Abstract
We have analyzed the pattern of gene expression in human primary CD34+ stem/progenitor cells. We identified 42,399 unique serial analysis of gene expression (SAGE) tags among 106,021 SAGE tags collected from 2.5 × 106 CD34+ cells purified from bone marrow. Of these unique SAGE tags, 21,546 matched known expressed sequences, including 3,687 known genes, and 20,854 were novel without a match. The SAGE tags that matched known sequences tended to be at higher levels, whereas the novel SAGE tags tended to be at lower levels. By using the generation of longer sequences from SAGE tags for gene identification (GLGI) method, we identified the correct gene for 385 of 440 high-copy SAGE tags that matched multiple genes and we generated 198 novel 3′ expressed sequence tags from 138 high-copy novel SAGE tags. We observed that many different SAGE tags were derived from the same genes, reflecting the high heterogeneity of the 3′ untranslated region in the expressed genes. We compared the quantitative relationship for genes known to be important in hematopoiesis. The qualitative identification and quantitative measure for each known gene, expressed sequence tag, and novel SAGE tag provide a base for studying normal gene expression in hematopoietic stem/progenitor cells and for studying abnormal gene expression in hematopoietic diseases.
Hematopoietic stem cells have self-renewal ability. They can differentiate into different hematopoietic lineages, including myelomonocytic, megakaryocytic, lymphoid, and erythroid cells (1, 2). Hematopoietic stem cells have been widely used in the treatment of hematopoietic disorders such as leukemia (3). Recent data show that hematopoietic stem cells are highly plastic. Under certain conditions, they can differentiate into nonhematopoietic cells such as brain, liver, and cardiac cells (4–6). These features suggest that hematopoietic stem cells can potentially be used for the treatment of nonhematopoietic disorders such as neural and cardiac diseases.
Although much knowledge about hematopoietic stem cells has been gained, we still do not know much about the genetic mechanisms determining their development. We initiated a genome-scale analysis to characterize the pattern of gene expression in human hematopoietic stem cells. Our goal in this study was to answer the following questions: (i) How many genes are expressed in hematopoietic stem cells? (ii) Which genes are expressed in these cells? (iii) What is the level of expression of each gene in these cells? (iv) What is the quantitative relationship among the genes expressed in these cells? In this study, we used the serial analysis of gene expression (SAGE; ref. 7) technique as the tool for the analysis to provide the broadest coverage for expressed genes and to provide quantitative information for each identified gene. We also used the generation of longer sequences from SAGE tags for gene identification (GLGI) technique (8, 9) to confirm the genes corresponding to the SAGE tags. In this report, we present the results of this analysis.
Materials and Methods
Cell Purification.
The CD34+ cells were purchased from Poietics (Gaithersburg, MD) with Institutional Review Board approval and donor consent. Cells were isolated from mononuclear cells of human bone marrow through positive immunomagnetic selection (CD34 Progenitor Cell Isolation kit, Miltenyi Biotec, Auburn, CA). The purity of the isolated cells was determined by fluorescence-activated cell sorter analysis. The CD34+ cells from three donors were pooled for the analysis.
SAGE Performance.
SAGE was performed with our modified SAGE protocol (10), and the data were processed by use of our procedure (11).
GLGI Performance.
The GLGI method was designed for two purposes. One is to identify the correct sequence from multiple sequences matched by a single SAGE tag, and the other is to generate a longer 3′ expressed sequence tag (EST) for a SAGE tag that does not match to known expressed sequences for further analysis (8). In the GLGI process, a SAGE tag sequence is used as the sense primer, and a universal antisense primer located at the 3′ end of cDNA is used as the antisense primer to amplify the original cDNA template from which the SAGE tag was derived. We developed the original GLGI method into a high-throughput GLGI procedure for large-scale conversion of SAGE tags into 3′ ESTs (9). In this study, we used the high-throughput procedure to identify the correct sequences for the multimatched SAGE tags with more than nine copies, and to convert novel SAGE tag sequences into 3′ ESTs for SAGE tags with more than four copies.
Results and Discussion
Experimental Design.
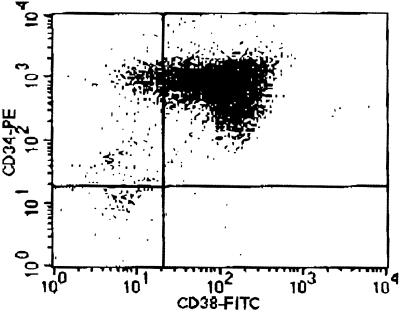
It is a challenge to perform a genome-level analysis of gene expression in human hematopoietic stem cells because of the rarity of stem cells in human bone marrow. We developed two strategies to overcome this obstacle. The first strategy was to use CD34+ cells for the analysis. CD34+ cells represent hematopoietic stem cells and progenitors for myeloid, erythroid, megakaryoid, and lymphoid cells. Normal bone marrow contains about 1–2% CD34+ cells (12, 13). This amount will provide a minimal number of cells for analysis. The second strategy is to modify the standard SAGE protocol to decrease the initial amount of mRNA required for SAGE analysis. Our modified SAGE protocol needs 100-fold less mRNA compared with the standard SAGE protocol. This modification enabled us to perform the entire SAGE analysis by using 2.5 × 106 CD34+ cells. The purity of the CD34+ cells was 96.4, 98.7, and 97.3% in the three samples as measured by fluorescence-activated cell sorter analysis (Fig. 1). We used the CD34+ cells directly for analysis without any in vitro culture, to reflect the original pattern of gene expression in the cells.
Figure 1.
Purity of CD34+ cells. The CD34+ cells were isolated from bone marrow with CD34 immunomagnetic beads. The purity of isolated cells was above 96%. This figure was obtained from one of the three samples used for the analysis.
Distribution of SAGE Tags in CD34+ Cells.
We collected a total of 106,021 SAGE tags, from which we identified 42,399 unique SAGE tags. These unique SAGE tags were matched to the SAGE database for gene identification (Tables 1 and 6–9, which are published as supporting information on the PNAS web site, www.pnas.org). We observed three features of the SAGE tag distribution.
Table 1.
Distribution of 42,400 unique SAGE tags in CD34+ stem cells
| Copy number of SAGE tags
|
Total | |||||
|---|---|---|---|---|---|---|
| >100 | 99 to 10 | 9 to 5 | 4 to 2 | 1 | ||
| Total unique tags* | 91 (0.2) | 771 (1.8) | 1,328 (3.1) | 7,757 (18.3) | 32,453 (76.5) | 42,399 (100) |
| Total novel tags† | 6 (6.5)§ | 85 (11.0) | 128 (9.6) | 2,123 (27.3) | 1,851 (57.0) | 20,853 (49.1) |
| Total matched tags | 85 (93.4) | 686 (89.0) | 1,200 (90.4) | 5,634 (72.6) | 13,941 (43.0) | 21,546 (50.9) |
| Single-matched tags‡ | 24 (28.2) | 307 (44.8) | 657 (54.8) | 3,360 (59.6) | 9,864 (70.8) | 14,212 (66.0) |
| Multiple-matched tags | 61 (71.8) | 379 (55.2) | 543 (45.3) | 2,274 (40.4) | 4,077 (29.2) | 7,334 (34.0) |
The number in parentheses is the percentage of tags in the total unique tag set.
The number in parentheses is the percentage of the tags in the total tags of each subgroup.
The number in parentheses is the percentage of tags within the total matched tags.
The number in bold represents the tags analyzed by GLGI.
(i) The quantitative distribution of SAGE tags.
In CD34+ cells, a few genes are expressed at high levels, and most genes are expressed at low levels. Only 91 (0.2%) SAGE tags were present in more than 100 copies; 9,085 (21.4%) SAGE tags had between 9 and 2 copies, whereas 32,453 (76.5%) SAGE tags were present as a single copy. This distribution is consistent with that observed in other mature somatic cell types (14), indicating the universal pattern of quantitative distribution of expressed genes between stem/progenitor cells and mature cells.
(ii) The quantitative distribution of matched and novel SAGE tags.
Of the unique SAGE tags, 21,546 (50.9%) matched existing known expressed sequences and 20,854 (49.1%) were novel SAGE tags without matches. The distribution of matched tags and novel SAGE tags shows a reciprocal relationship with their copy numbers. The matched tags tended to be the ones with more copies, whereas the novel SAGE tags tended to be the ones with fewer copies. This pattern supports our previous observation that a large number of genes in the human genome have not been identified (11), particularly for the genes expressed at low levels, and at different stages of development such as those in CD34+ cells.
(iii) The rate of multiple matches.
By using the matched UniGene cluster as the measure, we observed that 34% of matched SAGE tags matched more than one sequence located in different UniGene clusters. The low specificity of a SAGE tag representing a gene is largely caused by the short length of the SAGE tag sequence (15, 16). The distribution of these multiple-matched SAGE tags also paralleled their copy numbers. For example, 61 of 85 SAGE tags (71.8%) with more than 99 copies matched multiple sequences. Thus, it is highly unreliable to identify the correct genes for these SAGE tags based solely on a database search.
Identification of Correct Genes for SAGE Tags with Multiple Matches.
Because the SAGE tags with multiple matches tend to be the ones with more copies, we tried to identify the correct genes for 440 SAGE tags with more than 9 copies with multiple matches. By using the high-throughput GLGI method, we converted these 440 SAGE tags into 3′ ESTs and we used these longer sequences to search databases to identify their corresponding genes. We identified the correct gene for 385 (88%) of these 440 SAGE tags (Table 10, which is published as supporting information on the PNAS web site). Similar to other cell types, many housekeeping genes were among the highly expressed genes, including 55 ribosomal proteins. However, many genes with specified function were also expressed at high levels, such as v-fos, TNF, and Myeloperoxidase. Interestingly, there were many functional unknown genes among these highly expressed genes, including 77 ESTs, 13 hypothetical genes, and 10 KIAA protein genes. The high-level expression of these genes suggests their functional importance in the development of hematopoietic stem cell. Table 2 shows the top 60 genes after subtraction of 41 ribosomal protein genes and removal of 4 SAGE tags with poly(A) nucleotide. All of these 60 genes were identified from multimatched SAGE tags.
Table 2.
The top 60 genes expressed in CD34+ cells
| No. | SAGE tag | Copy | Matched UniGene cluster | GLGI confirmation | Gene |
|---|---|---|---|---|---|
| 1 | TGTGTTGAGA | 1711 | 6 | Hs.181165(X03558) | Translation elongation factor 1 alpha 1 |
| 2 | CCTGTAATCC | 516 | 430 | Hs.106004(AK025503) | Hypothetical protein FLJ22347 |
| 3 | GTGAAACCCC | 471 | 325 | Hs.225030(AI246594) | EST |
| 4 | TGGGCAAAGC | 377 | 2 | Hs.2186(BG222874) | Eukaryotic translation elongation factor 1 gamma |
| 5 | CCACTGCACT | 331 | 175 | Hs.293521(BE205895) | EST |
| 6 | GCCTCAGTTC | 256 | 3 | AL571622 | EST |
| 7 | AATGGATGAA | 253 | 3 | BG223362 | EST |
| 8 | AGCCCTACAA | 253 | 2 | BG214720 | EST |
| 9 | GTGAAACCCT | 239 | 155 | Hs.184376(BF692242) | Synaptosomal-associated protein, 23kD |
| 10 | CCAGAGAACT | 165 | 2 | Hs.6975(AW582859) | PRO1073 protein |
| 11 | TTTTTGATAA | 156 | 2 | Hs.181165(BG770933) | Eukaryotic translation elongation factor 1 alpha 1 |
| 12 | TAGGTTGTCT | 148 | 5 | Hs.279860(AW167109) | Tumor protein, translationally-controlled 1 |
| 13 | TTCATACACC | 147 | 3 | Hs.297184(AW193452) | EST |
| 14 | CACCTAATTG | 140 | 2 | AL583021 | EST |
| 15 | TGATTTCACT | 138 | 4 | AL583322 | EST |
| 16 | GCAAGCCAAC | 132 | 2 | BG687451 | EST |
| 17 | CATTTGTAAT | 131 | 2 | BE890421 | EST |
| 18 | ATTGTTTATG | 127 | 2 | Hs.181163(BF593317) | High-mobility group protein 17 |
| 19 | CTCATAAGGA | 126 | 3 | BG385900 | EST |
| 20 | GCTCCCCTTT | 126 | 2 | Hs.1817(AV736453) | Myeloperoxidase |
| 21 | AGGTCAGGAG | 122 | 47 | H77590 | EST |
| 22 | ACCCTTGGCC | 122 | 3 | AI880722 | EST |
| 23 | AAGGTGGAGG | 118 | 3 | BG056715 | EST |
| 24 | TGTAATCAAT | 117 | 3 | Hs.249495(BG655713) | Heterogeneous nuclear ribonucleoprotein A1 |
| 25 | TTGGCCAGGC | 109 | 96 | AI078409 | EST |
| 26 | TCACAAGCAA | 109 | 3 | Hs.32916 (BG271651) | Nascent-polypeptide-associated complex a polypeptide |
| 27 | GGGCATCTCT | 102 | 2 | Hs.76807(AU157203) | HLA-DRA |
| 28 | CCTGTAGTCC | 100 | 116 | Hs.314307(AA749235) | EST |
| 29 | TACCCTAAAA | 100 | 49 | BG271479 | EST |
| 30 | GTGTTAACCA | 98 | 2 | Hs.85301(BF941019) | Calcium binding protein P22 |
| 31 | GTTCGTGCCA | 96 | 3 | Hs.179666(BG236685) | Uncharacterized hypothalamus protein HSMNP1 |
| 32 | CCATTGCACT | 91 | 59 | AU117661 | EST |
| 33 | CTCATAGCAG | 84 | 2 | Hs.279860(BG654607) | Tumor protein, translationally-controlled 1 |
| 34 | ACTTTTTCAA | 76 | 46 | BG099326 | EST |
| 35 | AAAAGAAACT | 76 | 4 | Hs.172182(BG744897) | Poly(A)-binding protein, cytoplasmic 1 |
| 36 | GCTTTATTTG | 75 | 2 | Hs.288061(AA554747) | Actin, beta |
| 37 | GCCTTCCAAT | 74 | 3 | Hs.76053(BF941985) | DEAD/H box polypeptide 5 |
| 38 | TACCATCAAT | 72 | 6 | Hs.169476(BG370213) | Glyceraldehyde-3-phosphate dehydrogenase |
| 39 | ACTCCAAAAA | 72 | 5 | BG236559 | EST |
| 40 | GCAAAACCCC | 70 | 44 | BF931620 | EST |
| 41 | GCGAAACCCC | 68 | 39 | Hs.269899(AI079278) | EST |
| 42 | GCATTTAAAT | 64 | 4 | Hs.275959(BG655489) | Eukaryotic translation elongation factor 1 beta 2 |
| 43 | GTCTGGGGCT | 63 | 3 | Hs.75725(BF591438) | Transgelin 2 |
| 44 | ATTTGTCCCA | 61 | 2 | Hs.139800(AU160425) | High-mobility group protein isoforms I and Y |
| 45 | TCTGCTAAAG | 58 | 4 | Hs.274472(BF434300) | High-mobility group protein 1 |
| 46 | TGTACCTGTA | 58 | 3 | Hs.278242(BG222897) | Hypothetical protein MGC12992 |
| 47 | AACCCGGGAG | 56 | 19 | AA428792 | EST |
| 48 | GTTCCCTGGC | 55 | 3 | Hs.177415(BG271519) | FBR-MuSV |
| 49 | CCTGTAATCT | 53 | 44 | Hs.35088(BF515942) | EST |
| 50 | GGCTTTACCC | 51 | 2 | Hs.119140(BF432256) | Eukaryotic translation initiation factor 5A |
| 51 | CCTAGCTGGA | 50 | 4 | Hs.182937(BG655492) | Peptidylprolyl isomerase A (cyclophilin A) |
| 52 | TTGGCTTTTC | 50 | 2 | Hs.41569(BF475411) | Phosphatidic acid phosphatase type 2A |
| 53 | CCTATAATCC | 49 | 73 | Hs.25328(AL048825) | EST |
| 54 | GATGCTGCCA | 49 | 3 | Hs.129914(L21756) | AML1 oncogene |
| 55 | ATTTGAGAAG | 48 | 5 | Hs.169921(BE621880) | General transcription factor II |
| 56 | TAGAAAGGCA | 48 | 2 | Hs.78909(W37407) | Butyrate response factor 2 |
| 57 | TGTGTTAAGA | 47 | 4 | Hs.288036(AI804500) | TRNA isopentenylpyrophosphate transferase |
| 58 | GTGGCTCACA | 46 | 44 | Hs.120769(AI982685) | Homo sapiens cDNA FLJ20463 fis |
| 59 | TTGGTCAGGC | 45 | 34 | Hs.12094(AA650333) | Hypothetical protein |
| 60 | GTGCACTGAG | 45 | 5 | Hs.181244(D32129) | Major histocompatibility complex, class I, A |
The list was generated from the top expressed genes after removing 40 ribosomal protein genes and 4 SAGE tags with poly(A) nucleotides.
Conversion of Novel SAGE Tags into Novel 3′ ESTs.
The novel SAGE tags account for half of the unique SAGE tags detected in this analysis. The question arises whether these novel SAGE tags represent unidentified novel genes expressed in CD34+ cells. We used the GLGI method to convert 219 novel SAGE tags present in more than 4 copies into 3′ ESTs, and we matched these longer 3′ ESTs to the database. With 85% of sequence homology as the cut-off value to distinguish the known or novel sequences, a total of 198 sequences generated from 138 novel SAGE tags was confirmed to be novel 3′ ESTs. These ESTs range in length from 8 to 454 bp (mean = 152 bp) (Tables 3 and 11, which is published as supporting information on the PNAS web site). Sequences from 32 novel tags matched known expressed sequences, and reactions for 49 novel tags did not generate qualified sequences. This result, as well as our earlier study on CD15+ cells (11), indicates that the novel SAGE tags we have identified truly represent a large number of novel genes. The conversion of novel SAGE tags into 3′ ESTs provides an efficient way to identify novel genes on a large scale. Our strategy becomes especially important for novel gene identification, in view of the reports that the number of genes in the human genome has been seriously underestimated (17, 18).
Table 3.
Examples of novel 3′ ESTs from novel SAGE tags
| Copy | SAGE tag | GenBank accession no. | Sequence length, bp |
|---|---|---|---|
| 114 | GAGCGGCGCT | BI094690 | 38 |
| BI094691 | 257 | ||
| BI388635 | 226 | ||
| BI388636 | 47 | ||
| 104 | GTGCCACGGG | BI094692 | 127 |
| 97 | TGGCGTACGG | BI094693 | 204 |
| 83 | TAAGCGGCGC | BI388635 | 28 |
| 79 | AGAGCGGCGC | BI388636 | 59 |
| 75 | AAAGCGGCGC | BI094694 | 154 |
| 59 | GTGCCCACGG | BI388637 | 413 |
| 41 | CCGACGGGCG | BI094695 | 344 |
| BI094696 | 344 | ||
| 34 | GATGCCCCCC | BI388638 | 258 |
| 32 | GTGCCCCGGG | BI094697 | 300 |
| BI388639 | 90 | ||
| 30 | CTGAGCGGCG | BI094698 | 133 |
| BI388640 | 104 | ||
| 29 | CCACTTCTGG | BI094699 | 132 |
| 27 | GTGACCAAGG | BI094700 | 129 |
| 27 | GTGAAGCAGT | BI094701 | 359 |
| 26 | GGGACCACCG | BI094702 | 113 |
| 24 | CAACACCACA | BI388641 | 409 |
| BI388642 | 215 | ||
| 23 | AGCTCTGTAG | BI094703 | 233 |
| 23 | GGTCAGTCGG | BI094704 | 211 |
| BI094705 | 137 | ||
| 22 | AGCGGCGCTC | BI388643 | 37 |
| 21 | ACCCCGCCGG | BI388644 | 130 |
| 20 | GTGCCCAGGG | BI388645 | 163 |
| 19 | TAGAGCGGCG | BI388646 | 181 |
| 19 | CCAGAGGCTG | BI388647 | 114 |
| 18 | TGCACCGTTT | BI388648 | 143 |
| BI388649 | 157 | ||
| 17 | GGACCACGGG | BI094706 | 127 |
| 16 | ACTCCTGAAC | BI094707 | 301 |
| 16 | CTATAGCGGC | BI094819 | 281 |
| 15 | AGCTCTTCCT | BI094820 | 109 |
| 15 | TTACCCACAC | BI094821 | 65 |
| 15 | CAAGCGGCGC | BI094822 | 341 |
| 14 | GGGAAGCAGA | BI094823 | 90 |
| BI094824 | 35 | ||
| 14 | ATCAAAGGTG | BI094825 | 428 |
| 14 | AGCACCTTCA | BI094826 | 57 |
| BI094827 | 210 | ||
| 14 | GTTCCAGCCG | BI094828 | 16 |
| 14 | CTAAGCGGGG | BI094829 | 45 |
| BI094830 | 119 | ||
| 13 | GGTGACCACG | BI094831 | 129 |
| 13 | TAGGTTGCTA | BI094832 | 172 |
| BI094833 | 82 | ||
| 13 | GTTGTCCTAC | BI094834 | 49 |
| 12 | ATGGCGCCTC | BI094835 | 85 |
| 12 | ACCCGCCCGG | BI388650 | 168 |
| 12 | TCGCCGCGAC | BI388651 | 98 |
| 11 | ACCCAGGGAG | BI094836 | 82 |
| 11 | GCACGTGTCT | BI094837 | 329 |
| 11 | AACGAAAAAA | BI094838 | 16 |
| 11 | GTAAAGCCAG | BI094708 | 68 |
Known Genes Identified in CD34+ Cells.
Based on the UniGene clusters single-matched by SAGE tags, we identified 3,687 known genes expressed in CD34+ cells (Table 12, which is published as supporting information on the PNAS web site). This is the largest number of known genes identified in human hematopoietic CD34+ cells (19–23). Considering the identification of 42,399 unique SAGE tags and for the matches of 21,546 unique SAGE tags to known expressed sequences, the 3,687 known genes account for only 9% of the total unique SAGE tags and 17.6% of the matched SAGE tags. These data indicate that most of the genes expressed in CD34+ cells have not been identified or studied. Clarifying the function of such a large number of genes expressed in stem/progenitor cells is a serious challenge for stem cell biology.
Heterogeneity of 3′ Untranslated Region (UTR) Sequences.
When matching SAGE tags with expressed sequences, we frequently observed that different SAGE tags matched different sequences located within the same UniGene cluster (Table 4). One assumption would be that, if a SAGE tag matched sequences upstream of the most 3′ tag, this tag was most likely to be derived from partially digested cDNA templates in the process of SAGE library construction. Two lines of evidence fail to support this assumption. (i) The SAGE tag database was constructed through extracting the SAGE tag sequences after the last CATG site from expressed transcripts in the database (24). The match between an experimental SAGE tag and a tag in the SAGE database indicates the existence of an expressed transcript for this SAGE tag. (ii) We converted more than 1,000 SAGE tags into 3′ ESTs through the GLGI method. Examination of these 3′ ESTs shows that 97% of them do not have internal CATG, which is the restriction sequence of NlaIII used for SAGE library construction. This result strongly indicates that NlaIII restriction digestion of cDNA templates is very efficient. Because SAGE tags are located in the 3′ part of transcripts, the identification of different SAGE tags that match different sequences located in one UniGene cluster reflects the presence of transcripts from the same gene with different 3′ UTRs. The 3′ UTR plays important roles in regulating the function of expressed genes, such as mRNA stability and translational efficiency (25–29). Therefore, analyzing the heterogeneity of the 3′ UTR through SAGE tags will provide information for understanding the relationship between 3′ UTR structure and the function of the genes expressed during hematopoiesis.
Table 4.
Examples of transcripts with different 3′ UTRs detected by SAGE tags
| Gene | Tag | Gene | Tag |
|---|---|---|---|
| Transcriptional/translational factors | DNA structure | ||
| C-fos (Hs.25647) | ACATCAAAAA | Chromatin assembly factor-I p150 subunit (Hs.79018) | GACGGCTTCC |
| CTTTCAGACT | ACCCCTGAGA | ||
| ACTGTAATTG | High mobility group box (SSRP1) (Hs.79162) | TAAGGCCAGG | |
| C-jun (Hs.78465) | AGGAACCGCA | AAAGAATATG | |
| GCGAGGGGAG | Histone acetyltransferase (MORF) (Hs.27590) | TGGGCGGGTC | |
| GGGGGAGGGC | CTAAAGTTTT | ||
| CCTTTGTAAG | TAGTACAATG | ||
| CTAACGCAGC | Nucleosome assembly protein 2 (Hs.78103) | CTTTCTCAGT | |
| CREB (Hs.79194) | CCCGCTTCGC | AGGACGGGCT | |
| ATTCCTGGCG | ACGCAGGCGC | ||
| Forkhead protein (FKHR) (Hs.170133) | GATGGAGATA | Scaffold attachment factor A (Hs.103804) | TTTAGTAACC |
| AAGTCTAACA | AGTTTTATAG | ||
| AGGTCAGTAG | AGGTCAGCAT | ||
| Methyl-CpG binding protein MBD4 (Hs.35947) | TTTTGAGATT | CAACTGTGAG | |
| TGGTGAGACC | GAGCCCGGGA | ||
| NF-kappa-B (Hs.83428) | GTTACAATCA | TTTGGACCGG | |
| TATTACTTTA | GAGTCAGCAT | ||
| ATTATGGGCA | AATTGCATTA | ||
| Poly(A) binding protein II (Hs.117176) | TGTCACTAAG | AATCTGGTTG | |
| AATAAAGTTG | Telomeric repeat binding factor (TRF1) (Hs.194562) | ACAGTATTTT | |
| GTATTCCCCT | GGGCAAAATT | ||
| ATCACCGGGC | GAACCCAGCA | ||
| Serum response factor (Hs.155321) | GCGCTCGATG | ||
| GTCACAGTCC | Signal transduction | ||
| TTTCAGCCAG | Calmodulin 1 (CALM1) (Hs.177656) | GAGGCTACGA | |
| AATTATACAC | TAGGTCTAGT | ||
| Splicing factor SF3a60 (Hs.77897) | GTAGAGTTGG | TACACTGGCC | |
| CTGGCAGATT | ACTTGGAGCC | ||
| SWI/SNF complex 155-kDa subunit (Hs.172280) | TCTCTCAGTG | ACAAACTTAG | |
| TCTTTGATCT | CDC2L5 protein kinase (Hs.59498) | TAGAAGTGTT | |
| TTTCTCAGTG | CTGCCTGAAG | ||
| TFIID subunit TAFII55 (TAFII55) (Hs.155188) | AATGTTAGGT | ERK activator kinase (MEK1) (Hs.3446) | TGTTCTTTAT |
| ATGAGCTTCG | ATAGCTGGGG | ||
| TIF-IA (Hs.110103) | GACCATTTTA | G protein gamma-10 subunit (Hs.79126) | AGAAAAGAAC |
| CCATATGAAT | AAGAAAATAT | ||
| IL-1 receptor-associated kinase (Hs.182018) | GGAACGATTA | ||
| Cell cycle regulators | CCCCCGTGAA | ||
| CDC2 (Hs.184572) | CCAAAATTTG | K-ras (Hs.184050) | AACTGTACTA |
| GAAAGAAACT | CTATCCAGTA | ||
| CDC21 (Hs.154443) | GCTACTATTA | Protein tyrosine kinase 6 (PTK6) (Hs.51133) | AGTGTGCGTG |
| CTAAAGTAAG | GTCATTTTAA | ||
| Checkpoint suppressor 1 (Hs.211773) | GGCGACAGGG | Rab11a GTPase (Hs.75618) | CTATGGCTTC |
| GAAGAAGTTC | TTCCACCAAC | ||
| GAGAAGAAAT | CAGTAGAGTC | ||
| Cyclin protein (Hs.78996) | GAAACACAGA | CTTGTGGGCA | |
| TCGATAAAGA | CTCTGTAATT | ||
| Cyclin I (CYC1) (Hs.79933) | CAAAATGAGG | Serine/threonine kinase (KDS) (Hs.12040) | CAAAAAAATA |
| CCACTTTTAA | ATAACTGTCA | ||
| AATTTTAATT | CCAACAACTC | ||
| CAAAAATCAG | GAGAGCCTCA | ||
| ATGACCCCCG | Serine/threonine protein kinase MASK (Hs.23643) | GTAATAAATT | |
| CAAAATCAGG | AGTTGCAAAA | ||
| TTATAACTGA | TTATGGGTTA | ||
| ACCTTAAGAG |
Genes Known to Be Important for Hematopoiesis.
We analyzed the genes known to be important for hematopoiesis (Tables 5 and 13, which is published as supporting information on the PNAS web site). Many of these genes were identified in this study. Because SAGE provides a simultaneous quantitation for the expressed genes in a sample, the levels of expression among different genes can be compared directly. For example, seven HOX genes were identified from the HOX-A, B, and C clusters, including A3, A5, A9, B2, B7, B13, and C9 (30). All of these HOX genes were expressed at low levels. Some genes known to play roles in hematopoiesis were not detected in this analysis, such as PU.1 (31) and SCF (stem cell factor; ref. 32). This discrepancy may arise because of the difference in methodologies used for the analyses. SAGE analyzes gene expression in a “horizontal” way. That is, all of the transcripts expressed from different genes were simultaneously identified and quantified. In other studies, genes were identified based on the reverse-transcription (RT)-PCR method. RT-PCR analyzes gene expression in a “vertical” manner. That is, it can identify genes expressed at very low levels through millionfold amplification. Other explanations may be related to differences in the cell types used or differences in culture conditions. For example, a gene can be expressed in transformed cell lines or cells treated with various cytokines in in vitro culture conditions but may not be expressed in in vivo physiologic conditions.
Table 5.
Examples of genes known to be important for hematopoiesis
| Gene | UniGene ID | SAGE tag | Copy no. |
|---|---|---|---|
| Cathepsin B | Hs.249982 | GAAAAGGACA | 4 |
| GGGGTAACCA | 2 | ||
| ATTCTTTAAT | 1 | ||
| TGGGTAAGCC | 1 | ||
| AGGGGAAGGG | 1 | ||
| ATTAGCAGAG | 1 | ||
| Cathepsin C | Hs.10029 | CAAAATGCAA | 3 |
| CTGGCAACCT | 2 | ||
| CTATATTTTT | 2 | ||
| CACCCACCCA | 1 | ||
| Cathepsin D (lysosomal aspartyl protease) | Hs.79572 | TATTGGCCTG | 2 |
| GCAGCTCAGG | 2 | ||
| ATCTCAAAGA | 2 | ||
| TTAAGCATAA | 1 | ||
| Cathepsin S | Hs.181301 | ACCAGTGAAG | 2 |
| GTGGAGCCCC | 1 | ||
| C/EBP alpha | Hs.76171 | GGGGGTGAAG | 1 |
| C/EBP delta | Hs.76722 | CTCACTTTTT | 2 |
| CTCCCTTTTT | 1 | ||
| CD33 antigen (gp67) | Hs.83731 | GAAAACACCA | 1 |
| CD34 antigen | Hs.85289 | GCTTCCTCCT | 2 |
| GGACCAGGGT | 1 | ||
| GTCCTGCCTA | 1 | ||
| CD8 antigen, alpha polypeptide (p32) | Hs.85258 | ATTATTATTT | 1 |
| M-CSF1 | Hs.173894 | GTATCCAGCT | 2 |
| G-CSF3 receptor | Hs.2175 | CTCCATCCAG | 7 |
| Friend of GATA2 | Hs.106309 | GCTTCTATTT | 2 |
| GATA-binding protein 2 | Hs.760 | GACAGTTGTT | 1 |
| Hemoglobin, beta | Hs.155376 | GCAAAGAAAG | 1 |
| GCAAGAAAGT | 1 | ||
| Homeo box A3 | Hs.248074 | GACTATGGGG | 1 |
| Homeo box A5 | Hs.37034 | TGCGTGGAAG | 2 |
| Homeo box A9 | Hs.127428 | TACCTCACCA | 1 |
| Homeo box B13 | Hs.66731 | ACTCCCTGTT | 1 |
| Homeo box B2 | Hs.2733 | AAGCACAAGC | 1 |
| Homeo box B7 | Hs.819 | CTTGCAGCCT | 1 |
| Homeo box C9 | Hs.40408 | CCGCGGGCTG | 1 |
| Jun D proto-oncogene | Hs.2780 | ACCCCCCGGC | 1 |
| Macrophage migration inhibitory factor | Hs.73798 | AACGCCGGCA | 1 |
| AACGCGGTCA | 1 | ||
| Mesenchymal stem cell protein DSCD75 | Hs.25237 | GGAAAGCTGC | 1 |
| GGAAAGCTTG | 1 | ||
| Myeloid differentiation primary response gene | Hs.82116 | TTTTGTACGC | 12 |
| Myeloid leukemia factor 2 | Hs.79026 | CATTGAAGGG | 12 |
| GCAGGAGTAG | 3 | ||
| ACAGCTGGAG | 1 | ||
| Myeloperoxidase | Hs.1817 | TATGTGCGAA | 2 |
| Retinoid X receptor, alpha | Hs.288688 | CAGATGGACA | 1 |
| CCCGGCCGGC | 1 | ||
| Retinoid X receptor, beta | Hs.79372 | ATTTTTGCCC | 6 |
In summary, the data generated from this study provide an overview of the pattern of gene expression in normal human CD34+ stem/progenitor cells. With this information in hand, we are in a position to identify the genes important in hematopoietic stem cells, to understand the regulatory network of self-renewal and differentiation of hematopoietic stem cells into different lineages, and to identify the genes whose expression are abnormal in hematopoietic diseases such as leukemia.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants CA78862-01 (to J.D.R. and S.M.W.) and CA42557 (to J.D.R.), American Cancer Society Grant IRG-41-40 (to S.M.W.), and by the G. Harold and Lelia Y. Mathers Foundation (S.M.W.).
Abbreviations
- SAGE
serial analysis of gene expression
- GLGI
generation of longer cDNA fragments from SAGE tags for gene identification
- EST
expressed sequence tag
- UTR
untranslated region
References
- 1.Morrison S J, Wright D E, Cheshier S H, Weissman I L. Curr Opin Immunol. 1997;9:216–221. doi: 10.1016/s0952-7915(97)80138-0. [DOI] [PubMed] [Google Scholar]
- 2.Orkin S H. In: Stem Cell Biology. Marshak D R, Gardner R, Gottlieb D, editors. Plainview, New York: Cold Spring Harbor Lab. Press; 2001. pp. 289–301. [Google Scholar]
- 3.Weissman I L. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 4.Brazelton T R, Rossi F M, Keshet G I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell W M. Hepatology. 2000;32:1181. doi: 10.1016/s0270-9139(00)80043-9. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S M, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine D M, et al. Nature (London) 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Velculescu V E, Zhang L, Vogelstein B, Kinzler K W. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Rowley J D, Wang S M. Proc Natl Acad Sci USA. 2000;97:349–353. doi: 10.1073/pnas.97.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J., Lee, S., Zhou, G. & Wang, S. M. (2001) Genes Chromosomes Cancer, in press. [DOI] [PubMed]
- 10.Lee S, Chen J, Zhou G, Wang S M. BioTechniques. 2001;31:348–354. doi: 10.2144/01312st07. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Zhou G, Clark T, Chen J, Rowley J D, Wang S M. Proc Natl Acad Sci USA. 2001;98:3340–3345. doi: 10.1073/pnas.051013798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause D S, Fackler M J, Civin C I, May W S. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 13.D'Arena G, Musto P, Cascavilla N, Di, Giorgio G, Zendoli F, Carotenuto M. Haematologica. 1996;81:404–409. [PubMed] [Google Scholar]
- 14.Velculescu V E, Madden S L, Zhang L, Lash A E, Yu J, Rago C, Lal A, Wang C J, Beaudry G A, Ciriello K M, et al. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S., Clark, T., Chen, J., Zhou, G., Scott, L. R., Rowley, J. D. & Wang, S. M. (2001) Genomics, in press. [DOI] [PubMed]
- 16.Clark, T., Lee, S., Scott, L. R. & Wang, S. M. (2001) J. Comput. Biol., in press.
- 17.Wright, F. A., Lemon, W. J., Zhao, W. D., Sears, R., Zhuo, D., Wang, J. P., Yang, H. Y., Baer, T., Stredney, D., Spitzner, J., et al. (2001) Genome Biol.2, RESEARCH 0025. [DOI] [PMC free article] [PubMed]
- 18.Hogenesch J B, Ching K A, Batalov S, Su A I, Walker J R, Zhou Y, Kay S A, Schultz P G, Cooke M P, et al. Cell. 2001;106:413–415. doi: 10.1016/s0092-8674(01)00467-6. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Peterson K R, Stamatoyannopoulos G, Papayannopoulou T. Exp Hematol. 1996;24:605–612. [PubMed] [Google Scholar]
- 20.Claudio J O, Liew C C, Dempsey A A, Cukerman E, Stewart A K, Na E. Genomics. 1998;50:44–52. doi: 10.1006/geno.1998.5308. [DOI] [PubMed] [Google Scholar]
- 21.Mao M, Fu G, Wu J S, Zhang Q H, Zhou J, Kan L X, Huang Q H, He K L, Gu B W, Han Z G, et al. Proc Natl Acad Sci USA. 1998;95:8175–8180. doi: 10.1073/pnas.95.14.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q H, Ye M, Wu X Y, Ren S X, Zhao M, Zhao C J, Fu G, Shen Y, Fan H Y, Lu G, et al. Genome Res. 2000;10:1546–1560. doi: 10.1101/gr.140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu J, Zhang Q H, Huang Q H, Ren S X, Wu X Y, Ye M, Huang C H, Fu G, Zhou J, Niu C, et al. Hematol J. 2000;1:206–217. doi: 10.1038/sj.thj.6200020. [DOI] [PubMed] [Google Scholar]
- 24.Lal A, Lash A E, Altschul S F, Velculescu V, Zhang L, McLendon R E, Marra M A, Prange C, Morin P J, Polyak K, et al. Cancer Res. 1999;59:5403–5407. [PubMed] [Google Scholar]
- 25.Sonenberg N. Curr Opin Genet Dev. 1994;4:310–315. doi: 10.1016/s0959-437x(05)80059-0. [DOI] [PubMed] [Google Scholar]
- 26.Decker C J, Parker R. Curr Opin Cell Biol. 1995;7:386–392. doi: 10.1016/0955-0674(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 27.Wickens M, Anderson P, Jackson R J. Curr Opin Genet Dev. 1997;7:220–232. doi: 10.1016/s0959-437x(97)80132-3. [DOI] [PubMed] [Google Scholar]
- 28.Gallie D R. Gene. 1998;216:1–11. doi: 10.1016/s0378-1119(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 29.Varani G. Proc Natl Acad Sci USA. 2001;98:4288–4289. doi: 10.1073/pnas.091108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buske C, Humphries R K. Int J Hematol. 2000;71:301–308. [PubMed] [Google Scholar]
- 31.Klemsz M J, McKercher S R, Celada A, Van Beveren C, Maki R A. Cell. 1990;61:113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- 32.Martin F H, Suggs S V, Langley K E, Lu H S, Ting J, Okino K H, Morris C F, McNiece I K, Jacobsen F W, Mendiaz E A, et al. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.