Abstract
AIMS: To investigate the association between serum orexin concentrations and insulin resistance/sensitivity in a sample of patients with type 2 diabetes mellitus, and to study the effects of anti-hyperglycemic treatment on orexin concentrations over three months. METHODS: This study was designed as a randomized, open-label, clinical trial. Before allocation, sixty medication-naïve, newly-diagnosed, type 2 diabetes patients underwent a 75 g oral glucose tolerance test (OGTT). Afterwards, using a randomized trial design (IRCT201102275917N1) patients were allocated to either the metformin (1000 mg daily) or pioglitazone (30 mg daily) arm, and were reexamined after three months. Serum insulin, plasma glucose, and orexin concentrations were measured at baseline, during OGTT, and after three months. RESULTS: Orexin concentrations significantly decreased after OGTT (0 vs. 120 min: 0.63 ± 0.07 vs. 0.31 ± 0.03 ng/ml, p < 0.001). Insulin resistance determined by homeostasis model assessment of insulin resistance (HOMA-IR) was significantly and negatively correlated with orexin (r = -0.301, p = 0.024). Furthermore, orexin concentrations were significantly and positively correlated with the insulin sensitivity index derived from OGTT (r = 0.326, p = 0.014). Three-month treatment with metformin and pioglitazone significantly improved insulin sensitivity and increased orexin concentrations by 26% (p = 0.025) and 14% (p = 0.076), respectively. Between-group analysis showed that changes in orexin concentrations with metformin and pioglitazone were not significantly different (p = 0.742). CONCLUSIONS: There was a negative association between peripheral orexin concentrations and insulin resistance in type 2 diabetes patients. Three-month anti-hyperglycemic treatment with proportionate doses of metformin or pioglitazone increased orexin concentrations via amelioration of insulin resistance and improvement of glycemic control.
Keywords: type 2 diabetes, orexin A, insulin resistance, metformin, pioglitazone, HbA1c, glucose homeostasis, peripheral orexin, enterochromaffin cell
Abbreviations: ANCOVA - analysis of covariance; ANOVA - analysis of variance; BMI - body mass index; ELISA - enzyme-linked immunosorbent assay; FPG - fasting plasma glucose ; HbA1c - glycated hemoglobin A1c; HDL - high-density lipoprotein; HOMA-β - homeostasis model assessment of beta-cell function; HOMA-IR - homeostasis model assessment of insulin resistance; HPLC - high-performance liquid chromatography; ICTRP - International Clinical Trials Registry Platform; LDL - low-density lipoprotein; OGTT - oral glucose tolerance test; RIA - radioimmunoassay; SD - standard deviation; SPSS - statistical package for social sciences; T2DM - type 2 diabetes mellitus; WHO - World Health Organization
1. Introduction
Human orexin-A (orexin) is a 33-amino acid peptide initially described as a neuropeptide originating from neurons in the lateral and perifornical hypothalamic area [1]. The perifornical area mediates the functional interaction between brain and liver [2]. Orexin concentrations follow a daily rhythm peaking during wakefulness [3]. Orexin concentrations are controlled by the suprachiasmatic nucleus [4], the hypothalamic center responsible for maintaining the circadian rhythm; its functional disorder has been tightly linked to the development of insulin resistance and diabetes [5-7]. Orexin-knockout mice become susceptible to the age-related development of insulin resistance through the disruption of insulin signaling in both hypothalamus and peripheral tissues [8]. Furthermore, centrally produced orexin has been shown to be associated with the regulation of appetite and food intake via interacting with glucose-sensing neurons in the hypothalamus [9-11].
Irrespective of its centrally regulated functions, orexin occurs in several peripheral tissues, and is assumed to be involved in the maintenance of energy and glucose homeostasis via hormone-like functions [12, 13]. Orexin is also involved in pancreatic hormone secretion. It may thus be a modulator of insulin [13]. Immunostaining of the human pancreas has shown that about two-thirds of insulin-immunopositive cells are contemporaneously positive for orexin [14]. Peripheral orexin may originate from hypothalamus cells traveling through the blood-brain barrier [15], or may be produced peripherally by cells like the enterochromaffin cells of the intestines [16] and pancreatic islet cells [12].
Currently, our understanding of the cross-talk between peripheral orexin and insulin and the possible effects of orexin on insulin resistance remains limited. Experimental models in rats and humans have resulted in discrepant findings [17-20]. Moreover, the possible association of orexin with insulin resistance in type 2 diabetes mellitus (T2DM) has rarely been studied. Therefore, we aimed to investigate the association between serum orexin concentrations and insulin resistance in a sample of T2DM patients. In the present study, the following two-step approach was used:
We investigated how peripheral orexin concentrations change in response to an acute glycemic challenge in medication-naïve patients.
We delved into the effects of anti-hyperglycemic treatment on insulin resistance and orexin concentrations over a three-month period.
2. Methods and subjects
2.1 Study design and selection criteria
In the present study, we used blood samples derived from a randomized clinical trial conducted previously [21]. The original study was planned as a prospective, three-month, single-center, open-label, randomized clinical trial. Between March and June 2011, patients visiting the outpatient Diabetes Clinic of the Vali-Asr Hospital (a university-affiliated teaching hospital) were assessed for eligibility. Patients were considered eligible for inclusion in the study if they:
Were newly diagnosed with T2DM according to the American Diabetes Association diagnostic criteria [22]
Were 30 years or older
Had not taken anti-hyperglycemic medications for the management of diabetes
Had not been diagnosed with chronic diseases of the heart, kidneys, lungs, or liver
Were not pregnant or lactating
Using a simple randomization method, patient numbers 1-60 were generated in a manner that rendered them unrepeated and unsorted. With this random sequence, numbers smaller than 31 were allocated to the metformin arm, numbers 31-60 were assigned to the pioglitazone arm. In the metformin arm, subjects received 1000 mg metformin (500 mg tablets, twice daily). In the pioglitazone arm, patients took pioglitazone 30 mg (15 mg tablets, twice daily). After three months of therapy, a follow-up examination was carried out.
All procedures which included human subjects were conducted in accordance with the guidelines laid down in the recent revision of the Helsinki protocol. Written informed consent was obtained from each patient prior to enrollment. The Tehran University of Medical Sciences Ethics Committee approved the trial protocol. The trial has also been registered at the Iranian Registry of Clinical Trials (registration no. IRCT201102275917N1), which is a primary registry of the World Health Organization's (WHO) International Clinical Trials Registry Platform (ICTRP).
2.2 Assessments and definitions
At baseline visit, a detailed medical history was obtained, and physical examination was performed. Height was measured using a wall-mounted stadiometer, and was recorded to the nearest 0.1 cm. The patients' weight was measured with only light clothing using a calibrated digital scale (Beurer, GS49, Germany), and was documented to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight in kg divided by squared height in meters (kg/m2). Patients were stratified into normal weight, overweight, and obese according to WHO criteria.
Waist circumference was measured at the end of normal expiration mid-way between the lower costal margin and anterior superior iliac crest, and was recorded to the nearest 0.1 cm. Central (abdominal) obesity was defined as waist circumference ≥90 cm (for both men and women) based on the cut-off proposed for the Iranian population [23]. After the patients had relaxed for about 10 minutes, two readings of systolic and diastolic blood pressures were obtained from each patient within a 5-min interval using a standard sphygmomanometer (Riester, Big Ben adults, Germany). Patients were considered to be hypertensive if they had an average reading of ≥140 mmHg systolic and/or ≥90 mmHg diastolic or if they were taking anti-hypertensive medication.
2.3 Laboratory evaluations
Patients were instructed to perform a 12-hour overnight fast; fasting blood was obtained the next day. Additionally, in all patients, a standard 75 g fasting oral glucose tolerance test was performed, and two blood draws were conducted at 60 and 120 minutes. Orexin concentrations were obtained at time zero and at 120 minutes. Serum insulin and fasting plasma glucose (FPG) were determined at times 0, 60, and 120 minutes. All other laboratory parameters were determined at baseline only. Using a similar protocol, three months after initiation of treatment, a second fasting blood draw was performed.
Serum concentrations of FPG were determined using the glucose oxidize method. Fasting insulin was measured using the chemiluminescence radioimmunoassay (RIA) technique (Immunotech, Prague, Czech Republic). As a surrogate marker of insulin resistance, homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as FPG (mmol/l) multiplied by fasting insulin (mU/l), divided by 22.5. Homeostasis model assessment of beta-cell function (HOMA-β) was defined as fasting insulin (mU/l) times 20 divided by FPG (mmol/l) minus 3.5. Insulin sensitivity derived from the oral glucose tolerance test (OGTT) was calculated using the equation provided by Matsuda and DeFronzo [24], which is as follows: 10,000/sqr root of (FPG x fasting insulin x mean plasma glucose x mean insulin).
Mean plasma glucose (mg/dl) and insulin (mU/l) were calculated as the arithmetic average of three measurements (at 0, 60, and 120 minutes). The insulin sensitivity index derived from this equation correlates strongly with the direct measurement obtained using the euglycemic insulin clamp [24]. The percentage of glycated hemoglobin A1c (HbA1c) was determined using the high performance liquid chromatography (HPLC) method. Serum concentrations of lipids including total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides were measured with enzymatic methods (Pars Azmun commercial kits, Karaj, Iran). Serum creatinine concentrations were determined using the Jaffe kinetic method, and serum orexin concentrations were measured using the enzyme-linked immunosorbent assay (ELISA) method (Human Orexin-A ELISA kit, CSB-E08859h, Cusabio, Wuhan, China). The inter- and intra-assay coefficients of variation were <10% and <8%, respectively.
2.4 Statistical analysis
All analyses were performed using the statistical package for social sciences (SPSS) version 19.0 for windows (IBM Corporation, New York, United States). Continuous variables with normal distributions were expressed as mean ± standard deviation (SD) and categorical variables as proportion or ratio. Continuous variables with non-normal distributions were expressed as median (interquartile range). A logarithmic transformation was carried out to obtain normality, and hence to make parametric statistical tests plausible.
Comparisons of the mean levels of continuous variables across two categories were performed using the independent t-test. With more than two categories, analysis of variance (ANOVA) was employed and the p-value for the linear trend was calculated. The distribution of categorical variables across categories was assessed using the Chi-squared test. Associations between two continuous variables were evaluated using partial correlation and adjustment for the effects of possible confounders (i.e. age). The within-group changes in continuous variables between baseline and after three months, or before and after OGTT, were investigated using the paired t-test. The between-group efficacy of continuous variables between baseline and after three months was investigated using analysis of covariance (ANCOVA). In all tests, a p-value <0.05 was considered necessary to reject the null hypothesis.
3. Results
3.1 Baseline characteristics
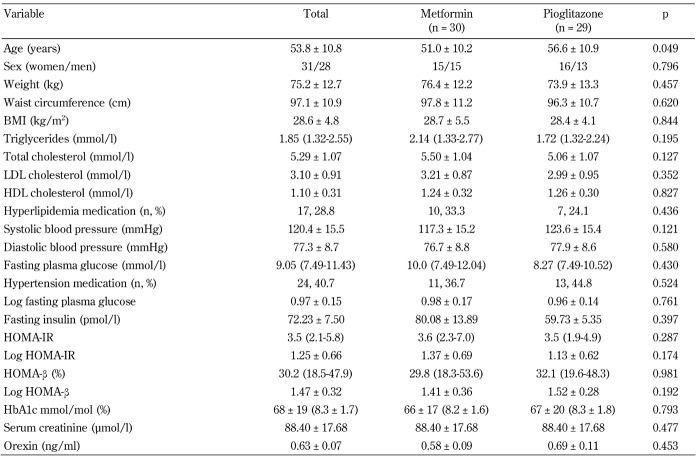
A total of 60 medication-naïve T2DM patients were enrolled. As the serum sample of one of the patients in the pioglitazone arm was missing, 59 patients (30 in the metformin arm and 29 in the pioglitazone arm) were included in the final analysis. Baseline characteristics of the patients are presented in Table 1. The mean age of the participants was 53.8 ± 10.8 years and ranged from 36 to 81 years. Women represented 52.5% of the subjects. Mean HbA1c concentrations were 68 ± 19 mmol/mol (8.3% ± 1.7%) with 39.0% of the patients having HbA1c concentrations >64 mmol/mol (>8.0%).
Table 1. Baseline characteristics of the trial participants by trial arm.
Legend: Variables with non-normal distributions are presented as median (interquartile range) followed by their log-transformed values. Abbreviations: BMI - body mass index; LDL cholesterol - low-density lipoprotein cholesterol; HDL cholesterol - high-density lipoprotein cholesterol; HOMA-IR - homeostasis model assessment of insulin resistance; HOMA-β - homeostasis model assessment of β-cell function; HbA1c - glycated hemoglobin A1c.
Two patients in the metformin arm (6.7%) reported gastrointestinal symptoms. They were advised to change the time of taking the medication. No significant adverse events requiring medication discontinuation were observed for either arm of the trial, and all patients were able to tolerate the prescribed doses. As a result, no dose titration or switching of medication was necessary.
After three months, dose adjustment was carried out based on HbA1c values and the physician’s assessment of glycemic control. In the metformin group, the mean daily dose after three months was 1335 ± 225 mg/day. In the pioglitazone group, metformin was added to the treatment regimen if necessary (mean dose 650 mg ± 295 mg/day).
3.2 Orexin concentrations at baseline and after OGTT
Mean orexin concentrations at baseline were 0.63 ± 0.07 ng/ml. Baseline orexin concentrations by sex, age, general and central obesity, and hypertension are shown in Table 2. A statistically significant inverse association between orexin concentration and age was found (p = 0.006). Orexin concentration in the youngest age quartile was about 2.3-fold higher than in the oldest quartile. No association between orexin concentration and gender, general obesity, central obesity, or hypertension was found (Table 2).
Table 2. Orexin concentration by age, sex, general and central obesity, and hypertension.
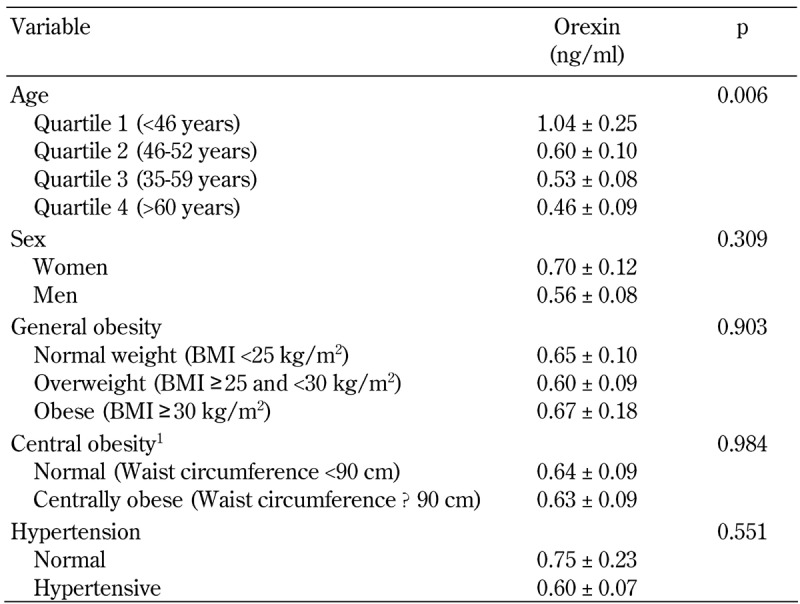
Legend: P-value for linear trend is calculated for age and general obesity.
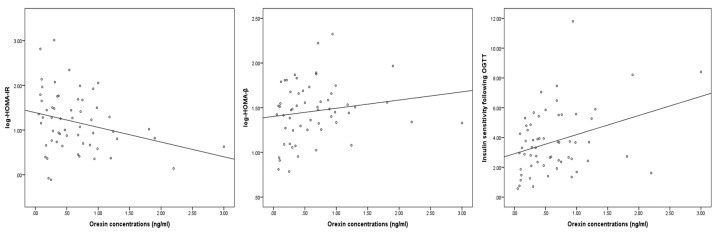
Associations between orexin concentrations and HOMA-IR, HOMA-β, and insulin sensitivity index following OGTT are shown in Figure 1 (A-C). As shown in the figure, a significantly negative correlation between orexin and log HOMA-IR was found (r = -0.301, p = 0.024). Orexin positively correlated with log HOMA-β, although it did not fully reach the critical threshold for statistical significance (r = 0.260, p = 0.053). Orexin concentrations at baseline significantly and positively correlated with insulin sensitivity calculated from OGTT (r = 0.326, p = 0.014). Following OGTT, serum orexin concentrations significantly decreased from 0.63 ± 0.07 to 0.31 ± 0.03 ng/ml (p < 0.001). In 86% of the patients (n = 51), orexin concentrations decreased after OGTT (ranging from -5.6% to -94.1%), whereas the remaining 8 patients experienced increments in orexin levels.
Figure 1. Correlations between serum orexin concentrations and indices of insulin resistance/sensitivity.
A: Significant and negative correlation between orexin and log HOMA-IR (r = -0.301, p = 0.024). B: Positive correlation between orexin and log HOMA-β (r = 0.260, p = 0.053). C: Significant and positive correlation between orexin and insulin sensitivity index following OGTT (r = 0.326, p = 0.014). Given the strong association between orexin concentration and age, all correlations are adjusted for age. Abbreviations: HOMA-IR - homeostasis model assessment of insulin resistance; HOMA-β - homeostasis model assessment of β-cell function; OGTT - oral glucose tolerance test.
3.3 Treatment with metformin and pioglitazone and their effect on orexin concentrations
Baseline characteristics of study participants by trial arms are presented in Table 1. Patients in the pioglitazone arm were on average 5.5 years older than their counterparts in the metformin arm (56.6 ± 10.9 vs. 51.0 ± 10.2 years, p = 0.049). All other demographic, clinical, and laboratory variables were comparable between the two trial arms (p > 0.05 in all tests). The mean baseline orexin concentrations in metformin and pioglitazone arms were 0.58 ± 0.09 and 0.69 ± 0.11 ng/ml, respectively (p = 0.453).
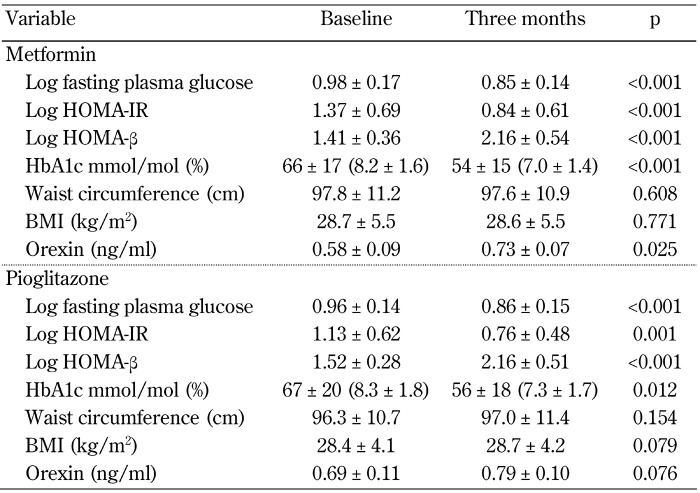
Within-group effects of metformin and pioglitazone on the indices of glycemic control, insulin resistance/sensitivity, obesity, and orexin concentration after three months are summarized in Table 3. In the metformin group, FPG, HOMA-IR, and HbA1c were significantly decreased, while HOMA-β was significantly increased. No significant changes in waist circumference and BMI were noted. Treatment with metformin resulted in a 26% increase in orexin concentrations over a period of three months (p = 0.025). In the pioglitazone group, similar changes in all indices of glycemic control were noted (Table 3). The changes in both waist circumference and BMI were not statistically significant in patients receiving pioglitazone (p = 0.154 and 0.079, respectively). Although not statistically significant, treatment with pioglitazone led to a 14% increase in orexin concentrations (p = 0.076).
Table 3. Within-group effects of metformin and pioglitazone on indices of glycemic control, obesity, and orexin concentration.
Legend: Variables with non-normal distribution were log-transformed. Abbreviations: BMI - body mass index; HbA1c - glycated hemoglobin A1c; HOMA-β - homeostasis model assessment of β-cell function; HOMA-IR - homeostasis model assessment of insulin resistance.
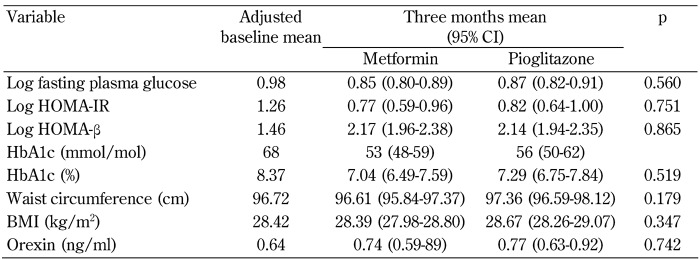
Comparison of the effect of each treatment on the indices of glycemic control, insulin resistance/sensitivity, anthropometric measures, and orexin concentration are presented in Table 4. ANCOVA models indicated that both medications are equally effective in improving FPG, HOMA-IR, HOMA-β, and HbA1c (p > 0.05 in all tests). Similarly, after controlling for age, no significant between-group differences with respect to three-month changes in waist circumference or BMI were documented (p > 0.05). Finally, treatment with metformin or pioglitazone resulted in comparable outcomes in term of orexin increment in medication-naïve patients with T2DM (p = 0.742, Table 4).
Table 4. Between-group effects of metformin and pioglitazone on indices of glycemic control, obesity, and orexin concentration.
Legend: All ANCOVA models are adjusted for age, due to significant age differences between the trial arms. Variables with non-normal distributions are log-transformed. Abbreviations: BMI - body mass index; CI - confidence interval; HbA1c - glycated hemoglobin A1c; HOMA-β - homeostasis model assessment of β-cell function; HOMA-IR - homeostasis model assessment of insulin resistance.
4. Discussion
In our sample of newly diagnosed patients with T2DM, we aimed to investigate the association between orexin concentration and insulin resistance/sensitivity. Our observations show a negative association between peripheral orexin and insulin resistance.
Before discussing the main findings in detail, a few corollary observations deserve mention. Orexin concentrations were negatively associated with age. In the youngest age category, orexin concentrations were more than twofold higher than in patients in the oldest age category. In line with our findings, in an experimental study, old (3-4 months) hybrid rats were shown to experience a greater than 40% loss in their orexin-immunoreactive neurons compared with young (26-28 months) rats [25]. Since peripheral orexin is in part produced centrally, and crosses the blood-brain barrier, the loss of orexigenic neurons in both medial and lateral sectors of the hypothalamus with age may explain the decline in peripheral orexin concentrations in older individuals.
Findings from studies investigating the relationship between peripheral orexin and obesity have been inconsistent. In a study of 23 healthy individuals with BMI ranging from 19.8 to 59 kg/m2, orexin concentrations were found to correlate negatively with BMI [26]. Morbidly obese individuals were found to have significantly lower orexin concentrations than their normal-weight counterparts. Conversely, Heinonen et al. have shown that peripheral orexin concentrations are markedly higher in morbidly obese bariatric surgery candidates than in normal-weight controls [27]. However, it should be noted that, despite an average weight loss of 39-48 kg over one year following gastric banding, no significant changes in plasma orexin concentrations were observed [27]. In our sample of patients, no significant association between orexin levels and either general or central obesity was found. In fact, orexin concentrations were comparable between normal and obese patients (0.65 vs. 0.67 ng/ml). Therefore, the relationship between obesity and peripheral orexin seems to be complex and non-linear; there may be some unknown confounders contributing to this association.
In the present study, OGTT resulted in a significant reduction in serum orexin concentrations after 120 min (from 0.63 to 0.31 ng/ml). In 86% of the patients, the net change in orexin levels was negative; only 8 subjects (14%) experienced increments in orexin concentrations. Our findings are in agreement with a previous study by Ouedraogo et al. where high glucose levels were shown to reduce orexin secretion from the islet cells in the pancreas [12]. Overall, it appears that peripheral orexin in the blood, at least in that originating from the pancreas, decreases following hyperglycemia and increases in response to hypoglycemia and fasting.
Two key findings in our study suggest a negative link between orexin and insulin resistance:
Orexin concentrations were negatively correlated with HOMA-IR, a surrogate measure of insulin resistance. Additionally, orexin concentrations were positively correlated with the insulin sensitivity index derived from OGTT.
The observation that three-month treatment with either metformin or pioglitazone resulted in significant improvements in glycemic control and glucose sensitivity is paralleled by an increase in peripheral orexin concentrations.
Compared with baseline, orexin concentrations were increased by about 26% in the metformin and 14% in the pioglitazone group three months after treatment. There were no significant differences between the two medications.
Recently, Park et al. have investigated the possible effects of exogenous orexin on glucose and insulin levels following acute glycemic challenge [28]. In their experimental model of diabetic mice, exogenous orexin delivered subcutaneously following a glucose load decreased blood glucose concentrations through an increase in glucose-stimulated insulin secretion from beta-cells, a reduction in glucagon secretion, and a delayed but sustained increase in leptin levels [28]. Similar findings have been reported previously by Tsuneki et al. where a decrease in blood glucose levels following injection of exogenous orexin-A and orexin-B in streptozotocin-induced diabetic mice was observed, although in the fasting state only [29].
Irrespective of its role in stimulating insulin secretion, orexin may also contribute to an amelioration of glucose intolerance through a direct effect on target organs like adipose tissue. In rats treated with orexin for four weeks, Skrzypski et al. showed that peripheral orexin directly enhances glucose uptake through translocation of the glucose transporter GLUT4 from the cytoplasm to the plasma membrane [30]. Furthermore, orexin enhanced the expression and secretion of insulin-sensitizing adipocytokine adiponectin from adipocytes [30]. Taken together, these observations confirm that the functions of peripheral orexin in reducing insulin resistance are multifaceted, and include actions in both the pancreas and target tissues.
Beside the role of orexin in the development of insulin resistance and T2DM, it may also confer important benefits later in the disease process and in preventing diabetes complications. In a recent study, Harada et al. have suggested a neuroprotective role for central orexin following ischemia. Based on their observations, direct administration of orexin in the hypothalamus prevents glucose-induced, post-ischemic neuronal damage in a dose-dependent manner [31]. More recently, they have shown that the protective effect is due to the reversal of increased hepatic gluconeogenesis and decreased hepatic insulin receptor expression [32].
To the best of our knowledge, our study is among the first to investigate the association between peripheral orexin and insulin resistance/sensitivity in T2DM. The present study was not designed to find a causal link between orexin and insulin resistance. As previous studies have suggested, orexin affects insulin secretion and serum glucose concentrations, which in turn play a key role in the regulation of orexin levels. The present study contributes to the understanding of orexin and its effects in the clinical context by showing that peripheral orexin concentrations are negatively associated with insulin resistance and that amelioration of insulin resistance by antidiabetic treatment increases orexin concentrations over time.
Metformin and pioglitazone represent common antidiabetic medications, but they improve glycemic control through distinct pathways and mechanisms. The comparability of orexin changes in patients treated with proportionate doses of either metformin or pioglitazone suggests that the elevation in orexin concentrations is mainly due to the overall improvement in hyperglycemia and insulin resistance; it does not seem to depend on the specific drug’s mechanism of action.
Acknowledgments
Disclosures
The authors reported no conflict of interests.
References
- 1.De Lecea L, Kilduff T, Peyron C, Gao XB, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett FN. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalsbeek A, Yi CX, La Fleur SE, Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab. 2010;21(7):402–410. doi: 10.1016/j.tem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, Mignot E. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27(4):619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121(6):2133. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, van Dijk KW, Romijn JA. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62(4):1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrzypski M, Khajavi N, Mergler S, Billert M, Szczepankiewicz D, Wojciechowicz T, Nowak KW, Strowski MZ. Orexin A modulates INS-1E cell proliferation and insulin secretion via extracellular signal-regulated kinase and transient receptor potential channels. J Physiol Pharmacol. 2016;67(5):643–652. [PubMed] [Google Scholar]
- 8.Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T, Sasaoka T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51(4):657–667. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 10.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264(1):101–104. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- 11.Otlivanchik O, Le Foll C, Levin BE. Perifornical hypothalamic orexin and serotonin modulate the counterregulatory response to hypoglycemic and glucoprivic stimuli. Diabetes. 2015;64(1):226–235. doi: 10.2337/db14-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouedraogo R, Näslund E, Kirchgessner AL. Glucose regulates the release of orexin-a from the endocrine pancreas. Diabetes. 2003;52(1):111–117. doi: 10.2337/diabetes.52.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Heinonen M, Purhonen A, Mäkelä K, Herzig K. Functions of orexins in peripheral tissues. Acta Physiol (Oxf) 2008;192(4):471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakabayashi M, Suzuki T, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Date F, Takeyama J, Darnel AD, Moriya T. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol. 2003;205(1):43–50. doi: 10.1016/s0303-7207(03)00206-5. [DOI] [PubMed] [Google Scholar]
- 15.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289(1):219–223. [PubMed] [Google Scholar]
- 16.Kirchgessner AL, Liu MT. Orexin Synthesis and Response in the Gut. Neuron. 1999;24(4):941–951. doi: 10.1016/s0896-6273(00)81041-7. [DOI] [PubMed] [Google Scholar]
- 17.Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK. Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci. 2000;66(5):449–454. doi: 10.1016/s0024-3205(99)00611-6. [DOI] [PubMed] [Google Scholar]
- 18.Ehrström M, Näslund E, Levin F, Kaur R, Kirchgessner A, Theodorsson E, Hellström P. Pharmacokinetic profile of orexin A and effects on plasma insulin and glucagon in the rat. Regul Pept. 2004;119(3):209–212. doi: 10.1016/j.regpep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Ehrström M, Gustafsson T, Finn A, Kirchgessner A, Grybäck P, Jacobsson H, Hellström P, Näslund E. Inhibitory effect of exogenous orexin a on gastric emptying, plasma leptin, and the distribution of orexin and orexin receptors in the gut and pancreas in man. J Clin Endocrinol Metab. 2005;90(4):2370–2377. doi: 10.1210/jc.2004-1408. [DOI] [PubMed] [Google Scholar]
- 20.Tsuneki H, Tokai E, Nakamura Y, Takahashi K, Fujita M, Asaoka T, Kon K, Anzawa Y, Wada T, Takasaki I. et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes. 2015;64(2):459–470. doi: 10.2337/db14-0695. [DOI] [PubMed] [Google Scholar]
- 21.Esteghamati A, Noshad S, Rabizadeh S, Ghavami M, Zandieh A, Nakhjavani M. Comparative effects of metformin and pioglitazone on omentin and leptin concentrations in patients with newly diagnosed diabetes: a randomized clinical trial. Regul Pept. 2013;182:1–6. doi: 10.1016/j.regpep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, Haghazali M, Asgari F. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007) Nutr Metab (Lond) 2010;7(26):2–8. doi: 10.1186/1743-7075-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–88. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam J, Menheere P, Van Dielen F, Soeters P, Buurman W, Greve J. Decreased plasma orexin-A levels in obese individuals. Int J Obes. 2002;26:274–276. doi: 10.1038/sj.ijo.0801868. [DOI] [PubMed] [Google Scholar]
- 27.Heinonen MV, Purhonen AK, Miettinen P, Pääkkönen M, Pirinen E, Alhava E, Åkerman K, Herzig KH. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130(1-2):7–13. doi: 10.1016/j.regpep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Shim HM, Na AY, Bae JH, Im SS, Song DK. Orexin A regulates plasma insulin and leptin levels in a time-dependent manner following a glucose load in mice. Diabetologia. 2015;58(7):1542–1550. doi: 10.1007/s00125-015-3573-0. [DOI] [PubMed] [Google Scholar]
- 29.Tsuneki H, Sugihara Y, Honda R, Wada T, Sasaoka T, Kimura I. Reduction of blood glucose level by orexins in fasting normal and streptozotocin-diabetic mice. Eur J Pharmacol. 2002;448(2-3):245–252. doi: 10.1016/s0014-2999(02)01936-2. [DOI] [PubMed] [Google Scholar]
- 30.Skrzypski M, Le T, Kaczmarek P, Pruszynska-Oszmalek E, Pietrzak P, Szczepankiewicz D, Kolodziejski PA, Sassek M, Arafat A, Wiedenmann B. et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia. 2011;54(7):1841–1852. doi: 10.1007/s00125-011-2152-2. [DOI] [PubMed] [Google Scholar]
- 31.Harada S, Yamazaki Y, Tokuyama S. Orexin-A suppresses postischemic glucose intolerance and neuronal damage through hypothalamic brain-derived neurotrophic factor. J Pharmacol Exp Ther. 2013;344(1):276–285. doi: 10.1124/jpet.112.199604. [DOI] [PubMed] [Google Scholar]
- 32.Harada S, Yamazaki Y, Koda S, Tokuyama S. Hepatic branch vagus nerve plays a critical role in the recovery of post-ischemic glucose intolerance and mediates a neuroprotective effect by hypothalamic orexin-A. Plos One. 2014;9(4):e95433. doi: 10.1371/journal.pone.0095433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]