Abstract
The effect of genistein on Bcl-2 and Bax protein expression in the ovarian tissue of rats with polycystic ovarian syndrome (PCOS) was evaluated. Sixty rats were divided into six groups. Rats in the Dose group received genistein at a concentration of either 5 (L-gen), 10 (M-Gen) or 20 (H-Gen) mg per kg of body weight per day. The expression of Bcl-2 mRNA and Bax mRNA was determined by in situ hybridization. Bcl-2 and Bax protein concentration was quantified by ELISA. The results showed that the expression of Bcl-2 mRNA and Bcl-2 protein was significantly higher in the high genistein Dose group (H-Gen) when compared to the Model group (MG) (P<0.05). Genistein induced higher expression of the Bcl-2 gene at the transcriptional and translational level. Treatment with genistein resulted in an improvement of ovarian function with Bcl-2 expression being enhanced and Bax expression being suppressed. These alterations may be due to the structural and functional modifications that take place in these cells, and could be related to apoptotic changes that occur in rats with PCOS.
Keywords: Bax, Bcl-2, genistein, ovary, polycystic ovarian syndrome
Polycystic ovarian syndromes (PCOS) are complaints of the reproduction that affect bovine, ovine, nervous, and endocrine system [12, 18,19,20, 24]. PCOS is the most common and complex endocrine complaint, which has influence on about 7–10% women in childbearing age. There is obviously complicated etiology and pathophysiology performance in PCOS patients. It is considered a syndrome not a disease, which has evidence of heterogeneous clinical symptoms. Some substantive problem of PCOS is reflected in many PCOS animal models [1, 4, 7, 25].
Fas system and the Bcl-2 family are generally considered the regulation of apoptotic signaling in the ovary [8, 10, 21]. One of the main regulatory proteins is members of the Bcl-2 family of proteins. They can be divided into those having either an antiapoptotic (e.g. Bcl-2, Bcl-W, Bcl-xL) or proapoptotic (Bax, Bad, Bim, Bcl-xS, Bod, Bok/Mtd) function. Bcl-2 protein locates in endoplasm omentum and mitochondrial membranes and it can prevent cell apoptosis by inhibiting the release of apoptosis inducing factors [6, 13]. Due to the inhibitor of apoptosis gene Bcl-2 can affect the apoptosis by affecting intracellular information conduction, many scholars believe it is the key of regulating factor in cell apoptosis. A number of hypotheses about the pathogenesis of PCOS have been proposed. However, the etiology and pathology of PCOS still has not been clarified clearly. Attention of researchers has been attracted by the expression of apoptosis signals in ovarian cell over the years. Among them, the well-known theory was that Bcl-2 could play an important role in inhibiting or delaying apoptosis [26]. So if a sort of drug or biological active ingredient could enhance the expression of Bcl-2 mRNA and inhibit the expression of Bax mRNA, then it should have important value for delaying or reducing cell apoptosis.
Genistein is an isoflavone that has received a great deal of attention over the last few years because of its potential to prevent the most currently prevalent chronic diseases, such as cardiovascular diseases, osteoporosis and hormone related cancers [15]. Genisteins are considered to be phytoestrogens, because they have been shown to bind to trans-activated estrogen receptors and to induce gene expression [14]. The molecular structure of genistein is similar to estradiol. There are two phenolic hydroxyl residues in their relative ambi-molecular poles, which can cause an estrogen-like effect by binding to the estrogen receptor. It is well known that there is a close relationship between the chemical structure and the biological activity of bioactive compounds. Thus, structural modification of genistein might alter its biological activity. Initial speculation about its efficacy was based on its estrogen-like properties and earlier research studies showing that the chemically synthesized structural drug derivatives of genistein, ipriflavone, exerted skeletal benefits [3]. In recent years, phytoestrogen supplements have become attractive as safer alternatives to estrogen, and their efficacy has been investigated in clinical trials. Hyperandrogenism is one of the main symptoms of PCOS. Can the estrogen-like effects of Gen regulate the hormones levels in patients or animals with PCOS? And what are the mechanisms and ways of genistein regulating it? These problems are worth of studying.
In the current study, we used ovaries of rats with PCOS as models to investigate the effects of genistein exposure on the expression of two apoptotic regulatory key members of the Bcl-2 family, Bcl-2 and Bax. The objective of the present study was to further clarify the mechanism of genistein in PCOS ovarian function by studying the effect of genistein on the expression of Bcl-2 and Bax mRNAs in PCOS ovarian rat tissue by in situ hybridization, as well as the expression of Bcl-2 and Bax proteins by ELISA.
MATERIALS AND METHODS
Chemicals and test compounds
Genistein (4′,5,7-trihydroxyisoflavone) was provided by Sigma company (America, the purity >99.9%). Diethylstilbestrol was provided by Hefei JIULIAN Pharmaceutical Co. (Hefei, China); HCG was provided by Sihuan pharmaceutical Co. (Beijing, China); Isophand insulin injection: Novo Nordisk (Rio de Janeiro,Brazil, drug license No.: H20091126); ELISA reagent kit was provided by Wuhan boster biological company (Wuhan, China); immunohistochemical antibody was provided by Zhenjiang biology science and technology company, Rabbit polyclonal to FSHR (No. PR-1170), Rabbit polyclonal to LHR (No.PR-1318).
Animals and PCOS animal model building
Female Wistar rats (about 220 g, n=60) aged 2 months were obtained from Harbin (Harbin medical university breeding and research center, Harbin, China). Rats were housed one per cage and were maintained under controlled conditions of temperature (20 ± 1°C), relative humidity (50–80%) and illumination (13 hr light, 11 hr darkness).
Insulin (INS) was administered in combination with the HCG molding method [2, 17]: rats were given sufficient food and 5% glucose. During the first 10 days, the rats in the Model group (MG), the Dose groups (L-Gen, M-Gen and H-Gen), and the Estrogen (EG) group, were gradually given increasing doses of intermediate-acting insulin injection; the dosage gradually increased from 0.5 IU/day to 6 IU/day. From the 11th to the 22nd day, the rats were given a fixed dose of 6 IU/day of HCG. Rats in the Control group (CG) were injected instead with the same dose of normal saline as a blank control. The selection standard used for this animal model was based on carrying out vaginal epithelial cell smears continuously for two sexual cycles (each cycle during 5 days). If vaginal keratinization of cells continued to appear this suggested that the model was successful. In addition, all the groups, excluding the CG, were given genistein by gastric gavage for 15 days.
All rats were divided into six groups of ten animals each, blank control group (CG), model group (MG), dose group (L-Gen, M-Gen, H-Gen, received genistein at 5, 10, 20 mg per kg body weight per day mg/kg) and estrogen group (EG, received Diethylstilbestrol at 0.5 mg per kg per day). It was worth mentioning that the rather different magnitudes of human receiving these so-called environmental estrogens: Exposure to estrogenic isoflavones was varied with dietary habits; estimates were between 1 and 100 mg per day for consumers on a typical western diet and those on a traditional soy rich Asian style diet, respectively.
As the content of genistein in isoflavone was about 2–3%, the conversion factor between human (70 kg) and rat (250 g) was 7, the effective dose of genistein was about 0.14–21 mg, and we also read the other authors’ articles for reference, so the doses of genistein (5, 10 and 20 mg/kg BW) were determined. Additionally, in order to compare the age of rats with women of childbearing age, we used 2 month old animals. The results of vaginal epithelial cell smears and sex hormone levels in this study proved that the rat models could simulate the PCOS patient’s.
All animal experiments were approved by the Committee on Animal Care of College of Animal Science and Veterinary Medicine of Heilongjiang Bayi Agricultural University and Use of the local institution and according to accepted veterinary medical practice.
Animals and treatment
All procedures were carried out according to the Guide for the Care and Use of Laboratory Animals [16]. All rats fed with diet which did not contain genistein of soybean and alfalfa (soy and alfalfa free diets, SAFD-Diet, Harbin, China). SAFD feed formulation: component rich in genistein and alfalfa was instead of corn, wheat and casein, aimed to highlight the factors of genistein on the impact of experimental results. Feeding material formula was as follows: corn flour 30.56%, corn oil 2%, wheat flour 27.27%, yeast 2%, fish meal (60% protein) 10%, AIN mineral salt 3%, crude wheat 10%, vitamin premix 0.05%, casein 7%, choline chloride 0.12%, skim milk powder 5%, corn protein 3%.
Rats anesthetized by ether and their blood was collected from the abdominal aorta, then serum was stored at −20°C until being used for hormone assays. Half of the ovaries were dissected and fixed in 10% (v/v) buffered formalin for 6 hr at 8°C and were washed in phosphate buffered saline (PBS). For light microscopy, fixed tissues were dehydrated in an ascending series of ethanol, cleared in xylene, and embedded in paraffin. Five micrometer-thick sections were mounted in slides previously treated with 3-aminopropyltriethoxysilane (Sigma-Aldrich, St. Louis, MO, U.S.A.) and were stained with haematoxylin-eosin for a previous observation. Follicular mean diameter, the granulosa and theca thickness were analyzed using specific tools of the software. The other half of rats’ ovaries were organized by adding the appropriate amount of saline mash, then 1,000 × g centrifuged for 10 min. The supernatant was collected.
Assay method
The determination method of bcl-2 mRNA and bax mRNA was by In situ hybridization (We took the bcl-2 mRNA as an example). The bcl-2 probe (sequence as follows: (1) 5ʹ-GATGAAGTACATCCATTATAAGCTGTCACA -3ʹ; (2)5ʹ-GCGCTCAGCCCTGTGCCACCTGTGGTCCAC-3ʹ;(3)5ʹ-GGGAGATGTCACCCCTGGTGGACAACATCG-3ʹ) and the bax probe (sequence as follows: (1) 5ʹ-CCACCAGCTCTGAGCAGATCATGAAGACAG -3ʹ; (2)5ʹ-AGGATGCGTCCACCAAGAAGGTGAGCGAGT-3ʹ;(3)5ʹ-AGCAAACTGGTGCTCAAGGCCCTGTGCAC-3ʹ) were synthesized and labeled with biotin at the 5′end by ShangHai Sangon Bioengineering Co. (Shanghai, China). First, the sections were deparaffinized in xylene and rehydrated. After endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide at 37°C, nonspecific binding was blocked. The sections were then dehydrated with 100% alcohol and air-dried. Next, the sections were incubated with the probe at the concentration of 16 µg/ml for 20 hr at 45°C. The sections were then consequently washed with 2 × SSC and 0.1 × SSC buffered saline containing 0.1% SDS and were incubated with Streptavidin–HRP for 30 min at 37°C. Finally, Diaminobenzidine (DAB) was used as a chromagen, and the sections were counterstained with hematoxylin. The probe was replaced with dilution solution as the negative control. The cells with a stained brown were defined as positive.
Evaluation standard of in situ hybridization results
The immunohistochemical stained area (IHCSA) was calculated as a percentage of total area, assessed by color segmentation analysis, which produces quantification by locating all objects of a specific color (brown stain). The IHCSA (black area) was calculated from at least 25 images of each area (granulosa, theca externa, and theca interna) in each slide.
Ovarian granulosa cell apoptosis factor Bcl-2 mRNA and Bax mRNA positive evaluation standard: according to the granule cell immune tissue sections of ovary and uterus tissue staining depth was divided into 4 levels, no staining judged as negative expression, dark brown judged as weak positive expression, brown-yellow judged as moderate positive expression and brown judged as strong positive expression.
In order to get convenient statistics, expression status was judged as semiquantitative scoring: 1, 2, 3, 4 respectively correspond to negative expression, weak positive expression, moderate positive expression and strong positive expression.
Enzyme-linked immunosorbent assay
Bcl-2 and Bax protein concentration was quantified by an enzyme-linked immunosorbent assay (ELISA). Fifty µl of standard diluent was added to the standard wells. The samples were diluted in a final ratio of 1:1 by mixing 50 µl of the sample with 50 µl of diluent and 50 µl of the diluted sample was added to the wells. Fifty µl of diluted biotinylated anti-bcl-2 (bax) was added to all the wells. Then the plate was covered and incubated for 1 hr at 37°C. After removing the cover the plate was washed three times. Eighty µl of streptavidin-HRP solution were aliquoted into each well, including the blank wells. Following, the plate was covered and incubated for 30 min at 37°C. Then the solution was removed from all the wells, and the samples in the micro well plate were washed according to the corresponding washing step and then immediately preceded to the next step. Fifty µl of substrate A and substrate B were added to each well, and then incubated for 10 min at 37°C. The enzyme-substrate reaction was stopped by quickly adding 50 µl of H2SO4. The absorbance of each well was recorded by a spectrophotometer at 450 nm as the primary wavelength and optionally at 620 nm (610 nm to 650 nm is acceptable) as the reference wavelength.
The standard curve equation of Bcl-2 and Bax protein, which was individually determined from the standard sample provided by the ELISA reagent kit is: y=0.085x + 0.0793 (R2=0.9933) and y=0.0026x + 0.2652 (R2=0.9998). The letter “x” represents the optical density value, and the letter “y” stands for Bcl-2 and bax protein level.
Statistical analysis
A statistical software package (SPSS 13.0 for Windows, SPSS Inc., Chicago, IL, U.S.A.) was used for performing the statistical tests. The statistical significance of differences was assessed by one-way ANOVA, followed by Duncan’s multiple range test as a multiple comparison test. P<0.05 values were considered significant. Results were expressed as mean ± SEM.
RESULTS
Ovary histomorphometry
Histomorphometric results are listed in Table 1. The average diameter of the cystic follicles was smaller in the Dose group than in the Model group. Compared with the MG, the area and the volume of rats’ ovaries in the Dose group decreased, and this decrease was statistically significant in the EG (P<0.05).
Table 1. The area and volume calculation of ovarian (n=10).
| Group | Ovarian area (cm2) | Ovarian volume (cm3) |
|---|---|---|
| CG | 0.515 ± 0.203 | 12.671 ± 2.244 |
| MG | 0.611 ± 0.076 | 14.053 ± 0.869 |
| L-Gen | 0.553 ± 0.069 | 13.229 ± 0.830 |
| M-Gen | 0.589 ± 0.064 | 13.984 ± 0.850 |
| H-Gen | 0.583 ± 0.066 | 13.827 ± 1.235 |
| EG | 0.515 ± 0.074a) | 12.800 ± 0.863b) |
The area and volume calculation of ovarian after 15 days of treatment with genistein. Experimental conditions and treatment procedures are given in materials and methods. Significant against model group (a: P<0.05; b: P<0.01), ANOVA test.
Ovary histology
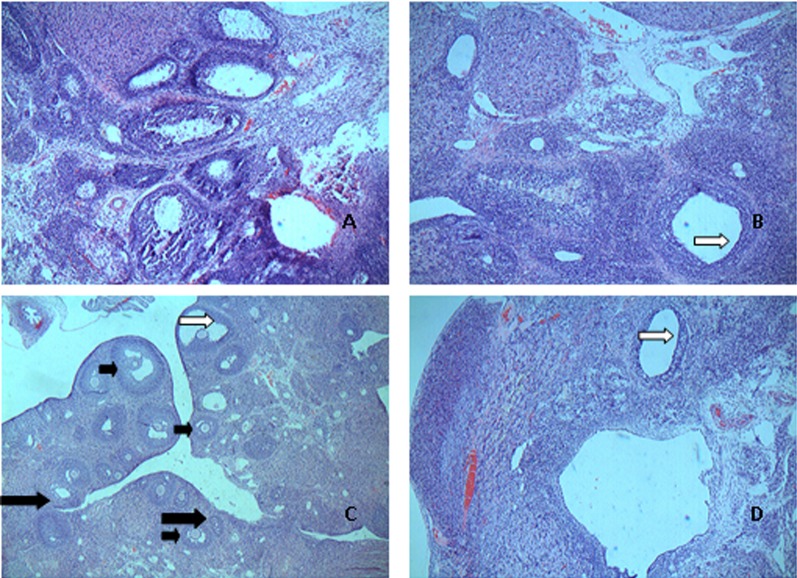
The rat ovarian anatomy and morphology was observed by light microscopy after HE staining (Fig. 1). In the CG, the ovary cell clusters were rough and there were a large number of corpus luteum cysts visible on the surface. The granulosa cell layers were multilayered and in good order. Compared with the CG, the ovarian area and volume of rats in the MG increased and got heavier, paler, with little luteal tissue. Different degrees of cystic changes were detected by microscopic observation in the Dose and Model groups. Follicular cystic dilatation, sparse granulosa cell arrangement, cystic sinus follicle and luteal tissue reduction was observed in the MG. Compared with the MG, the changes of rats in the H-Gen group were as follows: the ovarian theca cell layer got thinner, the layers of the granulosa cells increased, the general morphology and integrity of the ovarian tissue had a tighter arrangement. In addition, luteal tissue quantity also increased and presented more mature follicles. These changes were also observed in the EG.
Fig. 1.
Sections of ovaries from control group, model group, high dose group and estrogen group stained with hematoxylin and eosin (Original magnifieation: × 100). Different stages are indicated by different arrows (small black arrows, mature follicle; white arrows, ovarian theca cell; and large black arrows, corpus luteum). A: CG; B: MG; C: H-Gen; D: EG.
Bcl-2 mRNA and Bax mRNA expression in rat ovarian tissue
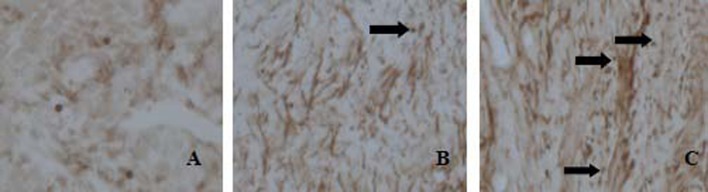
Expression of Bcl-2 mRNA is shown in Table 2 and Fig. 2. The positive expression of the Bcl-2 mRNA was mostly detected in the cytoplasm by immunohistochemical staining. Positive reactions for Bcl-2 mRNA were observed in 5 out of 10 cases (50%) in the CG, 3 out of 10 cases (30%) in the MG, 8 out of 10 cases (80%) in the L-Gen group, and in 10 out of 10 cases (100%) in the M-Gen, the H-Gen and the EG groups. Positive expression of Bcl-2 mRNA in the EG was the strongest as shown by the dark brown staining particles. Brown granules in the H-Gen group were also observed but with a moderate expression. Bcl-2 mRNA expression increased with increasing concentrations of genistein. In contrast to the EG and the H-Gen group, there were only a few brown-yellow granules present in the CG and the MG.
Table 2. Comparison of the expression of Bcl-2 mRNA in rats’ ovaries (n=10).
| Group | Genistein dose | Semiquantitative scoring of the positive expression of Bcl-2 mRNA | Positive | Positive reactions (%) |
|||
|---|---|---|---|---|---|---|---|
| Negative expression |
Weak positive expression |
Moderate positive expression |
Strong positive expression |
Integral | |||
| CG | 5 | 3 | 1 | 1 | 18 | 50 (5/10) | |
| MG | 7 | 1 | 2 | 0 | 15 | 30 (3/10) | |
| L-Gen | 5 mg/kg bw | 2 | 2 | 2 | 4 | 28 | 80 (8/10) |
| M-Gen | 10 mg/kg bw | 0 | 4 | 2 | 4 | 30 | 100 (10/10) |
| H-Gen | 20 mg/kg bw | 0 | 2 | 3 | 5 | 33a) | 100 (10/10) |
| EG | 0.5 mg/kg bw | 0 | 1 | 1 | 8 | 37a) | 100 (10/10) |
Expression of Bcl-2 mRNA and positive reactions in rats’ ovaries of Wistar rats after 15 days of treatment with genistein. Experimental conditions and treatment procedures are given in materials and methods. a) Significant against model group (P<0.05), ANOVA test.
Fig. 2.
Expressions of Bcl-2 mRNA in rats’ ovaries were detected by method of in situ hybridization. The staining depth of ovary tissue was divided into 4 levels, no staining is as negative expression, dark brown is as the weak positive expression, brown-yellow is as moderate expression, brown is as the strong positive expression (Original magnifieation: × 40). Positive expression is indicated by arrows. A: MG; B: H-Gen C: EG.
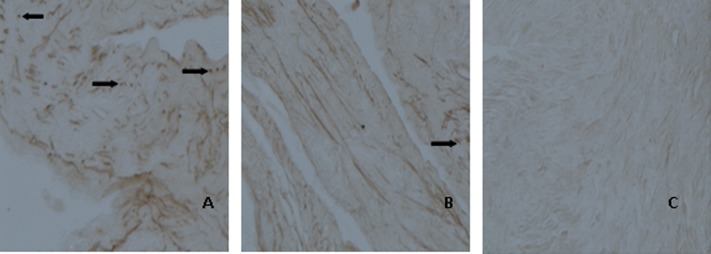
Expression of Bax mRNA is shown in Table 3 and Fig. 3. Positive expression of Bax mRNA was mainly detected in the cytoplasm. Positive reactions for Bax mRNA were observed in 9 out of 10 cases (90%) in the CG, 10 out of 10 cases (100%) in the MG, 9 out of 10 cases (90%) in the L-Gen group, 6 out of 10 cases (60%) in the M-Gen group, 4 out of 10 cases (40%) in the H-Gen group, and 3 out of 10 cases (30%) in the EG. Therefore, Bax mRNA levels decreased with increasing concentrations of genistein. In contrast with the MG, positive expression of Bax mRNA in the H-Gen and the EG groups was weak with less brown particles accounted for.
Table 3. The expression of Bax mRNA in rats’ ovaries (n=10).
| Group | Genistein dose | Semiquantitative scoring of the positive expression of Bax mRNA | Positive | Positive reactions (%) |
|||
|---|---|---|---|---|---|---|---|
| Negative expression |
Weak positive expression |
Moderate positive expression |
Strong positive expression |
Integral | |||
| CG | 1 | 1 | 3 | 5 | 32 | 90 (9/10) | |
| MG | 0 | 2 | 2 | 6 | 34 | 100 (10/10) | |
| L-Gen | 5 mg/kg bw | 1 | 2 | 3 | 4 | 30 | 90 (9/10) |
| M-Gen | 10 mg/kg bw | 4 | 1 | 3 | 2 | 23 | 60 (6/10) |
| H-Gen | 20 mg/kg bw | 6 | 2 | 1 | 1 | 17a) | 40 (4/10) |
| EG | 0.5 mg/kg bw | 7 | 1 | 1 | 1 | 16a) | 30 (3/10) |
Expression of Bax mRNA and positive reactions in rats’ ovaries of Wistar rats after 15 days of treatment with genistein. Experimental conditions and treatment procedures are given in materials and methods. a) Significant against model group (P<0.05), ANOVA test.
Fig. 3.
Expressions of Bax mRNA in rats’ ovaries were detected by method of in situ hybridization. The staining depth of ovary tissue was divided into 4 levels, no staining as negative expression, dark brown as the weak positive expression, brown-yellow as moderate expression, brown as the strong positive expression (Original magnifieation: × 40). Positive expression is indicated by arrows. A: MG; B: H-Gen C: EG.
Expression of Bcl-2 protein and Bax protein
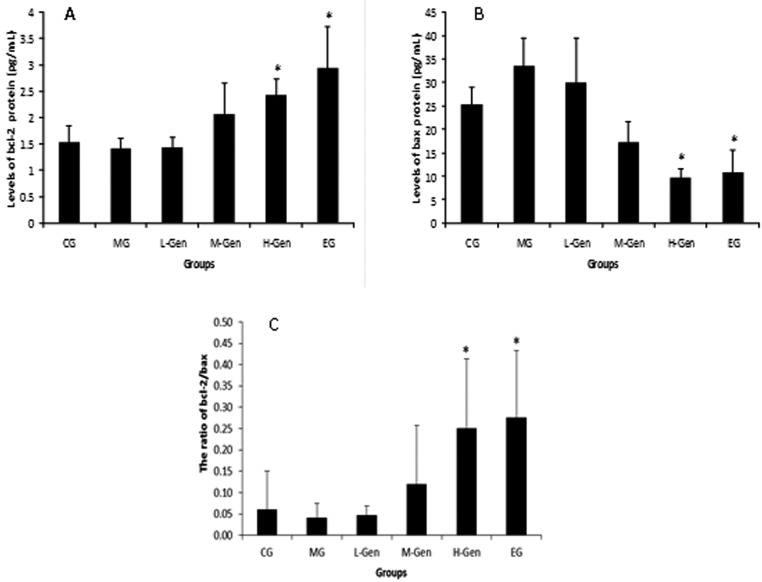
The results of the expression of Bcl-2 protein and Bax protein are shown in Fig. 4A and 4B. The expression of the Bcl-2 protein was higher in the H-Gen group (2.42 ± 0.32 pg/ml) and the EG (2.95 ± 0.78 pg/ml) than in the MG (1.41 ± 0.19 pg/ml). The expression of the Bax protein was significantly lower (P<0.05) in both the H-Gen group (9.67 ± 1.95 pg/ml) and the EG (10.69 ± 4.95 pg/ml) compared to the MG (33.64 ± 5.83 pg/ml).
Fig. 4.
Expressions of Bcl-2 protein, Bax protein (mean ± SE, n=10) (pg/ml) and the ratio of Bcl-2/Bax determined by ELISA. The expression of Bcl-2 protein protein in rats’ ovaries after 15 days of treatment with genistein. Experimental conditions and treatment procedures are given in materials and methods. Asterisk means significant against model group (*P<0.05), ANOVA test.
The ratio of Bcl-2/Bax
The result of Bcl-2/Bax ratio is shown in Fig. 4C. Bcl-2/Bax ratio has been proved to play a key role in the regulation of apoptosis. From the results we can see that with increased addition of genistein, the ratio of Bcl-2/Bax was also increased. The ratio of Bcl-2/Bax in the M-Gen (0.25 ± 0.16) and the EG (0.27 ± 0.15) groups were statistically higher (P<0.05) than in the MG (0.04 ± 0.03).
DISCUSSION
There are two main findings in the present study. Firstly, we demonstrate in vivo the positive effects of genistein administration on ovarian morphology and follicular distribution in rats with PCOS. Secondly, we demonstrate that administration of genistein influences the expression of both Bcl-2 and Bax proteins in the ovaries of rats with PCOS. These effects are worthy of further exploration when it comes to the study of ovulation disorders and its mechanisms in PCOS.
The ovary is one of the target organs for many chemicals [23], and is closely related with the female gonadal and reproductive function. Generally speaking, the ovarian surface of PCOS rats appears pale white, with a thickened membrane, increased volume and size, and with the presence of many cystic follicles. The present study showed that when compared with the MG, the ovarian area and the volume of the rats’ ovaries in the Dose group decreased. In addition, the results of rat ovarian anatomy and morphological test suggested that, compared with the Model group, the ovarian theca cell layer got thinner, layers of granulosa cells increased, and morphologically they displayed a more arranged pattern with the increase of the genistein dose. This suggests that genistein (at 20 mg per kg of body weight per day) could improve the morphology and function of the ovaries in rats with PCOS.
Some studies showed that positive and negative regulation is involved in the morphological changes of apoptotic cells, and these may vary depending on the signal transduction system used. Among these targets, members of the Bcl-2 family are considered as the primary regulators of apoptosis. The best characterized members are Bcl-2 and Bax. The expression level of the Bcl-2 gene in ovarian granulosa cells was over-expressed in transgenic mice [9]. To indicate the promoting action of Bcl-2 on apoptosis, other scholars used the transfection of exogenous Bcl-2 in chicken granulosa cells [27]. The Bcl-2 gene is also expressed in human ovarian tissues. However, the mechanism by which Bcl-2 inhibits apoptosis is still not clear. In our study, when different concentrations of genistein were used on rats’ ovarian granulosa cells, the expression of Bcl-2 mRNA increased with increasing genistein concentration, suggesting that cell apoptosis was reduced significantly.
Bax protein acts as an antagonist of Bcl-2 and is a related protein homologue of Bcl-2. Studies have shown that Bax expression in tissues and organs is more widespread than that of Bcl-2 in mice, and it is also expressed in the male testes and female ovaries [22]. The pathway of promoting apoptosis by Bax protein may be related to the anti-apoptotic protein Bcl-2, which can cause Bax to lose its pro-apoptotic effect. When there is excess of Bax that cannot be fully integrated over time, it leads to apoptosis. The results of the present study showed that when rats’ ovarian granulosa cells are treated with different concentrations of genistein, Bax expression reduces with increasing concentrations of genistein, suggesting a reduction of apoptosis.
Our study suggests that the mechanism of action of genistein in PCOS rats is mediated by the inhibition of ovarian granulosa cell apoptosis via the apoptosis signaling pathway of Bcl-2 and Bax. We found that the expression of Bcl-2 mRNA and Bcl-2 protein was significantly higher in the H-Gen group than in the Model group, which is consistent with previous studies [5, 11]. We observed a positive correlation between Bax levels and apoptosis, as well as a negative correlation between Bcl-2 levels and apoptosis.
To conclude, treatment with increasing doses of genistein resulted in an improvement of ovarian function by Bcl-2 enhancement and Bax reduction. Although much remains to be done in order to characterize the pathogenesis of cystic ovaries, we have confirmed that cellular apoptosis is altered in the cystic follicles of rats, which is in accordance with the findings of previous studies on related diseases in different species. These alterations may be due to structural and functional modifications that take place in these cells, and could be related to changes in apoptosis that happen in animals with this disease. Further studies will be needed to assess the specific role and regulation of each one of these cellular components and their participation in cystogenesis.
Acknowledgments
This study was supported by National Natural Science Foundation of China [81673170].
REFERENCES
- 1.Baravalle C., Salvetti N. R., Mira G. A., Pezzone N., Ortega H. H.2006. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch. Med. Res. 37: 830–839. doi: 10.1016/j.arcmed.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 2.Bogovich K.1991. Induction of ovarian follicular cysts in the pregnant rat by human chorionic gonadotropin. Biol. Reprod. 45: 34–42. doi: 10.1095/biolreprod45.1.34 [DOI] [PubMed] [Google Scholar]
- 3.Dai R., Ma Y., Sheng Z., Jin Y., Zhang Y., Fang L., Fan H., Liao E.2008. Effects of genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J. Bone Miner. Metab. 26: 342–349. doi: 10.1007/s00774-007-0830-4 [DOI] [PubMed] [Google Scholar]
- 4.Francou M., Durdos M., Salvetti N. R., Baravalle C., Rey F., Ortega H. H.2008. Characterization of pituitary cell populations in rats with induced polycystic ovaries. Cells Tissues Organs (Print) 188: 310–319. doi: 10.1159/000123202 [DOI] [PubMed] [Google Scholar]
- 5.Freier S., Weiss O., Eran M., Flyvbjerg A., Dahan R., Nephesh I., Safra T., Shiloni E., Raz I.1999. Expression of the insulin-like growth factors and their receptors in adenocarcinoma of the colon. Gut 44: 704–708. doi: 10.1136/gut.44.5.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gürsoy E., Ergin K., Başaloğlu H., Koca Y., Seyrek K.2008. Expression and localisation of Bcl-2 and Bax proteins in developing rat ovary. Res. Vet. Sci. 84: 56–61. doi: 10.1016/j.rvsc.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Hossain M. M., Cao M., Wang Q., Kim J. Y., Schellander K., Tesfaye D., Tsang B. K.2013. Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J. Ovarian Res. 6: 36–50. doi: 10.1186/1757-2215-6-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu S. Y., Hsueh A. J.2000. Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm. Physiol. Rev. 80: 593–614. doi: 10.1152/physrev.2000.80.2.593 [DOI] [PubMed] [Google Scholar]
- 9.Hsu S. Y., Lai R. J., Finegold M., Hsueh A. J.1996. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 137: 4837–4843. doi: 10.1210/endo.137.11.8895354 [DOI] [PubMed] [Google Scholar]
- 10.Hussein M. R.2005. Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update 11: 162–177. doi: 10.1093/humupd/dmi001 [DOI] [PubMed] [Google Scholar]
- 11.Ishijima N., Miki C., Ishida T., Kinoshita T., Suzuki H.1999. The immunohistochemical expression of BCL-2 oncoprotein in colorectal adenocarcinoma. Surg. Today 29: 682–684. doi: 10.1007/BF02483002 [DOI] [PubMed] [Google Scholar]
- 12.Jakimiuk A. J., Weitsman S. R., Yen H. W., Bogusiewicz M., Magoffin D. A.2002. Estrogen receptor alpha and beta expression in theca and granulosa cells from women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 87: 5532–5538. doi: 10.1210/jc.2002-020323 [DOI] [PubMed] [Google Scholar]
- 13.Johnson A. L., Bridgham J. T.2002. Caspase-mediated apoptosis in the vertebrate ovary. Reproduction 124: 19–27. doi: 10.1530/rep.0.1240019 [DOI] [PubMed] [Google Scholar]
- 14.Kuiper G. G., Shughrue P. J., Merchenthaler I., Gustafsson J. A.1998. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrinol. 19: 253–286. doi: 10.1006/frne.1998.0170 [DOI] [PubMed] [Google Scholar]
- 15.McCarty M. F.2006. Isoflavones made simple - genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses 66: 1093–1114. doi: 10.1016/j.mehy.2004.11.046 [DOI] [PubMed] [Google Scholar]
- 16.National Research Council Guide for the Care and Use of Laboratory Animals. 1996. National Academy Press, Washington, D.C. [Google Scholar]
- 17.Poretsky L., Clemons J., Bogovich K.1992. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism 41: 903–910. doi: 10.1016/0026-0495(92)90175-A [DOI] [PubMed] [Google Scholar]
- 18.Salvetti N. R., Canal A. M., Gimeno E. J., Ortega H. H.2004. Polycystic Ovarian Syndrome: temporal characterization of the induction and reversion process in an experimental model. Braz. J. Vet. Res. Anim. Sci. 41: 389–395. doi: 10.1590/S1413-95962004000600006 [DOI] [Google Scholar]
- 19.Salvetti N. R., Muller L. A., Acosta J. C., Gimeno J. E., Ortega H. H.2007. Estrogen receptors a and b and progesterone receptors in ovarian follicles of cows with cystic ovarian disease. Vet. Pathol. 44: 373–378. doi: 10.1354/vp.44-3-373 [DOI] [PubMed] [Google Scholar]
- 20.Silvia W. J., Hatler T. B., Nugent A. M., Laranja da Fonseca L. F.2002. Ovarian follicular cysts in dairy cows: an abnormality in folliculogenesis. Domest. Anim. Endocrinol. 23: 167–177. doi: 10.1016/S0739-7240(02)00154-6 [DOI] [PubMed] [Google Scholar]
- 21.Slot K. A., Voorendt M., de Boer-Brouwer M., van Vugt H. H., Teerds K. J.2006. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active caspase-3 in the rat ovary. J. Endocrinol. 188: 179–192. doi: 10.1677/joe.1.06165 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Lin Y., Li H., Liu J., Sheng X., Zhang W.2012. 2,5-Hexanedione induces human ovarian granulosa cell apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways. Arch. Toxicol. 86: 205–215. doi: 10.1007/s00204-011-0745-7 [DOI] [PubMed] [Google Scholar]
- 23.Terauchi K. J., Shigeta Y., Iguchi T., Sato T.2016. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 365: 197–208. doi: 10.1007/s00441-016-2371-4 [DOI] [PubMed] [Google Scholar]
- 24.Wild R. A., Carmina E., Diamanti-Kandarakis E., Dokras A., Escobar-Morreale H. F., Futterweit W., Lobo R., Norman R. J., Talbott E., Dumesic D. A.2010. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 95: 2038–2049. doi: 10.1210/jc.2009-2724 [DOI] [PubMed] [Google Scholar]
- 25.Yaba A., Demir N.2012. The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J. Ovarian Res. 5: 38–50. doi: 10.1186/1757-2215-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X.1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132. doi: 10.1126/science.275.5303.1129 [DOI] [PubMed] [Google Scholar]
- 27.Zhou D. R., Yang L. G., Jiang X. P., Yang C. F., And Zhang S. X.2001. Effect of bcl-2 gene and liposome on division and apoptosis of ovarian granular cells in laying hens. Nanjing Nongye Daxue Xuebao 24: 75–78. [Google Scholar]