 | 45 | 36 |
| 2001 | 73 | 66 |
| 2002 | 84 | 80 |
| 2003 | 69 | 64 |
| 2004 | 56 | 49 |
| 2005 | 62 | 55 |
| 2006 | 70 | 67 |
| 2007 | 71 | 70 |
| 2008 | 63 | 59 |
| 2009 | 68 | 67 |
| Total | 661 | 613 |
GWA Genome-wide association
Genotyping and quality control
Prior to quality control (QC) the data set consisted of individuals genotyped using the 670 K Axiom Equine Genotyping Array (n = 570) and the 670 K+ Axiom Equine Genotyping Array (n = 91). The data from the two arrays were subsequently merged based on SNP name, chromosome, and position, yielding a combined SNP data set of 611,888 SNPs for 661 horses. Iterative QC was then performed with the GenABEL package in R to remove poorly genotyped and noisy data using the following thresholds: minor allele frequency (MAF) (< 0.5%), missing genotypes per single nucleotide polymorphism (SNP) (> 5%), missing SNPs per sample (> 15%), and Hardy-Weinberg equilibrium (HWE) (first QC p < 1e− 10; second QC FDR < 0.2) [50].
Phenotyping
Pedigree and performance data for the 661 genotyped horses were structured for analyses using custom scripts written in Perl v5.20.1. Sex classifications were based on the official sex of the horse at the end of the study. Number of career starts was defined as the total number of competitive races for each individual (i.e. premie and qualification races were excluded). Number of gallops (NG) was defined as the total number of competitive races in which the horse was recorded as galloping at some point during the race. Career earnings (CE) were calculated as the total amount of prize money won for each horse as of 8 February 2017. Prize money won in Sweden (SEK) was converted to Norwegian currency (NOK) based on the average exchange rate for the year in which the race occurred [52]. Horses having participated in only premie or qualification races were given a value for career earnings of − 1 NOK in order to distinguish them from horses that had zero career earnings, despite having raced competitively. Best km time (BT), independent of starting method, was defined as the fastest average km time for a competitive race in which the horse was not recorded as galloping during the race (i.e. races in which a horse galloped, regardless of if the horse was disqualified, were excluded).
Genome-wide association analyses
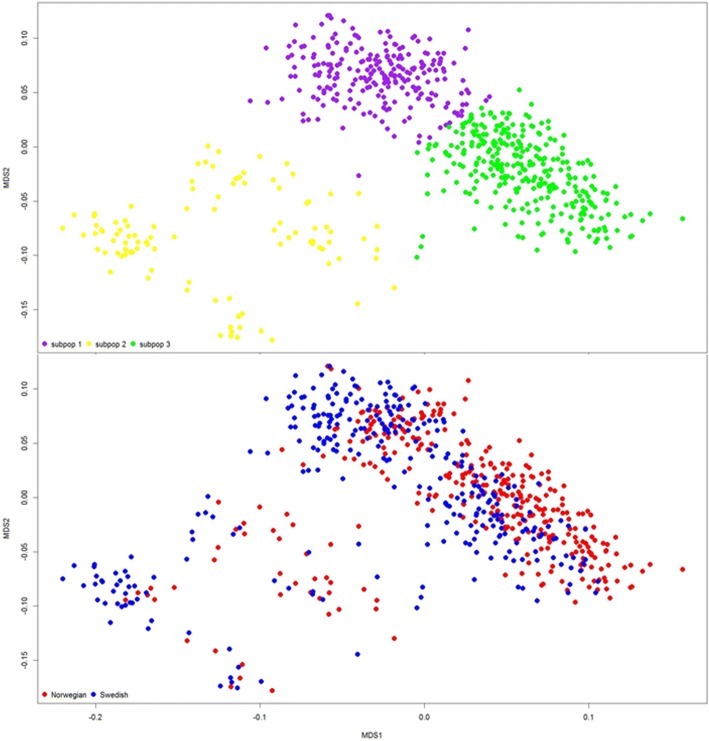
Genome-wide association (GWA) analyses were performed using the GenABEL package in R [50]. The package was used to compute an autosomal genomic kinship matrix as well as perform standard K-means clustering. K-means clustering with K = {1,2,…,10} were executed to determine the number of clusters (subpopulations). For each iteration, the sum of within-cluster sum of squares (∑WCSS) was calculated and subsequently plotted vs. K. To define the subpopulations, the number of clusters corresponding with the first inflection point (K = 3) was chosen [53]. The multidimensional scaling (MDS) plot yielded no apparent outliers and a visualization of the genomic-kinship matrix and subpopulations using MDS can be seen in Fig. 1.
Fig. 1.
Visualization of multidimensional scaling, stratified by country of birth and subpopulation, performed on the genomic-kinship matrix
To account for any population stratification, genome-wide association analyses of CE, BT, and NG were performed using a mixed model-structured association approach (“mmscore” function with the “strata” option in GenABEL). Preliminary analyses indicated significant effects of sex, birth year, and number of career starts on all three traits. Country of birth was also shown to influence CE. As a result, sex, birth year, number of career starts, and country of birth were included as co-variants in the final analyses accordingly. Genome-wide significance for each of the analyses was determined by Bonferroni correction (p < 1.39 × 10− 7, corrected for total number of SNPs post QC) while a “suggestive” genome-wide significance threshold was also set at 1.0 × 10− 5. Manhattan and quantile-quantile plots were generated in R while the extent of linkage disequilibrium (LD) was estimated by calculating r2 values between all pairs of SNPs with inter-SNP distances of less than 1 Mb using PLINK v1.09 (http://zzz.bwh.harvard.edu/plink/). The effB in the GenABEL result was regarded as the allele substitution effect and the proportion of phenotypic variance explained by each significant SNP was estimated as follows:
Where p and q are the allele frequencies, β is the estimated allele substitution effect, and S2 is the sample phenotypic variance. Stepwise regressions were then performed to estimate the total proportion of phenotypic variation explained by the multiple genome-wide associated SNPs (before and after pruning at r2 > 0.2; PLINK command –indep-pairwaise 100 25 0.2) for each trait using the lm function in R [50]. The bioinformatics database Ensembl (http://www.ensembl.org/) was used for candidate gene screening. Genomic coordinates of genome-wide significant and suggestive genome-wide signficant SNPs +/− 500 kb were used as inputs to generate a list of annotated genes using the Ensembl Biomart function. The PANTHER Classification system was then used to obtain an overview of the biological processes, molecular functions, and pathways known to be affected by these genes [54, 55].
Results
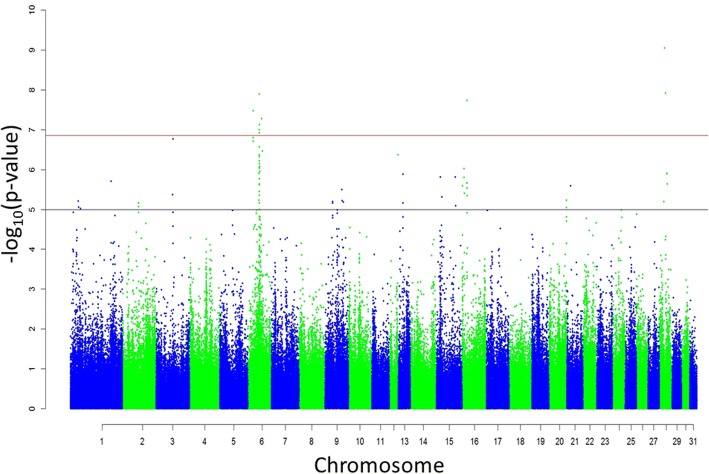
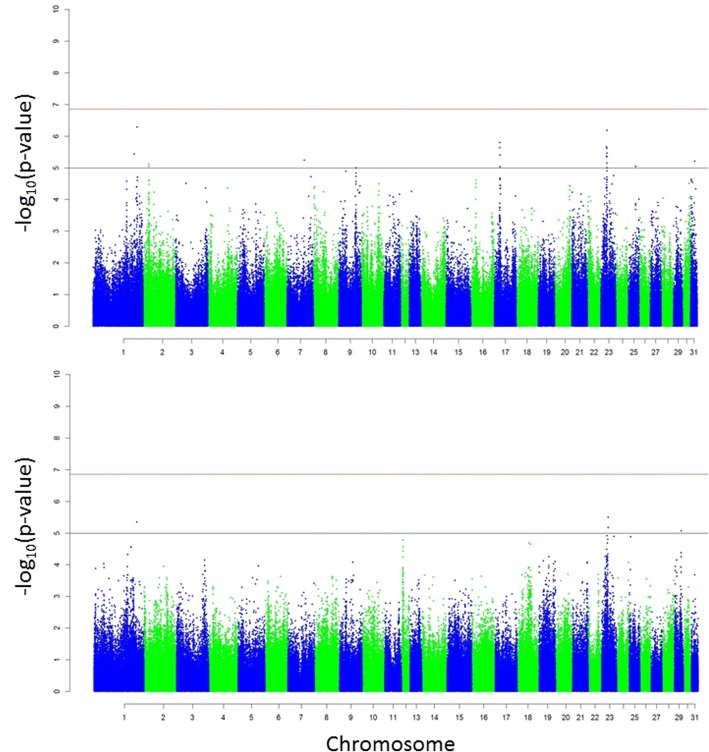
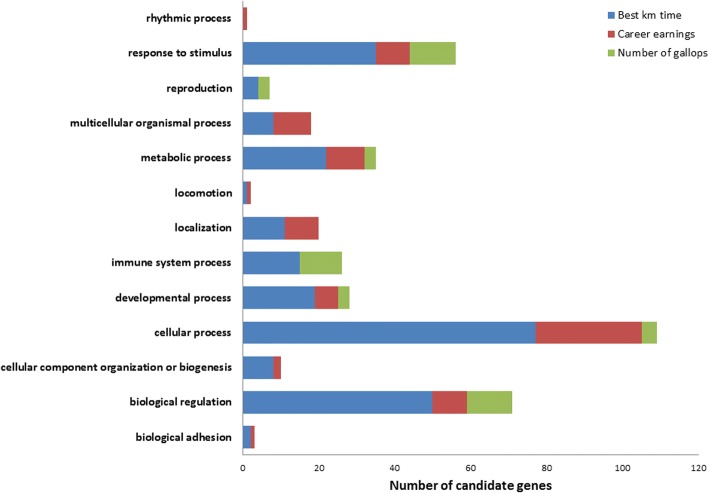
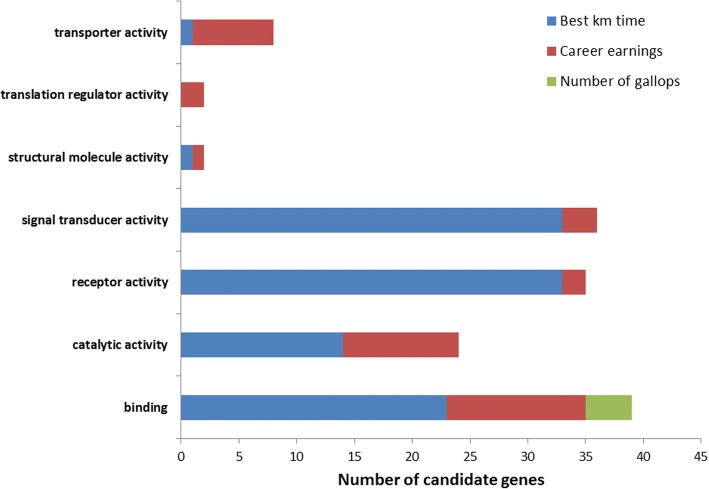
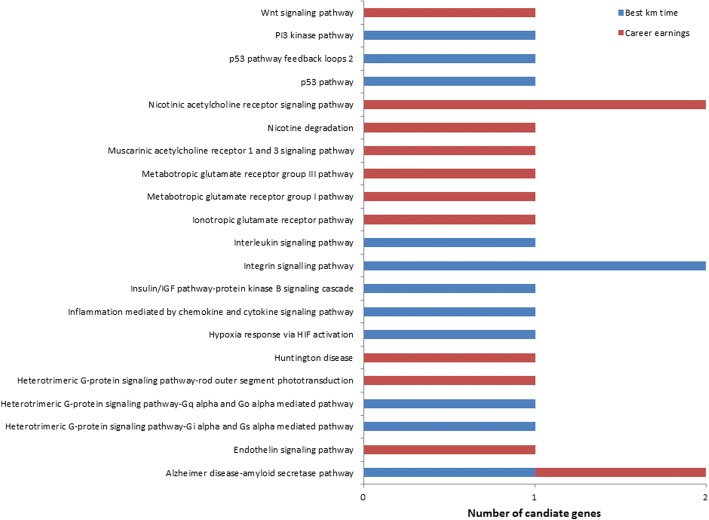
Following QC, 359,635 autosomal SNPs and 642 horses were available for association analyses. Of these individuals, 29 had only participated in non-competitive premie and/or qualification races. Initial association analyses with CE, combined with the vast array of reasons known to prevent a horse from competitive racing suggested the exclusion of these horses would help to reduce noise in the final analyses. As a result, only 613 horses, representing 120 sires (interquartile range [IQR] 1–5) and 547 dams (IQR 1–1), were included in the final GWA analyses (Table 1). Descriptive statistics for CE, BT, and NG in the final sample are presented in Table 2. Both CE and NG were not normally distributed and were subsequently log transformed for the GWA analyses. The extent of LD decayed faster across the final sample of horses with mean r2 dropping below 0.20 by 3 kb (Additional file 1). The GWA analysis of CE yielded multiple genome-wide significant SNPs (p < 1.39 × 10− 7), with the majority of these SNPs residing on Equus caballus chromosome (ECA) 6 (Fig. 2; Table 3; Additional file 2). Analyses of both BT and NG failed to result in genome-wide significant SNPs. However, two regions of interest were apparent based on the presence of slight peaks on ECA17 and ECA23 in the resulting Manhattan plots (Fig. 3; Additional file 2). Genome-wide significant SNPs for CE, suggestive genome-wide significant SNPs for BT and NG, and the nearest genes are shown in Table 3. The most significant SNP was detected at the 20,006,740 position on chromosome 28 (AX-104828170, p = 9.01E-10) and presented an estimated allele substitution effect of − 7079.46 NOK. The favourable allele appeared to be the T allele, with each C allele resulting in a negative effect on CE. Despite the fact that the frequency of the T allele was 98.2% and the frequency of the C allele was only 1.8%, the percentage of phenotypic variance explained by this SNP was 3.85%. The 32 SNPs in total were estimated to explain 18.34%, 18.71% and 33.17% of the variation for CE, NG and BT, respectively, in the population studied. Consequently, after LD pruning, only 17 SNPs remained – explaining 14.17%, 18.38% and 33.13% of the variation for CE, NG and BT, respectively (Table 3). Overall, 378, 144, and 23 candidate genes identified were associated with known biological processes, molecular functions, and pathways, respectively (Figs. 4, 5 and 6; Additional file 3).
Table 2.
Descriptive statistics of CE, BT, NG for the final sample of horses (n = 613)
| Min | 25th percentile | Median | Mean | 75th percentile | Max | |
|---|---|---|---|---|---|---|
| Career earnings (NOK) | 0 | 36,321 | 124,625 | 302,506 | 327,493 | 4,216,554 |
| Best km time (s) | 78.9 | 86.8 | 89.3 | 89.6 | 92.4 | 105.7 |
| Number of gallops | 0 | 5 | 11 | 14.4 | 21 | 123 |
CE career earnings, BT Best km time, NG number of gallops
Fig. 2.
Manhattan plot of the genome-wide association analysis of career earnings. The red horizontal line indicates the genome-wide significance level and the black horizontal line indicates the suggestive genome-wide significance level. Uncorrected λ = 1.0532
Table 3.
Genome-wide significant SNPs for CE and suggestive genome-wide significant SNPs for BT and NG
| SNP | Analysis | Location (ECA:bp) | MAF | β | SE | Var (%) | Nearest gene | Distance to nearest gene (bp) | Raw p-value |
|---|---|---|---|---|---|---|---|---|---|
| AX-104828170 | CE | 28:20,006,740 | 0.018 | −3.380 | 0.552 | 3.85 | NDUFA12 | 114,732 | 9.01E-10 |
| AX-103147507 | CE | 28:22,153,465 | 0.005 | −5.762 | 1.011 | 3.27 | ENSECAG00000025907 | 46,196 | 1.20E-08 |
| AX-104611735 | CE | 6:47,132,529 | 0.015 | −3.407 | 0.599 | 3.16 | PDE3A | 0 | 1.28E-08 |
| AX-104865129 | CE | 16:18,366,321 | 0.008 | −4.488 | 0.798 | 3.17 | ENSECAG00000003087/PROK2 | 138,149/138,791 | 1.87E-08 |
| AX-104494389 | CE | 6:20,020,914 | 0.006 | −4.557 | 0.825 | 3.32 | INPP5D | 0 | 3.36E-08 |
| AX-103248294 | CE | 6:49,512,490 | 0.016 | −3.166 | 0.582 | 3.11 | SOX5 | 0 | 5.28E-08 |
| AX-103090138 | CE | 6:42,063,985 | 0.018 | −3.007 | 0.559 | 2.46 | PLBD1 | 3191 | 7.48E-08 |
| AX-104711589 | CE | 6:41,462,481 | 0.012 | −3.497 | 0.657 | 2.59 | GRIN2B | 0 | 9.99E-08 |
| AX-104307051 | CE | 6:41,329,519 | 0.012 | −3.474 | 0.657 | 2.67 | GRIN2B | 0 | 1.21E-07 |
| AX-104144838 | NG | 23:23,333,501 | 0.278 | −0.214 | 0.046 | 1.51 | ENSECAG00000023609 | 187,643 | 3.18E-06 |
| AX-104568609 | NG | 1:159,285,045 | 0.004 | −1.343 | 0.293 | 1.14 | ENSECAG00000003696/ENSECAG00000022264 | 19,813/25,804 | 4.52E-06 |
| AX-102982528 | NG | 23:23,324,996 | 0.412 | −0.186 | 0.041 | 1.27 | ENSECAG00000023609 | 179,138 | 6.74E-06 |
| AX-103734745 | NG | 29:24,530,437 | 0.453 | −0.176 | 0.040 | 1.29 | ENSECAG00000004576 | 944,126 | 8.67E-06 |
| AX-104373992 | BT | 1:162,993,722 | 0.016 | 3.289 | 0.655 | 1.79 | ENSECAG00000023062/ENSECAG00000008721 | 32,728/51,254 | 5.10E-07 |
| AX-103261370 | BT | 23:22,522,071 | 0.409 | −0.910 | 0.183 | 0.87 | DOCK8 | 0 | 6.49E-07 |
| AX-104219924 | BT | 17:19,525,955 | 0.279 | 0.946 | 0.197 | 2.24 | WDFY2 | 0 | 1.57E-06 |
| AX-104634248 | BT | 23:21,857,316 | 0.108 | 1.346 | 0.284 | 1.21 | PIP5K1B/FAM122A | 14,893/18,794 | 2.20E-06 |
| AX-103287280 | BT | 23:21,064,571 | 0.117 | 1.270 | 0.268 | 1.49 | PTAR1 | 43,300 | 2.22E-06 |
| AX-104645782 | BT | 17:19,318,167 | 0.258 | 0.951 | 0.201 | 2.21 | ATP7B | 0 | 2.32E-06 |
| AX-103762427 | BT | 23:22,461,979 | 0.154 | −1.156 | 0.245 | 1.37 | DOCK8 | 0 | 2.45E-06 |
| AX-103530176 | BT | 23:22,464,604 | 0.156 | −1.139 | 0.246 | 1.25 | DOCK8 | 0 | 3.49E-06 |
| AX-103445942 | BT | 1:151,919,692 | 0.012 | 3.600 | 0.777 | 1.39 | ENSECAG00000012236/ENSECAG00000013533 | 74,605/78,356 | 3.63E-06 |
| AX-104538418 | BT | 17:21,083,126 | 0.279 | 0.905 | 0.196 | 2.29 | KCNRG | 0 | 3.99E-06 |
| AX-104268231 | BT | 23:21,689,609 | 0.385 | −0.823 | 0.179 | 0.79 | PIP5K1B | 0 | 4.25E-06 |
| AX-102964033 | BT | 23:22,423,197 | 0.121 | −1.259 | 0.275 | 0.95 | DOCK8 | 5699 | 4.59E-06 |
| AX-104117851 | BT | 7:65,266,179 | 0.010 | 3.724 | 0.821 | 1.76 | ENSECAG00000007398 | 162,661 | 5.78E-06 |
| AX-104642194 | BT | 31:14,300,483 | 0.006 | 4.612 | 1.021 | 1.37 | MTRF1L/FBXO5 | 108/14,491 | 6.31E-06 |
| AX-103166989 | BT | 23:22,496,787 | 0.116 | −1.270 | 0.283 | 0.99 | DOCK8 | 0 | 7.07E-06 |
| AX-103803214 | BT | 2:21,466,714 | 0.049 | 1.725 | 0.386 | 1.82 | AGO1 | 2494 | 7.72E-06 |
| AX-104450418 | BT | 2:21,311,680 | 0.087 | 1.351 | 0.304 | 1.93 | TEKT2/ADPRHL2 | 10,587/14,440 | 9.00E-06 |
| AX-103305676 | BT | 25:26,866,219 | 0.019 | 2.590 | 0.584 | 1.67 | OR1L3 | 492 | 9.11E-06 |
| AX-104591507 | BT | 17:20,813,164 | 0.249 | 0.898 | 0.203 | 1.84 | KCNRG | 227,34 | 9.36E-06 |
CE career earnings, BT Best km time, NG number of gallop, MAF Minor allele frequency, β Estimated allele substitution effect, Var (%) Percentage of phenotypic variance explained
Red line = Bonferroni threshold (P < 1.39 × 10−7)
Fig. 3.
Manhattan plots of the genome-wide association analyses of best km time and number of gallops. The red horizontal lines indicate the genome-wide significance levels and the black horizontal lines indicate the suggestive genome-wide significance levels. Top panel: Best km time analysis, uncorrected λ = 1.0902. Bottom panel: Number of gallops analysis, uncorrected λ = 1.0256
Fig. 4.
Biological process summary information from the functional classification analysis of candidate genes in PANTHER. PANTHER biological process classification: the function of the protein in the context of a larger network of proteins that interact to accomplish a process at the level of the cell or organism
Fig. 5.
Molecular function summary information from the functional classification analysis of candidate genes in PANTHER. PANTHER molecular function classification: the function of the protein by itself or with directly interacting proteins at a biochemical level
Fig. 6.
Pathway summary information from the functional classification analysis of candidate genes in PANTHER
Discussion
Knowing where, why, and how genes and athletic prowess intersect in a racehorse has long been the goal of countless researchers, veterinarians, breeders, trainers, and owners [4–38]. While great strides in this area have recently been made for gallop racing horses, similar advancements for harness racing horses have been limited [4–26]. Using the NSCT, the current study explored the genetic background for athletic prowess in a harness racing horse by performing GWA analyses and functional classification for three traits associated with harness racing success. These analyses resulted in a total of 32 SNPs of interest with 9 demonstrating genome-wide significance and 13 residing in genes. Subsequent functional classifications went on to provide further support of the complexity of harness racing success with several candidate genes involved in neurological, metabolic, and musculoskeletal regulation identified. Since a gene can be declared as a candidate gene if at least 1 out of the 4 following characteristics are present: 1) the gene has a known physiological role in the phenotype of interest, 2) the gene affects the trait in question based on studies of knockouts, mutations, or transgenics in other species, 3) The gene is preferentially expressed in organs related to the quantitative trait, or 4) the gene is preferentially expressed during developmental stages related to the phenotype, a large fraction of the genes identified in the current study can plausibly be considered as candidate genes [56]. As a result, the following discussion prioritizes genes that contained variants with significant or suggestive associations with our traits of interest. The rationale for this prioritization is simply that for associated variants that reside outside of annotated genes, it is in general more difficult to determine which gene(s) the variants act on.
Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B)
Two genome-wide significant SNPs associated with career earnings were located in the GRIN2B gene, a gene also identified in a previous study exploring pacing ability in Icelandic horses [57]. The gene has been shown to be involved in neural regulations in humans and laboratory species with mutations in the gene having been associated with neurodevelopmental disorders [58–60]. Considered to be an important factor for learning and memory, one can only speculate as to its association with career earnings in a harness racing horse. However, horses with a greater capacity to learn and adapt to the highly variable nuances of harness racing would conceivably be more likely to achieve racing success. On the other hand, the gene’s association with attention-deficit/hyperactivity disorder in humans suggests that perhaps certain horses lack the ability to focus on racing and training, thereby preventing or at least hindering their racing performance [61].
ATPase copper transporting beta (ATP7B)
A single suggestive genome-wide significant SNP associated with best time was located in the ATP7B gene on ECA17. The gene encodes a protein that functions as a monomer, exporting copper out of cells. Excess copper can cause serious toxicity with the process of excess copper disposal relying heavily on ATP7B [62, 63]. Over 500 mutations have been identified in the gene, 380 of which are considered to be disease causing mutations [64]. Elevated levels of copper in the body often result in muscle stiffness with acute muscle stiffness prior to a race having the potential to affect individual performance and chronic muscle stiffness likely to impact a horse’s conditioning, trainability, and overall capacity for speed [62, 63].
Potassium channel regulator (KCNRG)
The KCNRG gene on ECA17 was also identified by two SNPs demonstrating suggestive genome-wide significance for best time. The gene encodes a protein which regulates the activity of voltage-gated potassium channels with a study using songbirds suggesting potassium channels to be lineage specific [64, 65]. The same study also revealed that apart from broad expression in the brain a subset of potassium channel genes are selectively expressed, with the authors hypothesizing that the KCNRG gene may be associated with learning [65]. Although no previous studies of racehorse performance have specifically identified KCNRG as important for racing success, a large conserved haplotype on ECA17 has been advocated to have selective importance in Thoroughbreds and closely related breeds [4, 66].
Also of note is the role voltage-gate potassium channels play in Hyperkalemic periodic paralysis (HYPP), a genetic disorder predominantly seen in Quarter Horses. The condition, caused by a mutation in the sodium voltage-gated channel alpha subunit 4 (SCN4A) gene, manifests intermittently with clinical signs ranging from muscle fasciculation to signs of paresis [67–70]. Hyperkalemia, a term used to describe abnormally high levels of potassium in the blood, is often seen during or immediately after an attack. Voltage-gated potassium channels are thought to remain open, allowing continual potassium efflux, thereby promoting an open sodium channel configuration. As a result, an HYPP attack can be triggered or an already occurring attack can increase in severity [67, 70–72]. Horses with HYPP also tend to possess hypertrophic muscles; however, they have been reported as having a reduced tolerance to exercise with relatively more lactate being produced during exercise [73, 74].
Phosphatidylinositol-4-phosphate 5-kinase type 1 beta (PIP5K1B)
A single suggestive genome-wide significant SNP associated with best time was also located in the PIP5K1B gene on ECA23. Three widely expressed isoforms of PIP5K1 are responsible for the regulation of the major pools of cellular phosphatidylinostitols in mammalian tissues, with PIP5K1B negatively regulated in response to oxidative stress [75, 76]. Neurite outgrowth, a critical process for neuronal development, has also been shown as negatively regulated by PIP5K1A [77]. Since the current study is the first to suggest an association between PIP5K1B and racing success, understanding the roles PIP5K1 isoforms have on a horse’s capacity for speed remains a task for future studies. However, genes that influence cell differentiation processes, such as endocytosis, assuredly contribute in some way or another to the physical limitations and overall performance of any racehorse.
Dedicator of cytokinesis 8 (DOCK8)
Perhaps the most obvious candidate gene for harness racing success in the current study was DOCK8. Five suggestive genome-wide significant SNPs indicated the importance of DOCK8 to a horse’s best time, with 4 of the SNPs located in the gene. Mutations in DOCK8 result in a form of hyper-IgE syndrome; however, loss or mutations of DOCK8 have also been associated with intelligence and motor retardation [78–83]. While the importance of intelligence in a racehorse has been briefly discussed above, in the case of DOCK8 the significance of the gene may lie with its link to motor skills. DOCK8 is not only located on ECA23, the same chromosome as DMRT3, but multiple studies have hypothesized some sort of commonality or overlap between DMRT-(1,2,3) gene effects and DOCK8 [22, 82, 84]. Despite the established association between DMRT3 and harness racing performance, additional research of DMRT3 in horses strongly suggest that the mutation is unlikely to be the single cause of gaiting ability [22, 53, 85–88]. Therefore, it is conceivable that DOCK8 also significantly contributes to gaiting ability, ultimately playing some role in a harness racing horse’s propensity to exhibit speed at trot or pace.
Phosphodiesterase 3A (PDE3A)
The protein encoded by the PDE3A gene, a gene on ECA6 in which a single genome-wide significant SNP associated with career earnings is located, plays a critical role in cardiovascular function [89–91]. The encoded protein regulates vascular smooth muscle contraction and relaxation and has been linked to familial hypertension, cardiovascular disease, and fertility [89–92]. Healthy cardiovascular function is important for racing success as the act of racing undeniably requires a higher than resting-level of oxygen to support the horse’s increased muscle activity. A mutation in the PDE3A gene that ultimately alters cardiovascular function could potentially prevent a horse from meeting the higher metabolic demands of racing, thus decreasing his/her chances of winning and limiting his/her career earnings. On the contrary, an advantageous mutation in the gene could allow some horses to perform at an even greater cardiovascular level, increasing their likelihood of winning races and earning more prize money.
Inositol polyphosphate-5-phosphatase D (INPP5D) & SRY-box 5 (SOX5)
Also identified by single genome-wide significant SNPs on ECA6 were the INPP5D and the SOX5 genes. The INPP5D gene is an important regulator of immune cell signaling, while the SOX5 gene is involved in embryonic development and has been associated with multiple human diseases and disorders [93–99]. Moreover, both genes have been suggested as important in B cell activity indicating that their association with career earnings in the current study may be rooted in the immune response of a horse [95, 100]. However, mutations in SOX5 have also been theorized to disrupt neuronal development and function [101, 102].
Other candidate genes
Regions on ECA1, ECA7, and ECA16 have also previously been described as important for endurance performance traits, while regions on ECA14 and ECA18 associated with gallop racing in other studies do not appear to play a significant role in harness racing [4–8, 10–15, 19–21, 39, 66]. This likely suggest a greater demand for endurance in harness racing compared to gallop racing and is perhaps a sign of the different physiological demands for speed in trot versus speed in gallop. Candidate genes for harness racing success in the current study were also identified on ECA1, ECA2, ECA6, ECA7, ECA16, ECA17, ECA23, ECA25, ECA28, ECA29, and ECA31. However, it is important to note that the MAF threshold applied in the current study is slightly lower than is generally accepted. Although this may have inadvertently resulted in some SNP associations being simply by chance, it is also plausible that the lower MAF threshold allowed for the capture of candidate genes/regions that are perhaps the difference between an elite horse and a very, very good horse. Racing performance is undoubtedly complex and the unique history of the NSCT, being a blend of draught horse and racehorse, means that rare variants cannot be ruled out purely because they are rare – particularly when one considers the rarity of an elite racehorse. While not all candidate variants/genes are discussed above, the results of the current analyses clearly suggest that different molecular and cellular events mediate adaptive processes in the neuromusculoskeletal system in response to exercise. High intensity exercise (e.g. racing) is known to be associated with significant physiological adaptations in the neuromuscular system in equine athletes with prolonged and intense exercise potentially resulting in oxidative damage to cellular constituents [103]. Moreover, the importance of the central nervous system (CNS) as a critical “central governing” factor in sporting performance has been previously documented in endurance horses with exercise shown to induce several biological processes that regulate neurological functions that help to maintain good mental health [104]. Our results add to this line of thought, providing further evidence that genes involved in neural regulations (e.g. GRIN2B) likely play an important role in controlling the fundamental biological processes underlying adaptation to equine athletic performance.
Conclusions
After strict Bonferroni correction, 9 genome-wide significant and 23 suggestive genome-wide significant SNPs associated with harness racing success were identified. These SNPs were located on ECA1, ECA2, ECA6, ECA7, ECA16, ECA17, ECA23, ECA25, ECA28, ECA29, and ECA31 with eight genes (GRIN2B, DOCK8, ATP7B, KCNRG, PIP5K1B, PDE3A, INPP5D, SOX5) suggested as strong candidate genes for harness racing success. Apart from the physical attributes required to achieve racing success, multiple candidate genes identified in the current study also advocate learning ability and memory as critical to success. However, further analyses of these genes based on additional genetic and functional studies are required to explore this notion in greater detail. Moreover, future studies should also consider a validation study with an independent population as well as sequencing of candidate genes to better identify causal alleles.
Additional files
Extent of linkage disequilibrium of the final sample of horses. Pairwise r2 was calculated between each SNP within 1 Mb. (TIFF 15316 kb)
QQ plots for earnings, best km time, and number of gallops analyses. Top panel - corrected QQ plot for earnings analysis (Uncorrected λ = 1.0532); Middle panel – corrected QQ plot for best km time analysis (λ = 1.0902); Bottom panel – corrected QQ plot for number of gallops analysis (λ = 1.0256). (TIF 111 kb)
Functional classification gene list from PANTHER analysis. (XLSX 27 kb)
Acknowledgements
We want to thank Christina Olsson, Annica Edberg, Knut Larsen, Chameli Lilja Lawrence, Thorvaldur Árnason, the Swedish Trotting Association, and the Norwegian Trotting Association for providing their support and the data/samples for the study.
Funding
This work was supported by the Swedish-Norwegian Foundation for Equine Research, H-15-47-075, http://hastforskning.se (GL, ES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the Swedish Trotting Association (Stockholm, Sweden) and the Norwegian Trotting Association (Oslo, Norway), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of the Swedish Trotting Association (Stockholm, Sweden) and the Norwegian Trotting Association (Oslo, Norway).
Abbreviations
- ATP7B
ATPase copper transporting beta
- BT
Best km time
- CE
Career earnings
- CT
Coldblooded trotter
- DMRT3
Doublesex and mab-3 related transcription factor 3
- DOCK8
Dedicator of cytokinesis
- ECA
Equus caballus chromosome
- GRIN2B
Glutamate ionotropic receptor NMDA type subunit 2B
- GWA
Genome-wide association
- INPP5D
Inositol polyphosphate-5-phosphatase D
- KCNRG
Potassium channel regulator
- NG
Number of gallops
- NSCT
Norwegian-Swedish Coldblooded Trotter
- PDE3A
Phosphodiesterase 3A
- PIP5K1B
Phosphatidylinositol-4-phosphate 5-kinase type 1 beta
- QH
Quarter horse
- SB
Standardbred trotter
- SOX5
SRY-box 5
- TB
Thoroughbred
Authors’ contributions
BDV, KJF, CI, ES, and GL conceived and designed the experiments; KJF, MKR, and KHR contributed to sampling. GL and ES contributed the reagents and MKR extracted the DNA; BDV and MS analyzed the data and drafted the manuscript; KJF, MS, CI, ES, and GL discussed and contributed to data analysis; All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures and sample collection methods were approved by the Ethics Committee for Animal Experiments in Uppsala, Sweden [Number: C 121/14]. Samples used in the study were already available at either the Animal Genetics Laboratory at SLU in Uppsala, Sweden or the Department of Basic Sciences and Aquatic Medicine at the Norwegian University of Life Sciences in Oslo, Norway as they previously had been used for parentage testing. Permission to use the samples was granted from the Swedish Trotting Association and the Norwegian Trotting Association (the owners of the samples per the rules/guidelines of the industry).
Consent for publication
Not applicable.
Competing interests
The authors have the following interest: GL is a co-inventor on a granted patent concerning commercial testing of the DMRT3 mutation: A method to predict the pattern of locomotion in horses. PCT EP 12747875.8. European patent registration date: 2011-05-05, US patent registration date: 2011-08-03. There are no further patents, products in development, or marketed products to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United States Trotting Association. Charter, Bylaws, Rules and regulations. In: Rule book. United States trotting association. 2018. http://www.ustrotting.com/pdf/USTARuleBook.pdf. Accessed 10 Jan 2018.
- 2.Harness Racing Australia. Australian harness racing rules. Harness racing Australia Inc. 2017. http://www.harness.org.au/rules/AHRRules.pdf. Accessed 10 Jan 2018.
- 3.Union Européenne du Trot. International Agreement On Trotting Races. Union Européenne du Trot. 2018. http://www.uet-trot.eu/images/pdf-uet/en/publications/international_agreement_on_trotting_races.pdf. Accessed 10 Jan 2018.
- 4.Petersen JL, Mickelson JR, Rendahl AK, Valberg SJ, Andersson LS, Axelsson J, et al. Genome-wide analysis reveals selection for important traits in domestic horse breeds. PLoS Genet. 2013;9:e1003211. doi: 10.1371/journal.pgen.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill EW, McGivney BA, Gu J, Whiston R, MacHugh DE. A genome-wide SNP-association study confirms a sequence variant (g.66493737C>T) in the equine myostatin (MSTN) gene as the most powerful predictor of optimum racing distance for thoroughbred racehorses. BMC Genomics. 2010;11:552. doi: 10.1186/1471-2164-11-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen JL, Valberg SJ, Mickelson JR, McCue ME. Haplotype diversity in the equine myostatin gene with focus on variants associated with race distance propensity and muscle fiber type proportions. Anim Genet. 2014;45:827–835. doi: 10.1111/age.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozaki T, Hill EW, Hirota K, Kakoi H, Gamahara H, Miyake T, Sugita S, Hasegawa T, Ishida N, Nakano Y, Kurosawa M. A cohort study of racing performance in Japanese thoroughbred racehorses using genome information on ECA18. Anim Genet. 2011;43:42–52. doi: 10.1111/j.1365-2052.2011.02201.x. [DOI] [PubMed] [Google Scholar]
- 8.Shin DH, Lee JW, Park JE, Choi IY, Oh HS, Kim HJ, Kim H. Multiple genes related to muscle identified through a joint analysis of a two-stage genome-wide association study for racing performance of 1,156 thoroughbreds. Asian Australas J Anim Sci. 2015;28:771–781. doi: 10.5713/ajas.14.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon S, Lee JW, Shin D, Shin KY, Kim J, Choi IY, Kim J, Kim H. A genome-wide scan for selective sweeps in racing horses. Asian Australas J Anim Sci. 2015;28:1525–1531. doi: 10.5713/ajas.14.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tozaki T, Miyake T, Kakoi H, Gawahara H, Sugita S, Hasegawa T, Ishida N, Hirota K, Nakano Y. A genome-wide association study for racing performances in thoroughbreds clarifies a candidate region near the MSTN gene. Anim Genet. 2010;41:28–35. doi: 10.1111/j.1365-2052.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- 11.Hill EW, Gu J, Eivers SS, Fonseca RG, McGivney BA, Govindarajan P, Orr N, Katz LM, MacHugh DE. A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS One. 2010;5:e8645. doi: 10.1371/journal.pone.0008645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binns MM, Boehler DA, Lambert DH. Identification of the myostatin locus (MSTN) as having a major effect on optimum racing distance in the thoroughbred horse in the USA. Anim Genet. 2010;41:154–158. doi: 10.1111/j.1365-2052.2010.02126.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill EW, Fonseca RG, McGivney BA, Gu J, MacHugh DE, Katz LM. MSTN genotype (g.66493737C/T) association with speed indices in thoroughbred racehorses. J Appl Physiol. 2012:86–90. [DOI] [PubMed]
- 14.Tozaki T, Sato F, Hill EW, Miyake T, Endo Y, Kakoi H, Gawahara H, Hirota K, Nakano Y, Nambo Y, Kurosawa M. Sequence variants at the myostatin gene locus influence the body composition of thoroughbred horses. J Vet Med Sci. 2011;73:1617–1624. doi: 10.1292/jvms.11-0295. [DOI] [PubMed] [Google Scholar]
- 15.McGivney BA, Browne JA, Fonseca RG, Katz LM, MacHugh DE, Whiston R. Hill EW. MSTN genotypes in thoroughbred horses influence skeletal muscle gene expression and racetrack performance. Anim Genet. 2012;43:810–812. doi: 10.1111/j.1365-2052.2012.02329.x. [DOI] [PubMed] [Google Scholar]
- 16.Gu J, MacHugh DE, McGivney BA, Park SDE, Katz LM, Hill EW. Association of sequence variants in CKM (creatine kinase, muscle) and COX412 (cytochrome c oxidase, subunit 4, isoform 2) genes with racing performance in thoroughbred horses. Equine Vet J. 2010;42:569–575. doi: 10.1111/j.2042-3306.2010.00181.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill EW, Gu J, McGivney BA, MacHugh DE. Targets of selection in the thoroughbred genome contain exercise-relevant gene SNPs associated with elite racecourse performance. Anim Genet. 2010;41:56–63. doi: 10.1111/j.1365-2052.2010.02104.x. [DOI] [PubMed] [Google Scholar]
- 18.Meira CT, Fortes MRS, Farah MM, Porto-Neto LR, Kelly M, Moore SS, Pereira GL, Chardulo LAL, Curi RA. Speed index in the racing quarter horse: a genome-wide association study. J Equine Vet Sci. 2014;34:1263–1268. doi: 10.1016/j.jevs.2014.09.003. [DOI] [Google Scholar]
- 19.Dall’Olio S, Fontanesi L, Nanni Costa L, Tassinari M, Minieri L, Falaschini A. Analysis of horse myostatin gene and identification of single nucleotide polymorphisms in breeds of different morphological types. J Biomed Biotechnol. 2010;542945. [DOI] [PMC free article] [PubMed]
- 20.Negro Rama S, Valera M, Membrillo A, Gómez MD, Solé M, Menendez-Buxadera A, Anaya G, Molina A. Quantitative analysis of short- and long-distance racing performance in young and adult horses and association analysis with functional candidate genes in Spanish trotter horses. J Anim Breed Genet. 2016;133:347–356. doi: 10.1111/jbg.12208. [DOI] [PubMed] [Google Scholar]
- 21.Velie BD, Bas Conn L, Petäjistö K, Røed KH, Ihler CF, Strand E, Fegraeus KJ, Lindgren G. The importance of MSTN for harness racing performance in the Norwegian-Swedish coldblooded trotter and the Finnhorse. Proc 10th World Congr Genet Appl Livest Prod. 2018;11:36. [Google Scholar]
- 22.Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;30:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricard A. Does heterozygosity at the DMRT3 gene make French trotters better racers? Genet Sel Evol. 2015;47:10. doi: 10.1186/s12711-015-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jäderkvist K, Andersson LS, Johansson AM, Arnason T, Mikko S, Eriksson S, Anderson L, Lindgren L. The DMRT3 ‘gait keeper’ mutation affects performance of Nordic and Standardbred trotters. J Anim Sci. 2014;92:4279–4286. doi: 10.2527/jas.2014-7803. [DOI] [PubMed] [Google Scholar]
- 25.Jäderkvist Fegraeus K, Lawrence C, Petäjistö K, Johansson MK, Wiklund M, Olsson C, Andersson L, Andersson LS, Røed KH, Ihler CF, Strand E, Lindgren G, Velie BD. Lack of significant associations with early career performance suggest no link between the DMRT3 “gait keeper” mutation and precocity in coldblooded trotters. PLoS One. 2017;12:e01777351. doi: 10.1371/journal.pone.0177351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jäderkvist Fegraeus K, Johansson L, Mäenpää M, Mykkänen A, Andersson LS, Velie BD, Andersson L, Arnason T, Lindgren G. Different DMRT3 genotypes are best adapted for harness racing and riding in Finnhorses. J Hered. 2015;106:734–740. doi: 10.1093/jhered/esv062. [DOI] [PubMed] [Google Scholar]
- 27.Solé M, Valera M, Gómez MD, Sölkner J, Molina A, Mészáros G. Heritability and factors associated with number of harness race starts in the Spanish trotter horse population. Equine Vet J. 2017;49:288–293. doi: 10.1111/evj.12632. [DOI] [PubMed] [Google Scholar]
- 28.Thiruvenkadan AK, Kandasamy N, Panneerselvam S. Inheritance of racing performance of trotter horses: an overview. Livest Sci. 2009;124:163–181. doi: 10.1016/j.livsci.2009.01.010. [DOI] [Google Scholar]
- 29.Saastamoinen MT, Ojala MJ. Estimates of genetic and phenotypic parameters for racing performance in young trotters. Acta Agr Scand. 1991;41:427–436. doi: 10.1080/00015129109439925. [DOI] [Google Scholar]
- 30.Saastamoinen MT, Nylander A. Genetic and phenotypic parameters for age at starting to race and racing performance during early career in trotters. Livest Prod Sci. 1996;45:63–68. doi: 10.1016/0301-6226(95)00080-1. [DOI] [Google Scholar]
- 31.Suontama M, van der Werf JH, Juga J, Ojala M. Genetic parameters for racing records in trotters using linear and generalized linear models. J Anim Sci. 2012;90:2921–2930. doi: 10.2527/jas.2011-4526. [DOI] [PubMed] [Google Scholar]
- 32.Olsen HF, Klemetsdal G, Odegård J, Arnason T. Validation of alternative models in genetic evaluation of racing performance in North Swedish and Norwegian cold-blooded trotters. J Anim Breed Genet. 2012;129:164–170. doi: 10.1111/j.1439-0388.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 33.Gómez MD, Menendez-Buxadera A, Valera M, Molina A. Estimation of genetic parameters for racing speed at different distances in young and adult Spanish trotter horses using the random regression model. J Anim Breed Genet. 2010;127:385–394. doi: 10.1111/j.1439-0388.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 34.Thiruvenkadan AK, Kandasamy N, Panneerselvam S. Inheritance of racing performance of thoroughbred horses. Livest Sci. 2009;121:308–326. doi: 10.1016/j.livsci.2008.07.009. [DOI] [Google Scholar]
- 35.Velie BD, Hamilton NA, Wade CM. Heritability of racing performance in the Australian thoroughbred racing population. Anim Genet. 2015;46:23–29. doi: 10.1111/age.12234. [DOI] [PubMed] [Google Scholar]
- 36.Tozaki T, Miyake T, Kakoi H, Gawahara H, Hirota K, Nakano Y, Kurosawa M. Heritability estimates for racing performance in Japanese thoroughbred racehorses using linear and non-linear model analyses. J Anim Breed Genet. 2012;129:402–408. doi: 10.1111/j.1439-0388.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 37.Velie BD, Hamilton NA, Wade CM. Performance selection for thoroughbreds racing in Hong Kong. Equine Vet J. 2015;47:43–47. doi: 10.1111/evj.12233. [DOI] [PubMed] [Google Scholar]
- 38.Sharman P, Wilson AJ. Racehorses are getting faster. Biol Lett. 2015;11:20150310. doi: 10.1098/rsbl.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricard A, Robert C, Blouin C, Baste F, Torquet G, Morgenthaler C, Riviére J, Mach N, Mata X, Schibler L, Barrey E. Endurance exercise ability in the horses: a trait with complex polygenic determinism. Front Genet. 2017;8:89. doi: 10.3389/fgene.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frischnkecht M, Signer-Hasler H, Leeb T, Rieder S, Neuditschko M. Genome-wide association studies based on sequence-derived genotypes reveal new QTL associated with conformation and performance traits in the Franches-Montagnes horse breed. Anim Genet. 2016;47:227–229. doi: 10.1111/age.12406. [DOI] [PubMed] [Google Scholar]
- 41.Schröder W, Klostermann A, Stock KF, Distl O. A genome-wide association study for quantitative trait loci of show-jumping in Hanoverian warmblood horses. Anim Genet. 2012;43:392–400. doi: 10.1111/j.1365-2052.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 42.Lykkjen S, Dolvik NI, McCue ME, Rendahl AK, Mickelson JR, Røed KH. Equine developmental orthopaedic diseases—a genome-wide association study of first phalanx plantar osteochondral fragments in Standardbred trotters. Anim Genet. 2013;44:766–769. doi: 10.1111/age.12064. [DOI] [PubMed] [Google Scholar]
- 43.Teyssédre S, Dupuis MC, Guérin G, Schibler L, Denoix JM, Elsen JM, Ricard A. Genome-wide association studies for osteochondrosis in French trotter horses. J Anim Sci. 2012;90:45–53. doi: 10.2527/jas.2011-4031. [DOI] [PubMed] [Google Scholar]
- 44.McCoy AM, Beeson SK, Splan RK, Lykkjen S, Ralston SL, Mickelson JR, McCue ME. Identification and validation of risk loci for osteochondrosis in Standardbreds. BMC Genomics. 2016;17:41. doi: 10.1186/s12864-016-2385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lykkjen S, Dolvik NI, McCue ME, Rendahl AK, Mickelson JR, Roed KH. Genome-wide association analysis of osteochondrosis of the tibiotarsal joint in Norwegian Standardbred trotters. Anim Genet. 2010;41:111–120. doi: 10.1111/j.1365-2052.2010.02117.x. [DOI] [PubMed] [Google Scholar]
- 46.Dupuis MC, Zhang Z, Druet T, Denoix JM, Charlier C, Lekeux P, Georges M. Results of a haplotype-based GWAS for recurrent laryngeal neuropathy in the horse. Mamm Genome. 2011;22:613–620. doi: 10.1007/s00335-011-9337-3. [DOI] [PubMed] [Google Scholar]
- 47.Dupuis MC, Zhang Z, Durkin K, Charlier C, Lekeux P, Georges M. Detection of copy number variants in the horse genome and examination of their association with recurrent laryngeal neuropathy. Anim Genet. 2013;44:206–208. doi: 10.1111/j.1365-2052.2012.02373.x. [DOI] [PubMed] [Google Scholar]
- 48.Svensk Travsport: Kvalificerings- & premielopp. https://www.travsport.se/artikel/kvalificerings-_&_premielopp (2017). Accessed 1 Nov 2017.
- 49.Det Norske Travselskap: Lover & Reglement. https://www.travsport.no (2017). Accessed 1 Nov 2017.
- 50.R Development Core Team: R-A Language and Environment for Statistical Computing. https://www.r-project.org/ (2018). Accessed 13 Jan 2018.
- 51.Sargolzaei M, Iwaisaki H, Colleau JJ. Proceedings of the 8th world congress on genetics applied to livestock production. Brazil: Belo Horizonte; 2006. CFC, a tool for monitoring genetic diversity; pp. 27–28. [Google Scholar]
- 52.OFX: Historical Exchange Rates. https://www.ofx.com/en-au/forex-news/historical-exchange-rates/ (2017). Accessed 1 Nov 2017.
- 53.Everitt BS, Hothorn TA. Handbook of statistical analyses using R: Chapman & Hall/CRC; 2010.
- 54.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER version 11: expanded annotation data from gene ontology and Reactome pathways, and data analysis tool enhancments. Nucl Acids Res. 2016; 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed]
- 55.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller JI. Quantitative trait loci analysis in animals. 2. Oxfordshire: CABI; 2009. [Google Scholar]
- 57.Jäderkvist Fegraeus K, Hirschberg I, Arnason T, Andersson L, Velie BD, Andersson LS, Lindgren G. To pace or not to pace: a pilot study of four- and five-gaited Icelandic horses homozygous for the DMRT3 ‘Gait Keeper’ mutation. Anim Gent. 2017;48:694–697. doi: 10.1111/age.12610. [DOI] [PubMed] [Google Scholar]
- 58.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 59.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 60.Hu C, Chen W, Myers SJ, Yuan H, Traynelis SF. Human GRIN2B variants in neurodevelopmental disorders. J Pharmacol Sci. 2016;132:115–121. doi: 10.1016/j.jphs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JI, Yoo JH, Kim D, Jeong B, Kim BN. The effects of GRIN2B and DRD4 gene variants on local functional connectivity in attention-deficit/hyperactivity disorder. Brain Imaging Behav. 2017; 10.1007/s11682-017-9690-2. [DOI] [PubMed]
- 62.Concilli M, Iacobacci S, Chesi G, Carissimo A, Polishchuk R. A systems biology approach reveals new endoplasmic reticulum-associated targets for the correction of the ATP7B mutant causing Wilson disease. Metallomics. 2016;8:920–930. doi: 10.1039/C6MT00148C. [DOI] [PubMed] [Google Scholar]
- 63.Kieffer DA, Medici V. Wilson disease: at the crossroads between genetics and epigenetics-a review of the evidence. Liver Res. 2017;1:121–130. doi: 10.1016/j.livres.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usman H, Mathew MK. Potassium channel regulator KCNRG regulates surface expression of shaker-type potassium channels. Biochem Biophys Res Commun. 2010;391:1301–1305. doi: 10.1016/j.bbrc.2009.11.143. [DOI] [PubMed] [Google Scholar]
- 65.Lovell PV, Carleton JB, Mello CV. Genomics analysis of potassium channel genes in songbirds reveals molecular specializations of brain circuits for the maintenance and production of learned vocalizations. BMC Genomics. 2013;14:470. doi: 10.1186/1471-2164-14-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu J, Orr N, Park SD, Katz LM, Sulimova G, MacHugh DE, Hill EW. A genome scan for positive selection in thoroughbred horses. PLoS One. 2009;4:e5767. doi: 10.1371/journal.pone.0005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naylor JM. Hyperkalemic periodic paralysis. Vet Clin North Am Equine Pract. 1997;13:129–144. doi: 10.1016/S0749-0739(17)30260-2. [DOI] [PubMed] [Google Scholar]
- 68.Bowling AT, Byrns G, Spier S. Evidence for a single pedigree source of the hyperkalemic periodic paralysis susceptibility gene in quarter horses. Anim Genet. 1996;27:279–281. doi: 10.1111/j.1365-2052.1996.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 69.Naylor JM, Robinson JA, Bertone J. Familial incidence of hyperkalemic periodic paralysis in quarter horses. J Am Vet Med Assoc. 1992;200:340–343. [PubMed] [Google Scholar]
- 70.Hinchcliff KW, Kaneps AJ, Geor RJ. Musculoskeletal System. In: Equine sports medicine and surgery: Saunders Elsevier; 2014. p. 109–43.
- 71.Cannon SC, Brown RH, Jr, Corey DP. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991;6:619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- 72.Ricker K, Camacho LM, Grafe P, Lehmann-Horn F, Rüdel R. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 1989;12:883–891. doi: 10.1002/mus.880121103. [DOI] [PubMed] [Google Scholar]
- 73.Steele D, Naylor JM. Hyperkalemic periodic paralysis, plasma lactate and exercise tolerance. J Equine Vet Sci. 1996;202:933–937. [Google Scholar]
- 74.Maxson-Sage A, Parente EJ, Beech J, Lindborg S, May LL, Teleis DC. Effect of high-intensity exercise on arterial blood gas tensions and upper airway and cardiac function in clinically normal quarter horses and horses heterozygous and homozygous for hyperkalemic periodic paralysis. Am J Vet Res. 1998;59:615–618. [PubMed] [Google Scholar]
- 75.van den Bout I, Jones DR, Shah ZH, Halstead JR, Keune WJ, Mohammed S, D’Santos CS, Divecha N. Collaboration of AMPK and PKC to induce phosphorylation of Ser413 on PIP5K1B resulting in decreased kinase activity and reduced PtdIns(4,5)P2 synthesis in response to oxidative stress and energy restriction. Biochem J. 2013;455:347–358. doi: 10.1042/BJ20130259. [DOI] [PubMed] [Google Scholar]
- 76.Szalinski CM, Guerriero CJ, Ruiz WG, Docter BE, Rbaibi Y, Pastor-Soler NM, Apodaca G, Puthenveedu MA, Weisz OA. PIP5K1β selectively modulates apical endocytosis in polarized renal epithelial cells. PLoS One. 2013;8:e53790. doi: 10.1371/journal.pone.0053790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu T, Lee SY. Phosphatidylinositol 4-phosphate 5-kinase α negatively regulates nerve growth factor-induced neurite outgrowth in PC12 cells. Exp Mol Med. 2013;45:e16. doi: 10.1038/emm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin T, An Y, Liu C, Wu J, Dai R, Liu D, Li X, Jiang L, Wu D, Tang X, Song W, Wang T, Zhao X. Novel DOCK8 gene mutations lead to absence of protein expression in patients with hyper-IgE syndrome. Immunol Res. 2016;64:260–271. doi: 10.1007/s12026-015-8745-y. [DOI] [PubMed] [Google Scholar]
- 79.Biggs CM, Lu HY, Turvey SE. Monogenic immune disorders and severe atopic disease. Nat Genet. 2017;49:1162–1163. doi: 10.1038/ng.3925. [DOI] [PubMed] [Google Scholar]
- 80.Burbank AJ, Shah SN, Montgomery M, Peden D, Tarrant TK, Weimer ET. Clinically focused exome sequencing identifies an homozygous mutation that confers DOCK8 deficiency. Pediatr Allergy Immunol. 2016;27:96–98. doi: 10.1111/pai.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pedurupillay CR, Misceo D, Gamage TH, Dissanyayake VH, Frengen E. Post-zygotic breakage of a dicentric chromosome results in mosaicism for a telocentric 9p marker chromosome in a boy with developmental delay. Gene. 2014;533:403–410. doi: 10.1016/j.gene.2013.09.090. [DOI] [PubMed] [Google Scholar]
- 83.Griggs BL, Ladd S, Saul RA, DuPont BR, Srivastava AK. Dedicator of cytokinesis 8 is disrupted in two patients with mental retardation and developmental disabilities. Genomics. 2008;91:195–202. doi: 10.1016/j.ygeno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tassano E, Accogli A, Pavanello M, Bruno C, Capra V, Gimelli G, Cuoco C. Interstitial 9p24.3 deletion involving only DOCK8 and KANK1 genes in two patients with non-overlapping phenotypic traits. Eur J Med Genet. 2016;59:20–25. doi: 10.1016/j.ejmg.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 85.Jäderkvist K, Kangas N, Andersson LS, Lindgren G. Gaitedness is associated with the DMRT3 ‘Gait keeper’ mutation in Morgan and American curly horses. Anim Genet. 2014;45:908–909. doi: 10.1111/age.12228. [DOI] [PubMed] [Google Scholar]
- 86.Jäderkvist K, Holm N, Imsland F, Arnason T, Andersson L, Andersson LS, Lindgren G. The effect of the DMRT3 ‘Gait keeper’ mutation on riding traits and gaits in Standardbred and Icelandic horses. Livest Sci. 2015;176:33–39. doi: 10.1016/j.livsci.2015.03.025. [DOI] [Google Scholar]
- 87.Regatieri IC, Eberth JE, Sarver F, Lear TL, Bailey E. Comparison of DMRT3 genotypes among American Saddlebred horses with reference to gait. Anim Genet. 2016;47:603–605. doi: 10.1111/age.12458. [DOI] [PubMed] [Google Scholar]
- 88.Promerová M, Andresson LS, Juras R, Penedo MC, Reissmann M, Tozaki T, et al. Worldwide frequency distribution of the ‘Gait keeper’ mutation in the DMRT3 gene. Anim Genet. 2014;45:274–282. doi: 10.1111/age.12120. [DOI] [PubMed] [Google Scholar]
- 89.Vasan RS, Glazer NL, Felix JF, Lieb W, Wild PS, Felix SB, et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA. 2009;302:168–178. doi: 10.1001/jama.2009.978-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Netherton SJ, Jimmo SL, Palmer D, Tilley DG, Dunkerley HA, Raymond DR, Russell JC, Absher PM, Sage EH, Vernon RB, Maurice DH. Altered phosphodiesterase 3-mediated cAMP hydrolysis contributes to a hypermotile phenotype in obese JCR:LA-cp rat aortic vascular smooth muscle cells: implications for diabetes-associated cardiovascular disease. Diabetes. 2002;51:1194–1200. doi: 10.2337/diabetes.51.4.1194. [DOI] [PubMed] [Google Scholar]
- 91.Boda H, Uchida H, Takaiso N, Ouchi Y, Fujita N, Kuno A, Hata T, Nagatani A, Funamoto Y, Miyata M, Yoshikawa T, Kurahashi H, Inagaki H. A PDE3A mutation in familial hypertension and brachydactyly syndrome. J Hum Genet. 2016;61:701–703. doi: 10.1038/jhg.2016.32. [DOI] [PubMed] [Google Scholar]
- 92.Campos-Chillon F, Farmerie TA, Bouma GJ, Clay CM, Carnevale EM. Effects of aging on gene expression and mitochondrial DNA in the equine oocyte and follicle cells. Reprod Fertil Dev. 2015;27:925–933. doi: 10.1071/RD14472. [DOI] [PubMed] [Google Scholar]
- 93.Hamilton MJ, Halvorsen EC, LePard NE, Bosiljcic M, Ho VW, Lam V, Banáth J, Bennewith KL, Krystal G. SHIP represses lung inflammation and inhibits mammary tumor metastasis in BALB/c mice. Oncotarget. 2016;7:3677–3691. doi: 10.18632/oncotarget.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Somasundaram R, Fernandes S, Deuring JJ, de Haar C, Kuipers EJ, Vogelaar L, Middleton FA, van der Woude CJ, Peppelenbosch MP, Kerr WG, Fuhler GM. Analysis of SHIP1 expression and activity in Crohn’s disease patients. PLoS One. 2017;12:e0182308. doi: 10.1371/journal.pone.0182308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pauls SD, Ray A, Hou S, Vaughan AT, Cragg MS, Marshall AJ. FcyRIIB-independent mechanisms controlling membrane localization of the inhibitory phosphatase SHIP in human B cells. J Immunol. 2016;197:1587–1596. doi: 10.4049/jimmunol.1600105. [DOI] [PubMed] [Google Scholar]
- 96.Eijgelsheim M, Newton-Cheh C, Sotoodehnia N, de Bakker PI, Müller M, Morrison AC, et al. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum Mol Genet. 2010;19:3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfeufer A, van Noord C, Marciante KD, Arking DE, Larson MG, Smith AV, et al. Genome-wide association study of PR interval. Nat Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Della-Morte D, Beecham A, Rundek T, Wang L, McClendon MS, Slifer S, Blanton SH, Di Tullio MR, Sacco RL. A follow-up study for left ventricular mass on chromosome 12p11 identifies potential candidate genes. BMC Med Genet. 2011;12:100. doi: 10.1186/1471-2350-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hersh CP, Silverman EK, Gascon J, Bhattacharya S, Klanderman BJ, Litonjua AA, Lefebvre V, Sparrow D, Reilly JJ, Anderson WH, Lomas DA, Mariani TJ. SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am J Respir Crit Care Med. 2011;183:1482–1489. doi: 10.1164/rccm.201010-1751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rakhmanov M, Sic H, Kienzler AK, Fischer B, Rizzi M, Seidl M, et al. High levels of SOX5 decrease proliferative capacity of human B cells, but permit plasmablast differentiation. PLoS One. 2014;9:e100328. doi: 10.1371/journal.pone.0100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li A, Hooli B, Mullin K, Tate RE, Bubnys A, Kirchner R, Chapman B, Hofmann O, Hide W, Tanzi RE. Silencing of the Drosophila ortholog of SOX5 leads to abnormal neuronal development and behavioral impairment. Hum Mol Genet. 2017;26:1472–1482. doi: 10.1093/hmg/ddx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamb AN, Rosenfeld JA, Neill NJ, Talkowski ME, Blumenthal I, Girirajan S, et al. Haploinsufficiency of SOX5 at 12p12.1 is associated with developmental delays with prominent language delay, behavior problems, and mild dysmorphic features. Hum Mutat. 2012;33:728–740. doi: 10.1002/humu.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HJ, Baek SS. Role of exercise on molecular mechanisms in the regulation of antidepressant effects. J Exerc Rehabil. 2017;13:617–620. doi: 10.12965/jer.1735188.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extent of linkage disequilibrium of the final sample of horses. Pairwise r2 was calculated between each SNP within 1 Mb. (TIFF 15316 kb)
QQ plots for earnings, best km time, and number of gallops analyses. Top panel - corrected QQ plot for earnings analysis (Uncorrected λ = 1.0532); Middle panel – corrected QQ plot for best km time analysis (λ = 1.0902); Bottom panel – corrected QQ plot for number of gallops analysis (λ = 1.0256). (TIF 111 kb)
Functional classification gene list from PANTHER analysis. (XLSX 27 kb)
Data Availability Statement
The data that support the findings of this study are available from the Swedish Trotting Association (Stockholm, Sweden) and the Norwegian Trotting Association (Oslo, Norway), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of the Swedish Trotting Association (Stockholm, Sweden) and the Norwegian Trotting Association (Oslo, Norway).