Abstract
Background
It is assumed that mosquitoes surviving exposure to spatial repellents when attempting to bite a host will not have significant adverse impacts on their downstream biology. Therefore, a critical knowledge gap is understanding the extent to which sublethal exposure to volatile pyrethroids may damage the performance of mosquitoes that survive exposure to vapour-active pyrethroids. To address this, laboratory-reared Aedes aegypti (L.) and Ae. albopictus (Skuse) were exposed to one of three sublethal concentrations of transfluthrin before being offered a blood-meal, after which their survival, fecundity, fertility, and egg-laying behaviour was assessed.
Results
Both species expressed reduced skip-oviposition behaviour at all exposures. Both species also suffered a major reduction in viable eggs (50–75% reduction in viable eggs laid). A phenotype where eggs collapsed after laying was observed in Ae. aegypti, and this response increased with exposure concentrations. Dissected females of both species retained 50% or fewer of their eggs, with Ae. albopictus retaining a significant proportion of melanised oocytes following the highest exposure.
Conclusions
Our findings suggest that volatile pyrethroids can reduce skip-oviposition, which may improve source reduction outcomes during integrated management. The additional fecundity reduction caused by sublethal exposures to volatile pyrethroids improves our confidence in recommending them for urban vector management. Furthermore, we suggest that volatile pyrethroids should be adapted into delivery methods compatible with mosquito abatement programs.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-3065-4) contains supplementary material, which is available to authorized users.
Keywords: Repellent, Mosquito, Transfluthrin, Fecundity, Vector control
Background
The emergence of tropical pathogens, particularly Zika virus and its link to congenital disabilities [1, 2] renewed emphasis on domestic Aedes aegypti (L.) and Aedes albopictus (Skuse) as urban disease vectors in the USA following local transmission of Zika virus in Florida [3]. These mosquito species disperse eggs across many natural and artificial containers holding small quantities of water that permeate urban landscapes [4]. These simplified aquatic ecosystems virtually eliminate larval predation pressure, resulting in source reduction being the only long-term pressure to reduce densities of larval habitat used by container-inhabiting mosquitoes. In some environments, targeting key oviposition sites with source reduction has been effective in reducing container-inhabiting mosquito density [5, 6]. However, Ae. aegypti and Ae. albopictus bet-hedge by using a skip-oviposition strategy [7, 8], whereby eggs are distributed across multiple developmental sites per clutch. Because of skip-oviposition, it is difficult to eliminate all of the numerous mosquito oviposition sites from a peridomestic landscape. Oviposition sites also are often cryptic and difficult to access, further limiting the effectiveness of source reduction and cultural control [4]. This places reliance on continuing to conduct chemical adulticiding, such as through ultra-low volume space sprays or outdoor residual treatments, which have variable rates of success [9, 10]. Consequently, personal repellents are an essential part of integrated vector management because it allows an extra layer of prevention when other treatments fail [3].
In the current market, topical repellents form the core of personal protection guidelines against mosquito vectors [3]. However, many people do not use topical repellents because they find them inconvenient to apply or unpleasant in smell or feel when applied. Spatial repellent devices, such as ambient emanators, mosquito coils, vaporiser mats, and liquid vaporisers are an alternative to topical repellents that are frequently chosen by consumers [9]. Spatial repellents allow the dissemination of active ingredients on small scales, often serving as personal protection devices [11–13]. When such devices emit volatile pyrethroids, these tools can both reduce mosquito contact with humans [14] and kill mosquitoes outright [12, 13]. This is due to the primary mode of action of pyrethroids, in which the active ingredient binds to the voltage-gated sodium channel (VGSC) as the primary mode of toxicity. As reviewed in other work [15, 16], volatile pyrethroids can bind to the VGSC after inhalation. This property of inhalation is a clear benefit of some active ingredients, such as metofluthrin or transfluthrin, that lead to various acute and sub-acute outcomes. Because of the combined benefits of repellency and mortality, volatile pyrethroids have been advocated as a tool for urban vector management [12, 13].
While volatile pyrethroids are repellents that can also cause mortality, mosquitoes have many opportunities to survive exposure. Mosquitoes may escape the area before acquiring a lethal dose, or strong air movement may disperse the vapours. This range of exposures potentially produces a suite of sublethal outcomes depending on what point the mosquito escapes exposure [15, 16]. Sublethal impairment of mosquito reproduction by spatial repellents is relevant for container-inhabiting mosquitoes because they live in close association with humans and have relatively short-range oviposition site use [8]. This allows spatial repellents to impact container-inhabiting mosquitoes when host-seeking, gravid, or ready for oviposition. Some mosquitoes survive and escape exposure to spatial repellents, while others show signs of toxicity after escaping, summarised in Achee et al. [15] and Bibbs & Kaufman [16]. The degree to which sublethal exposure to volatile pyrethroids in spatial repellents can negatively affect downstream fecundity or oviposition behaviours is unknown.
In related work, a wide array of experimental and commercial skin repellent compounds have been shown to deter Ae. albopictus from ovipositing in containers fitted with a repellent treated barrier or repellent contaminated water [17, 18]. Furthermore, Xue et al. [19] found that when oviposition sites were contaminated with DEET, gravid mosquitoes retained eggs for as much as three weeks after the exposure. Furthermore, the viability of these retained eggs decreased as more time elapsed before mosquitoes were able to deposit their eggs [19] successfully. Choi et al. [20] exposed gravid Ae. aegypti to a volatile pyrethroid, transfluthrin, and found that bacteria-baited oviposition cups were twice as attractive to treated mosquitoes and that treated mosquitoes had a less overall dispersion of eggs despite being offered multiple containers. Whether the observed changes in oviposition were due to disrupted skip-oviposition patterns is not discussed by that study. However, the growing observations of sublethal effects should be applied to the peridomestic ecology of Ae. aegypti and Ae. albopictus to quantify how spatial repellents affect mosquitoes surviving exposure. We quantified the effects of exposure to sublethal concentrations of transfluthrin vapours on Ae. aegypti and Ae. albopictus fecundity and oviposition behaviour to test the extent to which these chemicals damage mosquito reproductive performance. We predicted that exposing Ae. aegypti and Ae. albopictus to sublethal concentrations of volatile pyrethroid vapours would reduce mosquito fecundity in a subsequent oviposition attempt. Additionally, exposure to sublethal concentrations of volatile pyrethroid vapours was predicted to decrease the occurrence of skip-oviposition behaviour in both species.
Methods
Mosquito rearing
Pyrethroid susceptible, 1952 Orlando, FL strain Aedes aegypti and 1992 Gainesville, FL strain Aedes albopictus were acquired from the United States Department of Agriculture, Agricultural Research Service, Center for Medical, Agricultural, and Veterinary Entomology (USDA-ARS-CMAVE) in Gainesville, Florida, USA. Colonies of the susceptible strain were not exposed to insecticides before evaluation and were not supplemented with wild-type introductions. Mosquitoes were kept at 26 ± 1 °C, 85 ± 5% relative humidity (RH), with a 14:10 (L:D) photoperiod. Batches of 2000 eggs were placed in larval pans containing 2500 ml of reverse osmosis (RO) water. Hatched larvae were fed 1–3 g of liver and yeast mixture at a 3:2 ratio ad libitum in a 50 ml suspension. Maturing adult mosquitoes were kept in 30 × 30 × 30 cm flight cages. Flight cages also were supplied with 10% sucrose solution and RO water. Mosquitoes used at the start of experimentation were non-blood-fed, 5–7 day-old females that were given the opportunity to mate without blood-feeding.
Bioassay design
Test cages were derived from single-use 473 ml clear polypropylene snap-lid cups. Container lids were modified to have a central 20 mm opening through which 20 female mosquitoes were aspirated into the container. The filter paper was cut into 5 mm widths and 40 mm lengths and pleated every 5 mm. Technical grade transfluthrin was serially diluted in acetone to create concentrations meeting LC10, LC20 and LC30 predicted dose responses using 24 h mortality data from Bibbs et al. [21]. The predicted concentrations from Bibbs et al. [21] were derived from acute toxicity dose responses after 2 h exposures, but the current concentrations were selected with the intent of avoiding acute mortality while still allowing signs of toxicity to be detected several days later. These concentrations in solution were 0.009% for LC10, 0.016% for LC20, and 0.026% for LC30 against Ae. aegypti and 0.012% for LC10, 0.020% for LC20, and 0.029% for LC30 against Ae. albopictus.
Paper strips were then treated with 40 μl of a transfluthrin solution. Treated strips were air dried for 6 min and then transferred into a mesh bag that was suspended within the test cage through the hole in the lid. The hole was sealed to prevent vapours from escaping during tests. Controls used paper strips treated with only acetone. Test cages were placed in an illuminated incubator maintained at 26 ± 1 °C, 85 ± 5% RH for a 2 h exposure period. The 2 h exposure was selected to adhere to the protocol of Bibbs et al. [21] and validate that the predicted concentrations abide the LC10, LC20 and LC30 acute toxicity outcomes.
Following the 2 h transfluthrin exposure, mosquitoes were removed into a clean container of the same design. Upon transfer to clean containment, both treatment and unexposed control cohorts were offered 100 ml of non-citrated bovine blood in an unsalted sausage casing after warming to 36 ± 0.5 °C using a hot water bath. Freshly warmed blood meals were supplied for 2 h per cohort, with blood replaced each hour. Post-blood-meal, mosquitoes lacking a fully engorged, red abdomen, as compared to control mosquitoes, were removed from the assay. The remaining mosquitoes were allowed 72 h post-blood meal to become gravid while held in the rearing conditions. Gravid mosquitoes for each cohort were confirmed with visual inspection of the abdominal membrane and transferred individually to polypropylene flight cages measuring 30 × 30 × 30 cm for oviposition (Bugdorm I # DP1000, MegaView Science Co. Ltd., Taichung, Taiwan). Groups of five flight cages, each with one mosquito per cage, were used for LC10, LC20 and LC30 treatments, and another group of five cages were used for control, totalling 20 cages per replicate. Each flight cage contained a source of 10% sucrose solution and six black, 118 ml oviposition cups (P400BLK, Dart Container Corporation, Mason, MI, USA). Each cup was filled with 50 ml of RO water and a 5 × 8 cm strip of oviposition paper (#6,512,981,311, Anchor Paper Co., Saint Paul, MN, USA). Gravid mosquitoes were allowed 72 h to oviposit across the array of cups. The parameters recorded from the oviposition bioassay included how many eggs were deposited, how many cups contained eggs, and the living or deceased status of the treated female mosquito. The flight-oviposition cages were randomly assigned a 0°, 90°, 180° or 270° orientation within an insectary at the beginning of the study to reduce the light effects and orientation biases.
Once oviposition bioassays were concluded, adult mosquitoes were immediately killed and dissected in 70% EtOH under a light microscope to examine egg retention. Dispersion of eggs laid across cups and quantity of eggs for each cup were recorded upon removal of adults for dissection. To facilitate embyronation of the eggs, oviposition papers were stored in rearing conditions and loosely enveloped in wax paper for a 24 h damp drying period. Eggs were inspected for any deformities following damp drying and then submerged in 118 ml cups filled with 50 ml of RO water. Larvae were reared out post-hatch with larval food provided ad libitum until all larvae pupated or perished. The number of larvae that hatched and subsequent natal mortality was recorded daily over the rearing period. Unhatched eggs were examined for deformities again once larval rearing from the selected paper was concluded. Eggs were denoted as non-viable if at either examination the chorion was collapsed. The progression required to complete a repetition of this bioassay appears in Fig. 1. This design was replicated across six different cohorts of mosquitoes for each species.
Fig. 1.
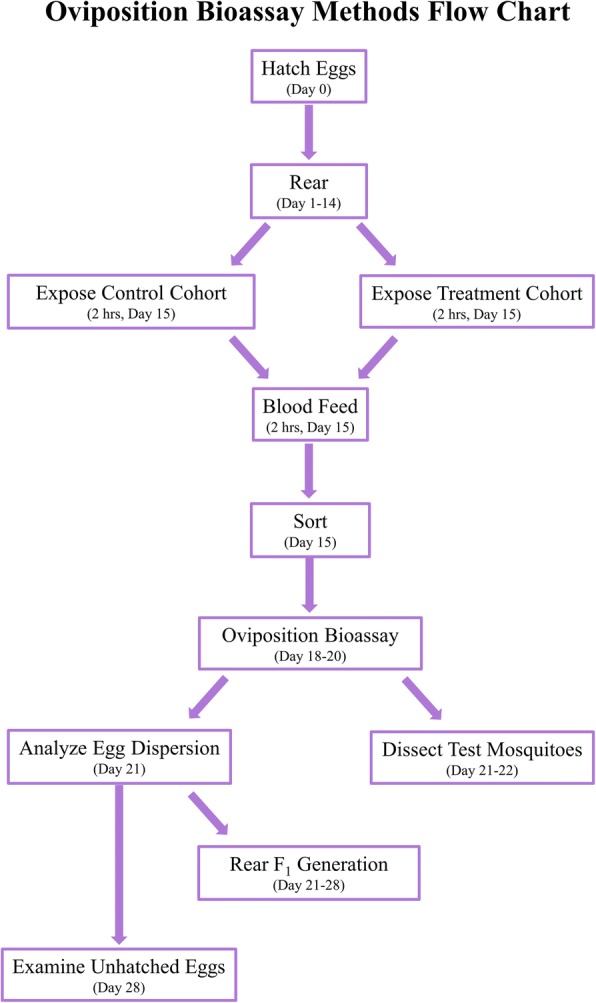
Flow chart: stages of the experiment with time estimates showing the step durations
Data analysis
Kruskal-Wallis analysis of variance and post-hoc Steel-Dwass tests were performed on averages of eggs collected, number of cups used by females, number of larvae successfully hatched and reared, number of eggs that failed to embryonate, and the number of eggs retained in dissected adult females after the bioassay to compare effects across treatments. Delayed toxicity of adult females for each treatment, whereby Ae. aegypti that were exposed to transfluthrin before blood-feeding were discovered in a prone position rigidly immobilised during the oviposition bioassay from 24 h to 6 d after transfluthrin exposure was compared to the controls using a Chi-square analysis. For Ae. albopictus, a melanisation effect observed in retained eggs also was analysed for each treatment using a Chi-square analysis. The severity of oocyte melanization in each female was qualitatively placed into one of five groups (0% of oocytes melanized; 1–25% of oocytes melanized; 26–50% of oocytes melanized; 51–75% of oocytes melanized; and 76–100% of visible oocytes melanized) and proportions of each group were compared among treatments. Statistical procedures were repeated for all concentrations and species in JMP 13.1.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Aedes aegypti
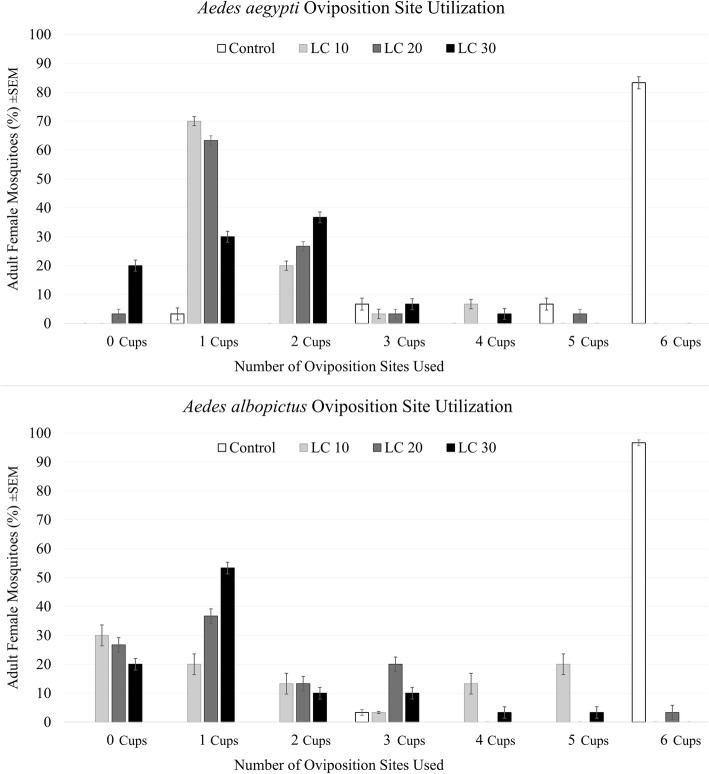
Exposure of female Ae. aegypti to sublethal concentrations of transfluthrin reduced the dispersion of eggs across the oviposition arena (Fig. 2, χ2(3) = 66.2, P < 0.0001). All transfluthrin exposure groups oviposited in fewer cups than the unexposed controls, but there were no differences in egg dispersion between our volatile-pyrethroid exposure groups (mean ranks from Kruskal-Wallis tests were 103.2 for controls, 46.6 for LC10, 46.3 for LC20, and 45.9 for LC30). There was also a significant reduction in the total number of eggs oviposited by Ae. aegypti females following sublethal transfluthrin exposure compared to unexposed controls (Fig. 3, χ2(3) = 187.6, P < 0.0001, n = 30), but there were no differences in total numbers of eggs oviposited among our volatile-pyrethroid exposure groups (mean ranks from Kruskal-Wallis tests were 524.5 for controls, 308.4 for LC10, 306.1 for LC20, and 303.0 for LC30).
Fig. 2.
Cluster graphs representing the mean percentage of adult Aedes aegypti (L.) (n = 30) and Ae. albopictus (Skuse) (n = 30) ovipositing in 0, 1, 2, 3, 4, 5 or 6 cups following exposure to three sublethal concentrations of transfluthrin (LC10, LC20, LC30). Figures shown with a standard error of the mean as I-bars
Fig. 3.
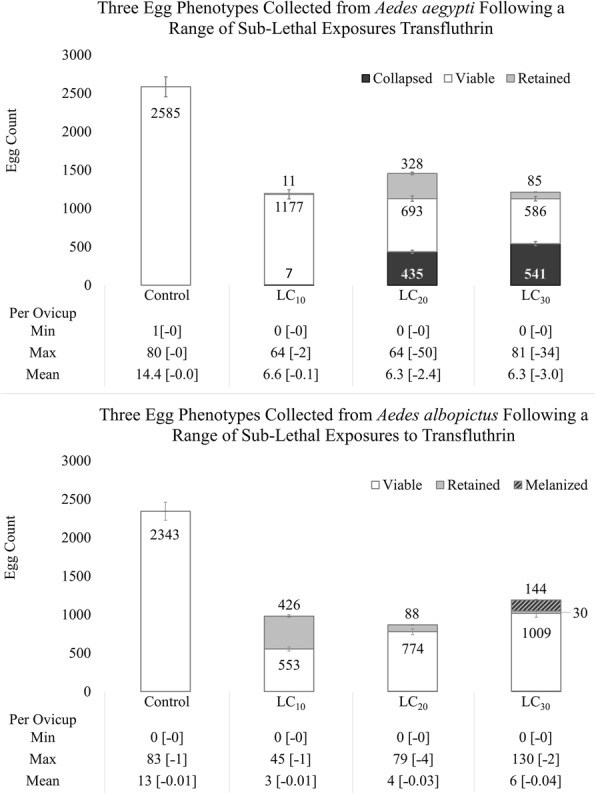
The LC10, LC20, and LC30 of transfluthrin were tested on Aedes aegypti (L.) (n = 30) and Ae. albopictus (Skuse) (n = 30). Compound bar graphs represent the counts of collapsed eggs (Ae. aegypti), viable eggs, eggs retained in the parent female, and melanisation of retained eggs (Ae. albopictus) following oviposition in a six-cup arena. The sums of: Collapsed or Melanized + Viable + Retained = total egg production, Collapsed + Viable = total eggs oviposited, Viable + Retained = yield that could recruit to the next generation. Figures are shown with 95% confidence as I-bars. Tables beneath graph detail range and mean counts per ovicup in the bioassay as ‘Viable [˗Collapsed]’
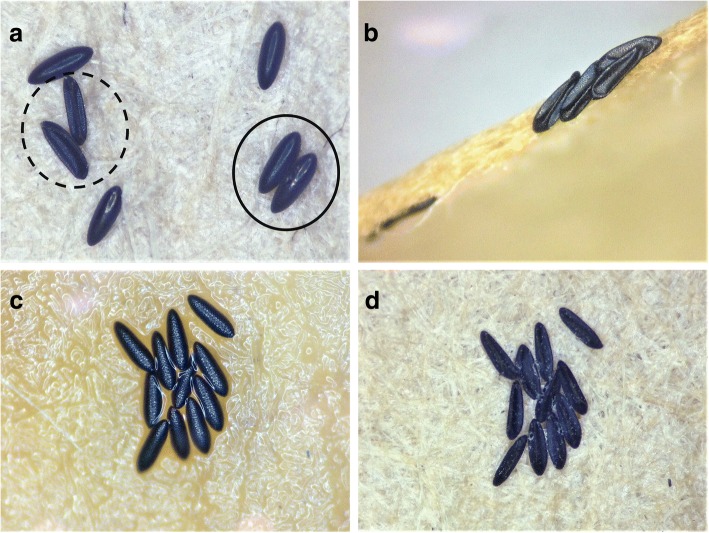
Female Ae. aegypti exposed to transfluthrin also experienced reduced egg viability. This was evident because many eggs were collapsed and few hatched in our sublethal exposure groups (Fig. 4b, d). Collapsed eggs tended to occur in clusters but were sometimes mixed with viable eggs (Fig. 4a). In ~30% of samples, the entire collection of eggs on an oviposition paper were collapsed by the end of the larval hatching period. The LC10 exposure group did not significantly differ in the number of collapsed eggs from the controls. However, the LC20 and LC30 exposures had significantly higher numbers of collapsed eggs than those observed in either the LC10 or control treatments (Fig. 3, χ2(3) = 76.0, P < 0.0001, n = 30; mean ranks from Kruskal-Wallis tests were 320.0 for controls, 393.6 for LC20, and 401.1 for LC30).
Fig. 4.
Egg collapse: Aedes aegypti (L.) eggs following exposure of the parent female mosquito to sublethal concentrations of transfluthrin. a Seed germination paper removed from an LC10 treatment oviposition bioassay cup, following a 24 h damp dry, and a full drying period. Solid line encircles embryonated, viable eggs. Dashed line encircles non-viable eggs with a collapsed chorion. b Cluster of collapsed eggs from an LC20 treatment oviposition bioassay, following a 24 h damp dry period, but not fully dried. c A cluster of eggs upon immediate removal from an LC30 treatment oviposition bioassay. d The same cluster of eggs, collapsed, following a 24 h damp dry and full drying period
Eggs that were not collapsed were considered potentially viable, therefore collapsed eggs were removed from the total, and only the remaining eggs were hatched out. Of these, the larval hatch was not significantly different across treatments and controls, but a weak visual trend indicated a negative correlation of hatch rate with exposures. In rearing the viable eggs, there was no difference in the survivorship to adulthood of hatched larvae between the control, LC10, and LC20 treatments. There was a weak trend suggesting that survivorship decreased 2% in larvae hatching from the eggs laid by LC30-exposed female mosquitoes (χ2(3) = 11.0, P < 0.0116), with a mean rank of 373.6 for control, 368.5 for LC10, 351.8 for LC20, and 348.1 for LC30. There were no effects on pupation, adult emergence, or subsequent blood-feeding and oviposition in the F1 generation.
There was significantly delayed toxicity at the higher exposure levels where Ae. aegypti that were exposed to transfluthrin before blood-feeding were discovered dead six days after exposure when concluding oviposition bioassays. The LC20 exposure generated 36.7% delayed toxicity in adult females during the oviposition bioassay, and the LC30 exposure generated 60.0% delayed toxicity (χ2(3) = 52.9, P < 0.0001). No delayed toxicity was observed in LC10 or the controls. Even though they may have died soon after, all females that died during the oviposition bioassay survived long enough to lay eggs in the provided arena. Both living and dead mosquitoes were dissected after the oviposition bioassay to determine whether they retained any matured eggs in their reproductive tracts or whether all matured eggs were laid. During dissections, control mosquitoes did not retain any mature eggs, while the egg retention in the LC10, LC20, and LC30 cohorts ranged from 0–3, 0–28, and 0–27 eggs per female, respectively. Females in the LC20 group retained more mature eggs than controls (Fig. 3, χ2(3) = 61.5, P < 0.0001, mean rank of 39.5 for control, 48.4 for LC10, 95.3 for LC20, and 58.8 for LC30). Although there was an observed trend towards egg retention in the LC30 group, this trend was not statistically detectably different from the control. Overall, when we combine each of the facets of reduced reproductive performance that we quantified in Ae. aegypti, mosquitoes exposed to the LC30 dose of transfluthrin vapours had ~70% reduction in viable eggs.
Aedes albopictus
Aedes albopictus also oviposited across significantly fewer sites than control mosquitoes when exposed to any of the sublethal concentrations of transfluthrin (Fig. 2, χ2(3) = 67.6, P < 0.0001, n = 30; Kruskal-Wallis mean ranks of 104.4 for control, 50.5 for LC10, 44.3 for LC20, and 42.8 for LC30). As above, there was a significant reduction in the total number of eggs deposited by mosquitoes exposed to any of the transfluthrin concentrations compared to controls (Fig. 3, χ2(3) = 242.7, P < 0.0001, n = 30; mean ranks of 550.5 for control, 315.0 for LC10, 286.1 for LC20, and 290.4 for LC30).
In contrast to Ae. aegypti, less than 1% of Ae. albopictus eggs were collapsed across all treatments and controls. Larval hatch was 98–100% successful for the control and all transfluthrin treatments, and larval survival after hatching was not significantly different across transfluthrin treatments and the control. Unlike Ae. aegypti, there was no delayed toxicity observed in adult Ae. albopictus as a result of exposure to transfluthrin vapours. When female mosquitoes were dissected to determine whether all matured eggs were laid, we found that Ae. albopictus controls did not retain any mature eggs, but the LC10 group collectively retained more mature eggs than either the control or higher-concentration transfluthrin treatments (Fig. 3, Fig. 5, χ2(3) = 54.8, P < 0.0001, n = 30; mean ranks of 32.0 for control, 90.3 for LC10, 50.2 for LC20, and 69.5 for LC30). Females in the LC30 treatment retained significantly more eggs than the control (Z = 5.5, P < 0.0001) and LC10 (Z = -3.1, P < 0.0109) groups, but were not statistically different from the LC20 group.
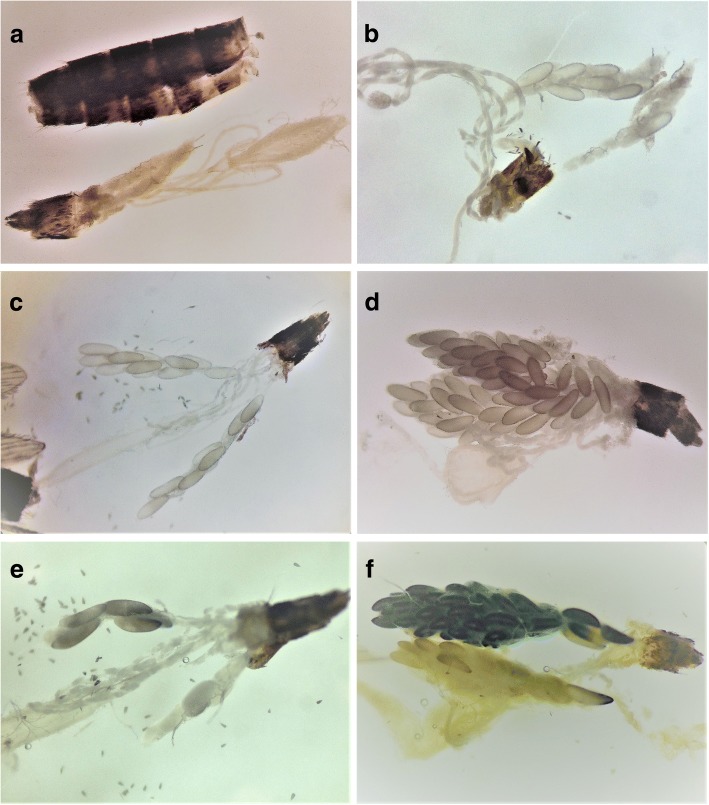
Fig. 5.
Egg melanization: dissected female Aedes albopictus (Skuse) reproductive tracts after exposure to sublethal concentrations of transfluthrin and upon conclusion of oviposition bioassays seven days post-exposure. a Control mosquito showing cleared oviducts and no late-stage development oocytes. b A reproductive tract following LC10 treatment with 10 or fewer retained eggs. c A reproductive tract following LC20 treatment with 20 or fewer retained eggs. d A reproductive tract following LC30 treatment with extreme egg retention. e A reproductive tract following LC30 treatment showing partial melanisation of retained eggs. f A reproductive tract following LC30 treatment showing full melanisation and tissue decay.
During dissections, we found that in the LC30 treatment group 70% of the females contained a proportion of oocytes retained in the reproductive tract that were melanized (Fig. 5e, f), but neither the control nor other sublethal exposure groups had melanized oocytes (Fig. 5a-d, χ2(12) = 74.6, P < 0.0001). The ratio of melanised eggs with respect to retained and oviposited eggs are displayed in Fig. 3. If melanised eggs were not viable, the combined effects that reduce reproductive performance in Ae. albopictus represent ~65% total reduction of viable eggs after exposure to the LC30 of transfluthrin vapours.
Discussion
Exposure to sublethal concentrations of transfluthrin vapours reduced female reproduction, including fecundity, fertility, and the dispersion of eggs across potential oviposition sites in both Aedes species. The breadth of impacts to reproductive performance add to the spectrum of outcomes against mosquitoes when using spatial repellents. Skip-oviposition, in particular, is a critical behaviour to interrupt because untreated Ae. aegypti and Ae. albopictus consistently spread eggs to 4–6 containers per gonotrophic cycle, as seen in our control mosquitoes [22–25]. However, skip-oviposition is susceptible to external pressures, it wanes if there are limited oviposition sites available [25], during seasonal changes [23], and in certain geographical localities [26]. Source reduction ideally should eliminate skip-oviposition behaviour by removing options for oviposition [25]. In practice, it has become evident that container-inhabiting mosquitoes find oviposition sites despite source reduction efforts, thereby confounding the sustainability of source reduction as a mosquito abatement strategy [6]. Volatile pyrethroids may provide an additional external pressure needed to manipulate skip-oviposition behaviour in Ae. aegypti and Ae. albopictus to facilitate source reduction impacts within an integrated approach.
Our results represent both a dramatic reduction of viable eggs and a favourable reduction of skip-oviposition behaviour by both Ae. aegypti and Ae. albopictus upon exposure to transfluthrin. The changes in oviposition behaviour in our work may be a result of behavioural modification by neuronal interference since transfluthrin binds to the VGSC. A complementary effect was discussed by Choi et al. [20] where Ae. aegypti displayed increased attraction to oviposition sites after sublethal exposure to transfluthrin. When paired with the reduced reproductive performance observed in the present study, spatial repellents appear to stimulate container-inhabiting mosquitoes to oviposit in nearby containers urgently. Therefore, volatile pyrethroids can synergise with source reduction programs by reducing the labour necessary to target key oviposition sites [5]. Additionally, there are other circumstances where mosquitoes may get unintentional sublethal exposures. It has suggested that the time it takes for the vapours to penetrate into surroundings can lead to reduced exposure of target mosquitoes [26, 27]. Our findings also support that mosquitoes that may escape spatial repellents can still incur various side-effects. A mosquito that approaches hosts protected by spatial repellents on multiple occasions may even experience several repeated sublethal exposures through its lifetime.
However, the current delivery methods for vapour-active pyrethroids as spatial repellents are restricted to managing small areas. With current delivery methods protecting large areas would be as labour intensive and costly as source reduction [5, 6]. Given the ecology of domestic mosquitoes and the growing spectrum of benefits that volatile pyrethroids have against container-inhabiting mosquitoes, volatile pyrethroids should be developed into tools that are more capable of addressing the needs of mosquito abatement programs. Recent field studies support the idea that vapour-active pyrethroids can be deployed in ultra-low volume sprays to suppress domestic mosquitoes [9, 10]. Vapour-active pyrethroids also may be compatible with other delivery formats that are useful for integrated vector management.
Although our results are proof of concept that spatial repellents can harm mosquito reproduction six days after exposure, many details are not well understood and will require further study. When breaking apart the fertility measurements, Ae. albopictus had an unusual pattern whereby all of the treatments had lower egg viability than the control, but there was an increasing pattern of egg viability with higher transfluthrin concentration. A possible explanation is hormesis [28], a well-documented occurrence in which an organism (e.g. insects) experiences sublethal exposure to a stressor (e.g. toxicant or xenobiotic) and correspondingly displays increased fitness (e.g. egg production) at low doses while being inhibited at high doses. [29, 30]. Hormesis is an effect observed in both field and laboratory test groups, and current evidence asserts that pesticide hormesis does not significantly differ in magnitude in the laboratory versus field colonies for Ae. aegypti [30]. Unfortunately, our range of concentrations was not broad enough to observe a drop in the effect after a peak, so we cannot confirm what caused the pattern in viability or what it means for the animal. The effect was minimal for overall reproduction, and the net effect still resulted in less oviposition compared to the control group. In contrast, the oocyte melanisation observed in Ae. albopictus could be explained as an immune-system cost of surviving exposure. Cellular responses to stress, such as upregulation of detoxification enzymes or immune responses, have been shown to trigger melanisation in the ovary and follicular apoptosis in Anopheles gambiae Giles [31]. In one Ae. albopictus female with 100% of the visible oocytes melanised, bacterial decomposition was visible in one of the ovaries of the otherwise surviving female mosquito. Stress responses could explain the observed melanisation and tissue decay, reinforcing that sublethal exposures to volatile pyrethroids could have substantial implications for mosquito populations.
Conclusions
There are many potential directions for continuing work on sublethal effects caused by volatile pyrethroids. Although we monitored for mortality and reproductive performance for one week after exposure and blood-feeding, there may be long-term effects of sublethal exposures beyond the first gonotrophic cycle that should be examined. Exposure concentrations also can be controlled by time and have been recognised as a relevant limiter for volatile pyrethroids when the vapours must travel and penetrate into areas of interest [26, 27]. To make the findings of our proof of concept study more relevant to field conditions, a valuable next step would be to test short exposure durations. Particularly with respect to the fact mosquitoes may only briefly encounter the toxicant when approaching hosts. Regardless, this study demonstrated that even despite hormetic gains in one facet of reproduction, Ae. aegypti and Ae. albopictus experienced reduced overall egg yield, viability, and skip-oviposition behaviour following exposure to transfluthrin at a low concentration.
Additional file
Acknowledgements
We would like to thank Carlye Mangum for managing the insectaries at the Anastasia Mosquito Control District of St Johns County during both mosquito rearing and post-exposure mosquito maintenance.
Funding
Funding for this research was provided in part by the Florida Department of Agriculture and Consumer Services: Florida Coordinating Council on Mosquito Control research subcommittee project 23583.
Availability of data and materials
All data analysed in this study are available in Additional file 1.
Abbreviations
- LC
Lethal concentration
- USDA-ARS-CMAVE
United States Department of Agriculture, Agricultural Research Service, Center for Medical, Agricultural and Veterinary Entomology
Authors’ contributions
CSB designed and conducted experiments, analysed data, and wrote the manuscript. DAH assisted experimental design, data analysis, and manuscript writing. PEK assisted data analysis and guided the manuscript writing. RDX provided facilities, assisted with data analysis, and guided manuscript writing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. Adult mosquitoes were blood-fed on purchased bovine blood for colony maintenance and experiments; no live animals were used in the care of mosquitoes during this project.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christopher S. Bibbs, Email: csbibbs@outlook.com, Email: cbibbsamcd@bellsouth.net, Email: chrisfish89@ufl.edu
Daniel A. Hahn, Email: dahahn@ufl.edu
Phillip E. Kaufman, Email: pkaufman@ufl.edu
Rui-de Xue, Email: xueamcd@gmail.com.
References
- 1.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;11:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo J. Studies using IPS cells support a possible link between ZIKA and microcephaly. Cell Biosci. 2016;6:28. doi: 10.1186/s13578-016-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(CDC) United States Centers for Disease Control and Prevention . Operational risk communication and community engagement plan. Atlanta: United States Department of Health and Human Services; 2016. Responding to local mosquito-borne transmission of Zika virus; pp. 1–22. [Google Scholar]
- 4.Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, Morrison AC. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc Natl Acad Sci USA. 2009;106:11530–11534. doi: 10.1073/pnas.0901369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciel-de-Freitas R, Lourenço-de-Oliveira R. Does targeting key-containers effectively reduce Aedes aegypti population density? Trop Med Int Health. 2011;16:965–973. doi: 10.1111/j.1365-3156.2011.02797.x. [DOI] [PubMed] [Google Scholar]
- 6.Faraji A, Unlu I. The eye of the tiger, the thrill of the fight: effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J Med Entomol. 2016;53:1029–1047. doi: 10.1093/jme/tjw096. [DOI] [PubMed] [Google Scholar]
- 7.Fay RW, Perry AS. Laboratory studies of oviposition preferences of Aedes aegypti. Mosq News. 1965;25:276–281. [Google Scholar]
- 8.Davis TJ, Kaufman PE, Tatem AJ, Hogsette JA, Kline DL. Development and evaluation of an attractive self-marking ovitrap to measure dispersal and determine skip oviposition in Aedes albopictus (Diptera: Culicidae) field populations. J Med Entomol. 2016;53:31–38. doi: 10.1093/jme/tjv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unlu I, Baker MA, Indelicato N, Drews D, Zeng Z, and Vaidyanathan R. Nighttime applications of two formulations of pyrethroids are effective against diurnal Aedes albopictus. J Am Mosq Control Assoc. 2018;34:158-62. [DOI] [PubMed]
- 10.Farajollahi A, Healy SP, Unlu I, Gaugler R, Fonseca DM. Effectiveness of ultra-low volume nighttime applications of an adulticide against diurnal Aedes albopictus, a critical vector of dengue and chikungunya viruses. PLoS One. 2012;7:e49181. doi: 10.1371/journal.pone.0049181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie SA, Devine GJ. Confusion, knock-down and kill of Aedes aegypti using metofluthrin in domestic settings: a powerful tool to prevent dengue transmission? Parasit Vectors. 2013;6:262. doi: 10.1186/1756-3305-6-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibbs CS, Xue RD. OFF! Clip-on repellant device with metofluthrin tested on Aedes aegypti (Diptera: Culicidae) for mortality at different time intervals and distances. J Med Entomol. 2015;52:480–483. doi: 10.1093/jme/tjv065. [DOI] [PubMed] [Google Scholar]
- 13.Bibbs CS, Fulcher AP, Xue RD. Allethrin based mosquito control device causing knockdown, morbidity, and mortality in four species of field-caught mosquitoes (Diptera: Culicidae) J Med Entomol. 2015;52:739–742. doi: 10.1093/jme/tjv065. [DOI] [PubMed] [Google Scholar]
- 14.Xue RD, Qualls WA, Smith ML, Weaver JR, Gaines MK, Debboun M. Field evaluation of the OFF! Clip-on mosquito repellent (metofluthrin) against Aedes albopictus and Aedes taeniorhynchus (Diptera: Culicidae) in northeastern Florida. J Med Entomol. 2012;49:652–655. doi: 10.1603/ME10227. [DOI] [PubMed] [Google Scholar]
- 15.Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, et al. Spatial repellents: from discovery and development to evidence-based validation. Malaria J. 2012;11:164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bibbs CS, Kaufman PE. Volatile pyrethroids as a potential mosquito abatement tool: a review of pyrethroid containing spatial repellents. J Integr Pest Manag. 2017;8:21. doi: 10.1093/jipm/pmx016. [DOI] [Google Scholar]
- 17.Xue RD, Barnard DR, Ali A. Laboratory evaluation of 18 repellent compounds as oviposition deterrents of Aedes albopictus and as larvicides of Aedes aegypti, Anopheles quadrimaculatus, and Culex quinquefasciatus. J Am Mosq Control Assoc. 2003;19:397–403. [PubMed] [Google Scholar]
- 18.Xue RD, Barnard DR, Ali A. Laboratory evaluation of 21 insect repellents as larvicides and as oviposition deterrents of Aedes albopictus (Diptera: Culicidae) J Am Mosq Control Assoc. 2006;22:126–130. doi: 10.2987/8756-971X(2006)22[126:LEOIRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Xue RD, Ali A, Barnard DR. Effects of forced egg-retention in Aedes albopictus on adult survival and reproduction following application of DEET as an oviposition deterrent. J Vec Ecol. 2004;30:45–48. [PubMed] [Google Scholar]
- 20.Choi DB, Grieco JP, Apperson CS, Schal C, Ponnusamy L, Wesson DM, et al. Effect of spatial repellent exposure on dengue vector attraction to oviposition sites. PLoS Negl Trop Dis. 2016;10:e0004850. doi: 10.1371/journal.pntd.0004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bibbs CS, Tsikolia M, Bernier UR, Bloomquist JR, Xue RD, Kaufman PE. Vapor toxicity of five volatile pyrethroids against Aedes aegypti, Ae. albopictus, Culex quinquefasciatus, and Anopheles quadrimaculatus (Diptera: Culicidae). Pest Manag Sci. 2018; 10.1002/ps.5088. [DOI] [PubMed]
- 22.Oliva LO, Correia JC, Albuquerque CMR. How does mosquito age and the type and color of oviposition sites modify skip-oviposition behavior in Aedes aegypti (Diptera: Culicidae)? J Insect Behav. 2014;27:81–91. doi: 10.1007/s10905-013-9407-3. [DOI] [Google Scholar]
- 23.Fonesca DM, Kaplan LR, Heiry RA, Strickman D. Density-dependent oviposition by female Aedes albopictus (Diptera: Culicidae) spreads eggs among containers during the summer but accumulates them in the fall. J Med Entomol. 2015;52:705–712. doi: 10.1093/jme/tjv060. [DOI] [PubMed] [Google Scholar]
- 24.Santos de Abreu FV, Morais MM, Ribeiro SP, Eiras AE. Influence of breeding site availability on the oviposition behaviour of Aedes aegypti. Mem Inst Oswaldo Cruz. 2015;110:669–76. [DOI] [PMC free article] [PubMed]
- 25.Harrington LC, Edman JD. Indirect evidence against delayed “skip-oviposition” behaviour by Aedes aegypti (Diptera: Culicidae) in Thailand. J Med Entomol. 2001;38:641–645. doi: 10.1603/0022-2585-38.5.641. [DOI] [PubMed] [Google Scholar]
- 26.Buhagiar TS, Devine GJ, Ritchie SA. Metofluthrin: investigations into the use of a volatile spatial pyrethroid in a global spread of dengue, chikungunya and Zika viruses. Parasit Vectors. 2017;10:270. doi: 10.1186/s13071-017-2219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buhagiar TS, Devine GJ, Ritchie SA. Effects of sublethal exposure to metofluthrin on the fitness of Aedes aegypti in a domestic setting in Cairns, Queensland. Parasit Vectors. 2017;10:274. doi: 10.1186/s13071-017-2220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutler GC. Insects, insecticides and hormesis: evidence and considerations for study. Dose Response. 2013;11:154–177. doi: 10.2203/dose-response.12-008.Cutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonio GE, Sánchez D, Williams T, Marina CF. Paradoxical effects of sublethal exposure to the naturally derived insecticide spinosad in the dengue vector mosquito, Aedes aegypti. Pest Manag Sci. 2009;65:323–326. doi: 10.1002/ps.1683. [DOI] [PubMed] [Google Scholar]
- 30.Bong LJ, Tu WC, Neoh KB, Huang CG, Ting RX. The effect of insecticidal stress on reproductive output of susceptible and field strains of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2017;55:36–42. doi: 10.1093/jme/tjx191. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed AM, Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiaevia follicular apoptosis. Microbes Infect. 2006;8:308–15. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed in this study are available in Additional file 1.