Abstract
Background
Recruitment and retention challenges are very common in mental health randomised trials. Investigators utilise different methods to improve recruitment or retention. However, evidence of the effectiveness and efficiency of these strategies in mental health has not been synthesised. This systematic review is to investigate and assess the effectiveness and cost-effectiveness of different strategies to improve recruitment and retention in mental health randomised trials.
Methods and materials
MEDLINE, EMBASE, the Cochrane Methodology Register and PsycINFO were searched from beginning of record up to July 2016. Randomised trials involving participants with mental health problems which compared different strategies for recruitment or retention were selected. Two authors independently screened identified studies for eligibility.
Results
A total of 5,157 citations were identified. Thirteen articles were included, 11 on recruitment and 2 on retention. Three randomised controlled trials compared different recruitment strategies, none of which found statistically significant differences between the interventional recruitment strategies and the routine recruitment methods. Retrospective comparisons of recruitment methods showed that non-web-based advertisement and recruitment by clinical research staff each have advantages in efficiency. Web-based adverts had the lowest cost per person recruited (£13.41 per person recruited). Specialised care referral cost £183.24 per person, non-web-based adverts cost £372.03 per patient and recruitment via primary care cost £407.65 for each patient. Financial incentives, abridged questionnaires and pre-notification had a positive effect on retention rates.
Conclusion
The recruitment studies included showed differences in strategies, clinical settings, mental health conditions and study design. It is difficult to assess the overall effectiveness of any particular recruitment strategy as some strategies that worked well for a particular population may not work as well for others. Paying attention to the accessibility of information and consent materials may help improve recruitment. More research in this area is needed given its important implications.
1. Introduction
Recruitment and retention in randomised clinical trials are challenging. [1] Delayed recruitment can give rise to a series of issues, such as additional costs or the need for an extension of the study period. Inadequate or ineffective recruitment may often result in reduced power of the study or even premature termination of a trial, and can lead to the trial being unable to answer important clinical questions. [2] Loss to follow-up and patient dropouts can also result in reduced study power, hindering the ability to detect potential differences between trial arms should they exist, as well as undermining the internal validity of the trial. Although an increasing amount of research has contributed to dealing with missing data in clinical trials, the risk of bias due to missing data cannot be avoided through the application of statistical techniques for missingness, such as multiple imputation, as these techniques require additional assumptions which may not be valid. It has been suggested that less than 5% loss to follow-up may lead to an unimportant level of bias, while 20% or greater loss to follow-up poses a substantial threat to a trial’s internal validity. [3] Some modern trials aim to reduce this risk by increasing the sample size by 20%, which addresses precision but not internal validity, and poses a further challenge to recruitment.
Although generally considered the gold standard of clinical research, randomised controlled trials often fail to recruit enough participants, particularly in the area of mental health. [4] Extensive collaboration is often required between researchers, patients, clinical professionals and institutions, and each party in a clinical trial has its unique expectations and concerns towards the trial. [4] Concerns from clinicians about mental health patients’ vulnerability and reduced decision-making ability may make recruitment difficult. [5] For patients particularly, doubts about getting involved in a trial primarily centre around their own health, that is, how they could benefit, or what potential issues they might be faced with in the treatment being investigated. During the consent process, where potential participants are introduced to the trial’s protocol, they could be put off by aspects of the study which may appear inconvenient, abstruse or irrelevant. [4]
The fundamental biological aetiology for some mental health conditions is still not well understood, and often the effects of psychiatric treatments are small and uncertain. Hence there may be scepticism that new treatments will be very helpful, which might make psychiatric trials less appealing. [6–8] High placebo response rates also highlight the importance of randomised trials in providing unbiased estimates of treatment effects. Patients with mental health problems often still consider their conditions as stigmatised (sadly often for good reason) and conceal their condition and treatment from public attention. Also, for some mental illnesses, there are ethical concerns when involving patients who are at high risk or have a history of aggression or self-harm. [9] These concerns make recruitment to mental health clinical trials challenging.
Retention is another pivotal component to a trial’s scientific success as it is key to a trial’s validity. Attrition may happen in drug trials because of side effects of the medication. Some patients may experience deterioration of their health during the follow-up period, making them reluctant to continue with treatment or the trial. In trials of complex interventions, such as cognitive behavioural therapy or early supported discharge, the absence of blinding in the control arm means that the participants know that they have not received the intervention, which may reduce engagement with trial follow-up or increase the risk of drop-out. It has been suggested that high drop-out rates are associated with larger sample sizes in antipsychotic trials, more specifically trials with multi-centre design. [10] However in modern trials, the sample size required often necessitates a multi-centre design as a single site would not provide enough participants and may not provide sufficiently generalisable results. This requirement for a multi-centre design might result in retention issues.
Previous systematic reviews by Treweek et al. and Brueton et al. investigated the efficacy of different strategies to improve recruitment and retention to randomised trials. [11,12] However evidence in the mental health trial population remains sparse. The review by Treweek et al. summarised the efficacy of different strategies to improve recruitment into randomised trials, but only included 3 eligible studies in mental health. No mental health studies were included in the systematic review by Brueton et al., which investigated the efficacy of different strategies to improve retention. Treweek et al. found that open trial design, and telephone reminders to people who do not respond to postal invitations may improve recruitment, whereas bespoke participant information materials helped little in recruitment.[13] Offering a small financial incentive for completing follow-up questionnaires appeared to help retain patients in the trials, as suggested by Brueton et al. [12] An increasing number of studies employ the use of a “study within a trial” (SWAT) method to assess the impact of technical or design innovations on a trial’s efficiency.[14] To date, most different recruitment strategies are usually employed in an ad hoc manner. Evidence on comparing recruitment strategies retrospectively and observationally can also provide some insight before SWATs are planned.
The aim of this review is to evaluate the evidence base for strategies to improve the recruitment and retention of patients to clinical trials in mental health. A secondary aim was to evaluate the cost-effectiveness of different recruitment and retention strategies, reported as the cost per patient recruited, or cost per patient retained.
2. Methods
2.1 Criteria for considering studies for this review
2.1.1 Types of studies
Two review authors independently screened titles and abstracts and any disagreements in selection were resolved through discussion. Studies that used randomised or observational methods to compare different recruitment strategies designed to recruit participants to randomised controlled trials of interventions for mental health problems were considered. Embedded randomised studies of different recruitment strategies were identified, but given the small number of such studies, we also included randomised controlled trials (RCTs) of mental health interventions which reported the effectiveness a range of strategies used in recruitment retrospectively (e.g. without randomising to different recruitment strategies). For retention, randomised trials of different retention strategies that were embedded in a randomised clinical trial (host trial), or within epidemiological studies such as cross-sectional surveys were included. A full description of the study protocol is available in S1 File.
2.1.2 Types of data
Studies comparing recruitment or retention that involved adult participants with mental health problems, regardless of gender, ethnicity or geographic location, were included. Of particular interest were trials including patients with serious mental illnesses (SMI), such as schizophrenia, but given the expectation of finding only a small number of studies involving these patients, the criteria were broadened to include common mental health problems such as depression and anxiety. Dementia and other organic mental health conditions were excluded, given the different context in which these trials are likely to be conducted. Studies on substance misuse were also excluded as this group of patients is likely to present different recruitment and retention challenges. Studies which did not report outcomes on recruitment or retention strategies for RCTs, studies in which mental illness was comorbid with other physical medical conditions (e.g. cardiovascular disease), and studies not involving adults (e.g. children or adolescents) were also excluded.
2.1.3 Types of methods
Strategies aimed at enhancing recruitment and retention included, but were not limited to:
-
·
Incentives for either or both of patients and clinicians
-
·
Advertising
-
·
Periodic phone call follow-up
-
·
Mailshots and newsletters
-
·
Customised or optimised consent materials
-
·
Amendments to protocol
-
·
Presentations to appropriate groups
-
·
Presentations at conferences
-
·
Trial material customised to specific sites
-
·
Resource manual for recruiters
2.2 Types of outcome measures
2.2.1 Primary outcome
For recruitment, the main outcomes of interest were the type of strategies employed in different studies and the number of patients recruited using each individual strategy. We also extracted data on how many potential participants were approached, if available, using each different strategy in each study. For studies comparing different retention strategies, the primary outcome was ‘response’, defined as the percentage of participants who were successfully engaged in follow-up assessments via each strategy out of the total number of people initially randomised to that strategy.
2.2.2 Secondary outcomes
We were also interested in the cost of each patient recruited/retained through a specific strategy (if any mentioned), namely, the cost-effectiveness of each strategy, defined as the mean cost per patient recruited or mean cost per patient retained, respectively.
2.3 Search strategy
We designed a search method for identifying published randomised trials that focused on improving recruitment and retention in mental health randomised trials. We did not apply any language restrictions apart from English language abstracts.
The search method was comprised of 4 components, each of which included both free-text terms and subject headings. The Boolean operator OR combined terms related to enhancing recruitment and improving retention. This was then combined using the AND operator with terms related to mental health conditions and randomised controlled trials. A brief search strategy is described as follows:
(informed consent OR recruit OR particip) OR (retention OR attrit OR retain)
AND
Randomi#ed controlled trials
AND
Mental health condition filters
Electronic databases searched included:
-
·
MEDLINE, Ovid (1946 to date of search, searched on 28 July 2016);
-
·
EMBASE, Ovid (1980 to date of search, searched on 28 July 2016);
-
·
PsycINFO, Ovid (1806 to date of search, searched on 28 July 2016);
-
·
Cochrane Methodology Review Group Specialised Register (CMR) (from inception until July 2012, searched on 28 July 2016).
The full search strategies for all of the 4 databases are included in S1 File.
2.4 Data extraction and analysis
2.4.1 Data extraction
Two reviewers extracted data from eligible studies. Data extracted for the recruitment trials and their corresponding host trials included:
For host trials: country, disease area, design, sample size, setting, primary outcomes, funding body;
For embedded randomised recruitment trials: strategies to which participants were randomised, number of participants in each arm who were recruited to the host trial;
For studies that compared recruitment strategies retrospectively: strategies used for recruitment, number of participants recruited and approached via each strategy.
For retention trials and their host trials, data extracted included:
For host trials: country, disease area, design, sample size, setting, primary outcomes, follow up period, funding body;
For retention trials: strategies to improve retention; retention rates for each strategy.
2.4.2 Assessment of risk of bias
We used the Critical Appraisal Checklist for Randomised Controlled Trials developed by the Joanna Briggs Institute (JBI) for the assessment of risk of bias for the eligible studies, and calculated the overall score based on the number of items checked for each assessment. Details of the risk of bias assessments are included in S2 File.
2.4.3 Data analysis
For randomised comparative studies, we used relative risk to describe the effect of each recruitment strategy. Non-randomised studies were categorised according to similarity of strategies, for instance, by combining optimised consent materials and incentives. We ranked the strategies based on the numbers of patients recruited and identified strategies that recruited most participants in each study. We also calculated the total number of patients randomised through each recruitment strategy for recruitment studies and the number of responses in each retention strategy for retention studies. Cost-effectiveness of strategies where cost data were available was measured by average cost per patient randomised or average cost per response, respectively. Cost information was first converted to the equivalent monetary value in 2016 using relevant inflation rates of the study country, and subsequently into GBP based on average exchange rates between each currency and GBP in 2016. (http://www.ukforex.co.uk/forex-tools/historical-rate-tools/yearly-average-rates). The average cost-effectiveness of each category of recruitment strategy was calculated using a weighted average approach, where the mean costs were weighted by the sample size.
For studies where cost data were not reported, we extracted relevant information on the processes and generated the cost using available reference cost information (from e.g. Personal Social Services Research Unit). Given the considerable uncertainty in this approach due to insufficient information on resources used, we also performed sensitivity analysis under different scenarios. For instance, the cost of recruiting using health care providers, or research staff, depends on the number of hours spent on recruitment. The assumptions made were: part-time (e.g. 3 hours/day) versus full-time (e.g. 7.5 hours/day). The costing mainly employed a bottom-up approach from a UK NHS/personal social services (PSS) perspective. The unit cost of each component mentioned during the recruitment process was multiplied by the recruitment duration, or study duration otherwise, before all the relevant cost components were summed to make the total recruitment cost. The cost-effectiveness of each strategy was obtained using the total cost divided by the total number of participants for each strategy.
3. Results
3.1 Results of the search
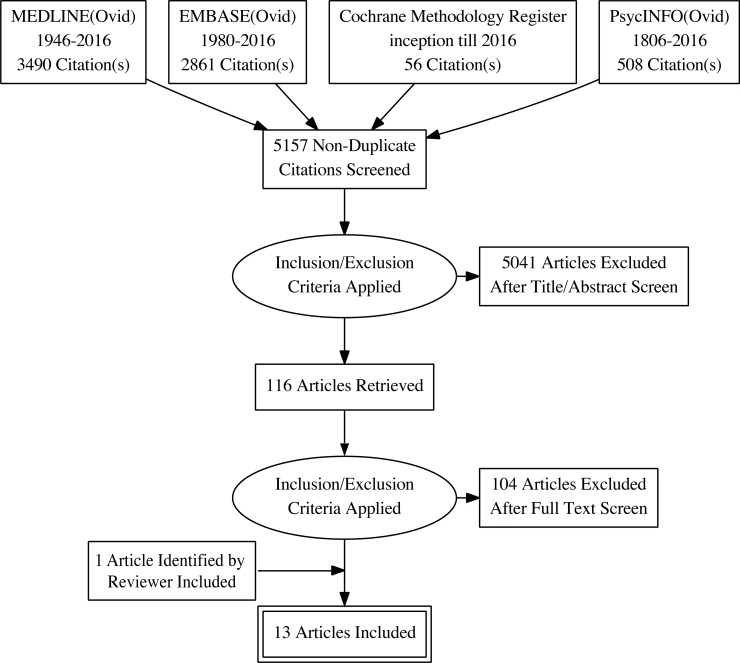
We identified 5157 abstracts, titles and other records from electronic databases from inception of records until July 2016. Of these, 116 were identified for full-text screening and 12 were found to be eligible for inclusion. One additional study (Hughes-Morley 2016) identified by one of the reviewers of this article was also included. (Fig 1) Out of the 13 included studies, 11 are studies on recruitment strategies in the mental health clinical trial context, and 2 focused on retention. Five out of 13 were randomised comparative trials of different recruitment or retention strategies, and 8 were observational comparisons of recruitment strategies embedded within a randomised trial.
Fig 1. A PRISMA flow chart of the article selection process (generated from http://prisma.thetacollaborative.ca/).
3.2 Recruitment strategies
3.2.1 Characteristics of the included studies
Table 1 describes the characteristics of the studies included which looked at recruitment strategies. Overall, three studies employed a randomised design for comparing recruitment strategies (Man 2015, Jeste 2009 and Hughes-Morley 2016). The other studies compared different recruitment strategies retrospectively without randomisation.
Table 1. Summary of the characteristics of included studies on recruitment strategies.
| Study ID | Trial design & intervention | Method of recruitment strategy comparison | Sample size (N)1 | Study duration | Recruitment strategies | No. Patients recruited/No. Patients approached or where contact was attempted | Country |
|---|---|---|---|---|---|---|---|
| Woolhouse 2014 [15] | RCT of mindfulness vs TAU in women of depression, anxiety or stress | retrospective | 32 | 6 weeks | a. researcher recruiting at clinic waiting room | 14/50 | Australia |
| b. mailed-out brochures | 16/2500 | ||||||
| c. recruitment via physiotherapy and childbirth education classes | 2 | ||||||
| Krusche 2014 [16] | RCT of mindfulness-based CBT vs TAU in preventing relapse in people with recurrent depression conducted in primary care | retrospective | 153 | 8 weeks | a. word of mouth | 16/46 | UK |
| b. information from charity | 2/8 | ||||||
| c. posters | 30/123 | ||||||
| d. web-based adverts | 37/300 | ||||||
| e. mental health care referral | 8/32 | ||||||
| f. radio adverts | 26/412 | ||||||
| g. GP referral | 18/116 | ||||||
| h. bus adverts | 2/4 | ||||||
| i. newspaper adverts | 11/101 | ||||||
| j. exhibition | 3/11 | ||||||
| Morgan 2013 [17] | RCT of email delivered self-help health promotion intervention for adults with subthreshold depression symptoms to prevent depression (patients were screened online using PHQ-9) | retrospective | 1699 | 6 weeks | a. Google advertising | 755 | Australia |
| b. Facebook adverts | 35 | ||||||
| c. online forums | unknown2 | ||||||
| d. links from mental health websites | unknown | ||||||
| e. online community noticeboards | unknown | ||||||
| f. group emails | unknown | ||||||
| Man 2015 [18] | RCT of a telephone support and computer-based self-management intervention vs. usual care in patients with depression in primary care |
RCT | 60 | 12 months | a. optimised written patient information material | 43/682 | UK |
| b. original patient information material | 27/682 | ||||||
| Rollman 2008 [19] | RCT of telephone-based collaborative care for treating patients with DSM-IV panic and anxiety disorders | retrospective | 369 | Not reported | a. electronic medical record reminder to primary care clinicians to approach eligible patients | 176/794 | US |
| b. waiting room recruitment by research staff | 193/8095 | ||||||
| Jeste 2009 [20] | Hypothetical RCT of a cognition-enhancing drug vs. placebo in patients with DSM-IV schizophrenia | RCT | 248 | 14 weeks | a. multimedia enhanced consent procedure | 31/62 | US |
| b. ordinary consent procedure | 29/66 | ||||||
| Daley 2007 [21] | RCT of an exercise intervention for women with postnatal depression |
retrospective |
38 | 12 weeks | a. recruitment via GP | 19/96 | UK |
| b. recruitment via specialised “mother and baby” unit | 9/28 | ||||||
| c. recruitment by health visitors | 7/10 | ||||||
| d. self-referral | 3/4 | ||||||
| Le 2008 [22] | RCT of an antenatal psycho-educational group intervention to prevent postpartum depression in patients with high risk3 | retrospective | 310 | 8 weeks | a. recruitment by community health centre staff | 276/553 | US |
| b. recruitment by clinical research staff at hospital-based clinic | 34/1349 | ||||||
| Debar 2009 [23] | RCT of a cognitive behavioural therapy-based guided self-help program on patients with DSM-IV Binge Eating Disorder | retrospective | 249 | not reported | a. mail invitation to comprehensive Eating Disorders Examination (EDE) assessment, $5 incentive for completing online screening questionnaire and $50 for baseline assessment b. mail invitation to abbreviated EDE assessment + telephone interview, $25 for baseline assessment (no payment for screening) |
US | |
| 70/11984 | |||||||
| 154/20810 | |||||||
| c. self-referral | 25/87 | ||||||
| Schlernit-zauer 1998 [24] | RCT of nortriptyline and interpersonal psychotherapy in elderly patients (age ≥ 65) with bereavement-related major depression (screened using HAM-D scale). |
retrospective | 65 | Not specified | a. adverts | 35/194 | US |
| b. obituary letter | 9/99 | ||||||
| c. acquaintance/friend | 9/54 | ||||||
| d. outpatient/in-house psychiatric referral | 7/47 | ||||||
| e. non-specific resources | 2/20 | ||||||
| f. non-mental health physicians | 3/11 | ||||||
| g. letters sent to medical community/health professionals | 0/7 | ||||||
| h. inpatient psychiatric referral | 0/5 | ||||||
| i. private mental health practitioner | 0/3 | ||||||
| j. other mental health facilities | 0/1 | ||||||
| Hughes-Morley 2016 [25] | EQUIP host trial–clustered RCT of a new user led training package to increase user and carer involvement in care planning for patients with a diagnosis of severe mental illness under community mental health teams | RCT and Retrospective4 |
480 | 30 months | a. leaflet sent to advertise patient and public involvement in research (PPIR) | 216/5382 | UK |
| b. control (without leaflet) | 148/2800 | ||||||
| c. leaflet sent to advertise PPIR + telephone follow up for non-responders | 129/4988 | ||||||
| d. control + telephone follow up for non-responders | 92/2580 |
Notes
1. For randomised recruitment trials, N = sample size of its host trial. For non-randomised studies, we assume that the sample size is the sum of number of patients recruited via each strategy.
2. According to the Morgan (2013), there was a total number of 94,808 approaches made in the study.
3. According to Le (2008), high risk = Epidemiologic Studies Depression Scale (CES-D) ≥ 16; all patients were self-reported.
4. In Hughes-Morley (2016), patients who were enrolled during telephone follow up (strategy c & d) were not included in the primary outcome as this was not the intervention for which this trial was designed to find evidence.
Four studies were carried out and funded in the UK, 5 in the US and 2 in Australia. One study involved recruitment to a preventive programme for depression, and one involved a relapse prevention trial in women with a history of post-partum depression. Two of the studies was conducted with people with severe mental illnesses. Five were carried out in a primary care setting. Four involved female participants only. Except for one RCT which was a study of recruitment to a hypothetical trial, the studies involved recruitment to randomised trials involving a range of interventions including mindfulness cognitive behavioural therapy (CBT), health promotion via email, telehealth intervention, exercise, antidepressants, interpersonal therapy and psycho-education. A brief description of the included studies is available in S1 Supplementary.
3.2.2 Randomised comparative studies
Of the included studies, Jeste 2009, Man 2015 and Hughes-Morley 2016 used a randomised approach to compare alternative recruitment strategies. (Table 2) Jeste et al. compared a multimedia consent process using a DVD to present key information from the consent form, with routine consent procedure plus a 10 min ‘control’ DVD giving general information about research. Man et al. used an ‘optimised’ version of the trial information sheet, with contrasting colour, larger fonts, bulleted lists, and accessible wording, compared with the original 8-page A5 patient information booklet. Hughes-Morley et al. investigated the impact of a strategy of providing a leaflet describing the patient and public involvement (PPI) in the trial on recruitment of people who had a diagnosis of severe mental illnesses. Using multimedia during the consent process did not significantly improve recruitment in patients with schizophrenia, whereas optimised written patient information material was superior to non-optimised information for recruitment of patients with depression in primary care, but this result may have occurred by chance. Finally, offering information on PPI collaboration on the trial was not found to have a positive impact on trial recruitment.
Table 2. Summary of randomised comparative studies on recruitment strategies.
| Study ID | Strategy comparison (intervention vs. control) | No. Patients recruited / No. Patients attempted (intervention) | No. Patients recruited / No. Patients attempted (control) | Relative Risk |
|---|---|---|---|---|
| Jeste 2009 | DVD multimedia consent with key information from consent form vs. routine consent procedure + 10 min control DVD on general information on the research | 41/62 | 44/66 | 0.9919 (p = 0.9487) |
| Man 2015 | optimised written patient information material vs. original patient information material | 43/682 | 27/682 | 1.5926 (p = 0.0520) |
| Hughes-Morley 2016 | Leaflet invitations sent to advertise PPIR vs. no leaflet invitations | 216/5382 | 148/2800 | Odds Ratio = 0.751 |
Note
1. Hughes-Morley (2016) reported ORs and used a random effects logistic regression, which yielded OR = 0.75, 95% CI: 0.53 to 1.07, p = 0.013
3.2.3 Non-randomised studies
Krusche et al. suggested that recruiting by adverts and posters showed no less efficacy than recruiting from GP referrals. In contrast, a study using electronic health records to remind GPs to approach potentially eligible patients was more efficient than recruitment by research staff in the clinic waiting room. The latter involved considerably more effort (more than 8000 patients were approached).[19] Le et al. also suggested that being contacted by clinical staff was more successful than being contacted by research staff. Among trials involving people with common mental disorders, GP referrals and contact by clinical staff were the most efficient and successful recruitment strategies and both resulted in an adequate number of patients for the size of a modern trial. Financial incentives are commonly used in commercially funded trials. The study done by DeBar suggested that neither different levels of financial incentives nor different lengths of assessment substantially affected recruitment rates. [23]
In an RCT of mindfulness versus treatment as usual (TAU) in women with depression, anxiety or stress, Woolhouse et al. used both more active (researcher approaching patients in clinic waiting room) and less active (invitations sent to potential participants) strategies.[15] The numbers of patients recruited were similar, despite 2,500 mailshots being sent compared with the researcher approaching 50 patients. A study comparing various forms of online recruitment for a preventive intervention for people with subthreshold depressive symptoms (as assessed by an online questionnaire) found that Google adverts recruited the highest number of participants (755 patients recruited). However, it was indicated that a total of 94,808 potential participants were approached, echoing findings by Krusche et al. suggesting that lower success rates may often be the case in recruitment via online advertisements. [16]
3.2.4 Cost effectiveness of recruitment strategies
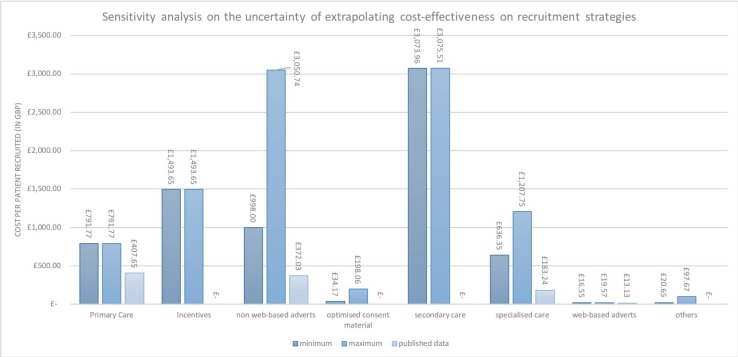
The results of the cost-effectiveness of recruitment strategies are reported in Table 3. The strategy with the lowest cost per patient recruited was web-based advertisement (£13.41 per patient), followed by recruiting via specialised care (£183.24 per patient), non-web-based adverts (£372.03 per patient) and recruitment via primary care (£407.65 per patient). The sensitivity analysis considered the variation in cost according to the different strategies used. For instance, the cost of recruiting using health care providers depends on how much time is spent on recruitment. The two assumed levels of time commitment were part-time (3 hours/day) versus full-time (7.5 hours/day). Fig 2 shows the results of the costing and sensitivity analyses, in comparison with the cost-effectiveness reported with original data. As each study reported different information on costing the recruitment, even for similar strategies across different studies, costs obtained from available sources showed considerable variation. Shown below is an example of how recruitment cost was obtained, using a study by Morgan et al. (Table 4). Further details and sensitivity analysis are given in S1 Data.
Table 3. Summary of recruitment strategies across studies.
| Recruitment strategy | Number of studies where strategy was used | Average cost per randomised participant (in GBP) with original data1 | Number of times recruiting the most within study |
|---|---|---|---|
| Web-based adverts2 | 2 | £13.41 | 2 |
| Via specialised care | 4 | £183.24 | 1 |
| Via secondary care | 2 | not reported | 1 |
| Non-web-based adverts | 3 | £372.03 | 1 |
| Financial incentives3 | 1 | not reported | 1 |
| Via primary care | 4 | £407.65 | 2 |
| Others | 4 | not reported | 0 |
Notes
1. The cost results account for the average exchange rates GBP/AUD and GBP/USD in year 2016, and inflation rates ofthe countries of publication from year of publication until 2016. (http://www.ukforex.co.uk/forex-tools/historical-rate-tools/yearly-average-rates; http://ination.stephenmorley.org/; U.S. Internal Revenue Service)
2. Results on web-based adverts included Morgan (2013), a study which used a number of different online resources to recruit patients.
3. Results on financial incentives included DeBar (2009), a study which used different incentives to recruit patients.
Fig 2. A sensitivity analysis on the uncertainty of extrapolating the cost-effectiveness on recruitment strategies.
Table 4. An example of the detailed costing for a strategy used in Morgan 2013.
| Study ID | Recruitment strategy | Description in original text | Resource used for costing | Calculation | Notes | Min. Cost (in GBP) | Max. Cost (in GBP) |
|---|---|---|---|---|---|---|---|
| Morgan 2013 | links from webpage | "A new page of supporters was created to accommodate this requirement. This page thanked each organization or website that had helped promote the study to participants. Some websites were generous and included a link and blurb on their home page; others listed the website within a section of their site that contained links to other interesting websites." | Clicking on the webpage link, assumed cost zero. | assuming from 2hrs/day to full time responsible for mailing and posting, salary Band 7 £38,786. (£52/hr) | recruitment from Feb2010 to March 2011(13 months). However, no information on how many hours dedicated to such strategy. | 25,740 | 38,786 |
3.3 Retention strategies
Table 5 summarises two studies identified that compared different strategies to improve postal response in surveys. On joining the trials, participants were randomised to followed up via different methods, and their response rates at follow-up were compared as a proxy for retention rates using the different methods (McLean 2014, Dirmaier 2007). McLean et al. investigated the effects of pre-notification (e.g. notifying participants in advance that they would be asked for information) and envelope ‘teaser’ (placement of a short message on the survey envelope) on increasing postal response rates in a bulimia nervosa mental health literacy survey. Dirmaier et al. conducted a randomised trial to find out whether small cash incentives and a shortened questionnaire helped increase postal response rates in a mailed follow-up survey one year after inpatient psychotherapeutic treatment for mental health patients. Both studies used a 2×2 factorial design to investigate the impact of strategies on postal response rates. Financial incentives, abridged questionnaire and pre-notification were suggested to be effective to increase postal response rates, but the effects were small.
Table 5. Summary of retention strategies.
| Study ID | Retention strategy | Study period | Numbers approached | Numbers responded | Response rates | Cost information | Relative risk |
|---|---|---|---|---|---|---|---|
| McLean 2014 [26] | Prenotification (+), envelope teaser (-) | not reported | 762 | 190 | 25% | $23.68/response | Marginal Prenotification RR = 1.165 (p = 0.027) Marginal Envelope Teaser RR = 0.955 (p = 0.508) |
| Prenotification (+), envelope teaser (+) | not reported | 747 | 167 | 22% | Not reported | ||
| Prenotification (-), envelope teaser (-) | not reported | 750 | 150 | 20% | $26.25/response | ||
| Prenotification (-), envelope teaser (+) | not reported | 747 | 154 | 21% | Not reported | ||
| Dirmaier 2007 [27] | Financial incentive (+), abridged questionnaire (-) | 1 year | 832 | 458 | 55% | Not reported | Marginal Incentive RR = 1.146 (p < 0.0001) Marginal abridged questionnaire RR = 1.073 (p = 0.021) |
| Financial incentive (+), abridged questionnaire (+) | 1 year | 845 | 500 | 59% | Not reported | ||
| Financial incentive (-), abridged questionnaire (-) | 1 year | 1045 | 502 | 48% | Not reported | ||
| Financial incentive (-), abridged questionnaire (+) | 1 year | 1103 | 569 | 52% | Not reported |
4. Discussion
The review identified only 3 eligible randomised comparative studies of alternative recruitment strategies in mental health clinical trials. None showed a statistically significant difference between using standard and optimised patient consent and information materials. Our findings were consistent with those of Treweek et al. The difference approached significance in one trial of recruitment using optimised patient information material compared with original patient material (Man 2015), although the effect was small. The 8 other studies included in the recruitment section of the review consisted of non-randomised, retrospective comparisons of different recruitment strategies. It is difficult to know whether the different strategies were employed in comparable ways in these studies, or for the same duration. Given the small number of randomised comparative studies identified, and the inconclusive results, this review suggests further research in this area may benefit trial recruitment. Two randomised studies comparing different retention strategies in mental health were identified (McLean 2014, Dirmaier 2007). Both involved different ways of maximising response rates to postal assessments. As follow-up assessment in RCTs is often carried out in the form of a questionnaire, the response rate to this type of assessment may be appropriate as a proxy for retention.
Prior to this review, we also piloted a search strategy that encompassed informed consent, recruitment, antipsychotics and randomised trials, attempting to review recruitment strategies in antipsychotic randomised trials. It generated approximately 2,000 records from MEDLINE, EMBASE, and CMR. However, after screening there was only 1 study (Jeste 2009) which met our criteria. Little attention has been paid to such methodological trials (e.g. using SWATs to increase the evidence base for trial decision-making) that endeavour to tackle some of the most common issues in mental health clinical trials.
The included studies showed substantial differences in strategies used, but also in clinical settings, mental health conditions and study design. We were not able to obtain a pooled estimate of recruitment efficacy of these strategies due to the non-randomised designs used, and the choice of analysis which could be used to assess the relative efficacy of different strategies was limited. It is therefore difficult to estimate the efficacy from beyond an individual level. Also, some included studies did not report numbers of potential participants approached by each strategy, e.g. the denominator for the efficiency measure of recruitment strategies (number recruited divided by number approached), and comparison between numerators should be made with caution, as some strategies have broader reach to the population and some studies required larger sample sizes. There were some interesting insights from the result of some recruitment studies, nevertheless. For instance, although clinical staff and GPs are often thought to be helpful in recruiting patients into randomised trials, here it was shown that they recruited no better than advertisements. The comparisons made were ad hoc, however, and in the absence of randomised controlled experiments, the area needs more rigorous investigation.
In this review, we also considered cost-effectiveness for each strategy based on numbers of participants recruited and cost incurred. It provided some useful information for public funded trials, which often work on a limited budget. However, it is worth noting that the choice of recruitment strategy should consider not solely cost-effectiveness, but also the study design, types of intervention and more importantly, population characteristics. For instance, we found that although using web-based advertisements showed merit in terms of efficacy and cost-effectiveness in recruitment, however the loss to follow-up in the population recruited via this method cannot be ignored. It is essential also to consider whether certain recruitment methods may identify a biased population. We also considered the uncertainty due to the inadequately reported cost information in the included studies, and performed sensitivity analysis of the costs obtained. The lack of a standard and transparent methodological framework for reporting the costs or resource use during recruitment has engendered considerable variations in the analysis and has led to challenges in interpreting the results. For instance, strategies that involved research assistants recruiting in clinic waiting rooms did not specify the total hours spent, therefore it was necessary to make assumptions regarding the numbers of hours spent per day on the recruitment task. Even for studies which employed similar recruitment strategies, reporting on resources used during recruitment varied tremendously, leading to considerable differences in costs obtained. Speich et al. also found in their systematic review that none of the included studies provided empirical resource use and cost data for all aspects of an RCT, and for trials that reported costs of recruitment, even similar recruitment strategies could cost different amounts across studies. Within a given category of recruitment strategies, for instance, the median cost of a mailed invitation was 228 USD, ranging from 15 to 1,116 USD per patient. [28]
Recently the ORRCA project (Online Resource for Recruitment research in Clinical triAls, www.orrca.org.uk) has attempted to bring together all the studies on recruitment into randomised trials by creating a searchable database. This initiative may help to inform trialists and recruiters of better ways to recruit patients into trials.[29] Also, Madurasinghe et al. provided guidelines for reporting embedded recruitment trials, for which a checklist based on the Consolidated Standards for Reporting Trials (CONSORT) statement 2010 was developed and several examples were listed. [30] Unlike the existing literature, this review has a focus on recruitment via different channels used as strategies described in the included studies, partly because of the inadequacy of the evidence available for mental health trials. It provides some evidence from a different perspective and makes suggestions regarding possible future research in this area. For instance, SWATs may be designed to compare the efficacy of recruitment by research staff with recruitment by clinical staff. Promoting the guidelines by Madurasinghe et al. will help to improve the quality of reporting for these methodological trials. Furthermore, it is also worth investigating the performance of different recruitment strategies with respect to other aspects of the trial, such as the population characteristics or adherence to the trial intervention, as these features also can determine a trial’s precision and efficiency. Some strategies may recruit a biased sample. For instance, using web-based adverts as a recruitment method in mental health trials may inadvertently recruit the “worried well” or those who do not sufficiently resemble real-world patients.
This study has the following limitations. Firstly, we only identified 3 randomised comparative studies of recruitment and two of retention. The rest compared different strategies without randomisation and this may diminish the internal validity of their findings. Secondly, out of 13 identified studies, the majority were in depression-related illnesses. The limited number of studies involving people with diagnoses of severe mental illnesses such as bipolar disorders or schizophrenia, reduces the generalisability of the review. It highlights the need for more research in this area, since there are many challenges to recruitment within this group of people. Moreover, there were no eligible RCTs aimed at improving retention within randomised controlled trials. We included 2 studies which focus on improving postal response rates in follow-up, despite the fact they were not set within a randomised clinical trial. However, since they used a randomised design to assess methods to enhance response rates, we believe they contribute useful information, although clearly more studies are needed to address retention issues in randomised trials and in studies that use face to face assessments rather than postal questionnaires. Lastly, lack of reported information on costs in many of the included studies means there is considerable uncertainty in our findings on cost-effectiveness.
5. Conclusion
This review discusses the different strategies to improve recruitment and retention in mental health clinical trials. The recruitment studies included showed substantial variation in strategies, clinical settings, mental health conditions and study design. It is difficult to assess the overall efficacy of any particular recruitment strategy as some strategies that worked well for a particular population may not work as well for others. Paying attention to the accessibility of information and consent materials (optimisation) may help improve recruitment. Recruitment by clinical staff and non-web-based adverts showed some efficiency and success in certain circumstances. Pre-notification, abridged questionnaires and financial incentives have small positive effects on retention rates in postal surveys. The limited number of eligible studies identified suggests that more research in this area is needed given its important implications.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We sincerely thank Miss Sophie Pattison, librarian at the Royal Free Medical Library, for commenting on the search strategy, and Dr. Caroline S. Clarke for proofreading the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Howard L, de Salis I, Tomlin Z, Thornicroft G, Donovan J. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp Clin Trials. 2009;30: 40–46. 10.1016/j.cct.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furlong EMJRP Bull GUJ. Barriers to Clinical Trial Recruitment and Possible Solutions: A Stakeholder Survey. 2015; [Google Scholar]
- 3.Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359: 781–785. 10.1016/S0140-6736(02)07882-0 [DOI] [PubMed] [Google Scholar]
- 4.Weisfeld V, English RA, Claiborne AB%@ 0309219302. Public Engagement and Clinical Trials: New Models and Disruptive Technologies: Workshop Summary National Academies Press; 2012. [PubMed] [Google Scholar]
- 5.Mason VL, Shaw A, Wiles NJ, Mulligan J, Peters TJ, Sharp D, et al. GPs’ experiences of primary care mental health research: A qualitative study of the barriers to recruitment. Fam Pract. 2007;24: 518–525. 10.1093/fampra/cmm047 [DOI] [PubMed] [Google Scholar]
- 6.Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA psychiatry. American Medical Association; 2014;71: 1409–1421%@ 2168–622X. 10.1001/jamapsychiatry.2014.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinon BJ, Potts AJ, Watson SB. Placebo response in clinical trials with schizophrenia patients. Curr Opin Psychiatry. LWW; 2011;24: 107–7367. [DOI] [PubMed] [Google Scholar]
- 8.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. Jama. American Medical Association; 2002;287: 1840–7484. [DOI] [PubMed] [Google Scholar]
- 9.Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. American Medical Association; 2005;62: 247–253%@ 0003–990X. 10.1001/archpsyc.62.3.247 [DOI] [PubMed] [Google Scholar]
- 10.Spineli LM, Leucht S, Cipriani A, Higgins JPT, Salanti G. The impact of trial characteristics on premature discontinuation of antipsychotics in schizophrenia. Eur Neuropsychopharmacol. 2013;23: 1010–1016. 10.1016/j.euroneuro.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 11.Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3: e002360—e002360. 10.1136/bmjopen-2012-002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brueton VC, Tierney J, Stenning S. Strategies to improve retention in randomised trials. {…} Database Syst Rev. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database of Systematic Reviews. 2018. 10.1002/14651858.MR000013.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial Forge Guidance 1: What is a Study Within A Trial (SWAT)? Trials. 2018. 10.1186/s13063-018-2535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolhouse H, Mercuri K, Judd F, Brown SJ. Antenatal mindfulness intervention to reduce depression, anxiety and stress: a pilot randomised controlled trial of the MindBabyBody program in an Australian tertiary maternity hospital. BMC Pregnancy Childbirth. London: BioMed Central; 2014;14: 369 10.1186/s12884-014-0369-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krusche A, von Rohr I, Muse K, Duggan D, Crane C, Williams JMG. An Evaluation of the Effectiveness of Recruitment Methods: The Staying Well after Depression Randomized Controlled Trial. Clin Trials. 2014;11: 141–149. 10.1177/1740774514521905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan AJ, Jorm AF, Mackinnon AJ. Internet-Based Recruitment to a Depression Prevention Intervention: Lessons From the Mood Memos Study. Eysenbach G, editor. J Med Internet Res. JMIR Publications Inc., Toronto, Canada: Gunther Eysenbach; 2013;15: e31 10.2196/jmir.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man M-S, Rick J, Bower P. Improving recruitment to a study of telehealth management for long-term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials. 2015;16: 309 10.1186/s13063-015-0820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollman BL, Fischer GS, Zhu F, Belnap BH. Comparison of Electronic Physician Prompts versus Waitroom Case-Finding on Clinical Trial Enrollment. J Gen Intern Med. New York: Springer-Verlag; 2008;23: 447–450. 10.1007/s11606-007-0449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeste D V, Palmer BW, Golshan S, Eyler LT, Dunn LB, Meeks T, et al. Multimedia Consent for Research in People With Schizophrenia and Normal Subjects: a Randomized Controlled Trial. Schizophr Bull. 2009;35: 719–729. 10.1093/schbul/sbm148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daley AJ, Winter H, Grimmett C, McGuinness M, McManus R, MacArthur C. Feasibility of an exercise intervention for women with postnatal depression: a pilot randomised controlled trial. Br J Gen Pract. Royal College of General Practitioners; 2008;58: 178–183. 10.3399/bjgp08X277195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le H-N, Lara MA, Perry DF. Recruiting Latino women in the U.S. and women in Mexico in postpartum depression prevention research. Arch Womens Ment Health. 2008;11: 159–169. 10.1007/s00737-008-0009-6 [DOI] [PubMed] [Google Scholar]
- 23.DeBar LL, Yarborough BJ, Striegel-Moore RH, Rosselli F, Perrin N, Wilson GT, et al. Recruitment for a guided self-help binge eating trial: Potential lessons for implementing programs in everyday practice settings. Contemp Clin Trials. 2009;30: 326–333. 10.1016/j.cct.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlernitzauer M, Bierhals AJ, Geary MD, Prigerson HG, Stack JA, Miller MD, et al. Recruitment Methods for Intervention Research in Bereavement-Related Depression: Five Years’ Experience. Am J Geriatr Psychiatry. Elsevier; 6: 67–74. 10.1097/00019442-199802000-00009 [DOI] [PubMed] [Google Scholar]
- 25.Hughes-Morley A, Hann M, Fraser C, Meade O, Lovell K, Young B, et al. The impact of advertising patient and public involvement on trial recruitment: Embedded cluster randomised recruitment trial. Trials. 2016;17 10.1186/s13063-016-1718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean SA, Paxton SJ, Massey R, Mond JM, Rodgers B, Hay PJ. Prenotification but not envelope teaser increased response rates in a bulimia nervosa mental health literacy survey: A randomized controlled trial. J Clin Epidemiol. 2014;67: 870–876. 10.1016/j.jclinepi.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 27.Dirmaier J, Harfst T, Koch U, Schulz H. Incentives increased return rates but did not influence partial nonresponse or treatment outcome in a randomized trial. J Clin Epidemiol. Elsevier; 60: 1263–1270. 10.1016/j.jclinepi.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 28.Speich B, von Niederhäusern B, Schur N, Hemkens LG, Fürst T, Bhatnagar N, et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. Journal of Clinical Epidemiology. 2018. pp. 1–11. 10.1016/j.jclinepi.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 29.Kearney, Anna and Harman, Nicola L and Bacon, Naomi and Daykin, Anne and Heawood, Alison J and Lane, Athene and Blazeby, Jane and Clarke, Mike and Treweek, Shaun and Williamson PR. Online resource for recruitment research in clinical trials research (ORRCA). BIOMED CENTRAL LTD 236 GRAYS INN RD, FLOOR 6, LONDON WC1X 8HL, ENGLAND; 2017.
- 30.Madurasinghe VW. Guidelines for reporting embedded recruitment trials. Trials. 2016;17: 27 10.1186/s13063-015-1126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.