Abstract
The serotonin (5-HT) 5-HT2A receptor (5-HT2AR) and 5-HT2C receptor (5-HT2CR) in the central nervous system are implicated in a range of normal behaviors (e.g., appetite, sleep) and physiological functions (e.g., endocrine secretion) while dysfunctional 5-HT2AR and/or 5-HT2CR are implicated in neuropsychiatric disorders (e.g., addiction, obesity, schizophrenia). Preclinical studies suggest that the 5-HT2AR and 5-HT2CR may act in concert to regulate the neural bases for behavior. Here, we utilize three distinct biophysical and immunocytochemistry-based approaches to identify and study this receptor complex in cultured cells. Employing a split luciferase complementation assay (LCA), we demonstrated that formation of the 5-HT2AR:5-HT2CR complex exists within 50 nm, increases proportionally to the 5-HT2CR:5-HT2AR protein expression ratio, and is specific to the receptor interaction and not due to random complementation of the luciferase fragments. Using a proximity ligation assay (PLA), we found that cells stably expressing both the 5-HT2AR and 5-HT2CR exhibit 5-HT2AR:5-HT2CR heteroreceptor complexes within 40 nm of each other. Lastly, bioluminescence resonance energy transfer (BRET) analyses indicates the formation of a specific and saturable 5-HT2AR:5-HT2CR interaction, suggesting that the 5-HT2AR and 5-HT2CR form a close interaction within 10 nm of each other in intact live cells. The bioengineered receptors generated for the LCA and the BRET exhibit 5-HT-mediated intracellular calcium signaling as seen for the native receptors. Taken together, this study validates a very close 5-HT2AR:5-HT2CR interaction in cultured cells.
Introduction
The metabotropic serotonin (5-HT) 5-HT2 receptor (5-HT2R) family consists of three isoforms (5-HT2AR, 5-HT2BR, 5-HT2CR) that share ~50% total sequence homology and ~80% sequence homology within their seven transmembrane domains [1]. The 5-HT2AR and 5-HT2CR localized within the central nervous system are implicated in a range of normal behaviors (e.g., appetite, sleep) and physiological functions (e.g., endocrine secretion) while dysfunctional brain 5-HT2AR and/or 5-HT2CR are implicated in neuropsychiatric disorders (e.g., addiction, obesity, schizophrenia). Antagonism of the 5-HT2AR is a common feature of atypical antipsychotics employed in schizophrenia [2] and is the mode of action for the recently FDA-approved pimavanserin in the treatment of psychosis in Parkinson’s disease [3]. Furthermore, the 5-HT2CR agonist lorcaserin is FDA-approved for the treatment of obesity [4]. Biochemical, behavioral and pharmacological studies indicate that the 5-HT2AR and 5-HT2CR interact in rodents in vivo [5–10] raising the possibility that the 5-HT2AR and 5-HT2CR may act in concert to regulate the neural bases for behavior (for reviews) [11, 12].
The 5-HT2AR and 5-HT2CR transcripts co-exist in brain regions particularly associated with the limbic-corticostriatal circuitry [13–16], while the protein for these receptors co-localizes to the same neurons in the rat medial prefrontal cortex (mPFC) [17]. We recently demonstrated that the two receptors form a protein complex in the mPFC assessed by co-immunoprecipitation [5]. Expression of this complex was associated with phenotypic levels of impulsivity in the rat [5], suggesting the behavioral significance of a 5-HT2AR:5-HT2CR protein complex. Based upon reported evidence of a 5-HT2AR and 5-HT2CR association [5–10], we conducted biophysical studies to further define and validate the interaction between these homologous receptors at the single cell level.
The 5-HT2AR and the 5-HT2CR are G protein-coupled receptors (GPCRs) that interact with Gαq/11 to activate the enzyme phospholipase Cβ which generates intracellular second messengers inositol-1,4,5-trisphosphate and diacylglycerol, leading to increased calcium release from intracellular stores (Cai2+) [18, 19]. The main functional GPCR unit for the 5-HT2AR [20, 21] and 5-HT2CR [22–25] is proposed to be their homomeric form. There is also evidence that 5-HT2AR can form protein complexes with other GPCRs, including the metabotropic glutamate mGlu2 receptor (mGlu2R) [26], dopamine D2 receptor (D2R) [27], cannabinoid CB1 receptor (CB1R) [28], and 5-HT1A receptor (5-HT1AR) [29]. Likewise, protein:protein interactions with the 5-HT2CR have been reported with the ghrelin receptor (growth hormone secretagogue receptor 1α, GHS1αR) [30], the melatonin MT2 receptor (MT2R) [31], and the N-methyl-D-aspartate (NMDA)-gated ion channel subunit GluN2A [32]. The detection of the 5-HT2AR:5-HT2CR protein complex in cellular models [8] and rodent brain [5, 17] led us to develop biophysical approaches to further define this interaction using in vitro cellular models. In the present study, we tested if a 5-HT2AR:5-HT2CR interaction occurs in cultured cells using three complementary biophysical techniques with increasing spatial resolution. Our findings indicate these receptors form a close biophysical interaction within 10 nm in living cells and provide validation [8] and novel insights into the 5-HT2AR:5-HT2CR heteromeric receptor interaction.
Materials and methods
Compounds and 3H-radioligands
All purchased compounds were >98% pure according to the manufacturers. Coelentrazine H (Thermo Scientific, Waltham, MA) was dissolved in ethanol as a stock solution (5 mM). Serotonin (5-HT) hydrochloride (Acros Organics, ThermoFischer Scientific, Pittsburgh, PA), d-luciferin (Gold Biotechnology, St. Louis, MO), and mianserin hydrochloride (Sigma Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO) (10 mM) and prepared fresh daily. [3H]-Mesulergine (84.7 Ci/mmol) and [3H]-ketanserin (47.3 Ci/mmol) were purchased from PerkinElmer Life Sciences (Waltham, MA).
DNA and plasmid constructs
The cDNAs encoding human 5-HT2AR and 5-HT2C-INIR (non-edited isoform) in pcDNA3.1+ vector were obtained from UMR cDNA Resource Center (Rolla, MO) and employed in the Cai2+ release assay and luciferase complementation assay (LCA). Split luciferase constructs for the LCA were created using template mammalian expression vectors and standard cloning techniques: the N-terminus of the human rapamycin-binding domain of mammalian target of rapamycin (FRB) fused to the N-terminus of luciferase (NLuc) (pcDNA3.1-V5_HIS TOPO; FRB-NLuc) and the N-terminus of human FK506-binding protein 12 (FKBP) fused to the C-terminus of luciferase (CLuc) (pEF6-V5_HIS TOPO; FKBP-CLuc) were obtained from Dr. David Piwinca-Worms [33, 34]. Briefly, the FRB was replaced with 5-HT2C-R by PCR amplification of the open reading frame of the 5-HT2CR followed by ligation into the FRB-NLuc fusion vector to create the 5-HT2CR-NLuc plasmid. The FKBP was similarly replaced with 5-HT2AR in the FKBP-CLuc fusion vector to create the 5-HT2AR-CLuc plasmid. The resultant plasmids expressed 5-HT2CR-NLuc or 5-HT2AR-CLuc (verified by Western blot; data not shown). Because the N-terminus of the 5-HT2AR and 5-HT2CR is located extracellularly, this attachment of the NLuc (or CLuc) to the C-terminus of either receptor results in intracellular expression of the luciferase fragments. Bioluminescence resonance energy transfer (BRET) plasmids were created and synthesized by Genscript (Piscataway, NJ) for the 5-HT2AR, 5-HT2CR and the β2-adrenergic receptor (β2-AR, control for specificity of the BRET interaction). The 5-HT2AR and β2-AR gene sequences were codon optimized, fused with an N-terminus signal hemagglutinin (HA) sequence, and a C-terminus Renilla luciferase (RLuc) sequence in the open reading frame [35]; the 5-HT2AR-RLuc and β2-AR-RLuc served as the donor constructs. The 5-HT2CR gene sequence was fused with a C-terminus enhanced yellow fluorescent (eYFP) protein sequence in the open reading frame [35]; the 5-HT2CR-eYFP served as the acceptor construct. All three BRET constructs were subcloned into pcDNA 3.1+ using 5’ BamH1 and 3’ Xba1 restriction sites. The coding regions of all plasmids were entirely sequenced and verified prior to use (Molecular Genomics Core, University of Texas Medical Branch, Galveston, TX).
Cell culture and transfection
Human embryonic kidney 293 cells (HEK293; CRL-1573™, ATCC, Washington, DC) were cultured as a monolayer in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen, Waltham, MA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, Waltham, MA) and 1% penicillin/streptomycin (Gibco, Invitrogen, Waltham, MA) in 6-well plates (Thermo Scientific, Waltham, MA). Cells were incubated at 37°C in a 5% CO2 and 85% relative humidity. HEK293 cells from passage 7 to 18 (passage one was defined as the first plate of cells from our liquid nitrogen stock) were transiently transfected at 80% confluency in a 6-well plate with 10 μL of Lipofectamine 2000 (Invitrogen, Waltham, MA; according to manufacturer’s protocol) in antibiotic-free Opti-MEM (Gibco, Invitrogen, Waltham, MA) supplemented with 5% dialyzed FBS (Gibco, Invitrogen, Waltham, MA) overnight. For luciferase complementation assays (LCA), varying ratios of 5-HT2CR-NLuc:5-HT2AR-CLuc plasmids (1:2, 1:1, 2:1, 3:1) with a total of 1 μg cDNA/well were transiently transfected into HEK293 cells. Similarly, 1 μg total cDNA/well was used to transfect cells for analyses of 5-HT2AR and 5-HT2CR signaling through intracellular calcium release (Cai2+) assays. For BRET assays, 100 ng of donor plasmid (5-HT2AR-RLuc or β2AR-Rluc) and increasing amounts (1–20 fold) of acceptor plasmid (5-HT2CR-eYFP) were added to each well of a 6-well plate.
We generated a dual stably-transfected cell line by transfecting 5-HT2AR-CHO cells with a 5-HT2CR construct (5-HT2A+2CR-CHO cells) which was employed in the proximity ligation assay (PLA). A Chinese hamster ovary cell line (CHO-K1) stably transfected with the 5-HT2AR (5-HT2AR-CHO; FA4 cells) [36–38] was a generous gift of Drs. Kelly A. Berg and William P. Clarke (University of Texas Health Science Center at San Antonio, San Antonio, TX). This line expresses the transfected 5-HT2AR in the p198-DHFR plasmid containing a hygromycin resistance gene. The parental CHO cell line did not express detectable amounts of any 5-HT2R mRNAs [39]. The 5-HT2AR-CHO cells were stably transfected with the 5-HT2CR in pcDNA3.1+ containing a G418 resistance gene to generate the dual-expressing 5-HT2A+2CR-CHO clonal cell line (“FA4E4”). Stably transfected 5-HT2A+2CR-CHO cells from passage 8 to 15 (passage 1 was defined as the first plate of cells stably transfected with both 5-HT2A+2CR) were cultured at 37°C, 5% CO2, and 85% relative humidity in GlutaMax α-MEM (Invitrogen, Carlsbad, CA), 5% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 100 μg/mL hygromycin (Mediatech, Manassas, VA) and G418 (Corning, Manassas, VA) and were passaged when they reached 70–80% confluency.
Intracellular calcium (Cai2+) release assay
Serotonin-evoked release of Cai2+ was employed to validate the signaling profile of wild-type and novel 5-HT2AR and 5-HT2CR constructs. At 24 hours post transfection, HEK293 transiently expressing the WT 5-HT2AR, 5-HT2AR-CLuc, 5-HT2AR-RLuc, WT 5-HT2CR, 5-HT2CR-NLuc or 5-HT2CR-eYFP were washed with PBS, trypsinized (Gibco, Invitrogen, Waltham, MA), harvested, and plated in phenol-free DMEM with 10% dialyzed FBS at a density of 60,000 cells/well into poly-L-lysine coated (Sigma Aldrich, Waltham, MA) 96-well black wall, clear bottom cell culture plates (Greiner Bio-One, Monroe, NC). The next day (~18 hours later), complete media was aspirated and replaced with 50 μl of Hank’s balanced salt solution (HBSS) and cells serum starved for one hour in an incubator. In preparation for measurement of Cai2+ release on a fluorescence imaging plate reader (FLIPR Tetra®; Molecular Devices, Sunnyvale, CA), 50 μl of 2X FLIPR® Calcium 5 dye (Molecular Devices, Sunnyvale, CA) with 2.5 mM probenecid was added to the cells and plates were returned to the incubator for one hour. Serial dilutions of 5-HT (10−12 to 10−5 M) were prepared at 5X final concentration and transferred to a 96-well source plate. Cell and 5-HT plates were placed in a fluorescence imaging plate reader (FLIPRTETRA; Molecular Devices, Sunnyvale, CA). The FLIPRTETRA was programmed to read baseline dye fluorescence for 10 sec followed by addition of 25 μl (5X) drug/well and read for an additional 180 sec (acquisition once/sec). The maximum fluorescence (ΔF) observed in each well during the first 40 seconds after 5-HT addition was determined using the FLIPRTETRA ScreenWorks 4.0 program and results normalized to the average of the baseline fluorescence (F) in each well (first 10 reads); data are presented as the percentage of the WT 5-HT2 response. Data from four independent experiments, conducted in technical triplicate were analyzed.
Luciferase complementation assay (LCA)
In the LCA reporter system, two complementary N- (NLuc) and C-terminus (CLuc) components of the enzyme luciferase, which have no activity on their own, are split in half and fused to the two receptor proteins of interest [33, 34, 40]. The detection of two proteins in close proximity in living cells is achieved as the association of the two proteins within < 50 nm brings the inactive luciferase fragments into close proximity and the luciferase enzyme activity is reconstituted [33, 34, 40]. At 24 hours post transfection, HEK293 cells co-expressing various ratios of 5-HT2AR-CLuc and 5-HT2CR-NLuc plasmids were washed with phosphate buffered saline (PBS), trypsinized (Gibco, Invitrogen, Waltham, MA), harvested, centrifuged (500 x g for four mins) and plated in phenol-free DMEM with 10% dialyzed FBS at a density of 60,000 cells/well into poly-L-lysine (Sigma Aldrich, Waltham, MA) coated white-walled clear bottom 96-well cell culture plates (Greiner Bio-One, Monroe, NC) and cultured overnight. Cells were serum starved for one hour in HBSS and luciferase activity was measured 48 hours post-transfection following the addition of 1.5 mg/mL d-luciferin (GoldBio, St Louis, MO) and 150 μM coenzyme A (Thermo Scientific, Waltham, MA). Total luminescence was measured with an H4 synergy reader (Biotek, Winooski, VT) for 40 mins following d-luciferin addition (time = -5 min). Maximal luminescence values from 45-min kinetic runs are plotted for comparison. Data from three independent experiments were analyzed and conducted in at least four technical replicates.
Saturation binding assay
At 24 hours post-transfection, transfection media was replaced with DMEM supplemented with 10% dialyzed FBS; 24 hours later, cells were collected by centrifugation at 4000 x g at 4°C for 25 mins in ice cold assay buffer containing 50 mM Tris HCl, 10 mM MgCl2 and 0.1 mM EDTA. Membranes were collected by centrifugation three times at 4500 x g at 4°C for 20 mins and stored at -80 °C until use. Saturation binding isotherms were performed in 96-well plates using similar methods to the psychoactive drug screening program (PDSP) [41]. For saturation binding assays, 0.2 to 20 nM of [3H]-ketanserin (PerkinElmer, Waltham, MA) for 5-HT2AR or [3H]-mesulergine (PerkinElmer, Waltham, MA) for 5-HT2CR was used to obtain affinity (KD) and protein concentration (BMAX) values following co-transient transfection with the 5-HT2CR-NLuc and 5-HT2AR-CLuc receptor constructs into HEK293 cells. Non-specific binding was determined in the presence of 10 μM of mianserin hydrochloride (Sigma Aldrich, St. Louis MO). The reaction mixtures were incubated at room temperature (RT) for 90 mins on a plate shaker to reach equilibrium, and then passed rapidly through a printed filtermat using a FilterMate Harvester (PerkinElmer, Waltham, MA). The printed filtermat containing bound [3H]-ketanserin or [3H]-mesulergine was microwaved for one min to dry, then a MeltiLex sheet was melted onto the printed filtermat via a hotplate. The contents were sealed and counted for scintillation using a MicroBeta 2 (PerkinElmer, Waltham, MA). Direct radioligand concentrations were measured by pipetting into 1 mL of Optiphase Supramix (PerkinElmer, Waltham, MA) and measured on a Tri-Carb 2910TR liquid scintillation analyzer (PerkinElmer, Waltham, MA). Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Waltham, MA) by measuring absorbance values (562 nm) on a H4 synergy reader (Biotek, Winooski, VT). Each experiment was performed in technical triplicates with a minimum of three biological replicates.
MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for measurement of metabolic activity, an indicator of cell viability and health, was performed, according to manufacturer’s protocols (Sigma Aldrich, St. Louis MO). At 24 hours post-transfection, HEK293 cells co-transiently transfected with 0.75 μg of 5-HT2CR-NLuc, 0.25 μg of 5-HT2AR-CLuc, and increasing concentrations (0–2 μg) of either WT 5-HT2CR, 5-HT2AR or empty pcDNA3.1+ vector were washed with PBS, trypsinized (Gibco, Invitrogen, Waltham, MA), harvested, and plated in DMEM with 10% dialyzed FBS at a density of 60,000 cells/well into poly-L-lysine coated (Sigma Aldrich, Waltham, MA) clear 96-well cell culture plates (Greiner Bio-One, Monroe, NC). Cells were serum starved for one hour in HBSS, or 500 μM of H2O2 (positive control), then 0.5 mg/ml MTT labeling reagent added for four hours at 37°C. Next, 100 μL of solubilization solution was added and incubated overnight at 37°C. The following day absorbance values at 550 nm and 590 nm were measured with an H4 synergy reader (Biotek, Winooski, VT). Each experiment was performed in technical triplicate with a minimum of three biological replicates.
Proximity ligation assay (PLA)
The PLA is a flexible and informative technology that expands upon traditional immunocytochemistry to include direct detection of low levels of individual proteins, the existence of protein:protein interactions (≤ 40 nm) as well as the subcellular localization of the protein:protein interaction with high specificity and sensitivity. The PLA was performed using the recommended manufacturer’s protocol with minor modification, similar to those previously reported [42]. The 5-HT2A+2CR-CHO cells were plated in poly-D-lysine coated 16-well chamber slides (Thermo Scientific, Nunc Lab-Tek) in 100 μl growth media at the density of 16K cells/well and placed overnight in the incubator. The following day (~18 hours later), cells were fixed in 100 μl of freshly made, cold paraformaldehyde (PFA) in PBS (pH 7.4) for a final concentration of 4% PFA. After 15 min incubation at RT, the walls of the chamber slides were removed and slides washed twice with PBS. Next, cells were permeabilized for 10 min in 100 uM digitonin (Crescent Chemical Co., Islandia, NY) in PBS, washed twice in PBS with 0.1% Tween 20 (PBS-T) and blocked with 4% normal donkey serum (NDS) (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS-T. Cells were then incubated with rabbit anti-5-HT2AR (1:500; LS-C172270, LifeSpan Biosciences, Seattle, WA) and/or mouse anti-5-HT2CR (1:50; sc-17797, Santa Cruz Biotechnology, Dallas, TX) in 4% NDS in PBS-T and left overnight in 4°C. After washing the slides 5X with RT PBS-T, the samples were processed for the PLA according to manufacturer’s protocol with minor modifications (Sigma, Duolink®); cells were incubated with the appropriate combination of secondary antibodies conjugated with oligonucleotide probes for the PLA (Mouse PLUS; Mouse MINUS, Rabbit PLUS, Rabbit MINUS; Sigma, Duolink® In Situ PLA Probes: DUO92001-2; DUO92004-5) in 4% NDS in PBS-T for one hour in an incubator. To remove unbound PLA probes, slides were washed twice in Duolink® washing buffer A (Sigma, Duolink® In Situ Wash Buffers, Fluorescence, DUO82049) at RT and incubated with Duolink® ligation solution (Sigma, Duolink®, In Situ Detection Reagents Red; DUO92008) for 30 min in an incubator. Cells were then washed twice with RT Duolink® washing buffer A and Duolink® amplification-polymerase solution was applied to the slides. After 100 min incubation in the dark, slides were washed with Duolink® washing buffer B (Sigma, Duolink® In Situ Wash Buffers, Fluorescence, DUO82049) at RT followed by rinsing with 0.01x RT Duolink® washing buffer B. On the dried slides, mounting media (Sigma, Duolink® In situ Mounting Medium with DAPI, DUO082040) was applied and cells were imaged the same day with a Leica True Confocal Scanner SPE and Leica Application Suite Advanced software (Leica Microsystems, Wetzlar, Germany). Four non-overlapping fields of view per well (20X magnification) were identified and photomicrographs acquired under each experimental condition. Images were acquired with Leica LASX Software and processed with NIH ImageJ [43, 44]. To discriminate PLA puncta from the background fluorescence, identical for all conditions, the manually selected threshold (70) was applied to all images. The number of nuclei (DAPI+; ~170 per field of view per condition) and total puncta (red spots) were counted using the Duolink® Image Tool Software (Olink Bioscience) from each of the four field of views and averaged for each experimental condition for statistical comparison, with a total of five biological replicates.
Bioluminescence resonance energy transfer (BRET) assay
BRET is a biophysical technique that utilizes energy transfer between Renilla luciferase (RLuc) and a fluorescent protein, such as yellow fluorescent protein (YFP), that enables the spatial resolution of protein:protein interactions to ≤ 10 nm in living cells. The BRET assay is commonly used to characterize protein:protein interactions with GPCRs and was adapted from previous publications with minor modifications [8, 45]. At 24 hours post transfection, HEK293 cells co-expressing various ratios of 5-HT2AR-RLuc or β2-AR-Rluc and 5-HT2CR-eYFP plasmids were washed with PBS, trypsinized (Gibco, Invitrogen, Waltham, MA), harvested, centrifuged (500 x g for four mins), and plated in phenol-free DMEM with 10% dialyzed FBS at a density of 60,000 cells/well into poly-L-lysine (Sigma Aldrich, Waltham, MA) coated white-walled, clear bottom 96-well cell culture plates (Greiner Bio-One, Monroe, NC) and cultured overnight. The following day, cells were serum starved for one hour in HBSS and BRET activity was measured 48 hours post-transfection following the addition of 50 μM (5 μM final concentration) coelentrazine H (Fisher Scientific, Waltham, MA). Luminescence and fluorescence were measured simultaneously with an H4 synergy reader (Biotek, Winooski, VT) using 528/20 and 460/40 filters, respectively, 15 mins following coelentrazine H addition. The BRET ratio was calculated as [(emission at 528 nm/ emission at 460 nm)–(background at 528 nm/ background at 460 nm)]; background corresponds to the signal in cells expressing the RLuc fusion protein alone under similar conditions. For improved readability, the results were displayed in milli-BRET units (mBRET), with one mBRET corresponding to the BRET ratio multiplied by 1,000. Each experiment was performed in technical quadruplicates with a minimum of three biological replicates.
Data analysis and statistics
All data were analyzed with GraphPad Prism 7.0 software (La Jolla, CA). Maximal luminescence values (± SEM) from the LCA and MTT assays were compared using a one-way analysis of variance (ANOVA) followed by a priori comparisons conducted with a Tukey’s test. We assessed the potency (pEC50) and efficacy (EMAX) of 5-HT to activate Cai2+ release for all novel receptor fusion proteins to ensure similar signaling properties relative to the wildtype receptors. Data from Cai2+ release assays are presented as half maximum (pEC50) and maximum (EMAX) values (mean ± SEM), representing potency and efficacy, respectively, as computed by GraphPad using a four parameter nonlinear regression curve-fitting algorithm. To assess the effect of different transfection ratios of receptor plasmids on the total receptor protein level, we compared Bmax values obtained from radioligand binding studies. We also determined the potential effect of different transfection ratios of receptor plasmids on receptor ligand affinity, by statistically comparing receptor affinity values (Kd). Data from saturation binding isotherms are presented as BMAX and Kd values (mean ± SEM), as computed by GraphPad using one site-specific binding nonlinear regression curve-fitting algorithm; a one-way ANOVA was conducted followed by a priori comparisons with Dunnett’s test. The total number of PLA puncta (mean ± SEM) from four individual fields of view under each experimental condition were averaged for each separate condition; a one-way ANOVA followed by a priori comparisons with a Tukey’s test or a Student’s t-test was conducted, as appropriate. Data from BRET assays are presented as mBRET50 and mBRETMAX values (mean ± SEM) as computed by GraphPad using specific binding with Hill slope nonlinear regression curve-fitting algorithm. The experiment wise error rate for all analyses was set at α = 0.05.
Results
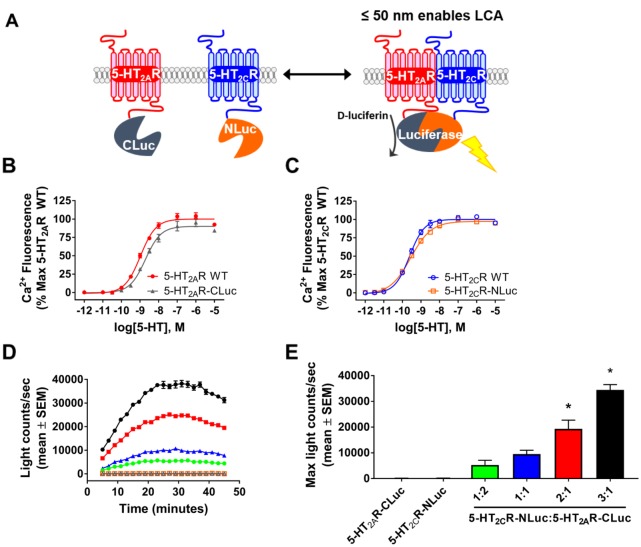
Luciferase complementation analysis indicates 5-HT2AR:5-HT2CR interaction within 50 nm
We developed a split luciferase complementation assay (LCA) in which half of luciferase is attached to the C-terminus of each receptor (5-HT2AR-CLuc and 5-HT2CR-NLuc, respectively) to test if the 5-HT2AR and 5-HT2CR interact in a complex in living cells. If the association of the two receptors occurs in close proximity (< 50 nm), then a functional luciferase is reconstituted and enzymatic activity assessed by emitted luminescence (Fig 1A) [33, 34]. We created 5-HT2AR-CLuc and 5-HT2CR-NLuc split luciferase constructs and initially tested the functional signaling activity of the engineered receptors in transfected HEK293 cells. Both 5-HT2AR and 5-HT2CR are Gαq/11-coupled receptors which, upon activation, stimulate Cai2+ release [18, 19]. Thus, concentration response curves (10−12 to 10−5) for 5-HT-mediated Cai2+ release were generated to determine the functional activity of the wildtype and engineered receptors (Fig 1B and 1C). Serotonin-mediated Cai2+ release was similar in cells transfected with the 5-HT2AR-CLuc (Fig 1B) or 5-HT2CR-NLuc (Fig 1C) relative to cells transfected with WT 5-HT2AR (Fig 1B) or WT 5-HT2CR (Fig 1C). The concentration of 5-HT required to induce calcium release (potency) and the magnitude of calcium released (efficacy) were both compared. The potency (pEC50 = 9.20 ± 0.27) and efficacy (EMAX = 91 ± 8%) of 5-HT for the 5-HT2AR-CLuc were not significantly different than the potency (pEC50 = 9.07 ± 0.09) and efficacy (EMAX = 100 ± 6%) of 5-HT for WT 5-HT2AR (p > 0.05; Fig 1B). Likewise, the potency (pEC50 = 9.41 ± 0.16) and efficacy (EMAX = 97 ± 8%) of 5-HT for the 5-HT2CR-NLuc were not significantly different than the potency (pEC50 = 9.54 ± 0.13) and efficacy (EMAX = 100 ± 8%) of 5-HT for the WT 5-HT2CR (p > 0.05; Fig 1C). Thus, these data demonstrate that the engineered 5-HT2AR-CLuc and 5-HT2CR-NLuc exhibit 5-HT-mediated signaling as seen for the native receptors when expressed in live cells.
Fig 1. Luciferase complementation analysis indicates 5-HT2AR:5-HT2CR interaction within 50 nm.
(A) Schematic of luciferase complementation assay (LCA) between 5-HT2AR-CLuc and 5-HT2CR-NLuc constructs (adapted from Luker et al., 2004) [33, 34]. (B) Representative concentration response curve for 5-HT-mediated intracellular calcium (Cai2+) release for WT 5-HT2AR (red) and 5-HT2AR-CLuc (grey) constructs transiently transfected in HEK293 cells. The 5-HT-mediated increase in Cai2+ dye fluorescence over baseline was calculated as the % maximal response WT 5-HT2AR. (C) Representative 5-HT concentration response curve for WT 5-HT2CR (blue) and 5-HT2CR-NLuc (orange) constructs transiently transfected in HEK293 cells. The 5-HT-mediated increase in Cai2+ dye fluorescence over baseline was calculated as the % maximal response of WT 5-HT2CR. (D) Representative luminescence trace in HEK293 cells using varying transfection ratios (1:1, 2:1, 2:1, 3:1; 1 μg total plasmid DNA) of 5-HT2CR-NLuc and 5-HT2AR-CLuc constructs, respectively. Light counts/sec are shown at 1:2 (green), 1:1 (blue), 2:1 (red), and 3:1 (black) transfection ratios of 5-HT2CR-NLuc to 5-HT2AR-CLuc, respectively. (E) The maximal luminescence value from three independent experiments in which varying 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratios were tested. *p < 0.05 vs. 1:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio.
We co-transfected HEK293 cells with 5-HT2CR-NLuc and 5-HT2AR-CLuc at various plasmid DNA ratios and assessed luciferase activity to determine if co-expression of 5-HT2AR-CLuc and 5-HT2CR-NLuc would reconstitute luciferase activity, which would indicate formation of a receptor complex. Bioluminescence light counts/sec are shown at 1:2 (green), 1:1 (blue), 2:1 (red), and 3:1 (black) transfection ratios of 5-HT2CR-NLuc to 5-HT2AR-CLuc plasmid DNA, respectively; luminescence was recorded once every four mins over a 45-min period (Fig 1D). The time course illustrates that robust luciferase complementation was observed at the 2:1 and 3:1 transfection ratios of 5-HT2CR-NLuc:5-HT2AR-CLuc (Fig 1D); no significant luminescence was detected following transfection of the negative controls 5-HT2CR-NLuc alone (orange) or 5-HT2AR-CLuc (grey) alone (Fig 1D). A main effect of transfection ratio on maximum light counts/sec was detected [F5, 12 = 49.79; p < 0.0001; Fig 1E]; the maximum light counts/sec was significantly elevated for the 2:1 and 3:1 transfection ratios of 5-HT2CR-NLuc:5-HT2AR-CLuc (p < 0.05 vs. 1:1 transfection ratio). To determine expressed receptor protein levels, we performed saturation radioligand binding for the 5-HT2AR (with [3H]-ketanserin) and 5-HT2CR (using [3H]-mesulergine) in membranes extracted from cells at 1:1, 2:1, and 3:1 transfection ratios of 5-HT2CR-NLuc:5-HT2AR-CLuc. A main effect of transfection ratio on total receptor level (BMAX) for the 5-HT2CR was detected [F2,6 = 5.67; p < 0.05; Table 1]; the 3:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio yielded a significant increase in the BMAX for 5-HT2CR vs. the 1:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio (p < 0.05; Table 1). A main effect of transfection ratio on total receptor level (BMAX) for the 5-HT2AR was detected [F2,6 = 6.16; p < 0.05; Table 1]; the 3:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio yielded a significant decrease in the BMAX for 5-HT2AR vs. the 1:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio (p < 0.05; Table 1). No main effect of 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio on the ligand binding affinity (Kd) for [3H]-ketanserin at the 5-HT2AR-CLuc [F2,6 = 0.07; p > 0.05] or the ligand binding affinity (Kd) for [3H]-mesulergine at the 5-HT2CR-NLuc was detected [F2,6 = 0.24; p > 0.05]. Moreover, the 3:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio resulted in a protein ratio of 0.408 (Table 1), indicating a molar excess of 5-HT2AR to 5-HT2CR protein. Ideally, a 1:1 protein ratio would be achieved for these studies, however, the 5-HT2AR-CLuc consistently expresses at a higher efficiency and level than the 5-HT2CR-NLuc in HEK293 cells, as observed in Table 1. Further conditions to attempt to achieve a 1:1 protein ratio (i.e., above the 3:1 plasmid ratio) negatively impacted the health of the cells using the MTT assay (data not shown). Thus, we were unable to obtain a higher expression of the 5-HT2CR without increasing the overall amount of transfected plasmid DNA and negatively impacting cell health. Taken together, these data suggest that a complex formation exists within 50 nm (limit of detection for the LCA) and that complex formation increases proportionally to the 5-HT2CR:5-HT2AR expression ratio.
Table 1. Protein levels and affinity values ascertained in HEK293 cells transiently co-transfected with the 5-HT2CR-NLuc and 5-HT2AR-CLuc at various ratios.
| Transfection Ratioa | 5-HT2CR-NLuc BMAX (fmol/mg)b |
[3H]-Mesulergine Kd (nM)b | 5-HT2AR-CLuc BMAX (fmol/mg)b |
[3H]-Ketanserin Kd (nM)b |
Protein Ratioc |
|---|---|---|---|---|---|
| 1:1 | 451 ± 48 | 2.5 ± 0.3 | 2512 ± 224 | 1.8 ± 0.1 | 0.180 |
| 2:1 | 588 ± 55 | 2.3 ± 0.2 | 1887 ± 121 | 1.9 ± 0.3 | 0.312 |
| 3:1 | 692 ± 49* | 2.2 ± 0.4 | 1697 ± 154* | 1.8 ± 0.2 | 0.408 |
a Different ratios of vectors (1:1, 2:1, 3:1) of 5-HT2CR-NLuc and 5-HT2AR-CLuc were co-transiently transfected into HEK293 cells and membranes were isolated.
b BMAX (fmol/mg protein) and Kd (nM) values (mean ± SEM) were determined for the 5-HT2CR-NLuc ([3H]-mesulergine) and 5-HT2AR-CLuc ([3H]-ketanserin).
c The protein ratio was calculated as the BMAX for 5-HT2CR-NLuc to the BMAX for 5-HT2AR-CLuc.
* p < 0.05 vs. 1:1 5-HT2CR-NLuc:5-HT2AR-CLuc transfection ratio
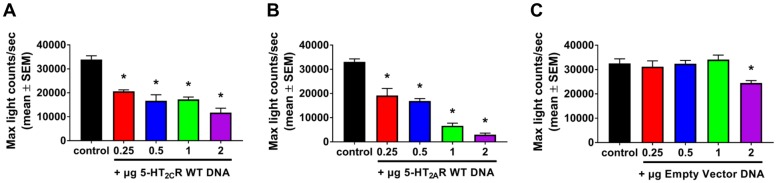
We next determined if the LCA interaction could be inhibited by co-transfection of the 5-HT2CR-NLuc:5-HT2AR-CLuc (3:1 transfection ratio, Fig 2, “control”) with increasing amounts of cDNA (0.25–2 μg) for untagged WT 5-HT2CR (Fig 2A), WT 5-HT2AR (Fig 2B) or empty vector (Fig 2C). We predicted that transfection of the untagged WT 5-HT2R would competitively interact with its respective 5-HT2R LCA construct for the heteromeric complex, thereby reducing the ability to reconstitute luciferase, resulting in a reduction in the luminescence signal. A main effect of the WT 5-HT2CR transfection condition on maximum light counts/sec was detected [F4, 12 = 21.13; p < 0.0001; Fig 2A]; the native WT 5-HT2CR at all amounts of cDNA co-transfected decreased the luminescence (mean ± SEM) of the 5-HT2CR-NLuc:5-HT2AR-CLuc (p < 0.05 vs. control; Fig 2A). A main effect of the WT 5-HT2AR transfection condition on maximum light counts/sec was detected [F4, 12 = 68.83; p < 0.0001; Fig 2B]; the native WT 5-HT2AR at all amounts of cDNA co-transfected decreased the luminescence of the 5-HT2CR-NLuc:5-HT2AR-CLuc (p < 0.05 vs. control; Fig 2B) A main effect of the empty vector transfection condition on maximum light counts/sec was detected [F4, 11 = 4.034; p < 0.05; Fig 2C]; the empty vector at 2 μg decreased the luminescence of the 5-HT2CR-NLuc:5-HT2AR-CLuc (p < 0.05 vs. control; Fig 2C). Taken together, these data indicate that the luciferase complementation between the 5-HT2AR:5-HT2CR is not due to a random complementation of the luciferase fragments.
Fig 2. Wildtype receptor attenuates the 5-HT2AR:5-HT2CR luciferase complementation.
The maximal luminescence value for the 5-HT2CR-NLuc:5-HT2AR-CLuc 3:1 transfection ratio (control) in the presence of varying amounts (0.25–2 μg) of (A) WT 5-HT2CR, (B) WT 5-HT2AR, or (C) empty vector. All results are maximal luminescence values from four independent experiments. *p < 0.05 vs. control.
We next employed an MTT cell viability assay to determine if cellular metabolic activity was affected following these various plasmid co-transfections (S1 Fig). A one-way ANOVA indicated a main effect of experimental condition on absorbance (F13,28 = 12.34; p < 0.0001); the positive control H2O2, which increases reactive oxygen species causing cell death, significantly suppressed absorbance, indicating a decrease in cell viability (S1A Fig). No effect of WT 5-HT2CR (S1A Fig), WT 5-HT2AR (S1B Fig) or empty vector (S1C Fig) on absorbance was detected (p > 0.05 vs. control), suggesting that DNA overexpression, at the levels in which inhibition of the LCA signal was observed, did not negatively affect cell viability.
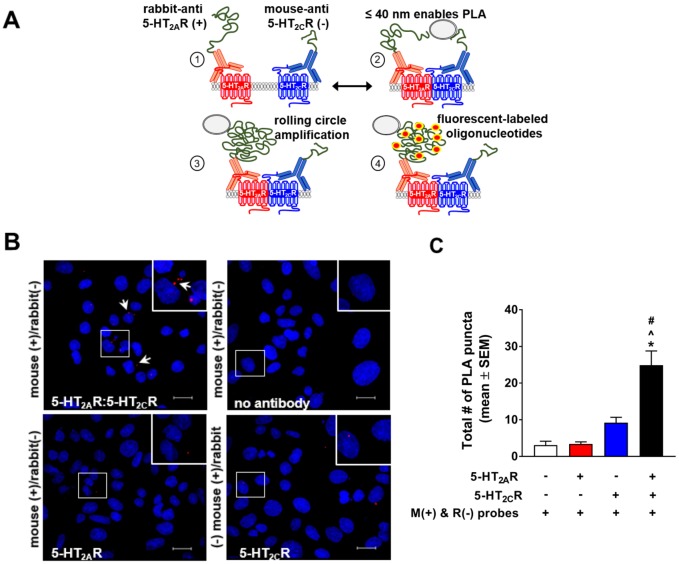
Proximity ligation analysis indicates WT 5-HT2AR:5-HT2CR interaction within 40 nm
We tested the hypothesis that the 5-HT2AR and 5-HT2CR interact using a proximity ligation assay (PLA) conducted in a cell line stably expressing both receptors (5-HT2A+2CR-CHO cells). In the PLA, when proteins of interest are in complex within close spatial proximity (≤ 40 nm), short DNA strands complement, ligate and allow rolling circle amplification to produce fluorescent signals [28, 42, 46, 47] (Fig 3A). The PLA was employed to identify and confirm expression of the 5-HT2AR (S2A Fig, top left) and 5-HT2CR (S2A Fig, top right) protomers in the dual-expressing cell line. Reverse transcription of RNA followed by qRT-PCR confirmed that 5-HT2A+2CR-CHO cells expressed both 5-HT2AR mRNA (ΔCt = 7.67 ± 0.10) and 5-HT2CR mRNA (ΔCt = 14.18 ± 0.13), but did not express 5-HT2BR mRNA (crossing threshold not determined). Similar to the transiently transfected HEK293 cells employed herein, the 5-HT2AR transcript was in greater abundance relative to the 5-HT2CR transcript in the stably expressing 5-HT2A+2CR-CHO cells. Experimental controls in which the appropriate primary antibody was omitted and the PLA probe included (e.g., rabbit+/rabbit-; mouse+/mouse-) demonstrated no puncta, as expected (S2A Fig, bottom). The total number of puncta specific to 5-HT2AR (p < 0.05; S2B Fig) or 5-HT2CR (p < 0.05; S2B Fig) was significantly higher relative to controls. We then quantified 5-HT2AR and 5-HT2CR co-localized in the native environment of the dual expressing 5-HT2A+2CR-CHO cells. We observed a distinct positive signal (red puncta spot) indicating that the 5-HT2AR and 5-HT2CR are in close proximity in the dual expressing cell line (Fig 3B, top). To provide sufficient controls and to ensure that observed fluorescent signal was caused by a receptor:receptor interaction, and not due to nonspecific binding of the antibodies, cells in separate wells were labeled no primary antibody, the anti-5-HT2AR antibody or the anti-5-HT2CR antibody alone (Fig 3B). A main effect of experimental conditions for the total number of puncta in the 5-HT2A+2CR-CHO cells was detected [F(3, 16) = 22,59; p < 0.05; Fig 3C]; the total number of puncta was significantly higher vs. all provided control conditions (p < 0.05; Fig 3C), indicating specificity of the antibody labeling. These data support the conclusion that WT 5-HT2AR and 5-HT2CR interact in cells within 40 nm of each other (limit of detection for PLA) [28, 42, 46, 47].
Fig 3. Proximity ligation assay indicates 5-HT2AR:5-HT2CR interaction within 50 nm.
(A) Schematic of proximity ligation assay (PLA) between 5-HT2CR and 5-HT2AR. (B) Representative 60X confocal photomicrographs of PLA signal from 5-HT2CR and 5-HT2AR heteromeric formation (red puncta) and associated negative controls. PLA was performed using 5-HT2AR (rabbit polyclonal) and 5-HT2CR (mouse monoclonal) primary antibody and oligonucleotide-linked PLA secondary probes [mouse, M(+) and rabbit, R(-)]. Scale bars represent 10 μm. (C) Quantification of puncta from 20X photomicrographs from five independent experiments. *p < 0.05 vs. M(+) and R(-) probes only; ^p < 0.05 vs. 5-HT2AR plus M(+) and R(-) probes; #p < 0.05 vs. 5-HT2CR plus M(+) and R(-) probes.
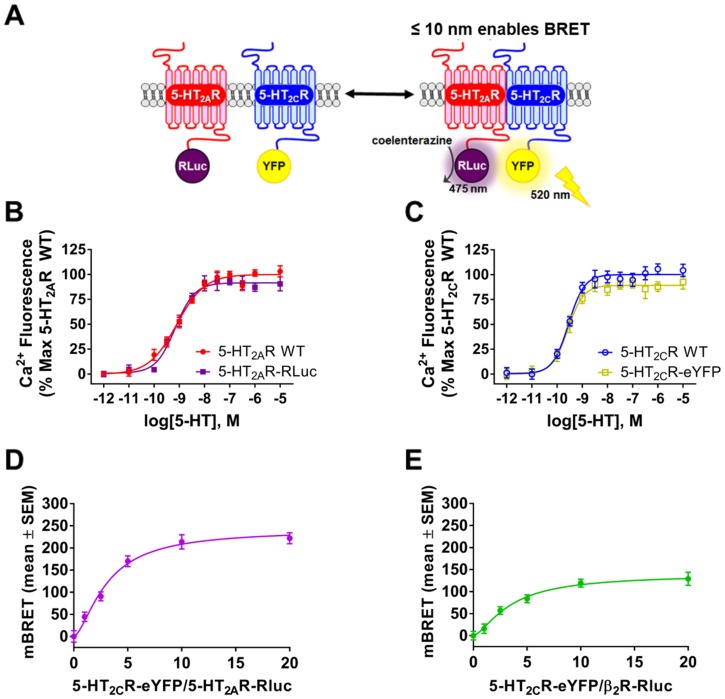
BRET analyses indicates 5-HT2AR:5-HT2CR interaction within 10 nm
To further resolve the spatial proximity of the receptors in live cells, we generated 5-HT2AR and 5-HT2CR constructs (donor: 5-HT2AR-RLuc; acceptor: 5-HT2CR-eYFP) for use in a BRET assay which requires interacting proteins be within 10 nm to generate resonance energy transfer (Fig 4A) [8]. To validate functionality of the engineered receptors, concentration response curves (10−12 to 10−5) for 5-HT-mediated Cai2+ release were generated (Fig 4B and 4C). Serotonin-mediated Cai2+ release was similar in cells transfected with the 5-HT2AR-RLuc (Fig 4B) or 5-HT2CR-eYFP (Fig 4C) relative to cells transfected with WT 5-HT2AR (Fig 4B) or WT 5-HT2CR (Fig 4C), respectively. The potency (pEC50 = 9.16 ± 0.08) and efficacy (EMAX = 94 ± 7%) of 5-HT for the 5-HT2AR-RLuc were not significantly different than the potency (pEC50 = 9.10 ± 0.05) and efficacy (EMAX = 100 ± 6%) of 5-HT for WT 5-HT2AR (p > 0.05; Fig 4B). Likewise, the potency (pEC50 9.57 ± 0.06) and efficacy (EMAX = 90 ± 9%) of 5-HT for the 5-HT2CR-eYFP were not significantly different than the potency (pEC50 = 9.58 ± 0.08) and efficacy (EMAX = 100 ± 5%) of 5-HT for the WT 5-HT2CR (p > 0.05; Fig 4C). Thus, these data demonstrate that the engineered 5-HT2AR-RLuc and 5-HT2CR-eYFP exhibit 5-HT-mediated signaling as seen for the native receptors when expressed in live cells.
Fig 4. BRET indicates 5-HT2AR:5-HT2CR interaction within 10 nm.
(A) Schematic of BRET assay between 5-HT2CR-RLuc and 5-HT2AR-eYFP constructs. (B) Representative concentration response curve for 5-HT-mediated intracellular calcium (Cai2+) release for WT 5-HT2AR (red) and 5-HT2AR-RLuc (purple) constructs transiently transfected in HEK293 cells. The 5-HT-mediated increase in Cai2+ dye fluorescence over baseline was calculated as the % maximal response of WT 5-HT2AR. (C) Representative concentration response curve for 5-HT-mediated Cai2+ release for WT 5-HT2CR (blue) and 5-HT2CR-eYFP (yellow) constructs transiently transfected in HEK293 cells. The 5-HT-mediated increase in Cai2+ dye fluorescence over baseline was calculated as the % maximal response of WT 5-HT2AR. (D) Representative BRET curve from HEK293 cells co-transfected with constant amount 5-HT2AR-RLuc (100 ng; donor construct) and increasing amounts of the 5-HT2CR-eYFP (1–20 fold excess; acceptor construct). (E) Representative BRET curve from HEK293 cells co-transfected with constant amount β2-AR-Rluc (100 ng; donor construct) and increasing amounts of the 5-HT2CR-eYFP (1–20 fold excess; acceptor construct). The mBRET signal was determined by calculating the ratio of light emitted at 528 nm to 460 nm (X 1000). Results obtained from four independent experiments.
A saturable BRET curve (mBRET50 = 3.0 ± 0.1; mBRETMAX = 231 ± 8 mBRET units) in cells expressing a constant amount of donor construct (100 ng of 5-HT2AR-RLuc) and increasing amounts acceptor construct (1–20 fold of 5-HT2CR-eYFP) was obtained (Fig 4D), indicating a specific and saturable receptor:receptor interaction. To further evaluate the specificity of this interaction, we used the β2-AR-RLuc as a donor construct control as β2-AR-RLuc minimally associates with the 5-HT2CR-eYFP [48]. A lower, more linear BRET saturation curve (mBRET50 = 3.4 ± 0.1; mBRETMAX = 135 ± 4 mBRET units) was observed in cells expressing a constant amount of donor construct (100 ng of β2-AR-RLuc) and increasing amounts of acceptor construct (1–20 fold of 5-HT2CR-eYFP) (Fig 4E). These results indicate the 5-HT2AR and 5-HT2CR form a close interaction within 10 nm of each other (limit of detection in BRET) in intact live cells.
Discussion
The present study used a tiered strategy employing three complementary biophysical techniques with increasing spatial resolution to confirm that the 5-HT2AR and 5-HT2CR form a cellular level protein-protein complex interaction. In the LCA assay, we demonstrated that formation of the 5-HT2AR:5-HT2CR complex exists within 50 nm and increases proportionally to the 5-HT2CR:5-HT2AR protein expression ratio. Using the PLA, we found that cells stably expressing both the 5-HT2AR and 5-HT2CR exhibit 5-HT2AR:5-HT2CR heteroreceptor complexes within 40 nm of each other. Lastly, BRET analyses signify a specific and saturable 5-HT2AR:5-HT2CR interaction, indicating that the 5-HT2AR and 5-HT2CR form a close interaction within 10 nm of each other in intact live cells. Importantly, the bioengineered receptors generated for the LCA and the BRET exhibit 5-HT-mediated intracellular calcium signaling as seen for the native receptors, assuring that the tagged constructs do not impede receptor function. Thus, these studies support the conclusion that a very close 5-HT2AR:5-HT2CR heteromeric receptor interaction occurs in cultured cells.
Energy transfer-based technologies, such as BRET, have been fundamental in the development of a database of knowledge concerning the homo- and heterodimerization of GPCRs [45, 49, 50]. Considerable research has implicated that the energy transfer from the donor to the acceptor is below 10 nm [45, 49, 50]. We demonstrated a specific BRET saturation curve in cells expressing a constant amount of the 5-HT2AR-RLuc donor construct and increasing amounts of the 5-HT2CR-eYFP acceptor construct. A lower, more linear BRET saturation curve was seen in cells expressing a constant amount of donor β2-AR-RLuc construct and increasing amounts of acceptor 5-HT2CR-eYFP construct, a receptor pair previously suggested to minimally associate in live cells [48]. Because the diameter of the seven transmembrane helical core is estimated at ~5 nm, the observed, positive BRET signal strongly suggests that oligomerization has occurred between the 5-HT2AR and 5-HT2CR [45, 49, 50]. Furthermore, a reciprocal interaction between these two receptors is corroborated by previous analyses of molecular and pharmacological properties of the 5-HT2AR:5-HT2CR heterocomplex in vitro [8]. Intriguingly, this formation of the 5-HT2AR:5-HT2CR complex did not modify the Gαq/11 coupling of the protomers, but rather the 5-HT2CR exerts dominance when in complex with the 5-HT2AR, such that only the 5-HT2CR couples with the G protein to generate intracellular signaling; the 5-HT2AR signaling is ‘masked’ [8]. Thus, the 5-HT2AR:5-HT2CR protein complex appears to be a distinct molecular species that contributes to the control of cellular signaling, triggering unique intracellular signaling properties when co-expressed in vitro [8].
The detection of a 5-HT2AR:5-HT2CR protein complex in transfected cells [present results, [8] and rodent brain [5] extends observations of the co-localization of these receptors in the single neurons of the rat mPFC [15–17]. Our previous studies indicate that the lowest expression levels of the 5-HT2AR:5-HT2CR complex assessed by co-immunoprecipitation in the mPFC associates with the highest level of phenotypic impulsive action in the rat and that the ratio of 5-HT2AR to 5-HT2CR protein expression in the rat mPFC predicts the inherent level of motor impulsivity in individual rats [5]. Engineered 5-HT2CR knockdown in mPFC resulted in increased motor impulsivity [5, 51] concomitant with elevated 5-HT2AR expression and greater potency of the 5-HT2AR antagonist M100907 to suppress motor impulsivity [5]. Intriguingly, rats exposed to a low-protein diet in utero exhibited reduced 5-HT2CR and elevated 5-HT2AR expression in the hypothalamus concomitant with impaired sensitivity to 5-HT-mediated appetite in adulthood [52]. Although not yet linked to the formation or action of heteromers, these previous studies support a potential role for the 5-HT2AR:5-HT2CR interaction in vivo [11, 12].
The oligomerization of GPCRs has emerged as a vital property of receptor structure and function with identified macromolecular complexes comprising of at least two different protomers with biochemical properties that are discernibly distinct from those of the individual protomers [8, 45, 53]. The main functional GPCR unit for the 5-HT2AR [20, 21] and 5-HT2CR [22–25] is proposed to be their homomeric form, and recent studies suggest that different interacting homomers may constitute heteromeric complexes [53]. Future studies are needed to clarify the degree to which downstream signaling pathways are recruited by the individual receptors vs. heteroreceptor complexes, especially in terms of the level of ligand-directed signaling [54] as well as the functional effects of the 5-HT2R and their underlying role in behavior. Furthermore, drug discovery efforts selectively targeting either receptor should take into account the formation of a heteromeric complex when analyzing physiological responses [8].
Supporting information
The absorbance value for the 5-HT2CR-NLuc:5-HT2AR-CLuc 3:1 transfection ratio (control) in the presence of varying amounts (0.25–2 μg) of (A) WT 5-HT2CR, (B) WT 5-HT2AR, or (C) empty vector. All results are absorbance at 550 nm minus absorbance at 590 nm from four independent experiments * p < 0.05 vs. control.
(TIF)
(A) Representative 60X confocal photomicrographs of PLA signal from 5-HT2AR (top left) and 5-HT2CR (top right) (red puncta) and associated negative controls (bottom). PLA was performed using 5-HT2AR (rabbit polyclonal) alone or 5-HT2CR (mouse monoclonal) primary antibody alone plus oligonucleotide-linked PLA secondary probes [rabbit, R(±); mouse, M(±)]. Scale bars represent 10 μm. (B) Quantification of puncta from 20X photomicrographs from five independent experiments. *p < 0.05 vs. M(±) probes only; ^p < 0.05 vs. R (±) probes only.
(TIF)
Acknowledgments
This work was supported by NIDA grants, P50 DA033935, T32 DA007287, K05 DA020087, and the Center for Addiction Research at the University of Texas Medical Branch. We thank Drs. Kelly A. Berg and William P. Clarke for the gift of cell lines, Dr. David Piwinica-Worms for the gift of plasmids expressing FRB-NLuc and FKBP-CLuc, Dr. Fernanda Laezza for technical guidance, Drs. Latham Fink, Patricia K. Seitz and Thressa D. Smith and Ms. Christina R. Merritt for their technical expertise. We also thank Drs. Claudia Soto and Marcy B. Jordan and Ms. Michelle Land for their helpful discussions. The authors declare no conflicts of interest.
Abbreviations
- 5-HT
serotonin
- 5-HT2AR
serotonin 5-HT2A receptor
- 5-HT2CR
serotonin 5-HT2C receptor
- ANOVA
analysis of variance
- β2-AR
β2-adrenergic receptor
- BMAX
protein concentration
- BRET
bioluminescence resonance energy transfer
- CHO
Chinese hamster ovary cells
- CLuc
C-terminal luciferase fragment
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- eYFP
enhanced yellow florescent protein
- FBS
fetal bovine serum
- FKBP
human FK506-binding protein 12
- FLIPR
fluorescence imaging plate reader
- FRB
rapamycin-binding domain of human mammalian target of rapamycin
- GPCR
G protein-coupled receptor
- GHS1αR
growth hormone secretagogue receptor 1α
- HBSS
Hank’s balanced salt solution
- Kd
affinity
- mBRET
milli-BRET units
- mPFC
medial prefrontal cortex
- HEK293
human embryonic kidney cells
- LCA
luciferase complementation assay
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NDS
normal donkey serum
- NLuc
N-terminal luciferase fragment
- PBS
phosphate buffered saline
- PBS-T
PBS with 0.1% Tween 20
- PLA
proximity ligation assay
- PDSP
Psychoactive Drug Screening Program
- pEC50
potency
- RLuc
Renilla luciferase
- RT
room temperature
Data Availability
All relevant data are within in the paper and its Supporting Information files.
Funding Statement
This work was supported by NIDA grants, P50 DA033935 (KAC), T32 DA007287 (DEF), K05 DA020087 (KAC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990;87(3):928–32. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, et al. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299(1):268–76. [PubMed] [Google Scholar]
- 3.Sahli ZT, Tarazi FI. Pimavanserin: novel pharmacotherapy for Parkinson’s disease psychosis. Expert Opin Drug Discov. 2018;13(1):103–10. 10.1080/17460441.2018.1394838 . [DOI] [PubMed] [Google Scholar]
- 4.Aronne L, Shanahan W, Fain R, Glicklich A, Soliman W, Li Y, et al. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med. 2014;126(6):7–18. 10.3810/pgm.2014.10.2817 . [DOI] [PubMed] [Google Scholar]
- 5.Anastasio NC, Stutz SJ, Fink LH, Swinford-Jackson SE, Sears RM, DiLeone RJ, et al. Serotonin (5-HT) 5-HT2A receptor (5-HT2AR):5-HT2CR imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem Neurosci. 2015;6(7):1248–58. 10.1021/acschemneuro.5b00094 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pockros LA, Pentkowski NS, Conway SM, Ullman TE, Zwick KR, Neisewander JL. 5-HT(2A) receptor blockade and 5-HT(2C) receptor activation interact to reduce cocaine hyperlocomotion and Fos protein expression in the caudate-putamen. Synapse. 2012;66(12):989–1001. 10.1002/syn.21592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton CL, Rizos Z, Diwan M, Nobrega JN, Fletcher PJ. Antagonizing 5-HT(2)A receptors with M100907 and stimulating 5-HT(2)C receptors with Ro60-0175 blocks cocaine-induced locomotion and zif268 mRNA expression in Sprague-Dawley rats. Behav Brain Res. 2013;240:171–81. 10.1016/j.bbr.2012.11.030 . [DOI] [PubMed] [Google Scholar]
- 8.Moutkine I, Quentin E, Guiard BP, Maroteaux L, Doly S. Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J Biol Chem. 2017;292(15):6352–68. 10.1074/jbc.M117.779041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, et al. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4:110–21. 10.1021/cn300072u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazovkina DV, Kondaurova EM, Naumenko VS, Ponimaskin E. Genotype-Dependent Difference in 5-HT2C Receptor-Induced Hypolocomotion: Comparison with 5-HT2A Receptor Functional Activity. Neural Plast. 2015;2015:846589 10.1155/2015/846589 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76 Pt B:460–78. 10.1016/j.neuropharm.2013.06.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67(1):176–97. 10.1124/pr.114.009514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Molecular Brain Research. 1994;23:163–78. [DOI] [PubMed] [Google Scholar]
- 14.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–73. 10.1002/cne.903510304 [DOI] [PubMed] [Google Scholar]
- 15.Vysokanov A, Flores-Hernandez J, Surmeier DJ. mRNAs for clozapine-sensitive receptors co-localize in rat prefrontal cortex neurons. Neurosci Lett. 1998;258(3):179–82. [DOI] [PubMed] [Google Scholar]
- 16.Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22(16):6846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA. Serotonin-2C and -2A receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience. 2015;297:22–37. 10.1016/j.neuroscience.2015.03.050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195(1):198–213. 10.1016/j.bbr.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 19.Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29(9):454–64. 10.1016/j.tips.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Iglesias A, Cimadevila M, Cadavid MI, Loza MI, Brea J. Serotonin-2A homodimers are needed for signalling via both phospholipase A2 and phospholipase C in transfected CHO cells. Eur J Pharmacol. 2017;800:63–9. 10.1016/j.ejphar.2017.02.028 . [DOI] [PubMed] [Google Scholar]
- 21.Iglesias A, Lage S, Cadavid MI, Loza MI, Brea J. Development of a Multiplex Assay for Studying Functional Selectivity of Human Serotonin 5-HT2A Receptors and Identification of Active Compounds by High-Throughput Screening. J Biomol Screen. 2016;21(8):816–23. 10.1177/1087057116644162 . [DOI] [PubMed] [Google Scholar]
- 22.Herrick-Davis K, Grinde E, Lindsley T, Teitler M, Mancia F, Cowan A, et al. Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells. Mol Pharmacol. 2015;87(4):660–73. 10.1124/mol.114.096636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrick-Davis K, Grinde E, Lindsley T, Cowan A, Mazurkiewicz JE. Oligomer size of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor revealed by fluorescence correlation spectroscopy with photon counting histogram analysis: evidence for homodimers without monomers or tetramers. J Biol Chem. 2012;287(28):23604–14. 10.1074/jbc.M112.350249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrick-Davis K, Grinde E, Weaver BA. Serotonin 5-HT(2C) receptor homodimerization is not regulated by agonist or inverse agonist treatment. Eur J Pharmacol. 2007;568(1–3):45–53. 10.1016/j.ejphar.2007.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem. 2006;281(37):27109–16. 10.1074/jbc.M604390200 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–7. 10.1038/nature06612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albizu L, Holloway T, Gonzalez-Maeso J, Sealfon SC. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;61(4):770–7. 10.1016/j.neuropharm.2011.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinals X, Moreno E, Lanfumey L, Cordomi A, Pastor A, de La Torre R, et al. Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015;13(7):e1002194 10.1371/journal.pbio.1002194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borroto-Escuela DO, Li X, Tarakanov AO, Savelli D, Narvaez M, Shumilov K, et al. Existence of brain 5-HT1A-5-HT2A isoreceptor complexes with antagonistic allosteric receptor-receptor interactions regulating 5-HT1A receptor recognition. ACS Omega. 2017;2(8):4779–89. 10.1021/acsomega.7b00629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schellekens H, Dinan TG, Cryan JF. Ghrelin Receptor (Ghs-R1a) -Mediated Signalling Is Attenuated Via Heterodimerization with the Serotonin 2c (5-Ht2c) Receptor: A Potential Role in Food Intake. Behav Pharmacol. 2012;23(5–6):634-. [Google Scholar]
- 31.Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi-Chaachoua A, Luka M, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem. 2015;290(18):11537–46. 10.1074/jbc.M114.559542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigford GE, Chaudhry NS, Keane RW, Holohean AM. 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-D-aspartate GluN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J Biol Chem. 2012;287(14):11049–59. 10.1074/jbc.M111.277806 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luker KE, Piwnica-Worms D. Optimizing luciferase protein fragment complementation for bioluminescent imaging of protein-protein interactions in live cells and animals. Methods Enzymol. 2004;385:349–60. 10.1016/S0076-6879(04)85019-5 [DOI] [PubMed] [Google Scholar]
- 34.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. ProcNatlAcadSciUSA. 2004;101(33):12288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K. Hallucinogenic 5-HT2AR agonists LSD and enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes. Biochem Biophys Res Commun. 2014;443(1):278–84. 10.1016/j.bbrc.2013.11.104 . [DOI] [PubMed] [Google Scholar]
- 36.Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RDG, Maayani S. 5-hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C- dependent and calcium/calmodulin-dependent mechanisms. MolPharmacol. 1994;45:826–36. [PubMed] [Google Scholar]
- 37.Berg KA, Maayani S, Clarke WP. 5-hydroxytryptamine2C receptor activation inhibits 5- hydroxytryptamine1B-like receptor function via arachidonic acid metabolism. Mol Pharmacol. 1996;50(4):1017–23. [PubMed] [Google Scholar]
- 38.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54(1):94–104. [PubMed] [Google Scholar]
- 39.Seitz PK, Bremer NM, McGinnis AG, Cunningham KA, Watson CS. Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in CHO cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci. 2012;13(1):25 10.1186/1471-2202-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shavkunov A, Panova N, Prasai A, Veselenak R, Bourne N, Stoilova-McPhie S, et al. Bioluminescence methodology for the detection of protein-protein interactions within the voltage-gated sodium channel macromolecular complex. AssayDrug DevTechnol. 2012;10(2):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–20. 10.1038/nature11691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng YJ, et al. Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci. 2013;33(4):1615–30. 10.1523/JNEUROSCI.2656-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):529 10.1186/s12859-017-1934-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayoub MA, Pfleger KD. Recent advances in bioluminescence resonance energy transfer technologies to study GPCR heteromerization. Curr Opin Pharmacol. 2010;10(1):44–52. 10.1016/j.coph.2009.09.012 . [DOI] [PubMed] [Google Scholar]
- 46.Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, et al. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques. 2011;51(2):111–8. 10.2144/000113719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro G, Cordomi A, Casado-Anguera V, Moreno E, Cai NS, Cortes A, et al. Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase. Nat Commun. 2018;9(1):1242 10.1038/s41467-018-03522-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrick-Davis K, Grinde E, Mazurkiewicz JE. Biochemical and biophysical characterization of serotonin 5-HT2C receptor homodimers on the plasma membrane of living cells. Biochemistry. 2004;43(44):13963–71. 10.1021/bi048398p [DOI] [PubMed] [Google Scholar]
- 49.Milligan G. Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci. 2004;21(4):397–405. 10.1016/j.ejps.2003.11.010 . [DOI] [PubMed] [Google Scholar]
- 50.Pfleger KD, Seeber RM, Eidne KA. Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat Protoc. 2006;1(1):337–45. 10.1038/nprot.2006.52 . [DOI] [PubMed] [Google Scholar]
- 51.Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, et al. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39(2):370–82. 10.1038/npp.2013.199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Gronert MS, Stocker CJ, Wargent ET, Cripps RL, Garfield AS, Jovanovic Z, et al. 5-HT2A and 5-HT2C receptors as hypothalamic targets of developmental programming in male rats. Dis Model Mech. 2016;9(4):401–12. 10.1242/dmm.023903 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, et al. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66(2):413–34. 10.1124/pr.113.008052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg KA, Maayani S, Goldfarb J, Clarke WP. Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci. 1998;861:104–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The absorbance value for the 5-HT2CR-NLuc:5-HT2AR-CLuc 3:1 transfection ratio (control) in the presence of varying amounts (0.25–2 μg) of (A) WT 5-HT2CR, (B) WT 5-HT2AR, or (C) empty vector. All results are absorbance at 550 nm minus absorbance at 590 nm from four independent experiments * p < 0.05 vs. control.
(TIF)
(A) Representative 60X confocal photomicrographs of PLA signal from 5-HT2AR (top left) and 5-HT2CR (top right) (red puncta) and associated negative controls (bottom). PLA was performed using 5-HT2AR (rabbit polyclonal) alone or 5-HT2CR (mouse monoclonal) primary antibody alone plus oligonucleotide-linked PLA secondary probes [rabbit, R(±); mouse, M(±)]. Scale bars represent 10 μm. (B) Quantification of puncta from 20X photomicrographs from five independent experiments. *p < 0.05 vs. M(±) probes only; ^p < 0.05 vs. R (±) probes only.
(TIF)
Data Availability Statement
All relevant data are within in the paper and its Supporting Information files.