Abstract
Campylobacteriosis is currently the most frequent zoonosis in humans and the main source of infection is contaminated poultry meat. As chickens are a natural host for Campylobacter species, one strategy to prevent infection in humans is to eliminate these bacteria on poultry farms. A study was conducted to evaluate the probiotic potential of 46 Lactobacillus isolates from chickens faeces or cloacae. All lactobacilli were able to produce active compounds on solid media with antagonistic properties against C. jejuni and C. coli, with L. salivarius and L. reuteri exhibiting particularly strong antagonism. The cell-free culture supernatants had a much weaker inhibitory effect on the growth of Campylobacter, and the neutralization of organic acids caused them to completely lose their inhibitory properties. The ability to produce H2O2 was exhibited by 93% of isolates; most of isolates had a hydrophobic surface, showed excellent survival at pH 2.0 or 1.5, and displayed tolerance to bile; 50% isolates displayed the ability to biofilm formation. Determination of MICs of various antibiotics showed that as much as 80.4% of Lactobacillus isolates were resistant to at least one antimicrobial agent. Seven ultimately selected isolates that met all the basic criteria for probiotics may have potential application in reducing Camylobacter spp. in chickens and thus prevent infections in both birds and humans.
Keywords: antibacterial activity, Campylobacter, chicken, Lactobacillus, probiotics
Campylobacter are Gram-negative, microaerophilic bacteria widespread throughout the world, causing a zoonotic disease in humans known as campylobacteriosis. It has been the most commonly reported gastrointestinal bacterial infection in humans in the E.U. since 2005, and over the last decade a significant upward trend has been observed. In 2013, the number of reported confirmed cases of human campylobacteriosis was 214,779, with an E.U. notification rate of 64.8 per 100,000 population (while there were 82,694 reported cases of salmonellosis) [13]. Campylobacteriosis in humans is induced mainly by Campylobacter jejuni (about 90% of cases), and the remaining fraction is induced predominantly by Campylobacter coli [18]. The characteristic symptoms of the disease are watery-mucous diarrhoea, often with blood in the stool, nausea, vomiting, abdominal pain, and fever lasting up to seven days or longer. Although such infections are generally self-limiting, serious consequences may arise, including bacteraemia, Guillain–Barré syndrome (GBS)−an autoimmune disease affecting the peripheral nervous system, reactive arthritis, inflammatory bowel disease, and irritable bowel syndrome [15]. The current cost associated with treating acute C. jejuni infections and GBS is estimated to be 2.4 billion € in the E.U. and $1.2 billion per year in the U.S.A. [27]. Campylobacter can be transmitted human-to-human by the faecal-oral route, but zoonotic or foodborne transmission predominates. Bacteria of the genus Campylobacter are widespread in the environment and have been detected in various animal reservoirs, but their prevalence is particularly high in chickens. Colonization of the gastrointestinal tract by Campylobacter usually does not produce any disease symptoms in chickens, although some studies have reported that challenged birds may exhibit distention of the jejunum, disseminated hemorrhagic enteritis or focal hepatic necrosis [35]. Raw poultry meat is often contaminated with Campylobacter, and eating undercooked chicken, or ready-to-eat foods that have been in contact with raw chicken, is the most common source of infection. In 2013, the presence of Campylobacter was detected in 31.4% of samples of fresh broiler meat collected at slaughter, during processing and at retail facilities in various countries of the E.U., and the pathogens were found at every sampling stage [13].
Many countries are currently working to prevent foodborne campylobacteriosis, and considerable progress has been made on numerous fronts during the past ten years. In 2011, the EFSA (European Food Safety Authority) Panel on Biological Hazards issued advice on reducing Campylobacter in chicken meat. Recommendations include controlling Campylobacter in primary broiler production before the bacteria spread from farms to humans by various pathways and implementation of GMP-HACCP during slaughter [11]. These measures may reduce colonization of broilers with Campylobacter and contamination of carcasses. Quantitative risk assessment based on data from several countries has concluded that there is a linear relationship between prevalence of Campylobacter in broiler flocks and public health risk. Campylobacter jejuni colonizes chickens at densities of 108 colony forming units (CFU)/gram of caecal contents or higher without causing disease. These bacteria rapidly spread throughout the flock and remain present throughout the bird’s lifespan. It is estimated that reducing the numbers of Campylobacter in the intestines at slaughter by 3 log10-units would reduce the public health risk by at least 90% [11, 27].
One of the strategies aimed at reducing the carriage of Campylobacter spp. among poultry includes the use of probiotic microorganisms that compete with pathogenic bacteria for colonization of the gut. The administration of probiotics is advantageous as compared to other strategies aimed at eliminating unwanted microflora (e.g. vaccination, antibiotic treatment, or chemical disinfection), as they are easy to administer and inexpensive to produce, and because they may persist in the animal. Bacteria of the genus Lactobacillus are recognized candidates for probiotics. They are non-pathogenic rods that naturally inhabit the mucous membranes of humans and animals, including chickens, and play an important beneficial role in the physiology of the host by providing a protective barrier in the gut [8]. Probiotic lactobacilli may eliminate unfavorable microflora by several possible mechanisms, including production of inhibitory substances, such as organic acids, hydrogen peroxide, bacteriocins and carbon peroxide, blocking of adhesion sites on intestinal epithelial surfaces, competition for nutrients, and stimulation of immunity. These beneficial properties of lactobacilli are largely dependent on their prolonged residence in the gastrointestinal tract and are dictated by adherence to the intestinal mucosa [22].
The objective of this study was to evaluate the probiotic potential of Lactobacillus isolates from chickens, with particular emphasis on their ability to inhibit the growth of C. jejuni and C. coli in vitro.
MATERIALS AND METHODS
Lactobacillus isolates
A total of 46 Lactobacillus isolates from the fresh faeces or cloacae of 17 healthy chickens (broilers and Green-legged Partridge hens) from 5 poultry farms located in Poland were included in this study. Lactobacilli were isolated on deMan Rogosa Sharpe medium (MRS, BTL, Łódź, Poland) at 37°C for 48 hr in 5% CO2 and identified to species level by MALDI-TOF mass spectrometry (MS) and in the case of ambiguous results, the 16S rDNA restriction analysis was additionally used, as previously described [5].
Campylobacter isolates
Campylobacter bacteria used in the experiment to evaluate the antibacterial activity of Lactobacillus sp. were isolated from the cloacae of 10 chickens exhibiting no disease symptoms, obtained from 3 poultry farms. Bacteria were cultured in microaerophilic atmosphere (5% O2, 10% CO2 and 85% N), as it was described in our previous paper [10]. Campylobacter isolates were identified using MALDI-TOF MS [10] and the multiplex PCR technique with species-specific primers according to protocol developed by Wieczorek and Osek [33]. Two reference Campylobacter strains-C. jejuni ATCC 29428 and C. coli ATCC 43479, were used as controls.
Detection of antibacterial activity of Lactobacilli-agar slab method
The suspensions of Lactobacillus isolates with an optical density of 0.5 measured at 600 nm (OD600=0.5, in 0.9% NaCl) were seeded onto MRS agar and incubated at 37°C in 5% CO2 for 24 hr. Then agar slabs (9 mm in diameter) were cut and placed on Columbia agar with 5% sheep blood inoculated with 0.5 ml of the Campylobacter indicator strain (OD600=0.5, in 0.9% NaCl). The plates were kept at 41.5°C in microaerophilic conditions. After 45 hr of incubation the plates were checked for inhibition zones. The experiment was performed in duplicate and the results are presented as the mean diameter of the inhibition zone ± SD [6].
Detection of antibacterial activity of Lactobacilli-well diffusion method
Lactobacillus isolates were grown in 1.2 ml of MRS broth for 24 hr (37°C, 5% CO2). The bacteria were separated from the medium by centrifugation and then each sample of medium was divided into 2 equal volumes. In half of the samples the pH was adjusted to 6.8–7.0 using 19 M NaOH. The Campylobacter isolates were inoculated on Columbia agar with 5% sheep blood according to the protocol described above. Cylindrical metal wells 8 mm in diameter were placed on the plates and filled with 100 µl of the cell-free supernatants. After 45 hr of incubation at 41.5°C in microaerophilic conditions, the plates were checked for inhibition zones [4].
Detection of H2O2 production by Lactobacilli
The ability of lactobacilli to produce H2O2 was determined by culture them on MRS agar supplemented with TMB (3,3′,5,5′-Tetramethylbenzidine, Sigma-Aldrich, Poznań, Poland) substrate (0.25 mg/ml,) and horseradish peroxidase (0.01 mg/ml, Sigma-Aldrich, Poznań, Poland). Inoculated plates were kept for 48 hr at 37°C, 5% CO2. Blue color of colonies indicated H2O2 production by the bacteria. Color intensity was designated as follows: –, +, ++, +++.
Measurement of bacterial hydrophobicity
Hydrophobicity of the bacteria was determined on the basis of microbial adhesion to hydrocarbon [4]. Isolates with hydrophobicity equal to or more than 50% were considered hydrophobic.
Tolerance for acidic pH
Lactobacillus isolates grown overnight on MRS broth were centrifuged at 10,000 × g for 5 min. Pellets were resuspended in 0.9% NaCl (OD600=3.0) and 30 µl of the suspension was added to 470 µl of MRS broth with pH 1.5, 2.0 or 6.8 (positive control). The bacteria were incubated for 60, 90 or 120 min at 37°C. Then the suspensions were centrifuged and the pellets were resuspended in fresh MRS medium (pH 6.8). Growth of the surviving bacteria was observed after 48 hr of culture at 37°C, 5% CO2.
Bile tolerance test
The tolerance of Lactobacillus isolates to bile salts was determined in microplate assay. MRS medium (200 µl) containing 2% bile (BTL, Łódź, Poland) was inoculated with 0.5 µl of fresh broth cultures of lactobacilli. Following 24 hr incubation at 37°C, 5% CO2, the optical density of the bacterial cultures was measured at 620 nm. Positive controls were bacterial cultures that grew without ox gall. The growth of each strain was expressed as a percentage of the OD620 value of the control samples.
Biofilm formation
Lactobacillus isolates cultured in MRS medium for 18–20 hr were diluted 1:50 in MRS broth to final volume of 200 µl in 96-well plates (MaxiSorb, Biokom, Janki, Poland). The bacteria were incubated 48 hr at 37°C, 5% CO2. Adherent cells were stained with crystal violet (1% w/v) for 15 min. Unbound dye was washed off with water, and cell-bound dye was dissolved in 20% acetone in ethanol for 10 min, and the absorbance (A570) was measured using a Microplate Reader 680 (Bio-Rad, Warszawa, Poland). Isolates were classified as the following four criteria: no biofilm producer (–: OD ≤ODc), weak biofilm producer (+: ODc <OD ≤2 × ODc), moderate biofilm producer (++: 2 × ODc <OD ≤4 × ODc) and strong biofilm producer (+++: 4 × ODc <OD), where the cut-off OD (ODc) was defined as the mean OD of the negative control.
Determination of minimal inhibitory concentration
Antibiotic susceptibility Lactobacillus isolates was determined by the broth microdilution assay using the LSM medium [7]. Inocula were prepared by suspending bacteria in 0.9% NaCl (OD600=0.5). Microdilution plates were inoculated with 50 µl of a 1:500-diluted (in LSM broth) inoculum and 50 µl of the appropriate antibiotic concentration. Plates were incubated 48 hr at 37°C in 5% CO2. The values of minimal inhibitory concentration (MIC) were read as the lowest concentration of an antimicrobial agent at which visible growth was inhibited.
Interpretation of the results for the antibiotic susceptibility of Lactobacillus isolates was based on the breakpoint values suggested by EFSA’s Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) [12] for ampicillin, tetracycline, erythromycin, streptomycin, gentamycin and chloramphenicol. For lincomycin we established cut-offs based on the distribution of MICs (bimodal/unimodal) and the breakpoint values suggested by Cauwerts et al. [3]−isolates were considered resistant if the lincomycin MIC was ≥32 µg/ml.
Statistical analysis
The mean diameters of the inhibition zones for Campylobacter isolates determined to be sensitive to various Lactobacillus species were compared by one-way analysis of variance. Normal distribution of data was tested using the Shapiro-Wilk test and the equality of variance was tested by the Brown-Forsythe test. Due to a lack of normal distribution and/or unequal variance of data, Kruskal-Wallis analysis of variance was used to analyse the differences between means. A level of P<0.05 was considered statistically significant. All statistical analyses were carried out using Statistica 10.0 software (StatSoft, Inc., Tulsa, OK, U.S.A.).
RESULTS
Identification of Lactobacillus and Campylobacter isolates
Lactobacillus bacteria were obtained from all samples, and 2–6 isolates of varying colony morphology were collected from each sample. A total of 46 isolates were classified by MALDI-TOF MS as bacteria of the genus Lactobacillus with a Biotyper log (score) equal to or greater than 1.70. Eight isolates, for which the two best matches with similar log (score) values (difference in log (score) <0.15) indicated different species, i.e. L. johnsonii and L. gasseri, were subjected to additional identification by restriction analysis of 16S rDNA using the MseI restriction enzyme. The study showed that eight isolates pre-classified as L. johnsonii/L. gasseri belonged to L. johnsonii species (data not showed).
Finally, the collected Lactobacillus isolates were classified into seven species, i.e. L. salivarius (n=15), L. johnsonii (n=11), L. crispatus (n=5), L. ingluviei (n=5), L. reuteri (n=5), L. oris (n=2) and L. saerimneri (n=3).
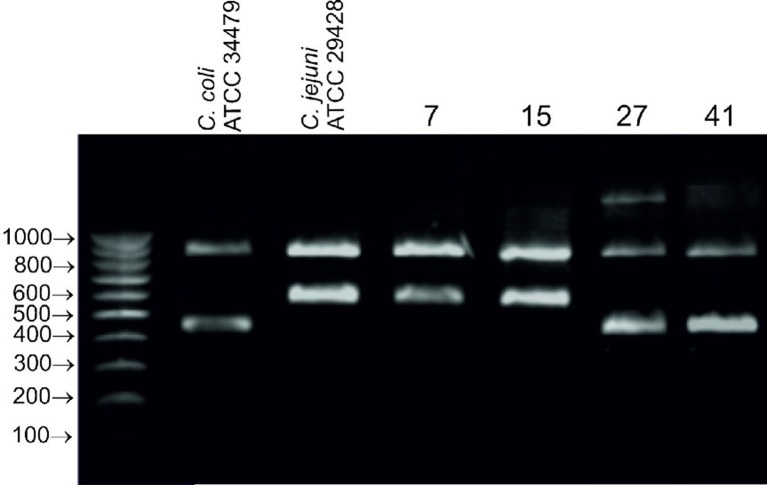
Campylobacter bacteria were isolated from 6 out of 10 samples, one isolate from each sample. Four isolates were identified as C. jejuni and 2 isolates as C. coli by MALDI-TOF MS (BioTyper log (score) values were greater than 2.00 for all isolates). The same identification results were obtained in species-specific PCR. The electrophoretic profiles of all isolates contained 2 bands-one corresponding to 16S rDNA (860 bp), and the other corresponding to the mapA gene (590 bp) coding for membrane-associated protein A specific for C. jejuni or the ceuE gene (460 bp) encoding an iron-chelating protein involved in siderophore transport specific for C. coli (Fig. 1). Two isolates of C. jejuni (7, 15) and two isolates of C. coli (27, 41) with different colony morphology were selected for further analysis.
Fig. 1.
Agarose gel (2%) showing the amplicon patterns obtained in multiplex PCR for reference and wild isolates (7, 15, 27, 41) of C. jejuni and C. coli; profiles contained the following amplicons: 16S rDNA 860 bp, mapA 590 bp, ceuE 460 bp.
Agar slab method
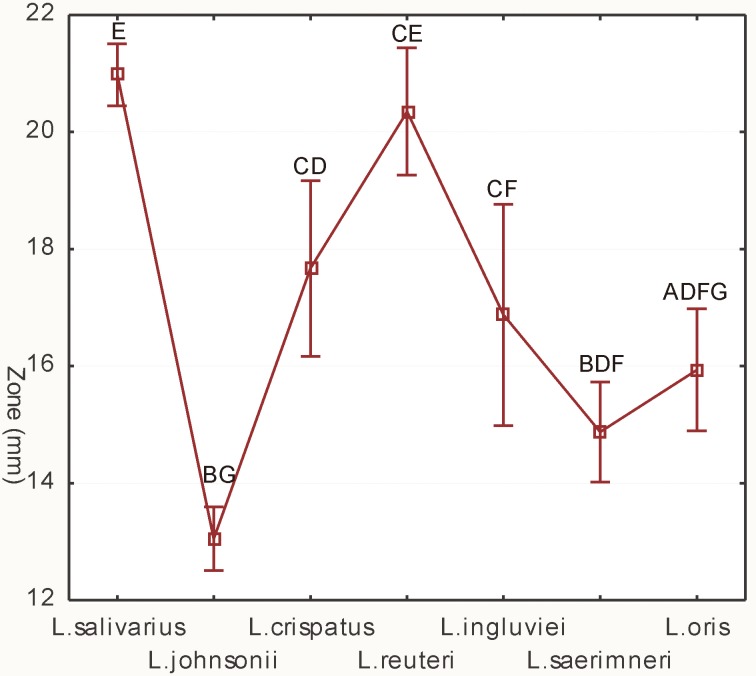
All Lactobacillus isolates tested showed an inhibitory effect towards Campylobacter (Supplementary Fig. 1). The diameter of the growth inhibition zones of the test bacteria induced by the lactobacilli ranged from 11.0 ± 0.0 to 26.5 ± 0.7 mm, where the diameter of the slab was 9 mm. The antimicrobial activity exhibited by lactobacilli was correlated with their species. The strongest inhibition of Campylobacter growth was exhibited by the isolates of L. salivarius (mean inhibitory zone 21.0 ± 2.0 mm) and L. reuteri (20.3 ± 2.3 mm). Isolates of the species L. johnsonii, L. saerimneri and L. oris exhibited weak antagonistic properties (≤15.9 ± 1.9 mm) (Fig. 2, Supplementary Table 1). However, individual isolates of some Lactobacillus species showed large differences in anti-Camylobacter activity. Particularly large heterogenity of the size of inhibitory zones was observed for isolates of the species L. crispatus and L. ingluviei (Table 1).
Fig. 2.
Effect of isolates of various Lactobacillus species on the growth of indicator bacteria, as determined by the agar slab method. Different capital letters (A–G) indicate significant differences (P<0.05) between mean diameters of growth inhibition zones caused by individual Lactobacillus species; vertical bars denote 0.95 CI.
Table 1. Anti-Campylobacter activity, tolerance to low pH and bile, hydrophobicity, production of H2O2 and biofilm formation of Lactobacillus isolates.
| Species | Strain | Results of agar slab method | Ability to survive at low pH | Ability to surviving in MRS + 2% bile | Ability to growth on MRS + 2% bile (%) | Ability to produce H2O2 | Hydrophobicity (%) | Biofilm formation | Resistance to any antibiotic | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average inhibition zone of C. jejuni (mm) | Average inhibition zone of C. coli (mm) | pH 1.5 | pH 2.0 | ||||||||||
| 1 hr | 3 hr | 1 hr | 3 hr | ||||||||||
| L. salivarius | 4a | 22.5 ± 2.1 | 21.0 ± 0.9 | + | + | + | + | + | no | – | 100 | +++ | + |
| 6a | 20.7 ± 1.1 | 18.5 ± 0.7 | + | + | + | + | – | no | + | 60 | – | + | |
| 6b | 21.7 ± 0.35 | 20.5 ± 1.4 | – | – | + | + | + | no | + | 100 | + | + | |
| 8b | 19.0 ± 0.0 | 18.5 ± 0.7 | + | + | + | + | + | no | + | 67 | – | + | |
| 9b | 22.2 ± 0.35 | 20.2 ± 1.1 | + | + | + | + | + | no | ++ | 60 | – | – | |
| 10a | 21.5 ± 2.1 | 20.0 ± 0.0 | + | + | + | + | + | no | + | 100 | – | + | |
| 10d | 18.2 ± 0.35 | 18.7 ± 1.1 | + | + | + | + | + | no | + | 100 | – | + | |
| 24a | 22.0 ± 0.0 | 20.0 ± 1.4 | + | + | + | + | + | no | + | 80 | + | + | |
| 24b | 22.5 ± 0.7 | 20.5 ± 0.7 | + | + | + | + | + | no | +++ | 100 | – | + | |
| 27e | 25.0 ± 0.0 | 23.5 ± 2.1 | + | + | + | + | + | 48.3 | ++ | 90 | + | + | |
| 37b | 23.5 ± 0.7 | 20.0 ± 0.0 | + | + | + | + | + | 6.5 | + | 100 | + | + | |
| 40a | 23.0 ± 0.0 | 18.5 ± 0.7 | + | + | + | + | + | 55.3 | + | 55 | – | + | |
| 48a | 23.7 ± 0.35 | 20.5 ± 0.7 | + | + | + | + | + | no | – | 100 | + | + | |
| 60b | 21 ± 1.4 | 19.2 ± 1.1 | + | + | + | + | + | no | – | 10 | + | + | |
| 60d | 23.5 ± 0.0 | 19.0 ± 0.0 | + | + | + | + | + | no | ++ | 100 | +++ | – | |
| L. johnsonii | 4b | 12.5 ± 0.7 | 13.5 ± 0.7 | + | + | + | + | – | no | +++ | 100 | + | – |
| 7d | 18.5 ± 0.7 | 16.5 ± 2.1 | + | + | + | + | + | 28.0 | +++ | 87 | ++ | + | |
| 8f | 14.5 ± 0.7 | 13.5 ± 0.7 | + | + | + | + | + | no | +++ | 100 | + | – | |
| 9d | 12.2 ± 0.35 | 12.5 ± 0.7 | + | + | + | + | – | no | +++ | 50 | + | – | |
| 13b | 12.0 ± 0.0 | 12.0 ± 0.0 | + | + | + | + | + | no | +++ | 70 | + | + | |
| 17c | 12.5 ± 0.7 | 12.5 ± 0.7 | + | + | + | + | + | no | +++ | 80 | + | + | |
| 25b | 11.7 ± 0.35 | 11.7 ± 0.3 | + | + | + | + | + | no | +++ | 60 | – | + | |
| 32a2 | 14.0 ± 1.4 | 13.4 ± 0.9 | + | + | + | + | + | no | +++ | 100 | – | + | |
| 37c | 11.5 ± 0.7 | 12.0 ± 0.0 | + | + | + | + | + | no | +++ | 70 | – | + | |
| 40b | 11.5 ± 0.7 | 11.5 ± 0.7 | + | + | + | + | + | no | +++ | 87 | + | + | |
| 44c | 14.0 ± 0.0 | 13.0 ± 0.0 | + | + | + | + | + | no | +++ | 100 | + | + | |
| L. crispatus | 1b | 15.0 ± 1.4 | 15.0 ± 0.7 | + | – | + | + | + | 23.0 | ++ | 100 | – | + |
| 17b | 18.2 ± 0.35 | 15.0 ± 1.4 | + | + | + | + | + | 8.9 | ++ | 80 | – | + | |
| 44b | 16.0 ± 0.0 | 17.0 ± 1.4 | + | + | + | + | + | no | ++ | 100 | – | + | |
| 49a | 22.2 ± 0.9 | 22.0 ± 0.8 | + | + | + | + | + | no | + | 100 | – | + | |
| 49b | 14.0 ± 1.4 | 13.2 ± 1.1 | + | + | + | + | + | 14.9 | +++ | 73 | – | – | |
| L. reuteri | 4c | 22.0 ± 0.0 | 19.0 ± 1.4 | + | + | + | + | + | 54.6 | ++ | 100 | ++ | + |
| 12d | 19.5 ± 0.7 | 18.0 ± 1.4 | + | + | + | + | + | 28.6 | +++ | 73 | + | + | |
| 14a | 24.5 ± 0.7 | 22.0 ± 1.4 | + | – | + | + | + | 69.0 | + | 89 | ++ | + | |
| 43a | 19.7 ± 2.5 | 17.2 ± 0.3 | + | + | + | + | + | 16.0 | ++ | 90 | – | + | |
| 45b | 22.0 ± 0.0 | 19.5 ± 0.7 | + | + | + | + | + | 26.4 | ++ | 100 | +++ | + | |
| L. ingluviei | 9e | 14.25 ± 0.5 | 12.4 ± 0.5 | + | – | + | – | + | no | +++ | 100 | – | – |
| 14e | 12.0 ± 0.0 | 12.0 ± 1.4 | + | + | + | + | + | no | ++ | 100 | – | + | |
| 18b | 14.0 ± 0.0 | 12.0 ± 0.0 | + | – | + | + | + | no | +++ | 100 | + | + | |
| 28c | 23.7 ± 0.3 | 21.7 ± 1.2 | + | + | + | + | + | no | +++ | 95 | – | + | |
| 43d | 22.7 ± 0.3 | 19.25 ± 0.3 | + | + | + | + | + | no | +++ | 100 | – | – | |
| L. saerimneri | 7b | 16.5 ± 0.7 | 15.5 ± 0.0 | + | – | + | + | + | no | +++ | 100 | – | + |
| 24c | 14.2 ± 1.1 | 14.0 ± 1.4 | – | – | + | + | – | no | +++ | 100 | – | + | |
| 44d | 15.7 ± 0.3 | 13.2 ± 1.1 | + | + | + | + | – | no | +++ | 100 | – | + | |
| L. oris | 40g | 16.7 ± 0.5 | 13.7 ± 1.5 | + | + | + | + | + | 90.5 | +++ | 91 | + | + |
| 50c | 17.9 ± 0.6 | 15.4 ± 1.9 | + | + | + | + | + | 88.4 | +++ | 100 | ++ | – | |
| Mean: | 18.3 ± 4.3 | 16.7 ± 3.7 | |||||||||||
Grey highlights indicate strains that meet all basic criteria for probiotics.
C. jejuni isolates tended to be more susceptible (mean inhibition zone 18.3 ± 4.3 mm) than C. coli ones (16.7 ± 3.7 mm) to the antagonistic substances produced by lactobacilli, but the differences were not statistically significant (Table 1, Supplementary Fig. 2).
The Lactobacillus isolates which showed the strongest antagonism towards the Campylobacter bacteria were chosen. Among the 10 selected isolates, which caused inhibition zones of ≥22 mm in the case of C. jejuni and ≥20 mm for C. coli, 7 belonged to the species L. salivarius (4a, 9b, 24a, 24b, 27e, 37b, 48a), one to the species L. crispatus (49a), one to the species L. reuteri (14a) and one to the species L. ingluviei (28c) (Table 1).
Well diffusion method
The pH of the cell free media obtained from the 24 hr culture of Lactobacillus isolates ranged from 3.0 to 4.5. The inhibition of growth of Campylobacter bacteria by native cell-free broth was much weaker than the inhibition observed in the agar slab method. The size of the inhibition zones caused by native acidified supernatants was up to 16.6 ± 0.5 mm for C. jejuni and 16.5 ± 0.7 mm for C. coli, where the well diameter was 8 mm. Cell-free supernatants with neutralized acids (pH 6.5–7.0) did not exhibit antagonistic activity towards the indicator bacteria (data not showed). Statistical analysis (Kruskal-Wallis test) showed no significant difference between the inhibitory effect of the cell-free culture supernatants of different species of Lactobacillus.
Production of H2O2
Among 46 Lactobacillus isolates tested, 43 (93%) produced H2O2. The highest rate of production (+++) was observed in 22 isolates, belonging mainly to the species L. johnsonii, L. ingluviei, L. saerimnerii and L. oris. Moderate hydrogen peroxide production (++) was noted in 11 isolates of different species, and the group with the lowest H2O2 production (+) comprised 10 isolates, including 8 isolates of L. salivarius, one isolate of L. crispatus and one of L. reuteri (Table 1, Supplementary Fig. 3).
Hydrophobicity
Forty-five out of 46 Lactobacillus isolates showed high affinity towards xylene. Their percentage of hydrophobicity (% H) was ≥50, and therefore these isolates were considered hydrophobic. Only one isolate (L. salivarius 60b) proved to be hydrophilic, as its % H was 10. For 69.6% Lactobacillus isolates the % H was as high as 87–100%, and for 30.4% it ranged between 50 and 80% (Table 1).
Resistance to low pH
Lactobacillus isolates showed excellent survival rates at pH values as low as 1.5 and 2.0. Forty-four out of 46 Lactobacillus isolates were able to survive pH 1.5 for 1 hr and 39 isolates survived as long as 3 hr at this pH. At pH 2.0, all Lactobacillus isolates survived 2-hr incubation, and only one (L. ingluviei 9e) was unable to survive 3 hr (Table 1).
Resistance to bile
Forty-one (89%) out of 46 Lactobacillus isolates tested were able to survive for 24 hr in the presence of 2% bile. Moreover, 13 isolates of various species demonstrated growth (8.9–90.5%) in the MRS broth containing 2% bile. The highest resistance to bile was demonstrated by 2 isolates of L. oris, whose growth intensity in the medium supplemented with bile salts was similar (~90%) to that of in a medium without bile (100%) (Table 1).
In vitro biofilm formation
Among the 46 isolates tested, 23 showed biofilm formation. Three isolates (L. salivarius 4a and 60d and L. reuteri 45b) were classified as strong biofilm-producers (+++), 4 isolates (L. johnsonii 7d, L. reuteri 4c and 14a and L. oris 50c) as moderate biofilm-producers (++) and 16 isolates of different species showed week ability (+) to biofilm formation. None of the isolates of L. crispatus and L. saerimneri exhibited the ability to biofilm formation (Table 1).
Antibiotic susceptibility
According to established criteria, 37 isolates (80.4%) were resistant to at least one antimicrobial agent, and 18 (39.1%) isolates showed multidrug resistance (resistance to 3 or more antibiotic groups). Only 9 (19.5%) Lactobacillus isolates, which were susceptible to all antibiotics tested, met the criteria for bacterial feed additives (L. salivarius 9b and 60d, L. johnsonii 4b, 8f, 9d, L. ingluviei 9e and 43d, L. crispatus 49b and L. oris 50c) (Table 2).
Table 2. Distribution of MICs of antibiotics tested among Lactobacillus species of chicken origin.
| Strain | AMP | TET | ERY | LIN | STR | GEN | CHL | |
|---|---|---|---|---|---|---|---|---|
| L. salivarius | 4a | 2 | 8 | 0.5 | 256 | >512 | 8 | 4 |
| 6a | 2 | 128 | 0.5 | 2 | 64 | 8 | 4 | |
| 6b | 0.5 | 128 | 0.5 | 2 | 64 | 8 | 4 | |
| 8b | 1 | 64 | 0.5 | 4 | 64 | 8 | 8 | |
| 9b | 1 | 2 | 0.5 | ≤2 | 64 | 8 | 4 | |
| 10a | 0.5 | 256 | >64 | >1,024 | 128 | 16 | 4 | |
| 10d | 1 | 256 | >64 | 1,024 | 64 | 8 | 8 | |
| 24a | 1 | 512 | >64 | 1,024 | 64 | 8 | 8 | |
| 24b | 4 | 512 | >64 | 1,024 | >1,024 | 256 | 8 | |
| 27e | 8 | 256 | >64 | 128 | 128 | 512 | 8 | |
| 37b | 2 | 256 | 0.5 | 32 | 64 | 8 | 4 | |
| 40a | 2 | 256 | 0.5 | 4 | 64 | 8 | 4 | |
| 48a | 1 | 2 | ≤0.125 | 4 | 128 | 16 | 2 | |
| 60b | 1 | 1 | 0.5 | 8 | 128 | 4 | 8 | |
| 60d | 1 | ≤1 | 0.25 | 4 | 64 | 8 | 4 | |
| L. johnsonii | 4b | 2 | ≤1 | ≤0.125 | 4 | 4 | 8 | 4 |
| 7d | 2 | ≤1 | ≤0.125 | 4 | 32 | 8 | 2 | |
| 8f | 2 | 2 | ≤0.125 | 4 | 4 | 4 | 4 | |
| 9d | 1 | ≤1 | ≤0.125 | 8 | ≤2 | 8 | 2 | |
| 13b | 1 | 64 | >64 | 1,024 | 32 | 4 | 4 | |
| 17c | 0.5 | 64 | >64 | 512 | 32 | 8 | 1 | |
| 25b | 2 | 256 | >64 | 128 | 16 | 8 | 4 | |
| 32a2 | 1 | 4 | >64 | 1,024 | ≤2 | 8 | 4 | |
| 37c | >64 | 128 | ≤0.125 | 8 | ≤2 | 4 | 4 | |
| 40b | >64 | 256 | >64 | 512 | 4 | 8 | 4 | |
| 44c | 1 | 128 | 0.25 | 256 | 4 | 4 | 8 | |
| L. crispatus | 1b | 1 | 2 | ≤0.125 | 8 | 8 | 64 | 4 |
| 3b | 1 | 64 | ≤0.125 | 64 | 16 | 4 | 2 | |
| 17b | 4 | 256 | >64 | 512 | ≤2 | 4 | 2 | |
| 44b | 4 | 64 | ≤0.125 | ≤2 | ≤2 | 2 | 2 | |
| 49a | 0.5 | ≤1 | 0.25 | 8 | 64 | 32 | 4 | |
| 49b | 0.5 | 1 | ≤0.125 | 8 | 4 | 8 | 4 | |
| L. reuteri | 4c | 8 | 16 | 1 | 256 | 64 | 8 | 4 |
| 12d | 1 | 256 | >64 | 512 | 16 | ≤1 | 4 | |
| 14a | 2 | 256 | ≤0.125 | 128 | 128 | 4 | 4 | |
| 43a | 2 | 512 | >64 | 128 | 8 | ≤1 | 16 | |
| 45b | 1 | 256 | 0.25 | 64 | 128 | ≤1 | 4 | |
| L. ingluviei | 9e | 0.5 | 8 | 0.25 | <0.5 | 64 | 8 | 4 |
| 14e | 2 | 512 | ≤0.125 | 32 | 64 | 4 | 4 | |
| 18b | 1 | 256 | >64 | 512 | 64 | 8 | 4 | |
| 28c | 0.5 | 512 | 0.25 | 64 | 64 | 4 | 4 | |
| 43d | 0.5 | ≤1 | ≤0.125 | ≤2 | 64 | 8 | 4 | |
| L. saerimneri | 7b | 0.5 | ≤1 | ≤0.125 | ≤2 | 32 | 16 | 2 |
| 24c | 1 | 128 | ≤0.125 | 32 | 64 | 32 | 2 | |
| 44d | 1 | 64 | >64 | 128 | 128 | 32 | 4 | |
| L. oris | 40g | >64 | 256 | >64 | 512 | 128 | 8 | 4 |
| 50c | 0.5 | 1 | ≤0.125 | 8 | 64 | 8 | 4 | |
Grey highlights indicate resistance; ampicillin (AMP), tetracycline (TET), erythromycin (ERY), lincomycin (LIN), streptomycin (STR), gentamicin (GEN) and chloramphenicol (CHL).
DISCUSSION
In the present study we demonstrated the probiotic potential of Lactobacillus isolates from chickens. In vitro tests showed that most of the lactobacilli were able to inhibit the growth of Campylobacter and survive the transit through the stomach and duodenum. Additionally, the high hydrophobicity of the isolates may indicate their ability to adhere to the mucosa, enabling them to colonize the intestine.
The use of the agar slab method allowed to shown that lactobacilli have an inhibitory effect on the growth of Campylobacter, which is the result of the production of certain antimicrobial substances on the solid medium. The strongest antagonism towards pathogenic bacteria was exhibited by L. salivarius and L. reuteri, as well as single isolates of L. ingluviei and L. crispatus. Antimicrobial in vitro activity of Lactobacillus bacteria from chickens against Campylobacter has been also observed by other authors, and similar to our studies, the most active isolates were generally L. salivarius and L. reuteri [23, 25, 36].
To determine the mechanism of the antimicrobial activity of Lactobacillus bacteria, we analyzed the activity of cell-free broth and the ability of the isolates to synthesize hydrogen peroxide. The results of the well diffusion method indicated that the reduced pH of the supernatant (due to lactic acid) play a key role in inhibiting pathogenic bacteria. The antimicrobial activity of organic acids produced by lactobacilli against Campylobacter has been observed by several authors. Neal-McKinney et al. [26] showed that lactic acid disrupts the membrane of C. jejuni and is responsible for inhibiting the growth of these bacteria in vitro and for reducing intestinal colonization in chickens. Antagonistic anti-Campylobacter activity of lactic and acetic acid produced by heterofermentative L. pentosus CWBI B78 has been demonstrated by Dubois-Dauphin et al. [9], while Bratz et al. [2] showed that anti-Campylobacter activity of cell-free supernatants of L. fermentum ATCC 1493, L. johnsonii BFE 663 and L. paracasei IMT 22353 was pH-dependent (pH<4.3). Similar findings were reported by Wang et al. [32], who demonstrated that the cell-free supernatant of selected lactic acid bacteria (LAB) characterized by the highest bactericidal capacity contained a high concentration of organic acid, and their inhibition effects against C. jejuni were pH sensitive. Several other authors have demonstrated that lactic acid may be effective in eliminating contamination of poultry meat by Campylobacter. Van Netten et al. [31] reported that decontamination with 1% lactic acid at pH 3.0 for at least 30 sec. was effective for C. jejuni, and Rasschaert et al. [28] showed that immersing the carcasses in a 1.5% lactic acid solution was far more effective in reducing the number of Campylobacter bacteria than spraying them with this acid. Protective effects of Lactobacillus strains of avian origin, especially L. salivarius, against C. jejuni in chickens have also been observed in experiments using animal models [17, 20, 24, 34].
Elimination of Campylobacter by probiotic LAB strains depends not only on production of antimicrobial substances, but also on the ability of these bacteria to adhere to the intestinal epithelium. The adhesion mechanism involves passive forces and electrostatic and hydrophobic interaction, as well as specific binding dependent on bacterial surface adhesins [19]. Our research showed that most of the Lactobacillus isolates were characterized by high hydrophobicity and several researchers have reported a positive correlation between hydrophobicity of Lactobacillus strains and their adhesion to epithelial cells [1, 14, 21, 30]. It has been demonstrated [16] that colonization of the caecum in chickens by Campylobacter sp. was reduced by adhesion of human probiotic strains to mucous in the chicken gut. Similar conclusions can be drawn from a study by Wang et al. [32], who showed in vitro that adhesion of Campylobacter to intestinal cells (HT-29) was inhibited by four selected L. plantarum and L. casei strains. The hydrophobicity of these isolates ranged from 38% to 56%, and L. casei ZL4, characterized by the highest hydrophobicity, displayed excellent ability to inhibit C. jejuni invasion in exclusion assays.
The results of the present study showed that Lactobacillus sp. isolates originating in chickens produce H2O2. However, production of this reactive oxygen species was not clearly correlated with the antimicrobial activity of lactobacilli observed in the agar slab method. All isolates of L. johnsonii, L. ingluviei and L. oris exhibited strong production of hydrogen peroxide, but their inhibition of the growth of C. jejuni and C. coli was generally weak. Moreover, some of the L. salivarius isolates that most strongly inhibited the growth of pathogens were marked as ‘+’ or ‘–’ in terms of H2O2 production. The lack of relationship between antimicrobial activity of poultry lactobacilli and the intensity of H2O2 production, were also observed in our previous research [4, 6].
Biofilm formation is an important feature for LAB enabling them to resist environmental conditions, leading to the successful colonization and maintenance of their population while displacing unfavorable microflora. A fundamental characteristic of biofilms is the production of an extracellular polysaccharide matrix, which provides protection against antibiotics and enzymes and supports the generation of a microenvironment for the metabolic interaction of the population [29]. We have shown that some lactobacilli have the ability to biofilm formation, but it should be taken into account that this activity depends on many factors, including environmental parameters such as texture of surface (rough or smooth), hydrophobicity, pH, nutrient concentration and temperature, and thus it may differ in vivo [29].
Despite the anti-Campylobacter activity and properties determining survival in the intestine, the majority of the Lactobacillus isolates tested cannot be directly used as feed additives due to their antibiotic resistances. According to the EFSA’s FEEDAP Panel, strains carrying acquired resistance should not be used as feed additives unless it can be demonstrated that the resistance is a result of chromosomal mutation(s). Only 9 isolates, for which the MIC did not exceed the established breakpoints, can be used without reservation as feed additives. Unfortunately, 2 (L. johnsoni 4b and 9d) of these 9 antibiotic susceptible isolates were sensitive to bile salts. Therefore, 7 isolates, i.e. L. salivarius 9b and 60d, L. johnsonii 8f, L. crispatus 49b, L. ingluviei 9e and 43d, L. oris 50c, met all the basic criteria for probiotics and were finally selected. Among these isolates, L. salivarius 9b and 60d displayed strong antagonism against Campylobacter, and 5 isolates (8f, 49b, 9e, 43d and 50c) of different species exhibited high resistance to low pH and bile as well as high hydrophobicity, which may enable the elimination of Campylobacter through stronger adhesion of these isolates to the intestinal mucosa. Moreover the isolates 60d, 50c and 8f displayed the ability to biofilm formation.
In summary, our research showed that seven finally selected Lactobacillus isolates may have potential application in reducing Camylobacter spp. in chickens and thus prevent infections in both birds and humans. Prior to commercialization, the efficiency as well as the safety of these isolates should be confirmed on animal models. In the case of the Lactobacillus isolates that has exceeded the MIC breakpoints, but strongly inhibit the growth of Camylobacter spp., more precise genetic testing can be conducted to determine whether the resistance to a given antibiotic is a caused by a genomic mutation or to exogenous DNA.
Supplementary Material
Acknowledgments
The authors thank Prof. Stanisław Winiarczyk and his team from Department of Epizootiology and Clinic of Infectious Diseases, University of Life Sciences in Lublin, for shering mass spectrometer and technician assistance during identification of lactobacilli using MALDI-TOF MS.
REFERENCES
- 1.Boris S., Suárez J. E., Vázquez F., Barbés C.1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 66: 1985–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratz K., Gölz G., Janczyk P., Nöckler K., Alter T.2015. Analysis of in vitro and in vivo effects of probiotics against Campylobacter spp. Berl. Munch. Tierarztl. Wochenschr. 128: 155–162. [PubMed] [Google Scholar]
- 3.Cauwerts K., Pasmans F., Devriese L. A., Martel A., Haesebrouck F., Decostere A.2006. Cloacal Lactobacillus isolates from broilers show high prevalence of resistance towards macrolide and lincosamide antibiotics. Avian Pathol. 35: 160–164. doi: 10.1080/03079450600598137 [DOI] [PubMed] [Google Scholar]
- 4.Dec M., Puchalski A., Nowaczek A., Wernicki A.2016. Antimicrobial activity of Lactobacillus strains of chicken origin against bacterial pathogenss. Int. Microbiol. 19: 57–67. [DOI] [PubMed] [Google Scholar]
- 5.Dec M., Puchalski A., Urban-Chmiel R., Wernicki A.2016. 16S-ARDRA and MALDI-TOF mass spectrometry as tools for identification of Lactobacillus bacteria isolated from poultry. BMC Microbiol. 16: 105. doi: 10.1186/s12866-016-0732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dec M., Puchalski A., Urban-Chmiel R., Wernicki A.2014. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poult. Sci. 93: 2464–2472. doi: 10.3382/ps.2014-04025 [DOI] [PubMed] [Google Scholar]
- 7.Dec M., Urban-Chmiel R., Stępień-Pyśniak D., Wernicki A.2017. Assessment of antibiotic susceptibility in Lactobacillus isolates from chickens. Gut Pathog. 9: 54. doi: 10.1186/s13099-017-0203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Cerbo A., Palmieri B., Aponte M., Morales-Medina J. C., Iannitti T.2016. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 69: 187–203. doi: 10.1136/jclinpath-2015-202976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois-Dauphin R., Sabrina V., Isabelle D., Christopher M., Andre T., Philippe T.2011. In vitro antagonistic activity evaluation of Lactic Acid Bacteria (LAB) combined with cellulase enzyme against Campylobacter jejuni growth in co-culture. J. Microbiol. Biotechnol. 21: 62–70. doi: 10.4014/jmb.1007.07006 [DOI] [PubMed] [Google Scholar]
- 10.Dudzic A., Urban-Chmiel R., Stępień-Pyśniak D., Dec M., Puchalski A., Wernicki A.2016. Isolation, identification and antibiotic resistance of Campylobacter strains isolated from domestic and free-living pigeons. Br. Poult. Sci. 57: 172–178. doi: 10.1080/00071668.2016.1148262 [DOI] [PubMed] [Google Scholar]
- 11.EFSA’s Scientific Opinion. 2011. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA9: 2105.
- 12.EFSA Guidance Document. 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA 10: 2740. [Google Scholar]
- 13.EFSA’s report. 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA 13: 3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrmann M. A., Kurzak P., Bauer J., Vogel R. F.2002. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 92: 966–975. doi: 10.1046/j.1365-2672.2002.01608.x [DOI] [PubMed] [Google Scholar]
- 15.Epps S. V., Harvey R. B., Hume M. E., Phillips T. D., Anderson R. C., Nisbet D. J.2013. Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 10: 6292–6304. doi: 10.3390/ijerph10126292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganan M., Martinez-Rodriguez A. J., Carrascosa A. V., Vesterlund S., Salminen S., Satokari R.2013. Interaction of Campylobacter spp. and human probiotics in chicken intestinal mucus. Zoonoses Public Health 60: 141–148. doi: 10.1111/j.1863-2378.2012.01510.x [DOI] [PubMed] [Google Scholar]
- 17.Ghareeb K., Awad W. A., Mohnl M., Porta R., Biarnés M., Böhm J., Schatzmayr G.2012. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 91: 1825–1832. doi: 10.3382/ps.2012-02168 [DOI] [PubMed] [Google Scholar]
- 18.Gibreel A., Taylor D. E.2006. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob. Chemother. 58: 243–255. doi: 10.1093/jac/dkl210 [DOI] [PubMed] [Google Scholar]
- 19.Grover S., Kumar A., Srivastava A. K., Batish V. K.2013. Probiotics as functional food ingredients for augmenting human health. pp. 396–397. In: Innovation in healthy and functional foods (Ghosh, D., Das, S., Bagchi, D. and Smarta, R. B. eds.), CRC Press Taylor & Francis Group, Boca Raton. [Google Scholar]
- 20.Kizerwetter-Świda M., Binek M.2009. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol. J. Vet. Sci. 12: 15–20. [PubMed] [Google Scholar]
- 21.Kos B., Susković J., Vuković S., Simpraga M., Frece J., Matosić S.2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 94: 981–987. doi: 10.1046/j.1365-2672.2003.01915.x [DOI] [PubMed] [Google Scholar]
- 22.Lebeer S., Vanderleyden J., De Keersmaecker S. C.2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72: 728–764. doi: 10.1128/MMBR.00017-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messaoudi S., Kergourlay G., Rossero A., Ferchichi M., Prévost H., Drider D., Manai M., Dousset X.2011. Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. Int. Microbiol. 14: 103–110. [DOI] [PubMed] [Google Scholar]
- 24.Morishita T. Y., Aye P. P., Harr B. S., Cobb C. W., Clifford J. R.1997. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis. 41: 850–855. doi: 10.2307/1592338 [DOI] [PubMed] [Google Scholar]
- 25.Nazef L., Belguesmia Y., Tani A., Prévost H., Drider D.2008. Identification of lactic acid bacteria from poultry feces: evidence on anti-Campylobacter and anti-Listeria activities. Poult. Sci. 87: 329–334. doi: 10.3382/ps.2007-00282 [DOI] [PubMed] [Google Scholar]
- 26.Neal-McKinney J. M., Lu X., Duong T., Larson C. L., Call D. R., Shah D. H., Konkel M. E.2012. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS One 7: e43928. doi: 10.1371/journal.pone.0043928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal-McKinney J. M., Samuelson D. R., Eucker T. P., Nissen M. S., Crespo R., Konkel M. E.2014. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS One 9: e114254. doi: 10.1371/journal.pone.0114254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasschaert G., Piessens V., Scheldeman P., Leleu S., Stals A., Herman L., Heyndrickx M., Messens W.2013. Efficacy of electrolyzed oxidizing water and lactic acid on the reduction of Campylobacter on naturally contaminated broiler carcasses during processing. Poult. Sci. 92: 1077–1084. doi: 10.3382/ps.2012-02771 [DOI] [PubMed] [Google Scholar]
- 29.Salas-Jara M. J., Ilabaca A., Vega M., García A.2016. Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms 4: 35. doi: 10.3390/microorganisms4030035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taheri H. R., Moravej H., Tabandeh F., Zaghari M., Shivazad M.2009. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 88: 1586–1593. doi: 10.3382/ps.2009-00041 [DOI] [PubMed] [Google Scholar]
- 31.Van Netten P., Huis in ’t Veld J. H., Mossel D. A.1994. The immediate bactericidal effect of lactic acid on meat-borne pathogens. J. Appl. Bacteriol. 77: 490–496. doi: 10.1111/j.1365-2672.1994.tb04392.x [DOI] [PubMed] [Google Scholar]
- 32.Wang G., Zhao Y., Tian F., Jin X., Chen H., Liu X., Zhang Q., Zhao J., Chen Y., Zhang H., Chen W.2014. Screening of adhesive lactobacilli with antagonistic activity against Campylobacter jejuni. Food Control 44: 49–57. doi: 10.1016/j.foodcont.2014.03.042 [DOI] [Google Scholar]
- 33.Wieczorek K., Osek J.2005. Multiplex PCR assays for simultaneous identification of Campylobacter jeiuni and Campylobacter coli. Med. Weter. 61: 797–799. [Google Scholar]
- 34.Willis W. L., Reid L.2008. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci. 87: 606–611. doi: 10.3382/ps.2006-00458 [DOI] [PubMed] [Google Scholar]
- 35.Workman S. N., Mathison G. E., Lavoie M. C.2005. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. J. Clin. Microbiol. 43: 2642–2650. doi: 10.1128/JCM.43.6.2642-2650.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang G., Ma L., Doyle M. P.2007. Potential competitive exclusion bacteria from poultry inhibitory to Campylobacter jejuni and Salmonella. J. Food Prot. 70: 867–873. doi: 10.4315/0362-028X-70.4.867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.