Abstract
In comparison to procedures used for the separation of individual cell types from other organs, the process of human pancreatic islet isolation aims to digest the pancreatic exocrine matrix completely without dispersing the individual cells within the endocrine cell cluster. This objective is unique within the field of tissue separation, and outlines the challenge of islet isolation to balance two opposing priorities. Although significant progress has been made in the characterization and production of enzyme blends for islet isolation, there are still numerous areas which require improvement. The ultimate goal of enzyme production, namely the routine production of a consistent and standardized enzyme blend, has still not been realized. This seems to be mainly the result of a lack of detailed knowledge regarding the structure of the pancreatic extracellular matrix and the synergistic interplay between collagenase and different supplementary proteases during the degradation of the extracellular matrix. Furthermore, the activation of intrinsic proteolytic enzymes produced by the pancreatic acinar cells, also impacts on the chance of a successful outcome of human islet isolation. This overview discusses the challenges of pancreatic enzymatic digestion during human islet isolation, and outlines the developments in this field over the past 5 decades.
Keywords: diabetes, collagenase, islet isolation, neutral protease, thermolysin, tryptic-like activity, clostripain, extracellular matrix protein, basement membrane
1. Introduction
Collagenases are collagenolytic proteases that are widely used in the food and cosmetic industries, in biotechnology and healthcare. Medical applications of collagenases include wound debridement [1], treatment of Peyronie's disease [2], fibrotic encapsulation of implants [3], and the treatment of Dupuytren’s contracture [4, 5].
Collagenolytic enzymes used for therapeutic applications are mainly produced by anaerobic or aerobic microorganisms which can be pathogenic or non-pathogenic. In contrast to vertebrate collagenases, microbial collagenases have the capability to cleave native and denatured collagen at multiple sites [6]. This unique attribute makes microbial collagenases to the reagent of choice for manifold applications in medical care and research [7]. Among other microorganisms, Clostridia strains are the most thoroughly investigated sources of collagenase. In particular, the bacterium Clostridium histolyticum has been established as the most widely used specie for collagenase production in different fields [8].
Another area of application of collagenases includes the isolation of cells from different organs and tissues. These include chondrocytes [9], cells of the immune system [10, 11], mesenchymal stem cells [12], myocytes [13], hepatocytes [14-16], and islets of Langerhans [17-19]. In contrast to the procedures used for the separation of cells from other organs, the isolation of islets from the pancreas essentially aims to completely digest the pancreatic stromal matrix whilst preserving the intact clusters of endocrine cells [20]. This objective is unique in the field of tissue separation, and reflects the challenge of islet isolation to achieve these contrasting goals [21]. In order to achieve the delicate equilibration between complete dissociation of the exocrine tissue and preservation of islet integrity, a substantial proportion of research efforts in this field has been devoted to the assessment, selection, and combination of suitable collagenolytic and proteolytic enzymes. Since 1965, when Moskalewski first introduced collagenase for isolating guinea-pig islets [22], most subsequent studies of collagenase digestion in the islet field have been performed as 'trial and error' approaches comparing different lots of enzymes or different enzyme products from different manufacturers, until acceptable numbers of islets could be isolated repeatedly from a donor pancreas. However, few of these studies have taken up the challenge of accurately defining the substrate.
Since our first overview of collagenase for islet isolation, published twenty years ago [21], significant progress has been achieved in enzyme purification [23, 24], biochemical characterization [25-28] and in the production of intact collagenase isoforms [29]. However, little advance until today has been made in enzyme standardization or consistency. At present, islet isolation teams are still not released from the obligation of having to test several enzyme batches ('batch testing') every time an old batch has been exhausted. This seems to be partly a result of the lack of detailed knowledge at the molecular level regarding enzymatic degradation of the pancreatic extracellular matrix (ECM) by infused collagenase and supplementary proteases. It may be that the enormous variability of the ECM, depicted by 28 different types of collagen [30] and at least 15 isoforms of laminin [31], may never allow the complete and detailed analysis of, for instance, the interaction between the peri-islet basement membrane, the islets themselves, and the administered enzyme blends [32, 33]. Moreover, the ECM is a dynamic system remaining under the influence of numerous donor variables such as age, and can also substantially change during the acute and chronic course of pancreatitis [34-36]. The specific production of high activities of proteolytic enzymes in the acinar cells additionally increases the complexity when multiple determinants of success or failure of human islet isolation and transplantation are included.
This review aims to provide the most recent information about the characteristics of different proteases used for the purpose of human pancreas digestion and islet isolation. Another aim is to emphasize the significance of understanding the mechanisms of enzymatic ECM degradation.
2. Enzyme purification
In the past, the collagenolytic enzyme products for islet isolation were mixtures of 12 or more different enzymes and bacterial byproducts such as pigments and endotoxin that are filtered and extracted from Clostridium histolyticum cultures [21, 37]. Some of these compounds have a strong proinflammatory character and can impair engraftment of transplanted islets [38, 39]. Perhaps not surprisingly, it has been demonstrated that enzyme-separated islets are metabolically less active and less potent than microdissected islets [40]. These crude products have been erroneously labeled as collagenase, although this particular enzyme represents only a minor proportion of the entire protein mass within these enzyme blends, as demonstrated in high-performance liquid chromatograms (HPLC) (Figure 1) [37, 41].
Figure 1. HPLC of crude collagenase NB-4 and potential contaminants.
HPLC data were kindly provided by Serva Electrophoresis GmbH [21].
As a result of these impurities, crude collagenase blends have been associated with substantial 'lot to lot' variability and inconsistency [21, 22, 42-44]. For clarity, the term 'collagenase' should be specifically used for different isoforms of enzymes cleaving different types of native collagen [45]. Products composed of combinations of collagenase and other enzymes should be termed 'enzyme blends'.
Basic experiments performed by the Groningen group in rats in the 1990s clearly demonstrated that only collagenase class I (CC-I), collagenase class II (CC-II) and a supplementary protease are essentially required for effective pancreas digestion and islet release [46, 47]. Because of the presence of numerous enzymes and other compounds with similar physical and chemical characteristics the purification of collagenolytic enzyme mixtures is challenging and costly [6, 37]. Nevertheless, to achieve reproducibility in the isolation of islets from human donor pancreases a commercially available purified enzyme blend was first developed and introduced by Roche Indianapolis in 1995 [23]. Liberase HI was standardized with respect to CC-I, CC-II, and two other proteases [48]. The efficiency of this product was confirmed in several follow-up studies [49, 50], and may have contributed to the significantly increased number of human islet allotransplantations during this period of time [51]. The success of the Liberase HI subsequently triggered the interest of other companies such as Nordmark-Serva [52] or Vitacyte [53] to develop and launch purified enzyme blends for human islet isolation. The purification of enzymes in collagenase manufacturing was also the essential prerequisite to develop donor-specific purified enzyme mixtures specifically formulated for the isolation of islets from different species such as dog, pig, rat, and mouse [42, 54-56].
3. Enzyme characterization
Early studies revealed that purified collagenase cannot disperse different tissues from the rat. Only when supplemented with another protease such as trypsin, collagenase quickly digests tail tendon, adipose tissue, or cardiac muscle [57]. Complete separation and efficient release of islets from within the acinar tissue of the pancreas also requires the synergistic interplay between CC-I, CC-II, and a supplementary neutral protease such as that from Clostridium histolyticum (CNP) or thermolysin (TL) from Bacillus thermoproteolyticus rokko [46, 47, 58]. Previous studies in the rat identified CC-II as the most relevant isoform for pancreas digestion when compared with CC-I [47, 59]. In agreement with the observations of the Groningen group [60], we found that human pancreases are efficiently digested even in the absence of CC-I as long as sufficient activities of supplementary neutral proteases are present [61, 62]. Nevertheless, for optimal collagenase function, a certain amount of CC-I has to be present within the enzyme blend. When calculating the proportion of CC-II and CC-I protein within different enzyme blends as measured by 'area under the curve' (AUC) in HPLC profiles, the ideal ratio between the CC-II and CC-I isoforms seems to be in a range between 1.0 and 0.64 [63, 64]. This ratio also seems to be effective for hepatocyte isolation [65]. Interestingly, investigating the effects of recombinant CC-I and CC-II, it was found that islets can be successfully isolated from the human pancreas using a broad range of ratios of CC-II and CC-I. It should be mentioned that this study applied different collagenase activity assays, and did not consider the HPLC profiles of enzyme blends to calculate this variable [66].
The delicate ratio between CC-II and CC-I seems also to determine the activity of neutral proteases required for efficient pancreas dissociation. A nearly ideal proportion of CC-I reduces the demand on supplementary proteases to a minimum which has significant implications for the viability of isolated islets [67]. In addition to observations made in the rat [46, 68], we described the detrimental effect of increased CNP activities on integrity and viability of isolated human islets [69]. In fact, it seems to be possible to isolate human islets using only a marginal amount of CNP [62, 70]. These observations raise the question of whether the identification of an effective replacement for clostridial CNP could improve the quality and functional potency of isolated islets. In human islet isolation, TL has been mostly used as alternative to CNP. TL is an essential component of Liberase HI, and is still combined with Mammalian Tissue-Free (MTF) Liberase (Roche, Penzberg, Germany) and Collagenase HA (Vitacyte, Indianapolis, USA). Because the specific activity of TL is multifold higher than CNP [28], it is associated with extensive islet fragmentation and loss of islets during culture if not carefully adjusted [71].
Electron microscopy studies of isolated rat islets have demonstrated that the complete loss of the basement membrane resulted in the subsequent disruption of the plasma membrane of peripheral islet cells exposing and releasing the intracellular content and subcellular components [72]. Several studies comparing enzyme blends supplemented with CNP or TL suggested that neutral protease-isolated human islets are characterized by a lower rate of apoptosis, higher secretory capacity and higher viability compared to islets released by means of TL [52, 73, 74]. A large-scale study, assessing 101 human pancreases digested with Liberase HI plus TL and 96 pancreases digested by means of collagenase NB-1 plus neutral protease, revealed that Liberase-isolated islets secreted higher amounts of TNF-α which may explain the lower secretory capacity and intracellular insulin content measured in these islets [75]. As a consequence of these observations, the Minneapolis group replaced TL as supplementary protease for Collagenase HA by CNP from Serva, and achieved consistently higher yields of islets fulfilling the release criteria for clinical islet transplantation [18].
To minimize the negative effects of collagenolytic enzyme blends, TL was replaced by dispase in enzyme mixtures specifically blended for porcine islet isolation [54]. Dispase is a neutral protease extracted from Bacillus polymyxa, and has been shown to be less harmful during dispersion of isolated rat islets than other proteases [76]. Most recently, dispase was identified as suitable supplementary protease for the digestion of human pancreases. However, a limited number of isolations performed in a split pancreas model did not detect significant differences between CNP and dispase with respect to islet yield, viability, and in vitro function [66].
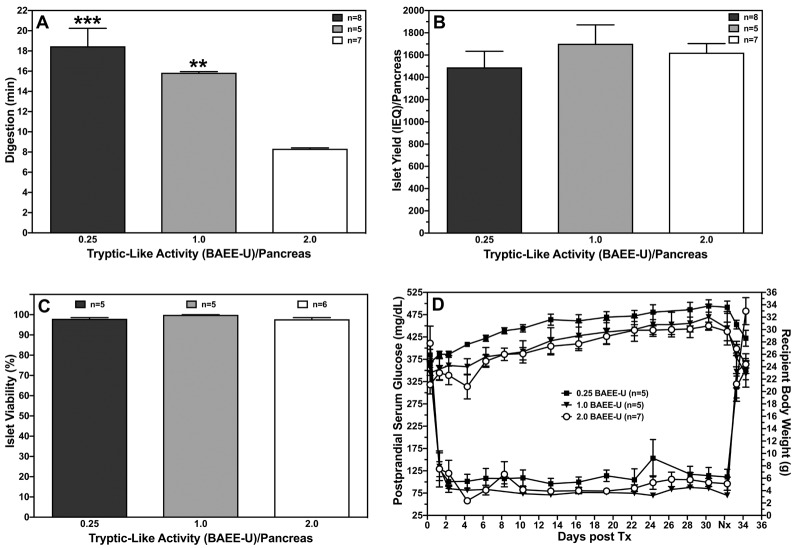
Another neutral protease of strong interest is clostripain. This enzyme is responsible for the tryptic-like activity (TLA) in enzyme blends [77]. Early studies in the porcine pancreas indicated that purifed enzyme fractions with high collagenase and high TLA but low CNP levels were most effective to release islets from juvenile pig pancreases [78]. Experiments in the rat clearly demonstrated that higher proportions of TLA/clostripain significantly reduce pancreas digestion time without affecting islets yield, viability, in vitro function, or post-transplant function in diabetic nude mice (Figure 2).
Figure 2. Effect of TLA on islet isolation parameters.
The figure shows the effect of TLA on rat pancreas digestion time (A), islet yield (B), viability (C), and graft function of isolated rat islets transplanted beneath the kidney capsule of diabetic nude mice (D). TLA was supplemented at a concentration of 0.25 (n = 5), 1.0 (n = 5) and 2.0 BAEE-U/pancreas combined with 0.4 DMC-U of neutral protease activity and 20 PZ-U of collagenase activity. Nephrectomy (Nx) was performed at day 32 post-transplant as indicated. **p < 0.01, ***p < 0.001 vs. 2.0 BAEE-U.
Likewise, retrospective analysis of human islet isolations also suggests that higher TLA reduces recirculation time and increase islet yield without deteriorating islet viability, purity, and recovery after culture. Consequently, incrementally increased TLA correlates with a higher percentage of islet preparations, fulfilling the quality release criteria for clinical islet transplantation [79]. A recent study in rats confirmed these previous observations, and found nearly triplicated islet yields when collagenase and neutral protease were additionally supplemented with clostripain. Interestingly, combining clostripain with TL did not enhance islet yield, and resulted in increased islet fragmentation in contrast to the combination with neutral protease [80]. An incompatibility between TL and clostripain extracted from different species is assumed to cause the problem. However, this hypothesis could not be confirmed when human islets were isolated by means of clostripain added as a third component to collagenase and TL. This protocol significantly enhanced isolation outcome and increased the rate of transplantability from 46% in controls to 100% in the treatment group [81]. The question whether islet release from the pancreas can be impaired by using collagenolytic proteases from different species is therefore still open.
The exact mechanisms of clostripain function are not completely understood until today. It has been speculated that the positive effect of clostripain is related to its capability to convert pro-elastase into elastase which may assist the degradation of elastin as component of the ECM [45]. Elastin fibers are strongly expressed in tissues continuously exposed to stretching forces such as vessels, lung, and heart, but have not been analyzed in detail in the pancreas [82]. Therefore, the importance and contribution of elastin to the pancreatic ECM is speculative today. A possible anatomical location might be within the walls of the vascular and ductal system. However, considering the harmful effects of activated elastase on pancreatic tissue (discussed below), a negative impact rather than a positive effect would be expected if clostripain is administered for islet isolation.
Irrespective of the exact specifity of clostripain, we are not aware of any study that has revealed a detrimental effect of clostripain on islet viability and function except one publication that found an inverse correlation of islet yield with clostripain and TLA [83]. However, the assessed enzyme lots that contained high clostripain-related activities were also contaminated with high activities of neutral protease, making a final conclusion from this study difficult.
4. Collagenase degradation
Any modification of the composition of an enzyme blend, in particular the balance between CC-I, CC-II, and the supplementary neutral protease, alters its characteristics. This particularly concerns the critical ratio between CC-II and CC-I, as discussed in section 3 [63, 64].
The analysis of 24 Liberase HI lots of different efficiencies revealed a stable content of CC-II, while an inconsistency in intact CC-I, associated with varying amounts of CC-Ib, was measured [25, 84]. As CC-Ib is a product of CC-I degradation, it is characterized by a reduction in the initial molecular weight of 116/115 kDa to 100 kDa, corresponding to the loss of one collagen-binding domain [45]. This has significant implications for islet isolation outcome concerning pancreas digestion time, islet yield, and post-transplant function [29]. Intra-lot variability with respect to protein mass, salt content, enzyme activities, and residual water clearly indicated a manufacturing problem, presumably related to the lyophilization process [25].
It has to be noted that the presence of CC-Ib in an enzyme blend was not solely a characteristic of Liberase HI. Two extensive studies detected a substantial proportion of degraded CC-I in several lots of collagenase NB-1, which became a significant issue during the NIH-sponsored Clinical Islet Transplantation (CIT) trial [19]. In comparison to intact collagenase HA blends from Vitacyte (Indianapolis, Indiana, USA), these collagenase NB-1 lots released lower islet yields from allogeneic and autologous pancreases, and were associated with a lower success rate after transplantation into diabetic nude mice [18, 29]. Remarkably, a suitable and cost-effective substitute for low-effective collagenase NB-1 lots was found in crude Sigma V enzyme blends that passed a three-step filtration process to remove endotoxin, pigments, and other harmful components [44].
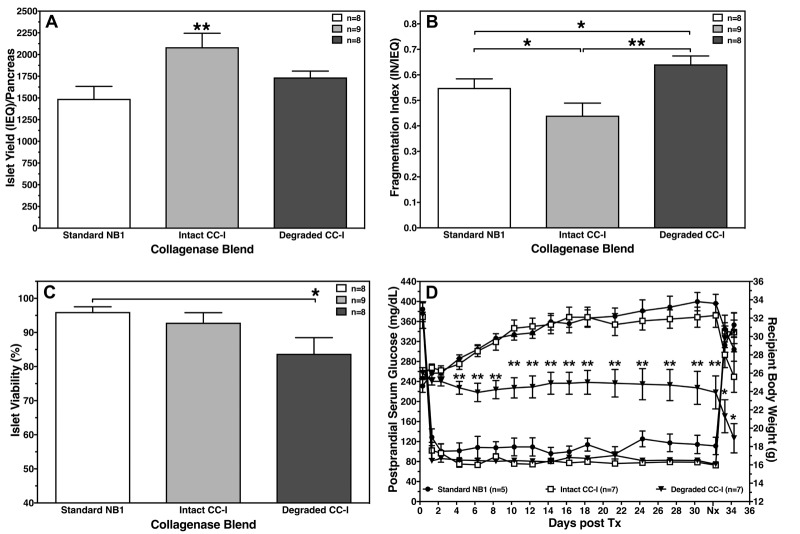
When low-molecular weight CC-I and CC-II isomers were chromatographically separated and combined in an enzyme blend, a detrimental effect on islet viability and post-transplant function was noted. Islet transplantation reversed hyperglycemia in streptozotocin-treated nude mice, but revealed the absence of weight gain when islets were isolated utilizing degraded CC-I (Figure 3) [85]. No data are available to clarify whether this finding was related to an increased internalization of smaller CC-I isomers, as observed with commercially available Liberase HI and collagenase NB-1, followed by local pro-inflammatory and pro-apoptotic processes [86, 87].
Figure 3. Effect of low molecular weight CC-I isomers on islet isolation parameters.
The figure shows the effect of low molecular weight CC-I isomers on islet yield (A), fragmentation index (B), viability (C), and graft function of isolated rat islets transplanted beneath the kidney capsule of diabetic nude mice (D). Islets were isolated utilizing 20 PZ-U of collagenase activity and 0.4 DMC-U of CNP activity. Nephrectomy (Nx) was performed at day 32 post-transplant as indicated. A-C: *p < 0.05, **P < 0.01, as indicated. D: *p < 0.05, **p < 0.01 vs. standard NB-1 and intact CC-I blend.
The loss of intact CC-I does not only have an effect on the CC-II-to-CC-I ratio as an important variable for collagenase efficiency [63, 64], it also determines the amount of neutral proteases required for efficient pancreas dissociation [67]. In agreement with observations by the Groningen group [60], we found that human pancreases are efficiently digested, even in the absence of intact CC-I, as long as sufficient activities of supplementary proteases such as CNP or clostripain are present [61, 62]. While CNP or TL may compensate the reduced activity of CC-I, these enzymes have a detrimental effect on islet viability, as discussed in section 3.
5. Endogenous enzymes
The detrimental effect of trypsin on islet morphological integrity and functional capacity of beta-cells has been described for decades [88]. The activation of intrinsic enzymes in the pancreas has been mainly related to the interruption of oxygen supply during pancreas retrieval, cold storage, and subsequent pancreas digestion. The increased production of lactate by anaerobic glucose breakdown during hypoxia/anoxia causes intracellular acidosis, which is one of the main inducers of premature intracellular auto-activation of trypsinogen and subsequent activation of the enzyme cascade in acinar cells [89]. Since 90% of the proteins synthesized by acinar cells are digestive enzymes, short periods of ischemia provide ‘ideal’ conditions to trigger autolytic processes within the pancreas [90-92]. The relevance of endogenous pancreatic proteases for islet function has also been extensively assessed in ischemia/reperfusion-induced pancreatitis after organ preservation [93-95]. Islet survival during ischemia is aggravated because islets are surrounded from acinar cells that are characterized by a higher density of zymogen granules compared to tele-insular cells. This histological arrangement makes islets particularly vulnerable to proteolytic damage [96, 97].
Several attempts had been undertaken to reduce the content of endogenous enzymes. Pretreatment of rats with pilocarpine induced a maximum discharge of enzyme granules that increased islet yield after isolation several-fold [98, 99]. At least in one study, pilocarpine treatment was applied in large mammals by injecting this compound into the pancreatic duct of pig pancreases prior to enzyme administration via the same route. This protocol confirmed the findings in rats by quadruplicating pig islet yield [100].
Observations in the same species revealed the harmful effect of activated trypsin on islet isolation outcome. When specific trypsin inhibitors such as Pefobloc were added to the intraductally perfused enzyme solution at a concentration of 1-4 mmol/l, the morphological integrity of islets could be preserved during digestion, and higher islet yields were frequently isolated from the pig pancreas [101-104]. The protective effect of Pefabloc was also observed in isolated rat islets cultured in supernatants collected from rat pancreas digestion procedures. When 0.4 mmol/l Pefabloc was added to the digest supernatants, a time-dependent decrease of islet viability and morphological integrity could be prevented. Surprsingly, islet yield was not significantly improved by Pefabloc [105]. Using the same concentration of Pefabloc in human islet isolation neither trypsin inhibition nor increased islet yield was observed. Nevertheless, Pefabloc seems to be effective in inhibiting chymotrypsin and elastase activity [106, 107]. However, no correlation between endogenous enzymes released during human pancreas digestion and subsequent islet yield was found [108], which is in agreement with the observation that digest supernatants do not damage incubated pig and rat islets [109]. Another study from the Edmonton group indicated that inhibition of trypsin by Pefabloc is effective in human pancreases when applied after prolonged cold ischemia [110]. In contrast, when islets were isolated after prolonged cold storage of rat pancreases, a dose-dependent detrimental effect of Pefabloc on islet yield was measured [111]. Whether the discrepancies between different studies can be related to species-specific differences or to the perfusion of human pancreases with organ preservation solutions such as UW-solution remains to be clarified.
Apart from the ischemia-induced activation of the intrinsic enzyme cascade, it has to be discussed whether certain components of collagenase enzyme blends have the capability to activate trypsin. In rat islet isolation, it was shown that TL strongly activates trypsin and chymotrypsin during pancreas digestion. Unexpectedly, CNP had no activating effect on these endogenous proteases [80]. These findings may serve as another proof for the superiority of CNP over TL [18]. Remarkably, when TL and neutral protease were combined with clostripain, a significant synergistic effect on the activation of endogenous proteases was measured [80]. Unfortunately, the study by Dendo et al. did not assess the activation of trypsin and chymotrypsin when clostripain was used without addition of other supplementary proteases. In our own studies, we observed a significant improvement of islet yield and islet function measured in vitro and post-transplant when CNP was substantially reduced for the digestion of long-term stored pig pancreases compared with freshly processed pancreases [112].
Although trypsin seems to be the most important enzyme for subsequent activation of intrinsic pancreatic enzymes [113], other enzymes have to be considered for islet damage as well [114]. In this context, it should be mentioned that elastase was identified as the most harmful endogenous pancreatic enzyme when comparing the toxic potency of lipase, phospholipase A2, trypsin, and chymotrypsin in the rat pancreas [115]. Early experiments in the rat demonstrated that trypsin can be utilized as supplementary protease without affecting islet post-transplant function when the concentration of crude collagenase was simultaneously reduced [116]. This finding raised the question whether the islet-damaging effect of trypsin was overestimated, while neglecting the toxicity of the other components of the enzyme cascade. Nevertheless, recent studies in cultured human islets confirmed the detrimental effects of activated chymotrypsin and elastase on morphological and functional integrity of islets [117].
Regardless of whether intrinsic or supplemented enzymes are the most harmful ones for islet integrity, most of the data available clearly suggest that prolonged pancreas digestion/recirculation time correlates inversely with isolation outcome in terms of yield and viability [105, 108, 118-120]. Although the variables that define the harmfulness of the pancreas digestion milieu have not been fully characterized, it can be assumed that the main determinants of islet quality are hypoxia/anoxia inducing a rapid loss of intraislet ATP [105], the release of pro-apoptotic free fatty acids [121, 122], and the subsequent formation of peroxidation products [123, 124], particularly when lipase and phospholipase A are present in the digest [125]. An effective way to protect islets from damage is to minimize exposure time to enzyme and acinar metabolites and to immediately remove free islets from the digestion device, as demonstrated in pig and human islets [126, 127].
6. Extracellular matrix degradation
An efficient and highly active enzyme blend quickly disperses all exocrine, ductal, and vascular components of the pancreas except the peri-insular basement membrane composed of different ECM proteins [20, 128, 129]. The basement membrane serves as an interface between islets, endothelial cells, and acinar cells via integrins and other cellular receptors [32, 130, 131], forming a protective barrier to preserve the morphological integrity of underlying islets during pancreas digestion [132]. In the human pancreas, collagen-IV and laminin-511/521 have been characterized as the major components of the islet basement membrane. Laminin-511 and collagen-IV networks are connected by nidogen bridging [133]. Collagen-IV is frequently interacting with collagen-VI to stabilize basement membranes in different tissues [134]. In own studies, we identified collagen-VI as the major subtype among several other peri-islet collagen subtypes assessed [135]. This finding is particularly remarkable since collagen-VI is resistant toward the activity of bacterial collagenases [136, 137], suggesting a distinct protective morphological wall during enzyme-mediated pancreas digestion. Moreover, collagen-V, the second most strongly expressed collagen subtype in the human peri-islet basement membrane, can be degraded by CC-I, but not by CC-II [138]. This finding is in clear contradiction to the proposition that CC-II is the most relevant isoform for islet release when compared with CC-I [47, 59]. Even so, previous and recent histological studies have clearly demonstrated that the basement membranes of isolated mouse and human islets are substantially disrupted during islet isolation, implying that supplementary proteases are mainly involved in basement membrane degradation rather than different isoforms of collagenase. The major ECM proteins are completely lost after pancreas digestion, and are not restored during subsequent islet culture, particularly if performed in serum-free culture media [139-141]. However, as nearly all information about function of collagenolytic enzymes has been gathered during in vitro experiments using collagen as an isolated substrate, our understanding about degradation of the pancreatic ECM is only unidimensional [142-144]. Currently, it is assumed that the digestion of ECM proteins within the pancreas is a gradual process that has to be initiated by neutral protease. Exposed native collagen is then cleaved by CC-I, which results in a loss of the triple helical structure of collagen. In the next step, CC-II digests the collagen molecules denatured by CC-I. Finally, collagen fragments are further degraded by neutral protease [21, 45].
Whether this hypothetic scenario gives a realistic picture of collagenase function and that of other collagenolytic enzymes is questionable, because it does not consider the presence and degradation of ECM proteins other than collagen. A large gap exists in the knowledge about the digestion of components such as laminin, perlecan, and nidogen. Detailed studies about the degradation of the human peri-islet basement membrane, which intend to fill this gap, have just started [141]. In accordance with a recent report from the Tohoku group, we found that substantial differences exist between TL, CNP, and TLA to cleave recombinant laminin-511 in vitro. While neither CC-I nor CC-II used alone or in combination could degrade laminin-511, strong degradation was observed after treatment with TL and CNP. Surprisingly, the laminin-511 molecule remained nearly intact when treated with TLA alone [80, 145] (Figure 4).
Figure 4. Degradation of laminin-511 treated with different enzymes.
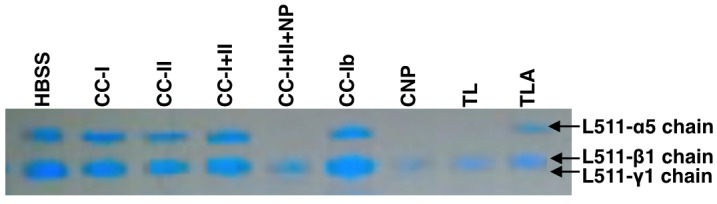
Laminin-511 incubated with Hank's balanced salt solution (HBSS) served as control. The picture is representative for three individual experiments [145].
As the enzymatic degradation of laminin-511 concerns the cleavage of the side chains (particularly the α5-chain), anti-apoptotic signaling between basement membranes and integrins or other cellular receptors is interrupted after islet isolation [32, 131, 139]. As a result, cells in general, and particularly islets detached from the basement membrane, rapidly undergo apoptosis and lose functional capacity after isolation and subsequent culture [146-148]. In contrast, islets that are still embedded in a rim of acinar tissue seem to be characterized by a lower apoptosis rate and improved function in vitro and post-transplant [149-151]. The careful selection of enzymes that save essential components of the peri-islet ECM may be important for the frequent isolation of islets with preserved basement membrane. One potential approach for this purpose may be the combination of clostripain, preserving the integrity of laminin-511, and dispase that acts specifically toward collagen-IV and fibronectin [152].
7. Donor-specific enzyme blending
The specific characteristics of the collagen network within the pancreas of man, dog, pig, and rats, as described in initial histological studies [20, 129], encouraged the development of enzyme blends specifically formulated by Roche for islet isolation from different species [23, 42, 54-56]. The enzyme composition was modified with respect to the ratio between protease and collagenase activity [153] adjusted according to the differentially expressed basement membrane which is fragmentary and weak in the pig, intermediate in the human pancreas, and strongly expressed over the entire perimeter of canine islets [128]. As a consequence of the morphological characteristics this 'protease-to-collagenase ratio' is highest for the canine blend Liberase CI and low for the porcine mixture Liberase PI [153]. Furthermore, Liberase PI was blended by adding dispase, a supplementary protease with a narrower specifity toward different collagen types than TL [28, 54, 76, 152].
While the manufacturing of species-specific enzyme blends may be important for research and pre-clinical studies, the development of enzyme blends, especially formulated according to different human donor variables, has not been realized until today. The non-existence of human donor-specific enzyme blends seems to be related to the lack of knowledge about the interaction between enzyme blends and pancreatic ECM, as discussed above.
Donor variables, which may be of particular interest for specific enzyme blending, are prolonged cold ischemia time, fibrotic pancreatic tissue, body mass index, or donation after cardiac death. However, the consideration of donor age seems to be most relevant for clinical islet isolation. The difficulties of isolating sufficient numbers of islets from younger donor pancreases [119] potentially reduces the chances of being able to successfully reverse type 1 diabetes mellitus by transplanting islets as islets from older donors are characterized by a lower metabolic potency than islets from younger donors [154-156]. Although similar yields of islets can be released from the pancreas of young and older donors, a substantial proportion of islets from young donors are still attached to exocrine tissue and lost during purification [157].
The reason for the incomplete separation of islets from acinar tissue is unknown, but it is evident that the enzyme activity used for older donors is insufficient when applied in younger donors. As a consequence, the Baylor group doubled the enzyme concentration for intraductal administration by reducing the volume of the perfusion solution, and obtained a lower percentage of mantled islets [158], which is in accordance with the linear relationship between collagenase concentration and activity [159, 160]. The Milan group used a similar technique for the isolation from younger donors, injecting 44% of the enzyme solution prior to cold pancreas storage and 56% after arrival of the gland in the isolation facility. The standard treatment group received only the second enzyme bolus [161]. For the isolation of islets from pediatric donors, Balamurugan et al. increased the exposure time to the enzyme blend, and reduced substantially the mechanical dissociation of the pancreas at the same time. In case of substantial exocrine embedding, a second incubation with enzymes was performed [162]. Another interesting approach was published by the Montreal group. When pancreases from donors ≤25 years were digested by means of Liberase CI, a significantly higher islet yield was isolated than with Liberase HI. The main difference between the enzyme blends was a collagenase activity reduced by 50%, while TL activity was increased by 30% in Liberase CI [163]. This observation demonstrates that a suitable formulation of collagenase and supplementary proteases can facilitate islet isolation even from difficult donor tissues.
Nevertheless, the successful application of higher enzyme concentrations or longer incubation times appear to be in clear contradiction to biochemical findings in human pancreases, demonstrating a significant increase of total collagen content with age [36]. Another histo-morphological change, which can be generally observed in different tissues of healthy humans, concerns a continuous and substantial increase in the basement membrane thickness during ageing [164-166]. Whether the increased thickness of the peri-islet basement membrane is associated with an altered composition and structure is unknown, but initial comparisons of young and older pancreas donors did not reveal a significant difference regarding the proportional collagen-VI content [135]. Nevertheless, findings in other tissues found an age-dependent change in the basement membrane content of collagen-IV and laminin [165, 166]. The age-related alterations in the ECM are multifactorial and not completely understood. In principle, ageing is associated with an imbalance between regenerative capacity and oxidative stress caused by the increased release of reactive oxygen species [167]. A strong oxidation of susceptible amino acid residues within the ECM proteins can result in a cleavage of peptide bonds, and an instability and fragmentation of the ECM proteins [168, 169]. These structural changes may increase the accessibility of collagenase and supplementary proteases to ECM proteins, thereby contributing to the higher isolability of human islets from older donors.
8. Future perspectives
Although significant progress has been made in the characterization and production of enzyme blends for islet isolation [23-29], numerous opportunities for improvement still exist in this field. The final goal of enzyme production, the long-term production of a consistent and standardized enzyme blend, has not been achieved to date. The most efficient way to control the production of Clostridium histolyticum collagenase and different neutral proteases is by the cloning and overexpression of individual proteins in suitable micro-organisms [170]. The principal functionality of an enzyme blend, containing recombinant collagenase for human islet isolation, has been demonstrated in an initial pilot study more than a decade ago [171]. Unfortunately, the optimization of this preliminary product was not pursued due to marketing reasons. Nevertheless, the production of recombinant enzyme blends has been recently revived by Vitacyte. Remarkably, the Minneapolis group found that low concentrations of recombinant collagenase are required to isolate human islets successfully, even if varying proportions of CC-I and CC-II are administered [66].
Ideally, the high standardization and stability of recombinant enzymes should be combined with a maximum of flexibility. The temporary availability of a triple component product, individually providing natural CC-I, CC-II, and TL, demonstrated the potential of a modular enzyme product individually blended according to the scope of application [58, 64]. A recombinant modular enzyme product offers ideal options to create enzyme mixtures specifically formulated according to different donor variables, as discussed above. It is obvious that the efficient composition of three or even four different enzymes according to certain donor variables requires either an enormous amount of experiences generated during numerous and costly 'trial and error' experiments or detailed instructions based on structured research about the human pancreatic ECM.
Even if these optimized enzyme products become available, the environment generated during pancreas digestion will still be defined by an abundance of toxic metabolites such as pro-apoptotic free fatty acids [121, 122], peroxidation products [123, 124], and anoxia [172, 173]. As the absence of oxygen is a significant contributor to islet cell death, pancreas digestion can be regarded as severe warm ischemia. Studies in rats and in the sensitive pig model clearly revealed that yield and viability of islets isolated from ischemically pre-damaged pancreases is significantly improved when the gland is digested at 20ºC and 30ºC, respectively [174, 175]. According to the Q10 temperature coefficient [176], the metabolic activity of islet cells at 30ºC and 20ºC is approximately 50% and 25%, respectively, when compared with normothermia at 37ºC [177, 178]. Because hypothermia mainly affects the preferred mitochondrial pathways of glucose breakdown, the demand for oxygen is also strongly decreased, and can slow-down ischemically induced damage of hypoxic islets [179].
The collagenase activity at 30% and 20ºC is decreased by approximately 40-50% and 65-75%, respectively [174, 180, 181]. The reduced collagenase activity may be compensated by doubling and quadruplicating the amount of enzymes normally used at 37ºC digestion temperature. A less expensive and more rationale mean of pancreas digestion at 20ºC or even lower temperatures may be the application of collagenase from marine species living in a hypothermic environment. The maximum collagenase activity extracted from muscle tissue of several fish species such as rainbow trout [182] or different cod species [183] was measured at a temperature of 20ºC. Another attractive enzyme for low temperature digestion is represented by a collagenolytic protease isolated from the marine bacterium Vibrio vulnificus. In comparison with the enzyme activity at 37ºC, the remaining protease activity at 10ºC reaches still 70% and offers the possibility to perform pancreas digestion at temperatures normally used for cold organ storage [184].
Acknowledgments
Disclosures
The authors declare no conflict of interests.
References
- 1.Patry J, Blanchette V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int Wound J. 2017 doi: 10.1111/iwj.12760. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang KK, Bennett N. The History of Collagenase Clostridium Histolyticum. Sex Med Rev. 2015;3(4):289–297. doi: 10.1002/smrj.54. [DOI] [PubMed] [Google Scholar]
- 3.Fischer S, Hirche C, Diehm Y, Nuutila K, Kiefer J, Gazyakan E, Bueno EM, Kremer T, Kneser U, Pomahac B. Efficacy and safety of the collagenase of the bacterium Clostridium histolyticum for the treatment of capsular contracture after silicone implants: ex-vivo study on human tissue. Plos One. 2016;11(5):e0156428. doi: 10.1371/journal.pone.0156428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syed F, Thomas AN, Singh S, Kolluru V, Emeigh Hart SG, Bayat A. In vitro study of novel collagenase (XIAFLEX(R)) on Dupuytren's disease fibroblasts displays unique drug related properties. Plos One. 2012;7(2):e31430. doi: 10.1371/journal.pone.0031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degreef I. Collagenase Treatment in Dupuytren Contractures: A Review of the Current State Versus Future Needs. Rheumatol Ther. 2016;3(1):43–51. doi: 10.1007/s40744-016-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal GK, Suresh PV. Microbial collagenases: challenges and prospects in production and potential applications in food and nutrition. RCSC Adv. 2016;6:33763–33780. [Google Scholar]
- 7.Alipour H, Raz A, Zakeri S, Djadid ND. Therapeutic applications of collagenase (metalloproteases): a review. Acta Pac J Trop Biomed. 2016;6(11):975–981. [Google Scholar]
- 8.Duarte AS, Correia A, Esteves AC. Bacterial collagenases - A review. Crit Rev Microbiol. 2016;42(1):106–126. doi: 10.3109/1040841X.2014.904270. [DOI] [PubMed] [Google Scholar]
- 9.Vedicherla S, Buckley CT. Rapid Chondrocyte Isolation for Tissue Engineering Applications: The Effect of Enzyme Concentration and Temporal Exposure on the Matrix Forming Capacity of Nasal Derived Chondrocytes. Biomed Res Int. 2017;2017:2395138. doi: 10.1155/2017/2395138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He X, de Oliveira VL, Keijsers R, Joosten I, Koenen HJ. Lymphocyte Isolation from Human Skin for Phenotypic Analysis and Ex Vivo Cell Culture. J Vis Exp. 2016;2016(110):e52564. doi: 10.3791/52564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vremec D. The Isolation and Enrichment of Large Numbers of Highly Purified Mouse Spleen Dendritic Cell Populations and Their In Vitro Equivalents. Methods Mol Biol. 2016;1423:61–87. doi: 10.1007/978-1-4939-3606-9_5. [DOI] [PubMed] [Google Scholar]
- 12.Knapinska AM, Amar S, He Z, Matosevic S, Zylberberg C, Fields GB. Matrix metalloproteinases as reagents for cell isolation. Enzyme Microb Technol. 2016;93-94:29–43. doi: 10.1016/j.enzmictec.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sincennes MC, Wang YX, Rudnicki MA. Primary Mouse Myoblast Purification using Magnetic Cell Separation. Methods Mol Biol. 2017;1556:41–50. doi: 10.1007/978-1-4939-6771-1_3. [DOI] [PubMed] [Google Scholar]
- 14.Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272(3):201–223. doi: 10.1111/j.1365-2796.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibars EP, Cortes M, Tolosa L, Gomez-Lechon MJ, Lopez S, Castell JV, Mir J. Hepatocyte transplantation program: Lessons learned and future strategies. World J Gastroenterol. 2016;22(2):874–886. doi: 10.3748/wjg.v22.i2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy RC. Purified tissue dissociation enzymes for human hepatocyte isolation. 2009. Available at: http://www.vitacyte.com. [DOI] [PubMed]
- 17.Brandhorst D, Brandhorst H, Hering BJ, Federlin K, Bretzel RG. Islet isolation from the pancreas of large mammals and humans: 10 years of experience. Exp Clin Endocrinol Diabetes. 1995;103:3–14. doi: 10.1055/s-0029-1211386. [DOI] [PubMed] [Google Scholar]
- 18.Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, Soltani SM, Radosevich DM, Yuasa T, Tiwari M. et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J. et al. National Institutes of Health - Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes. 2016;65(11):3418–3428. doi: 10.2337/db16-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Deijnen JH, Van Suylichem PT, Wolters GH, Van Schilfgaarde R. Distribution of collagens type I, type III and type V in the pancreas of rat, dog, pig and man. Cell Tissue Res. 1994;277(1):115–121. doi: 10.1007/BF00303087. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PR, White SA, London NJ. Collagenase and human islet isolation. Cell Transplant. 1996;5(4):437–452. doi: 10.1177/096368979600500403. [DOI] [PubMed] [Google Scholar]
- 22.Moskalewski S. Isolation and Culture of the Islets of Langerhans of the Guinea Pig. Gen Comp Endocrinol. 1965;44:342–353. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- 23.Fetterhoff TJ, Cavanagh TJ, Wile KJ, Wright MJ, Dwulet FE, Gill J, Ellis B, Smith ME, Critser JK, Zieger M. et al. Human pancreatic dissociation using a purified enzyme blend. Transplant Proc. 1995;27(6):3282–3283. [PubMed] [Google Scholar]
- 24.Gill JF, Chambers LL, Baurley JL, Ellis BB, Cavanaugh TJ, Fetterhoff TJ, Dwulet FE. Safety testing of Liberase, a purified enzyme blend for human islet isolation. Transplant Proc. 1995;27(6):3276–3277. [PubMed] [Google Scholar]
- 25.Barnett MJ, Zhai X, LeGatt DF, Cheng SB, Shapiro AM, Lakey JR. Quantitative assessment of collagenase blends for human islet isolation. Transplantation. 2005;80(6):723–728. doi: 10.1097/01.tp.0000174133.96802.de. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, Dwulet FE. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplant Proc. 2008;40(2):339–342. doi: 10.1016/j.transproceed.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy RC. What is the appropriate assay to assess collagenase prior to use in cell isolation procedures? 2009. Available at: http://www.vitacyte.com.
- 28.Breite AG, Dwulet FE, McCarthy RC. Tissue dissociation enzyme neutral protease assessment. Transplant Proc. 2010;42(6):2052–2054. doi: 10.1016/j.transproceed.2010.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, Dwulet FE, McCarthy RC, Hering BJ. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89(8):954–961. doi: 10.1097/TP.0b013e3181d21e9a. [DOI] [PubMed] [Google Scholar]
- 30.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120(Pt 12):1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 31.Durbeej M. Laminins. Cell Tissue Res. 2010;339(1):259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 32.Kragl M, Lammert E. Basement membrane in pancreatic islet function. Adv Exp Med Biol. 2010;654:217–234. doi: 10.1007/978-90-481-3271-3_10. [DOI] [PubMed] [Google Scholar]
- 33.Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng Part B Rev. 2011;17(4):235–247. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 34.Johnsson C, Hallgren R, Tufveson G. Role of hyaluronan in acute pancreatitis. Surgery. 2000;127(6):650–658. doi: 10.1067/msy.2000.106587. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy RH, Bockman DE, Uscanga L, Choux R, Grimaud JA, Sarles H. Pancreatic extracellular matrix alterations in chronic pancreatitis. Pancreas. 1987;2(1):61–72. doi: 10.1097/00006676-198701000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Bedossa P, Lemaigre G, Bacci J, Martin E. Quantitative estimation of the collagen content in normal and pathologic pancreas tissue. Digestion. 1989;44(1):7–13. doi: 10.1159/000199886. [DOI] [PubMed] [Google Scholar]
- 37.Cavanagh TJ, Fetterhoff TJ, Lonergan SC, Dwulet FE, Gill JF, McCarthy RC. In: Lanza RP, Chick WL (eds.) Landes Co.; 1994. Collagenase selection; pp. 39–51. [Google Scholar]
- 38.Vargas F, Vives-Pi M, Somoza N, Armengol P, Alcalde L, Marti M, Costa M, Serradell L, Dominguez O, Fernandez-Llamazares J. et al. Endotoxin contamination may be responsible for the unexplained failure of human pancreatic islet transplantation. Transplantation. 1998;65(5):722–727. doi: 10.1097/00007890-199803150-00020. [DOI] [PubMed] [Google Scholar]
- 39.Berney T, Molano RD, Cattan P, Pileggi A, Vizzardelli C, Oliver R, Ricordi C, Inverardi L. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation. 2001;71(1):125–132. doi: 10.1097/00007890-200101150-00020. [DOI] [PubMed] [Google Scholar]
- 40.Atkins T, Matty AJ. Metabolic viability of freehand microdissected and collagenase-isolated islets of Langerhans. J Endocrinol. 1970;46(2):17–18. [PubMed] [Google Scholar]
- 41.Breite A. Revealing the collagenase characteristics in crude collagenase mixtures. 2009. Available at: http://www.vitacyte.com.
- 42.Lakey JR, Cavanagh TJ, Zieger MA, Wright M. Evaluation of a purified enzyme blend for the recovery and function of canine pancreatic islets. Cell Transplant. 1998;7(4):365–372. doi: 10.1177/096368979800700404. [DOI] [PubMed] [Google Scholar]
- 43.Toledo-Pereyra LH, Zammit M, Malcom S, Cromwell P. Inconsistency of collagenase activity for isolation of islet cells for transplantation. Transplantation. 1979;27(3):222. doi: 10.1097/00007890-197903000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Paushter D, Wang S, Barbaro B, Harvat T, Danielson K, Kinzer K, Zhang L, Qi M, Oberholzer J. Highly Purified versus Filtered Crude Collagenase: Comparable Human Islet Isolation Outcomes. Cell Transplantation. 2011;20(11-12):1817–1825. doi: 10.3727/096368911X564994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy RC, Breite AG, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: factors to consider in setting enzyme acceptance criteria. Transplantation. 2011;91(2):137–145. doi: 10.1097/TP.0b013e3181ffff7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolters GH, Vos-Scheperkeuter GH, van Deijnen JH, van Schilfgaarde R. An analysis of the role of collagenase and protease in the enzymatic dissociation of the rat pancreas for islet isolation. Diabetologia. 1992;35(8):735–742. doi: 10.1007/BF00429093. [DOI] [PubMed] [Google Scholar]
- 47.Wolters GH, Vos-Scheperkeuter GH, Lin HC, van Schilfgaarde R. Different roles of class I and class II Clostridium histolyticum collagenase in rat pancreatic islet isolation. Diabetes. 1995;44(2):227–233. doi: 10.2337/diab.44.2.227. [DOI] [PubMed] [Google Scholar]
- 48.Dwulet FE, Ellis BE, Gill JF, Jacobsen LB, Smith ME, Waters DG, inventors. A purified mixture of collagenase I, collagenase II and two other proteases. 09/042295. Patent Application. 1998
- 49.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46(7):1120–1123. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- 50.Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of langerhans using Liberase enzyme blend. Hum Immunol. 1999;60(12):1303–1309. doi: 10.1016/s0198-8859(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 51.Brendel MD, Hering BJ, Schultz AO, Bretzel RG. Newsletter No. 9. International Islet Transplant Registry. 2001;8(1):1–20. [Google Scholar]
- 52.Bucher P, Mathe Z, Morel P, Bosco D, Andres A, Kurfuest M, Friedrich O, Raemsch-Guenther N, Buhler LH, Berney T. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation. 2005;79(1):91–97. doi: 10.1097/01.tp.0000147344.73915.c8. [DOI] [PubMed] [Google Scholar]
- 53.Caballero-Corbalan J, Friberg AS, Brandhorst H, Nilsson B, Korsgren O, Brandhorst D, Andersson HH, Felldin M, Foss A, Salmela K. et al. Vitacyte collagenase HA: a novel enzyme blend for efficient human islet isolation. Transplantation. 2009;88(12):1400–1402. doi: 10.1097/TP.0b013e3181bd1441. [DOI] [PubMed] [Google Scholar]
- 54.Cavanagh TJ, Lakey JR, Dwulet F, Wright MJ, Wile K, Albertson T, Fetterhoff T. Improved pig islet yield and post-culture recovery using Liberase PI purified enzyme blend. Transplant Proc. 1998;30(2):367. doi: 10.1016/s0041-1345(97)01311-0. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Gunther L, Drognitz O, Neeff H, Adam U, Hopt UT. Persistent normoglycemia in the streptozotocin-diabetic rat by syngenic transplantation of islets isolated from a single donor with Liberase. Pancreas. 2006;32(1):9–15. doi: 10.1097/01.mpa.0000191647.40044.58. [DOI] [PubMed] [Google Scholar]
- 56.Yesil P, Michel M, Chwalek K, Pedack S, Jany C, Ludwig B, Bornstein SR, Lammert E. A new collagenase blend increases the number of islets isolated from mouse pancreas. Islets. 2009;1(3):185–190. doi: 10.4161/isl.1.3.9556. [DOI] [PubMed] [Google Scholar]
- 57.Kono T. Roles of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta. 1969;178(2):397–400. doi: 10.1016/0005-2744(69)90410-0. [DOI] [PubMed] [Google Scholar]
- 58.O'Gorman D, Kin T, McGhee-Wilson D, Shapiro AM, Lakey JR. Multi-lot analysis of custom collagenase enzyme blend in human islet isolations. Transplant Proc. 2005;37(8):3417–3419. doi: 10.1016/j.transproceed.2005.09.139. [DOI] [PubMed] [Google Scholar]
- 59.Fujio A, Murayama K, Yamagata Y, Watanabe K, Imura T, Inagaki A, Ohbayashi N, Shima H, Sekiguchi S, Fujimori K. et al. Collagenase H is crucial for isolation of rat pancreatic islets. Cell Transplant. 2014;23(10):1187–1198. doi: 10.3727/096368913X668654. [DOI] [PubMed] [Google Scholar]
- 60.Vos-Scheperkeuter GH, van Suylichem PT, Vonk MW, Wolters GH, van Schilfgaarde R. Histochemical analysis of the role of class I and class II Clostridium histolyticum collagenase in the degradation of rat pancreatic extracellular matrix for islet isolation. Cell Transplant. 1997;6(4):403–412. doi: 10.1177/096368979700600407. [DOI] [PubMed] [Google Scholar]
- 61.Brandhorst H, Asif S, Andersson K, Monch J, Friedrich O, Ramsch-Gunther N, Ramsch C, Steffens M, Lambrecht J, Schrader T. et al. The effect of truncated collagenase class I isomers on human islet isolation outcome. Transplantation. 2010;90(3):334–335. doi: 10.1097/TP.0b013e3181e49bd7. [DOI] [PubMed] [Google Scholar]
- 62.Brandhorst H, Kurfurst M, Johnson PR, Korsgren O, Brandhorst D. Comparison of neutral proteases and collagenase class I as essential enzymes for human islet isolation. Transplant Direct. 2016;2(1):e47. doi: 10.1097/TXD.0000000000000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandhorst H, Raemsch-Guenther N, Raemsch C, Friedrich O, Huettler S, Kurfuerst M, Korsgren O, Brandhorst D. The ratio between collagenase class I and class II influences the efficient islet release from the rat pancreas. Transplantation. 2008;85(3):456–461. doi: 10.1097/TP.0b013e31816050c8. [DOI] [PubMed] [Google Scholar]
- 64.Kin T, Zhai X, O'Gorman D, Shapiro AM. Detrimental effect of excessive collagenase class II on human islet isolation outcome. Transplant Int. 2008;21(11):1059–1065. doi: 10.1111/j.1432-2277.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 65.Gramignoli R, Green ML, Tahan V, Dorko K, Skvorak KJ, Marongiu F, Zao W, Venkataramanan R, Ellis EC, Geller D. et al. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplant. 2012;21(6):1245–1260. doi: 10.3727/096368911X600939. [DOI] [PubMed] [Google Scholar]
- 66.Balamurugan AN, Green ML, Breite AG, Loganathan G, Wilhelm JJ, Tweed B, Vargova L, Lockridge A, Kuriti M, Hughes MG. et al. Identifying effective enzyme activity targets for recombinant class I and class II collagenase for successful human islet isolation. Transplant Direct. 2016;2(1):e54. doi: 10.1097/TXD.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandhorst H, Alt A, Huettler S, Raemsch-Guenther N, Kurfuerst M, Bretzel RG, Brandhorst D. The ratio between class II and class I collagenase determines the amount of neutral protease activity required for efficient islet release from the rat pancreas. Transplant Proc. 2005;37(1):215–216. doi: 10.1016/j.transproceed.2004.12.256. [DOI] [PubMed] [Google Scholar]
- 68.Bucher P, Bosco D, Mathe Z, Matthey-Doret D, Andres A, Kurfuerst M, Ramsch-Gunther N, Buhler L, Morel P, Berney T. Optimization of neutral protease to collagenase activity ratio for islet of Langerhans isolation. Transplant Proc. 2004;36(4):1145–1146. doi: 10.1016/j.transproceed.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 69.Brandhorst H, Brendel MD, Eckhard M, Bretzel RG, Brandhorst D. Influence of neutral protease activity on human islet isolation outcome. Transplant Proc. 2005;37(1):241–242. doi: 10.1016/j.transproceed.2004.12.254. [DOI] [PubMed] [Google Scholar]
- 70.Kin T, O'Gorman D, Senior P, Shapiro AM. Experience of islet isolation without neutral protease supplementation. Islets. 2010;2(5):278–282. doi: 10.4161/isl.2.5.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caballero-Corbalan J, Brandhorst H, Asif S, Korsgren O, Engelse M, de Koning E, Pattou F, Kerr-Conte J, Brandhorst D. Mammalian tissue-free Liberase: a new GMP-graded enzyme blend for human islet isolation. Transplantation. 2010;90(3):332–333. doi: 10.1097/TP.0b013e3181e117e3. [DOI] [PubMed] [Google Scholar]
- 72.Morini S, Braun M, Onori P, Cicalese L, Elias G, Gaudio E, Rastellini C. Morphological changes of isolated rat pancreatic islets: a structural, ultrastructural and morphometric study. J Anat. 2006;209(3):381–392. doi: 10.1111/j.1469-7580.2006.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Gorman D, Kin T, Imes S, Pawlick R, Senior P, Shapiro AM. Comparison of Human Islet Isolation Outcomes Using a New Mammalian Tissue-Free Enzyme Versus Collagenase NB-1. Transplantation. 2010;90(3):255–259. doi: 10.1097/TP.0b013e3181e117ce. [DOI] [PubMed] [Google Scholar]
- 74.Shimoda M, Noguchi H, Naziruddin B, Fujita Y, Chujo D, Takita M, Peng H, Tamura Y, Olsen GS, Sugimoto K. et al. Assessment of human islet isolation with four different collagenases. Transplant Proc. 2010;42(6):2049–2051. doi: 10.1016/j.transproceed.2010.05.093. [DOI] [PubMed] [Google Scholar]
- 75.Brandhorst H, Friberg A, Nilsson B, Andersson HH, Felldin M, Foss A, Salmela K, Tibell A, Tufveson G, Korsgren O, Brandhorst D. Large-scale comparison of Liberase HI and collagenase NB1 utilized for human islet isolation. Cell Transplant. 2010;19(1):3–8. doi: 10.3727/096368909X477507. [DOI] [PubMed] [Google Scholar]
- 76.Josefsen K, Stenvang JP, Kindmark H, Berggren PO, Horn T, Kjaer T, Buschard K. Fluorescence-activated cell sorted rat islet cells and studies of the insulin secretory process. J Endocrinol. 1996;149(1):145–154. doi: 10.1677/joe.0.1490145. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy RC. rypsin-like activity (TLA) in purified tissue dissociation enzymes: what is it? What impact does it have for achieving consisting purified tissue dissociation enzyme products for human islet isolation? 2009. Available at: http://www.vitacyte.com.
- 78.Klock G, Kowalski MB, Hering BJ, Eiden ME, Weidemann A, Langer S, Zimmermann U, Federlin K, Bretzel RG. Fractions from commercial collagenase preparations: use in enzymic isolation of the islets of Langerhans from porcine pancreas. Cell Transplant. 1996;5(5):543–551. doi: 10.1177/096368979600500504. [DOI] [PubMed] [Google Scholar]
- 79.Brandhorst H, Friberg A, Andersson HH, Felldin M, Foss A, Salmela K, Lundgren T, Tibell A, Tufveson G, Korsgren O, Brandhorst D. The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation. 2009;87(3):370–375. doi: 10.1097/TP.0b013e31819499f0. [DOI] [PubMed] [Google Scholar]
- 80.Dendo M, Maeda H, Yamagata Y, Murayama K, Watanabe K, Imura T, Inagaki A, Igarashi Y, Katoh Y, Ebina M. et al. Synergistic Effect of Neutral Protease and Clostripain on Rat Pancreatic Islet Isolation. Transplantation. 2015;99(7):1349–1355. doi: 10.1097/TP.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 81.Stahle M, Foss A, Gustafsson B, Lempinen M, Lundgren T, Rafael E, Tufveson G, Korsgren O, Friberg A. Clostripain, the Missing Link in the Enzyme Blend for Efficient Human Islet Isolation. Transplant Direct. 2015;1(5):e19. doi: 10.1097/TXD.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Bertuzzi F, Garancini P, Socci TC, Nano R, Taglietti MV, Santopinto M, Di Carlo V, Davalli AM. Lessons from in vitro perifusion of pancreatic islets isolated from 80 human pancreases. Cell Transplant. 1999;8(6):709–712. doi: 10.1177/096368979900800616. [DOI] [PubMed] [Google Scholar]
- 84.Antonioli B, Fermo I, Cainarca S, Marzorati S, Nano R, Baldissera M, Bachi A, Paroni R, Ricordi C, Bertuzzi F. Characterization of collagenase blend enzymes for human islet transplantation. Transplantation. 2007;84(12):1568–1575. doi: 10.1097/01.tp.0000295719.88525.60. [DOI] [PubMed] [Google Scholar]
- 85.Brandhorst H, Raemsch-Guenther N, Raemsch C, Friedrich O, Kurfuerst M, Korsgren O, Brandhorst D. Degraded collagenase deteriorates islet viability. Transplant Proc. 2008;40(2):370–371. doi: 10.1016/j.transproceed.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Balamurugan AN, He J, Guo F, Stolz DB, Bertera S, Geng X, Ge X, Trucco M, Bottino R. Harmful delayed effects of exogenous isolation enzymes on isolated human islets: relevance to clinical transplantation. Am J Transplant. 2005;5(11):2671–2681. doi: 10.1111/j.1600-6143.2005.01078.x. [DOI] [PubMed] [Google Scholar]
- 87.Cross SE, Hughes SJ, Partridge CJ, Clark A, Gray DW, Johnson PR. Collagenase penetrates human pancreatic islets following standard intraductal administration. Transplantation. 2008;86(7):907–911. doi: 10.1097/TP.0b013e318186df87. [DOI] [PubMed] [Google Scholar]
- 88.Krause U, Puchinger H, Wacker A. Inhibition of glucose-induced insulin secretion in trypsin-treated islets of Langerhans. Horm Metab Res. 1973;5(5):325–329. doi: 10.1055/s-0028-1093936. [DOI] [PubMed] [Google Scholar]
- 89.Gorelick FS, Otani T. Mechanisms of intracellular zymogen activation. Baillieres Best Pract Res Clin Gastroenterol. 1999;13(2):227–240. doi: 10.1053/bega.1999.0021. [DOI] [PubMed] [Google Scholar]
- 90.Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13(2):213–225. doi: 10.1053/bega.1999.0020. [DOI] [PubMed] [Google Scholar]
- 91.Piton G, Barbot O, Manzon C, Moronval F, Patry C, Navellou JC, Belle E, Capellier G. Acute ischemic pancreatitis following cardiac arrest: a case report. JOP. 2010;11(5):456–459. [PubMed] [Google Scholar]
- 92.Wu D, Xu Y, Zeng Y, Wang X. Endocrine pancreatic function changes after acute pancreatitis. Pancreas. 2011;40(7):1006–1011. doi: 10.1097/MPA.0b013e31821fde3f. [DOI] [PubMed] [Google Scholar]
- 93.Wahlberg J, Southard JH, Belzer FO. Preservation-induced pancreatitis in an isolated perfused pancreas model in the dog. Transpl Int. 1989;2(3):165–167. doi: 10.1007/BF02414603. [DOI] [PubMed] [Google Scholar]
- 94.Jonsson P, Kallen R, Montgomery A, Borgstrom A. Protease activation in the porcine pancreatic allograft during preservation. Pancreas. 1995;11(3):256–260. doi: 10.1097/00006676-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Dembinski M, Pabianczyk M, Stachura J, Konturek SJ. Ischemic preconditioning reduces the severity of ischemia/reperfusion-induced pancreatitis. Eur J Pharmacol. 2003;473(2-3):207–216. doi: 10.1016/s0014-2999(03)01994-0. [DOI] [PubMed] [Google Scholar]
- 96.Trimble ER. In: Lanza R P, Chick W L. Pancreatic islet transplantation. 1994. Pancreatic islet-acinar relationships; pp. 19–25. [Google Scholar]
- 97.Aughsteen AA, Kataoka K. Immunohistochemical localization of amylase in peri- and tele-insular acinar cells of the human exocrine pancreas. Hiroshima J Med Sci. 1994;43(4):115–118. [PubMed] [Google Scholar]
- 98.Kuo WN, Hodgins DS, Kuo JF. Adenylate cyclase in islets of Langerhans. Isolation of islets and regulation of adenylate cyclase activity by various hormones and agents. J Biol Chem. 1973;248(8):2705–2711. [PubMed] [Google Scholar]
- 99.Vrbova H, Theodorou NA, Tyhurst M, Howell SL. Transplantation of islets of Langerhans from pilocarpine-pretreated rats: a method of enhancing islet yield. Transplantation. 1979;28(5):433–435. doi: 10.1097/00007890-197911000-00020. [DOI] [PubMed] [Google Scholar]
- 100.Arendarczyk W, Lukaszuk K, Wojcikowski C. Influence of pilocarpine on the efficiency isolation of Langerhans islets from porcine pancreas. Horm Metab Res. 1996;28(1):57. [Google Scholar]
- 101.Heiser A, Ulrichs K, Muller-Ruchholtz W. Prophylactic trypsin inhibition during the isolation procedure guarantees reproducible, high porcine islet yields. Xenotransplantation. 1994;1:66–68. doi: 10.1002/jcla.1860080611. [DOI] [PubMed] [Google Scholar]
- 102.Basir I, van der Burg MP, Scheringa M, Tons A, Bouwman E. Improved outcome of pig islet isolation by Pefabloc inhibition of trypsin. Transplant Proc. 1997;29(4):1939–1941. doi: 10.1016/s0041-1345(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 103.Bai RX, Fujimori K, Koja S, Sekiguchi S, Doi H, Tsukamoto S, Satake M, Ohkohchi N, Satomi S. Effect of prophylactic administration of trypsin inhibitors in porcine pancreas islet isolation. Transplant Proc. 1998;30(2):349–352. doi: 10.1016/s0041-1345(97)01300-6. [DOI] [PubMed] [Google Scholar]
- 104.Shimoda M, Noguchi H, Fujita Y, Takita M, Ikemoto T, Chujo D, Naziruddin B, Levy MF, Kobayashi N, Grayburn PA, Matsumoto S. Improvement of porcine islet isolation by inhibition of trypsin activity during pancreas preservation and digestion using alpha1-antitrypsin. Cell Transplant. 2012;21(2-3):465–471. doi: 10.3727/096368911X605376. [DOI] [PubMed] [Google Scholar]
- 105.Tsukada M, Saito T, Ise K, Kenjo A, Kimura T, Satoh Y, Saito T, Anazawa T, Oshibe I, Suzuki S. et al. A model to evaluate toxic factors influencing islets during collagenase digestion: the role of serine protease inhibitor in the protection of islets. Cell Transplant. 2012;21(2-3):473–482. doi: 10.3727/096368911X605385. [DOI] [PubMed] [Google Scholar]
- 106.Rose NL, Palcic MM, Helms LM, Lakey JR. Evaluation of Pefabloc as a serine protease inhibitor during human-islet isolation. Transplantation. 2003;75(4):462–466. doi: 10.1097/01.TP.0000046537.47139.CE. [DOI] [PubMed] [Google Scholar]
- 107.Rose NL, Palcic MM, Lakey JR. Evaluating the effect of serine proteases on collagenase activity during human islet isolation. Cell Transplant. 2002;11(8):821–826. [PubMed] [Google Scholar]
- 108.Rose NL, Palcic MM, Shapiro AM, Lakey JR. Endogenous pancreatic enzyme activity levels show no significant effect on human islet isolation yield. Cell Transplant. 2004;13(2):153–160. [PubMed] [Google Scholar]
- 109.van Suylichem PT, de Vos P, de Haan BJ, Vonk MW, van Schilfgaarde R. Similar effect of enzymes on pig and rat islets. Transplant Proc. 1998;30(2):358. doi: 10.1016/s0041-1345(97)01305-5. [DOI] [PubMed] [Google Scholar]
- 110.Lakey JR, Helms LM, Kin T, Korbutt GS, Rajotte RV, Shapiro AM, Warnock GL. Serine-protease inhibition during islet isolation increases islet yield from human pancreases with prolonged ischemia. Transplantation. 2001;72(4):565–570. doi: 10.1097/00007890-200108270-00003. [DOI] [PubMed] [Google Scholar]
- 111.Lu WT, Lakey JR, Juang JH, Hsu BR, Rajotte RV. Effect of pefabloc on islet isolation from cold preserved rat pancreas. Transplant Proc. 2002;34(7):2700–2701. doi: 10.1016/s0041-1345(02)03381-x. [DOI] [PubMed] [Google Scholar]
- 112.Brandhorst D, Iken M, Tanioka Y, Brendel MD, Bretzel RG, Brandhorst H. Influence of collagenase loading on long-term preservation of pig pancreas by the two-layer method for subsequent islet isolation. Transplantation. 2005;79(1):38–43. doi: 10.1097/01.tp.0000146550.55596.48. [DOI] [PubMed] [Google Scholar]
- 113.Raraty MG, Petersen OH, Sutton R, Neoptolemos JP. Intracellular free ionized calcium in the pathogenesis of acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13(2):241–251. doi: 10.1053/bega.1999.0022. [DOI] [PubMed] [Google Scholar]
- 114.White SA, Djaballah H, Hughes DP, Roberts DL, Contractor HH, Pathak S, London NJ. A preliminary study of the activation of endogenous pancreatic exocrine enzymes during automated porcine islet isolation. Cell Transplant. 1999;8(3):265–276. doi: 10.1177/096368979900800307. [DOI] [PubMed] [Google Scholar]
- 115.Niederau C, Fronhoffs K, Klonowski H, Schulz HU. Active pancreatic digestive enzymes show striking differences in their potential to damage isolated rat pancreatic acinar cells. J Lab Clin Med. 1995;125(2):265–275. [PubMed] [Google Scholar]
- 116.Henriksson C, Bergmark J, Claes G. Use of trypsin for isolation of islets of Langerhans in the rat. Eur Surg Res. 1977;9(6):427–431. doi: 10.1159/000127964. [DOI] [PubMed] [Google Scholar]
- 117.Loganathan G, Dawra RK, Pugazhenthi S, Guo Z, Soltani SM, Wiseman A, Sanders MA, Papas KK, Velayutham K, Saluja AK. et al. Insulin Degradation by Acinar Cell Proteases Creates a Dysfunctional Environment for Human Islets Before/After Transplantation: Benefits of alpha-1 Antitrypsin Treatment. Transplantation. 2011;92(11):1222–1230. doi: 10.1097/TP.0b013e318237585c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brandhorst H, Brandhorst D, Hering BJ, Bretzel RG. Significant progress in porcine islet mass isolation utilizing liberase HI for enzymatic low-temperature pancreas digestion. Transplantation. 1999;68(3):355–361. doi: 10.1097/00007890-199908150-00006. [DOI] [PubMed] [Google Scholar]
- 119.Lakey JR, Warnock GL, Rajotte RV, Suarez-Alamazor ME, Ao Z, Shapiro AM, Kneteman NM. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61(7):1047–1053. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 120.Sakuma Y, Ricordi C, Miki A, Yamamoto T, Pileggi A, Khan A, Alejandro R, Inverardi L, Ichii H. Factors that affect human islet isolation. Transplant Proc. 2008;40(2):343–345. doi: 10.1016/j.transproceed.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eitel K, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Haring HU, Kellerer M. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem Biophys Res Commun. 2002;299(5):853–856. doi: 10.1016/s0006-291x(02)02752-3. [DOI] [PubMed] [Google Scholar]
- 122.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M. et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51(5):1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 123.Tsujimura T, Kuroda Y, Churchill TA, Avila JG, Kin T, Shapiro AM, Lakey JR. Short-term storage of the ischemically damaged human pancreas by the two-layer method prior to islet isolation. Cell Transplant. 2004;13(1):67–73. doi: 10.3727/000000004772664914. [DOI] [PubMed] [Google Scholar]
- 124.Salehi P, Mirbolooki M, Kin T, Tsujimura T, Shapiro AM, Churchill TA, Lakey JR. Ameliorating injury during preservation and isolation of human islets using the two-layer method with perfluorocarbon and UW solution. Cell Transplant. 2006;15(2):187–194. doi: 10.3727/000000006783982070. [DOI] [PubMed] [Google Scholar]
- 125.Nevalainen TJ. The role of phospholipase A in acute pancreatitis. Scand J Gastroenterol. 1980;15(6):641–650. doi: 10.3109/00365528009181510. [DOI] [PubMed] [Google Scholar]
- 126.Arbet-Engels C, Darquy S, Capron F, Pueyo ME, Dimaria S, Poitout V, Reach G. A one-step, operator-independent method for isolating islets of Langerhans from the porcine pancreas. Artif Organs. 1994;18(8):570–575. doi: 10.1111/j.1525-1594.1994.tb03381.x. [DOI] [PubMed] [Google Scholar]
- 127.Balamurugan AN, Chang Y, Fung JJ, Trucco M, Bottino R. Flexible management of enzymatic digestion improves human islet isolation outcome from sub-optimal donor pancreata. Am J Transplant. 2003;3(9):1135–1142. doi: 10.1046/j.1600-6143.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- 128.van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267(1):139–146. doi: 10.1007/BF00318700. [DOI] [PubMed] [Google Scholar]
- 129.van Suylichem PT, van Deijnen JE, Wolters GH, van Schilfgaarde R. Amount and distribution of collagen in pancreatic tissue of different species in the perspective of islet isolation procedures. Cell Transplant. 1995;4(6):609–614. doi: 10.1177/096368979500400610. [DOI] [PubMed] [Google Scholar]
- 130.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(Suppl):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 131.Jiang FX, Naselli G, Harrison LC. Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem. 2002;50(12):1625–1632. doi: 10.1177/002215540205001206. [DOI] [PubMed] [Google Scholar]
- 132.Mahler R, Franke FE, Hering BJ, Brandhorst D, Brandhorst H, Brendel MD, Federlin K, Schulz A, Bretzel RG. Evidence for a significant correlation of donor pancreas morphology and the yield of isolated purified human islets. Journal of Molecular Medicine. 1999;77(1):87–89. doi: 10.1007/s001090050308. [DOI] [PubMed] [Google Scholar]
- 133.Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181–1191. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 134.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272(42):26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 135.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81(3):423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 136.Aumailley M, von der Mark H, Timpl R. Size and domain structure of collagen VI produced by cultured fibroblasts. FEBS Lett. 1985;182(2):499–502. doi: 10.1016/0014-5793(85)80362-8. [DOI] [PubMed] [Google Scholar]
- 137.Kuo HJ, Keene DR, Glanville RW. Orientation of type VI collagen monomers in molecular aggregates. Biochemistry. 1989;28(9):3757–3762. doi: 10.1021/bi00435a020. [DOI] [PubMed] [Google Scholar]
- 138.Shima H, Inagaki A, Imura T, Yamagata Y, Watanabe K, Igarashi K, Goto M, Murayama K. Collagen V Is a Potential Substrate for Clostridial Collagenase G in Pancreatic Islet Isolation. J Diabetes Res. 2016;2016:4396756. doi: 10.1155/2016/4396756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999;47(4):499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- 140.Irving-Rodgers HF, Choong FJ, Hummitzsch K, Parish CR, Rodgers RJ, Simeonovic CJ. Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: impact for allograft rejection. Cell Transplant. 2014;23(1):59–72. doi: 10.3727/096368912X659880. [DOI] [PubMed] [Google Scholar]
- 141.Cross SE, Vaughan RH, Willcox AJ, McBride AJ, Abraham AA, Han B, Johnson JD, Maillard E, Bateman PA, Ramracheya RD. et al. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant. 2017;17(2):451–461. doi: 10.1111/ajt.13975. [DOI] [PubMed] [Google Scholar]
- 142.Mandl I, Zipper H, Ferguson LT. Clostridium histolyticum collagenase: its purification and properties. Arch Biochem Biophys. 1958;74(2):465–475. doi: 10.1016/0003-9861(58)90017-1. [DOI] [PubMed] [Google Scholar]
- 143.Kono T. Purification and partial characterization of collagenolytic enzymes from Clostridium histolyticum. Biochemistry. 1968;7(3):1106–1114. doi: 10.1021/bi00843a031. [DOI] [PubMed] [Google Scholar]
- 144.French MF, Bhown A, Van Wart HE. Identification of Clostridium histolyticum collagenase hyperreactive sites in type I, II, and III collagens: lack of correlation with local triple helical stability. J Protein Chem. 1992;11(1):83–97. doi: 10.1007/BF01025095. [DOI] [PubMed] [Google Scholar]
- 145.Bateman PA, Devereux-Cooke KJ, Johnson JD, Brandhorst H, Brandhorst D, Gray DW, Cross SE, Hughes SJ, Johnson PRV. Degradation of laminin and laminin-511 in the human peri-islet extracellular matrix is targeted by neutral protease and thermolysin, but not collagenase. Transplantation. 2013;96(S6):S18. [Google Scholar]
- 146.Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126(2):393–398. [PubMed] [Google Scholar]
- 147.Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 2010;31(7):1676–1682. doi: 10.1016/j.biomaterials.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 148.Banerjee M, Virtanen I, Palgi J, Korsgren O, Otonkoski T. Proliferation and plasticity of human beta cells on physiologically occurring laminin isoforms. Mol Cell Endocrinol. 2012;355(1):78–86. doi: 10.1016/j.mce.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 149.Ricordi C, Alejandro R, Rilo HH, Carroll PB, Tzakis AG, Starzl TE, Mintz DH. Long-term in vivo function of human mantled islets obtained by incomplete pancreatic dissociation and purification. Transplant Proc. 1995;27(6):3382. [PubMed] [Google Scholar]
- 150.Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126(2):299–304. [PubMed] [Google Scholar]
- 151.Huh KH, Lee JI, Kim JY, Jeong JH, Fang Y, Park YJ, Kang CM, Kim YS. Functional improvement of pig islet with exocrine encapsulation. Transplant Proc. 2009;41(1):323–325. doi: 10.1016/j.transproceed.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 152.Stenn KS, Link R, Moellmann G, Madri J, Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93(2):287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- 153.Roche-Diagnostics. Liberase research grade enzyme blends. Calculation of working concentrations and conversion guide compared to traditional collagenases. 2010. pp. 1–7. [Google Scholar]
- 154.Ihm SH, Matsumoto I, Sawada T, Nakano M, Zhang HJ, Ansite JD, Sutherland DE, Hering BJ. Effect of donor age on function of isolated human islets. Diabetes. 2006;55(5):1361–1368. doi: 10.2337/db05-1333. [DOI] [PubMed] [Google Scholar]
- 155.Niclauss N, Bosco D, Morel P, Demuylder-Mischler S, Brault C, Milliat-Guittard L, Colin C, Parnaud G, Muller YD, Giovannoni L. et al. Influence of donor age on islet isolation and transplantation outcome. Transplantation. 2011;91(3):360–366. doi: 10.1097/tp.0b013e31820385e6. [DOI] [PubMed] [Google Scholar]
- 156.Zeng Y, Torre MA, Karrison T, Thistlethwaite JR. The correlation between donor characteristics and the success of human islet isolation. Transplantation. 1994;57(6):954–958. doi: 10.1097/00007890-199403270-00031. [DOI] [PubMed] [Google Scholar]
- 157.Meier RP, Sert I, Morel P, Muller YD, Borot S, Badet L, Toso C, Bosco D, Berney T. Islet of Langerhans isolation from pediatric and juvenile donor pancreases. Transpl Int. 2014;27(9):949–955. doi: 10.1111/tri.12367. [DOI] [PubMed] [Google Scholar]
- 158.Shimoda M, Noguchi H, Naziruddin B, Fujita Y, Chujo D, Takita M, Peng H, Tamura Y, Olsen GS, Sugimoto K. et al. Improved method of human islet isolation for young donors. Transplant Proc. 2010;42(6):2024–2026. doi: 10.1016/j.transproceed.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 159.Yagisawa S, Morita F, Nagai Y, Noda H, Ogura Y. Kinetic studies on the action of collagenase. J Biochem (Tokyo) 1965;58(4):407–416. doi: 10.1093/oxfordjournals.jbchem.a128218. [DOI] [PubMed] [Google Scholar]
- 160.Ryhanen L, Rantala-Ryhanen S, Tan EM, Uitto J. Assay of collagenase activity by a rapid, sensitive, and specific method. Coll Relat Res. 1982;2(2):117–130. doi: 10.1016/s0174-173x(82)80028-9. [DOI] [PubMed] [Google Scholar]
- 161.Socci C, Davalli AM, Vignali A, Pontiroli AE, Maffi P, Magistretti P, Gavazzi F, De Nittis P, Di Carlo V, Pozza G. A significant increase of islet yield by early injection of collagenase into the pancreatic duct of young donors. Transplantation. 1993;55(3):661–663. [PubMed] [Google Scholar]
- 162.Balamurugan AN, Chang Y, Bertera S, Sands A, Shankar V, Trucco M, Bottino R. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia. 2006;49(8):1845–1854. doi: 10.1007/s00125-006-0318-0. [DOI] [PubMed] [Google Scholar]
- 163.Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation. 2008;85(7):950–955. doi: 10.1097/TP.0b013e3181683df5. [DOI] [PubMed] [Google Scholar]
- 164.Xi YP, Nette EG, King DW, Rosen M. Age-related changes in normal human basement membrane. Mech Ageing Dev. 1982;19(4):315–324. doi: 10.1016/0047-6374(82)90015-x. [DOI] [PubMed] [Google Scholar]
- 165.Vazquez F, Palacios S, Aleman N, Guerrero F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas. 1996;25(3):209–215. doi: 10.1016/s0378-5122(96)01066-3. [DOI] [PubMed] [Google Scholar]
- 166.Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29(5):402–410. doi: 10.1016/j.matbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 167.Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Prev Med. 2012;54(Suppl):S50–S56. doi: 10.1016/j.ypmed.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 168.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3-4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 169.Degendorfer G, Chuang CY, Hammer A, Malle E, Davies MJ. Peroxynitrous acid induces structural and functional modifications to basement membranes and its key component, laminin. Free Radic Biol Med. 2015;89:721–733. doi: 10.1016/j.freeradbiomed.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 170.Hesse F, Burtscher H, Popp F, Ambrosius D. Recombinant enzymes for islet isolation: purification of a collagenase from Clostridium histolyticum and cloning/expression of the gene. Transplant Proc. 1995;27(6):3287–3289. [PubMed] [Google Scholar]
- 171.Brandhorst H, Brandhorst D, Hesse F, Ambrosius D, Brendel M, Kawakami Y, Bretzel RG. Successful human islet isolation utilizing recombinant collagenase. Diabetes. 2003;52(5):1143–1146. doi: 10.2337/diabetes.52.5.1143. [DOI] [PubMed] [Google Scholar]
- 172.Tanioka Y, Tanaka T, Gotoh T, Kakinoki K, Li S, Yoshikawa T, Sakai T, Fujino Y, Suzuki Y, Kuroda Y. Augmentation of tissue ATP level during digestion using preoxygenated perfluorocarbon results in high islet yield-new strategy for islet isolation. Transplant Proc. 2005;37(1):220–222. doi: 10.1016/j.transproceed.2004.12.232. [DOI] [PubMed] [Google Scholar]
- 173.Goto T, Tanioka Y, Sakai T, Terai S, Kamoda Y, Li S, Tanaka T, Tsujimura T, Matsumoto I, Fujino Y. et al. Application of the two-layer method on pancreas digestion results in improved islet yield and maintained viability of isolated islets. Transplantation. 2007;83(6):754–758. doi: 10.1097/01.tp.0000256338.53305.a9. [DOI] [PubMed] [Google Scholar]
- 174.Dono K, Gotoh M, Monden M, Kanai T, Fukuzaki T, Mori T. Low temperature collagenase digestion for islet isolation from 48-hour cold-preserved rat pancreas. Transplantation. 1994;57(1):22–26. doi: 10.1097/00007890-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 175.Mirenda V, Le Mauff B, Huvelin JM, Sigalla J, Auget JL, Soulillou JP. Improvement of adult porcine islet recovery by early collagenase injection and low temperature digestion. Transplant Proc. 1997;29(4):2265. doi: 10.1016/s0041-1345(97)00325-4. [DOI] [PubMed] [Google Scholar]
- 176.Heldmaier G, Ruf T. Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol B. 1992;162(8):696–706. doi: 10.1007/BF00301619. [DOI] [PubMed] [Google Scholar]
- 177.Escolar JC, Hoo-Paris R, Castex C, Sutter BC. Effect of low temperatures on glucose-induced insulin secretion and glucose metabolism in isolated pancreatic islets of the rat. J Endocrinol. 1990;125(1):45–51. doi: 10.1677/joe.0.1250045. [DOI] [PubMed] [Google Scholar]
- 178.Brandhorst D, Brandhorst H, Hering BJ, Bretzel RG. Long-term survival, morphology and in vitro function of isolated pig islets under different culture conditions. Transplantation. 1999;67(12):1533–1541. doi: 10.1097/00007890-199906270-00006. [DOI] [PubMed] [Google Scholar]
- 179.Tamarit-Rodriguez J, Idahl LA, Gine E, Alcazar O, Sehlin J. Lactate production in pancreatic islets. Diabetes. 1998;47(8):1219–1223. doi: 10.2337/diab.47.8.1219. [DOI] [PubMed] [Google Scholar]
- 180.Mallya SK, Mookhtiar KA, Van Wart HE. Kinetics of hydrolysis of type I, II, and III collagens by the class I and II Clostridium histolyticum collagenases. J Protein Chem. 1992;11(1):99–107. doi: 10.1007/BF01025096. [DOI] [PubMed] [Google Scholar]
- 181.Teruel SR, Simpson BK. Characterization of the collagenolytic enzyme fraction from winter flounder (Pseudopleuronectes americanus) Comp Biochem Physiol. 1995;112(1):131–136. [Google Scholar]
- 182.Saito M, Sato K, Kunisaki N, Kimura S. Characterization of a rainbow trout matrix metalloproteinase capable of degrading type I collagen. Eur J Biochem. 2000;267(23):6943–6950. doi: 10.1046/j.1432-1033.2000.01807.x. [DOI] [PubMed] [Google Scholar]
- 183.Sovik SL, Rustad T. Effect of season and fishing ground on the activity of cathepsin B and collagenase in by-products from cod species. Food Sci Technol. 2006;39(1):43–53. [Google Scholar]
- 184.Kang SI, Jang YB, Choi YJ, Kong JY. Purification and properties of a collagenolytic protease produced by marine bacterium Vibrio vulnificus CYK279H. Biotechnol Bioprocess Eng. 2005;10(6):593–598. [Google Scholar]