Abstract
Background
Oxygen therapy has been widely used for RAO (retinal artery occlusion) patients; however, inconsistent results have been reported.
Methods
PubMed, Web of Science, EMBASE, Medline (OvidSP), Cochrane, China National Knowledge Infrastructure (CNKI), and Wanfang Database were examined. The primary endpoint was visual acuity (VA), and RevMan software 5.3 was used to statistically analyze the outcomes.
Results
Seven randomized controlled trials (RCTs) met the inclusion criteria. Patients who received oxygen therapy exhibited probability of visual improvement about 5.61 times compared with the control group who did not receive oxygen therapy (OR = 5.61; 95% CI, 3.60–8.73; p < 0.01). No statistically significant difference was observed between oxygen inhalation methods (Chi2 = 0.18, df = 1, p = 0.67), combined therapy (Chi2 = 0.21, df = 1, p = 0.64), or RAO type (Chi2 = 0.06, df = 1, p = 0.81). Conversely, 100% oxygen (Chi2 = 4.55, df = 1, p < 0.05) and hyperbaric oxygen (Chi2 = 4.55, df = 1, p < 0.05) significantly improved VA in RAO patients. Better effect was showed in period within 3 months (Chi2 = 5.76, df = 1, p < 0.05). The most effective treatment length was over 9 hours (Chi2 = 6.58, df = 1, p < 0.05).
Conclusion
Oxygen therapy demonstrated beneficial effects in improving VA in RAO patients, particularly when patients were treated with 100% hyperbaric oxygen and for over 9 hours.
Introduction
Retinal artery occlusion (RAO) is a serious event, which causes restriction in eyesight [1]. Central retinal artery occlusion (CRAO) was first reported in 1859 [2]. The ophthalmic artery originates from the internal carotid artery, and the central retinal artery (CRA) is a small important branch of the ophthalmic artery. The blood supply of the inner layer of the retina comes from the CRA and its branches; occlusion of the branch leads to a branch retinal artery occlusion (BRAO) [3]. The aetiology of RAO includes thrombosis, embolus, arteritis, vasospasm [4]. Clinically, the consequences of this vascular accident are dramatic, and delayed treatment may cause blindness; RAO is more common in hypertensive arteriosclerosis patients and occurs occasionally patients with endocarditis [5, 6]. Visual loss is a major symptom in CRAO, while limited vision field has been described in BRAO. Despite great developments in diagnostic, surgical and medical ophthalmology fields within recent years, retinal artery occlusion (RAO) remains a disease without approved therapy. Retinal cells exhibited the highest oxygen consumption in organs, which makes the retina extremely susceptible to ischaemia [7]. The inner retinal layers are normally supported by retinal circulation and typically lose viability, leading to vision loss. However, providing sufficient amounts of oxygen may improve visual acuity [8]. Traditional treatments for RAO include ocular massage, haemodilution, anterior chamber paracentesis, intravenous acetazolamide, oxygen therapy, transluminal Nd:YAG laser, intra-arterial thrombolytic therapy, intravenous fibrinolytic therapy and among all conventional conservative methods, most have not shown significant improvement [9–16]. Intravenous fibrinolytic therapy may also induce serious haemorrhagic events, and the time of symptom onset is critical to be safe and effective [17]. In terms of oxygen therapy, the outcome remains inconsistent [18–20]. Because there are few randomized controlled trials (RCTs) and many case reports in the literature, there has been no meta-analysis on oxygen therapy in RAO patients. Thus, we report this meta-analysis to provide a treatment reference for the use of oxygen therapy in RAO patients.
Materials and methods
Search strategy
We searched the literature in PubMed, Web of Science, EMBASE, Medline (OvidSP), Cochrane, China National Knowledge Infrastructure (CNKI), and Wanfang Database for articles published between the inception of the database to May 16, 2018. No language criteria were applied, and the following keywords were used: “normobaric oxygen” or “hyperbaric oxygen” or “oxygen” AND “retinal artery occlusion” OR “RAO”.
Inclusion and exclusion criteria
The inclusion criteria were as follows: A) research subjects should be patients diagnosed with RAO; B) all studies must be RCTs; C) the intervention group received oxygen therapy; and D) the best corrected visual acuity (VA) was compared between the oxygen therapy group and non-oxygen therapy group. The exclusion criteria were as follows: A) animal models; B) not related to the disease of RAO; C) not an intervention of oxygen therapy; and D) VA was not an endpoint.
Data extraction and risk of bias in included studies
Two investigators (Xiaodong Wu and Shuangshaung Chen) independently selected studies according to the abovementioned criteria. The following information was reported: first author’s name, year of publication, time from onset, oxygen pressure, oxygen inhalation method, length of treatment, combined therapy. Each risk of bias item was independently evaluated with the risk of bias software produced by the Cochrane Collaboration [21]. Any ambiguity or disagreement was resolved by a third investigator (Yang Xu).
Statistical analysis
Forest plots and funnel plots were generated to analyse the outcomes, and publication bias was detected using the Cochrane Collaboration’s RevMan 5.3 software. For each study, ORs and corresponding 95% CIs were estimated. A fixed-effect model was used for this meta-analysis to reduce errors for more accurate results. I2 reflects the heterogeneity of the proportion of total variation in the amount of the effect. According to I2, the degree of heterogeneity can be divided into three levels: 0 indicates no heterogeneity; 0–50% indicates low heterogeneity; 50%-75% indicates a moderate level of heterogeneity; 75%-100% indicates a high level of heterogeneity. Subgroup analysis was produced to search for the source if significant heterogeneity occurs. Chi-square test for metrological data analysis applied to subgroup analysis, p < 0.05 was considered a significant difference in the compared groups.
Results
Study inclusion and study characteristics
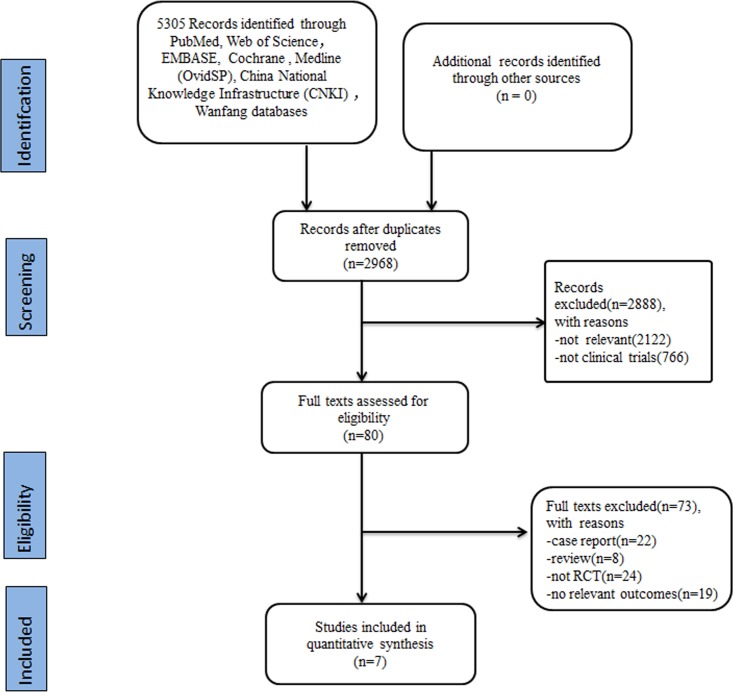
(Fig 1) shows a schematic of the study design. The search strategies initially identified 5305 papers. After removing duplications, 2968 articles were found. Next, 2888 papers were excluded for the following specific reasons: not relevant (n = 2122), no clinical trials (n = 766). Among the remaining 80 articles, 73 articles were removed due to being case reports (n = 22), being reviews (n = 8), not being an RCT (n = 24), or not reporting relevant outcomes (n = 19). Seven studies were finally included in this meta-analysis [22–28].Three studies were published in English [23–25], three studies were published in Chinese [26–28], and one study was published in German [22]. (Table 1) showed the main characteristics of the 7 studies.
Fig 1. Flow diagram of studies retrieval and screening.
Table 1. Statistical characteristics of included studies.
| Author | Language | Year | Patient | Time from onset | Oxygen treatment | Oxygen inhalation method | Total length of treatment | Outcome | Patients | Recovery, No.(%) | Time point of vision evaluation |
Combined Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neal H. Atebara | English | 1995 | CRAO | ≤24h | 1ATA, 95%O2 + 5%CO2 | Face mask | 4 hours | VA | 40 | 4, 10% | 4months-5years | Anterior chamber paracentesis |
| Zhang | Chinese | 2000 | CRAO | 4h-5d | 2.4ATA, 100% | Face mask | 16.5h | VA | 32 | 28, 87.5% | 1.5months | VitB1,B12 |
| S Aisenbrey | German | 2000 | CRAO + BRAO | 4-12h | 2.4ATA, N | N/A | 16.5h | VA | 18 | 12, 66.7% | 3months | Ocular massage acetazolamide |
| Beiran I | English | 2001 | CRAO + BRAO | ≤8h | 2.8 ATA, 100% | N/A | 9h | VA | 35 | 29, 82.9% | Discharge | Ocular massage, acetazolamide, retrobulbar block, |
| paracentesis | ||||||||||||
| He | Chinese | 2009 | CRAO | 2h-5d | 2.4ATA, | Face mask | 17h | VA | 20 | 18, 90% | 1month | TMP,VitB1 |
| Johannes | English | 2012 | CRAO | ≤12h | 2.4ATA, 100%, | Face mask | 7.5h | VA | 51 | 30, 58.8% | Discharge | Haemodilution therapy |
| Wang | Chinese | 2016 | CRAO | 0.5h-2d | 2.0–2.5ATA, 100% | Face mask | 24h | VA | 55 | 45, 81.8% | 10-12days | None |
ATA: atmosphere absolute; TMP: tetramethylpyrazine.
Overall efficacy
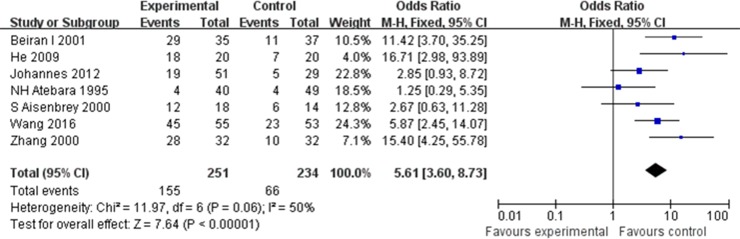
Seven studies that include 251 patients described VA improvement treated with oxygen therapy. Oxygen therapy exhibited significant VA improvement in RAO patients compared with the non-oxygen therapy group (OR 5.61; 95% CI, 3.60–8.73) in a fixed-effect model, with heterogeneity (I2 = 50%, p = 0.06) (Fig 2). Patients who received oxygen therapy exhibited probability of visual improvement about 5.61 times compared with the control group who did not receive oxygen therapy.
Fig 2. Forest plots for overall analyses: Oxygen therapy vs Control, outcome: Visual acuity.
Prespecified sub-group analyses
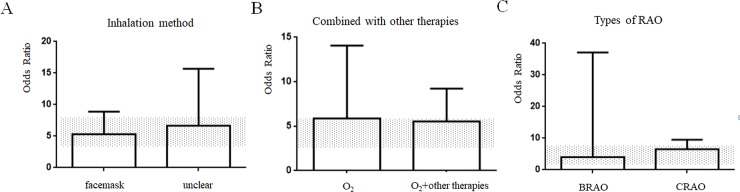
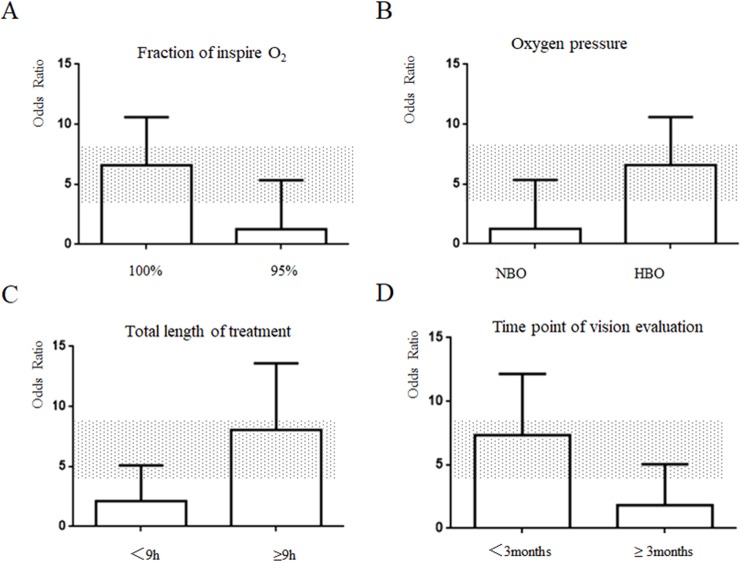
To exclude the effect of confounding factors, we divided the included studies into 6 subgroups according to oxygen inhalation method (mask or unclear way), treatment therapy (oxygen therapy only or combined with other therapies), type of RAO (BRAO or CRAO), fraction of inspiration O2 (FiO2) (100% or 95%), treatment length (≤ 9 h or > 9 h), pressure of oxygen (normobaric oxygen or hyperbaric oxygen). Five studies [23, 25–28] used a facemask providing oxygen compared with two studies [22, 24] providing oxygen in an unclear way. The inhalation method of using a facemask was not significantly different than using unclear methods (Chi2 = 0.18, df = 1, p = 0.67) (Fig 3A). There was no statistically significant difference observed between oxygen therapy alone and oxygen therapy combined with other therapies in the included literature (Chi2 = 0.21, df = 1, p = 0.64) (Fig 3B). Types of RAO (BRAO or CRAO) showed little difference on VA outcome (Chi2 = 0.06, df = 1, p = 0.81) (Fig 3C). Additionally, 100% oxygen was associated with a significant increase in VA improvement compared with methods using 95% oxygen (Chi2 = 4.55, df = 1, p < 0.05) (Fig 4A). A significant difference was observed between the normobaric oxygen (NBO) group and hyperbaric oxygen (HBO) group, demonstrating that oxygen pressure played an important role in VA improvement (Chi2 = 4.55, df = 1, p < 0.05) (Fig 4B). Over 9 hours of oxygen treatment exhibited a better effect compared with treatments shorter than 9 hours, and this trend was significant (Chi2 = 6.58, df = 1, p < 0.05) (Fig 4C). Better effect was showed after treatment in period within 3 months, it showed significant statistical difference (Chi2 = 5.76, df = 1, p < 0.05) (Fig 4D)
Fig 3.
(A) Inhalation method, (B) Combined with other therapies, (C) Types of RAO. The 95% CI for the global estimate is presented as a grey stripe.
Fig 4.
(A) Fraction of inspire O2, (B) Oxygen pressure, (C) Length of treatment. (D) Time point of vision evaluation. The 95% CI for the global estimate is presented as a grey stripe.
Risk of bias in included studies
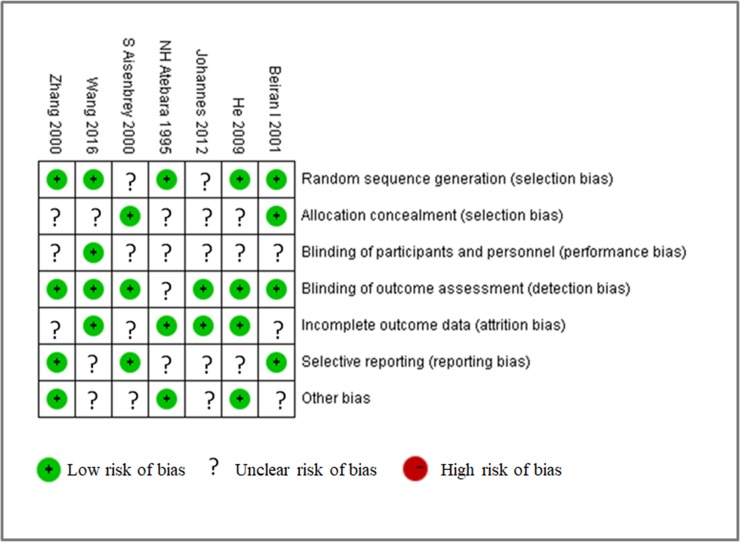
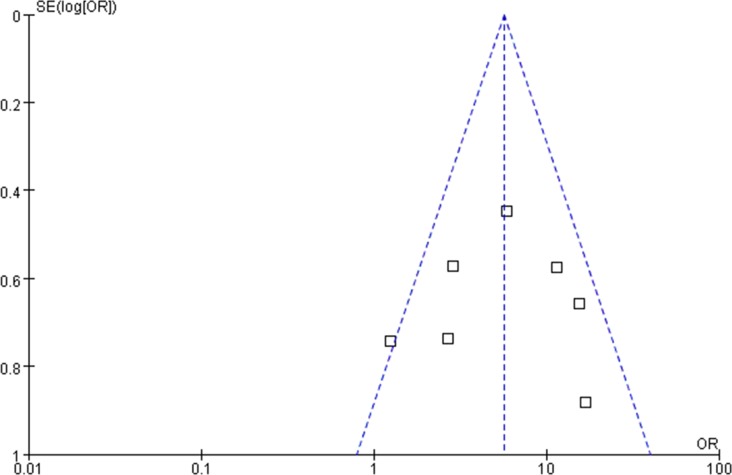
The quality of the 7 included studies was assessed using the Cochrane risk-of-bias tool, RevMan software 5.3 (Fig 5). Most studies showed low risk of bias. In terms of selection bias or detection bias, 86% of the included studies showed low risk, and 14% exhibited unclear risk. Moreover, three studies showed low risk and four studies showed unclear risks in reporting bias or other bias. Furthermore, 57% and 43% of the studies showed low and unclear risks of attrition bias, respectively. One study showed low risk of performance bias, and the other six studies showed an unclear risk of performance bias. All included studies showed the presence of publication bias (Fig 6).
Fig 5. Risk of bias summary.
Fig 6. Funnel plot evaluating publication bias.
Discussion
In this meta-analysis, seven studies that met the inclusion criteria focused on the effectiveness of oxygen therapy in RAO patients. The studies showed that RAO patients treated with oxygen demonstrated improvement in VA compared with the non-oxygen therapy group. Based on these results, oxygen therapy is considered an effective method of treatment for RAO diseases. RAO exhibits similar vascular risk factors with stroke and is caused by various aetiologies. Hayreh found that marked improvement in visual acuity and visual field can occur without treatment and is determined by different factors [29]. Retinal tissue is not tolerant of hypoxia [30]. The inner retinal layers may receive sufficient oxygen through the diffusion of the choroidal circulation to sustain viability if increased FiO2 is supported. Normally, choroidal circulation supplies most of the oxygen to the retina. In the above analyses, no difference was observed in the method of oxygen inhalation, combined therapies, or types of RAO. Oxygen therapy plays a key role in these patients when combined with other therapies [22–26, 28].
It has been reported that HBO can be used to treat the disease and achieved good results [24, 31–35]. Oxygen therapy reduced the risk of retinal infarction by increasing tissue oxygen saturation [36]. A previous study showed that hyperoxic conditions can provide 100% of the oxygen demanded by the retina [37]. Hyperbaric oxygen rapidly increases blood oxygen tension and blood oxygen content, effectively improving the hypoxia status of retinal tissue and preventing retinal inner cells loss, thereby contributing to the recovery of reversible lesions. An animal experiment in a CRAO mice model showed that hyperbaric oxygen therapy diminished cell loss from 58% to 30%, which was related to increased survival of cells in the retinal inner layer [38]. In terms of visual field defect in BRAO, a study showed BRAO observed within one week from onset, inferior nasal accounted for 29%, more than central scotoma, superior sector and central inferior altitudinal defect. The natural history was central and peripheral visual field defect improved in 47% and 52%, respectively [39].
All the included 7 studies obtained individual-level data from 251 patients who received oxygen therapy with a particular focus on defining treatment length. The study showed that over 9 hours of treatment length increased the therapeutic effect. More than 9 hours of treatment are effective for restoring a patient’s vision. A case presentation revealed that a CRAO patient who was treated with hyperbaric oxygen therapy for a total of 13.5 hours gained marked visual acuity [40]. The time between onset and starting oxygen therapy is critical in RAO. There is a threshold of time beyond which the inner retinal cells can no longer recover from a hypoxic event, even if reperfusion occurs [37]. In the included 7 articles, the timing of treatment from onset cannot be statistically analyzed because the patient selection criteria in all RCTs required a time window within a specific time point. Nevertheless, a study showed that five patient cases with CRAO lasting over one and one-half hours demonstrated restored obvious improvement in visual acuity [41]. This finding further proves our viewpoint described above. Short-term effect showed higher probability of visual improvement than long-term effect, it pointed out RAO patient should be intervened as soon as possible.
This analysis has some limitations. The search identified only seven studies, with small sample sizes, which may affect the result. Three of the included studies were published in Chinese, which may cause publication bias. One study did not mention the number of patients, and thus, we used the number of affected eyes as an alternative measure [27]. Time point of vision evaluation involved in one study was from 4 months to 5 years, we used the median mentioned in the study, which was 19 months [23]. Furthermore, the search strategy was uncertain to include all of the studies, which caused potential publication bias.
Conclusion
From a clinical perspective, oxygen therapy is a promising decongestive treatment to achieve VA improvement and favourable clinical outcomes in RAO patients. Taking all evidence into account, 100% hyperbaric oxygen and over 9 hours of treatment length is an effective clinical course.
Supporting information
(DOC)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the National Natural Science Foundation of China (81701161) and the Scientifc Research Fund Project for Talent Introduction of Yijishan Hospital, Wannan Medical College, Anhui, China (Grant Nos. YR201802).
References
- 1.Jung YH, Ahn SJ, Hong JH, Park KH, Han MK, Jung C, et al. Incidence and Clinical Features of Neovascularization of the Iris following Acute Central Retinal Artery Occlusion. Korean Journal of Ophthalmology Kjo. 2016;30(5):352–9. 10.3341/kjo.2016.30.5.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graefe AV. Ueber Embolie der Arteria centralis retinae als Ursache plötzlicher Erblindung. Archiv Für Ophthalmologie.5(1):136–57. [Google Scholar]
- 3.Dattilo M, Biousse V, Newman NJ. Update on the Management of Central Retinal Artery Occlusion. Neurologic Clinics. 2017;35(1):83 10.1016/j.ncl.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal Artery Occlusion: Associated Systemic and Ophthalmic Abnormalities. Ophthalmology. 2009;116(10):1928 10.1016/j.ophtha.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu CW, Wang J, Zhou DD, Hao JL, Liang LL, Li XH, et al. Central retinal artery occlusion associated with persistent truncus arteriosus and single atrium: a case report. Bmc Ophthalmology. 2015;15(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong JH, Sohn SI, Kwak J, Yoo J, Ahn SJ, Woo SJ, et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. Plos One. 2017;12(6):e0177663 10.1371/journal.pone.0177663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertzog LM, Meyer GW, Carson S, Strauss MB, Hart GB, Hertzog LM, et al. Central retinal artery occlusion treated with hyperbaric oxygen. 1992. [Google Scholar]
- 8.Murphy-Lavoie H, Butler F, Hagan C. Central retinal artery occlusion treated with oxygen: a literature review and treatment algorithm. Undersea & Hyperbaric Medicine Journal of the Undersea & Hyperbaric Medical Society Inc. 2012;39(5):943–53. [PubMed] [Google Scholar]
- 9.Weiss JN. Hyperbaric oxygen treatment of nonacute central retinal artery occlusion. Undersea & Hyperbaric Medicine Journal of the Undersea & Hyperbaric Medical Society Inc. 2009;36(6):401. [PubMed] [Google Scholar]
- 10.Stone R, Zink H, Klingele T, Burde RM. Visual recovery after central retinal artery occlusion: two cases. Annals of ophthalmology. 1977;9(4):445–50. [PubMed] [Google Scholar]
- 11.Agarwal N, Gala NB, Baumrind B, Hansberry DR, Thabet AM, Gandhi CD, et al. Endovascular Management of Central Retinal Arterial Occlusion. Vascular & Endovascular Surgery. 2016;50(8):579. [DOI] [PubMed] [Google Scholar]
- 12.Hwang K. Hyperbaric Oxygen Therapy to Avoid Blindness From Filler Injection. Journal of Craniofacial Surgery. 2016;27:1 10.1097/SCS.0000000000002387 [DOI] [PubMed] [Google Scholar]
- 13.Fraser S, Siriwardena D. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 2009;1(1):CD001989. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao SF, Huang YH. Partial vision recovery after iatrogenic retinal artery occlusion. BMC Ophthalmology,14,1(2014-10-11). 2014;14(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man V, Hecht I. Treatment of retinal artery occlusion using transluminal Nd:YAG laser: a systematic review and meta-analysis. 2017;255(10):1869–77. [DOI] [PubMed] [Google Scholar]
- 16.Chen YC, Wu HM, Chen SJ, Lee HJ, Lirng JF, Lin CJ, et al. Erratum: Intra-Arterial Thrombolytic Therapy Is Not a Therapeutic Option for Filler-Related Central Retinal Artery Occlusion. Facial plastic surgery: FPS. 2018;34(3):e1 10.1055/s-0038-1656550 [DOI] [PubMed] [Google Scholar]
- 17.Dumitrascu OM, Shen JF, Kurli M, Aguilar MI, Marks LA, Demaerschalk BM, et al. Is Intravenous Thrombolysis Safe and Effective in Central Retinal Artery Occlusion? A Critically Appraised Topic. Neurologist. 2017;22(4):153–6. 10.1097/NRL.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 18.Soares A, Gomes NL, Mendonça L, Ferreira C. Case Report: The efficacy of hyperbaric oxygen therapy in the treatment of central retinal artery occlusion. Bmj Case Rep. 2017;2017:bcr-2017-220113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wang W, Li J, Yu Y, Li L, Lu N. Fundus artery occlusion caused by cosmetic facial injections. Chinese medical journal. 2014;127(8):1434–7. [PubMed] [Google Scholar]
- 20.Gokce G, Metin S, Erdem U, Sobaci G, Durukan AH, Cagatay HH, et al. Late hyperbaric oxygen treatment of cilioretinal artery occlusion with nonischemic central retinal vein occlusion secondary to high altitude. High Altitude Medicine & Biology. 2014;15(1):84–8. [DOI] [PubMed] [Google Scholar]
- 21.Green HS. Cochrane handbook of systematic reviews of interventions. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2008;5(2):S38. [Google Scholar]
- 22.Aisenbrey S, Krott R, Heller R, Krauss D, Rössler G, Heimann K. [Hyperbaric oxygen therapy in retinal artery occlusion]. Der Ophthalmologe Zeitschrift Der Deutschen Ophthalmologischen Gesellschaft. 2000;97(7):461 [DOI] [PubMed] [Google Scholar]
- 23.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102(12):2029 [DOI] [PubMed] [Google Scholar]
- 24.Beiran I, Goldenberg I, Adir Y, Tamir A, Shupak A, Miller B. Early hyperbaric oxygen therapy for retinal artery occlusion. European journal of ophthalmology. 2001;11(4):345–50. [DOI] [PubMed] [Google Scholar]
- 25.Menzel-Severing J, Siekmann U, Weinberger A, Roessler G, Walter P, Mazinani B. Early Hyperbaric Oxygen Treatment for Nonarteritic Central Retinal Artery Obstruction. American Journal of Ophthalmology. 2012;153(3):454–9. 10.1016/j.ajo.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 26.H He Y., J Wang P. Therapeutic effect of ligustrazine and hyperbaric oxygen on central retinal artery occlusion. World Health Digest. 2009;6(34):61–. [Google Scholar]
- 27.F Wang L. Therapeutic effect of hyperbaric oxygen chamber on central retinal artery occlusion. China Health Care & Nutrition. 2016;26(26). [Google Scholar]
- 28.F Zhang X., Liu P., Shan X F. Hyperbaric oxygen treatment of central retinalartery embolism. Chinese Journal of Critical Care Medicine. 2000;20(1):32–. [Google Scholar]
- 29.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–91. 10.1016/j.ajo.2005.03.038 [DOI] [PubMed] [Google Scholar]
- 30.Elkordy AM, Sato K, Inoue Y, Mano Y, Matsumoto Y, Takahashi A, et al. Central Retinal Artery Occlusion after the Endovascular Treatment of Unruptured Ophthalmic Artery Aneurysm: A Case Report and a Literature Review. Nmc Case Report Journal. 2016;3(3):71 10.2176/nmccrj.cr.2015-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger AW, Siekmann UP, Wolf S, Rossaint R, Kirchhof B, Schrage NF. [Treatment of Acute Central Retinal Artery Occlusion (CRAO) by Hyperbaric Oxygenation Therapy (HBO)—Pilot study with 21 patients]. Klinische Monatsbltter Für Augenheilkunde. 2002;219(10):728. [DOI] [PubMed] [Google Scholar]
- 32.Canan H, Ulas B, Altanyaycioglu R. Hyperbaric oxygen therapy in combination with systemic treatment of sickle cell disease presenting as central retinal artery occlusion: a case report. Journal of Medical Case Reports. 2014;8(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celebi AR, Kilavuzoglu AE, Altiparmak UE, Cosar CB, Ozkiris A. Hyperbaric oxygen for the treatment of the rare combination of central retinal vein occlusion and cilioretinal artery occlusion. Diving & Hyperbaric Medicine. 2016;46(1):50–3. [PubMed] [Google Scholar]
- 34.Beiran I, Reissman P, Scharf J, Nahum Z, Miller B. Hyperbaric oxygenation combined with nifedipine treatment for recent-onset retinal artery occlusion. European journal of ophthalmology. 1993;3(2):89 [DOI] [PubMed] [Google Scholar]
- 35.Cope A, Eggert JV, O'Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving & Hyperbaric Medicine. 2011;41(3):135. [PubMed] [Google Scholar]
- 36.Wallyn CR, Jampol LM, Goldberg MF, Zanetti CL. The use of hyperbaric oxygen therapy in the treatment of sickle cell hyphema. Investigative Ophthalmology & Visual Science. 1985;26(8):1155. [PubMed] [Google Scholar]
- 37.Li, Helen K., Dejean, Baptiste J., Tang, Rosa A. Reversal of Visual Loss with Hyperbaric Oxygen Treatment in a Patient with Susac Syndrome. Ophthalmology. 1996;103(12):2091 [DOI] [PubMed] [Google Scholar]
- 38.Gaydar V, Ezrachi D, Dratviman-Storobinsky O, Hofstetter S, Avraham-Lubin BC, Goldenberg-Cohen N. Reduction of apoptosis in ischemic retinas of two mouse models using hyperbaric oxygen treatment. Investigative Ophthalmology & Visual Science. 2011;52(10):7514–22. [DOI] [PubMed] [Google Scholar]
- 39.Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116(6):1188–94.e1-4. 10.1016/j.ophtha.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemos JA, Teixeira C, Rui C, Fernandes T. Combined Central Retinal Artery and Vein Occlusion Associated with Factor V Leiden Mutation and Treated with Hyperbaric Oxygen. Case Reports in Ophthalmology. 2015;6(3):462–8. 10.1159/000442788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins SA, Magargal LE, Augsburger JJ, Sanborn GE. The idling retina: reversible visual loss in central retinal artery obstruction. Annals of ophthalmology. 1987;19(1):3–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.