Abstract
Objective
Our goal is to develop a tele-colposcopy platform for primary care clinics to improve screening sensitivity and access. Specifically, we developed a low-cost, portable Pocket colposcope and evaluated its performance in a tertiary healthcare center in Perú.
Design and setting
Images of the cervix were captured with a standard-of-care and Pocket colposcope at la Liga Contra el Cáncer in Lima, Perú.
Population
200 Peruvian women with abnormal cytology and/or HPV positivity were enrolled.
Methods
Images were collected using acetic acid and Lugol’s iodine as contrast agents. Biopsies were taken as per standard-of-care procedures.
Main outcome measures
After passing quality review, images from 129 patients were sent to four physicians who provided a diagnosis for each image.
Results
Physician interpretation of images from the two colposcopes agreed 83.1% of the time. The average sensitivity and specificity of physician interpretation compared to pathology was similar for the Pocket (sensitivity = 71.2%, specificity = 57.5%) and standard-of-care colposcopes (sensitivity = 79.8%, specificity = 56.6%). When compared to a previous study where only acetic acid was applied to the cervix, results indicated that adding Lugol’s iodine as a secondary contrast agent improved the percent agreement between colposcopes for all pathological categories by up to 8.9% and the sensitivity and specificity of physician interpretation compared to pathology by over 6.0% and 9.0%, respectively.
Conclusions
The Pocket colposcope performed similarly to a standard-of-care colposcope when used to identify pre-cancerous and cancerous lesions using acetic acid and Lugol’s iodine during colposcopy exams in Perú.
Keywords: Cervical Cancer, Uterine Cervical Diseases, Squamous Intraepithelial Lesions of the Cervix, Primary Health Care, Diagnostic Imaging, Technology, Biomedical
Introduction
Cervical cancer prevention is based on well-established interventions including human papillomavirus (HPV) vaccination and screening followed by treatment of pre-invasive disease (1–4). In the United States, cervical cancer incidence has decreased by 70% over the last 60 years due to screening with the Pap smear (5) and, more recently, co-testing for the HPV virus (1, 2); however, women living in low- and middle-income countries (LMICs) experience a disproportionately high burden of cervical cancer (6, 7).
In Perú, women are screened through visual inspection with acetic acid (VIA); screen-positive are referred to colposcopy-guided biopsy, which, if positive, requires another visit for treatment (8–10). Colposcopy-guided biopsy is the gold-standard for diagnosing suspected precancerous lesions of the cervix and involves the use of a low magnification microscope. However, colposcopes are expensive, and thus, women are referred to one of a handful of facilities that provide this service. Consequently, colposcopy is often inaccessible to the many women in LMICs who are at greatest risk for developing cervical cancer (11–19). Being able to bring colposcopy to the screening setting could eliminate one step in the referral process. Further, replacing VIA with colposcopy at the initial screening could improve ensitivity (6, 10, 20).
Our ultimate goal is to introduce a tele-colposcopy platform into the primary care setting to reduce multiple visits and improve screening sensitivity. Specifically, we have invented a low-cost, battery-operated Pocket colposcope that weighs less than half a pound (21). While traditional colposcopes visualize the cervix from outside the speculum, our design is used inside the speculum. The proximity of the colposcope to the cervix enables high-quality imaging of the cervix using consumer-grade light sources and cameras. Additionally, the Pocket colposcope can be connected to a smart phone for easy visualization of the image. Based on an exploratory study conducted at the Duke University Medical Center, physician interpretation of aceto-whitened cervix images acquired with the Pocket colposcope was comparable to a standard-of-care colposcope (22, 23).
Prior to introducing the Pocket colposcope into a screening setting in Perú, it is important to demonstrate that it can achieve comparable performance to a conventional standard-of-care colposcope in a referral setting within the same health care environment for which it is intended. Therefore, the performance of the Pocket and standard-of-care colposcopes was compared in patients undergoing colposcopy at la Liga Contra el Cáncer, a non-governmental organization that provides wide-scale screening and referral services for cervical cancer.
Methods
Funding
This study was funded by the National Institutes of Health Grants 1R01CA195500 and 1R01CA19338001, which included internal peer review for scientific quality. The funder did not play a role in conducting research or writing the manuscript.
The Pocket colposcope
The Pocket colposcope has gone through several iterations of speculum-based (21, 23) and speculum-free designs (24). This study was conducted using the latest generations of the speculum-based Pocket colposcope, which feature a waterproof design that enable the user to submerge the probe in chemical agents for high-level disinfection between patient uses (Figure 1A, B).
Figure 1.
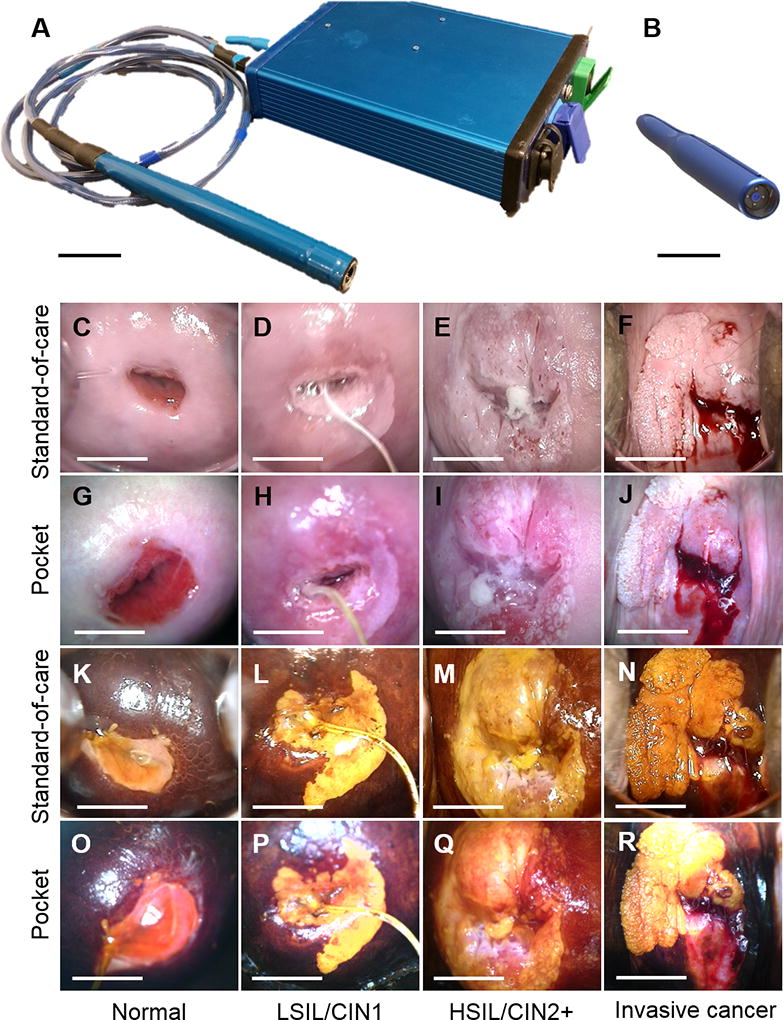
(A) Generation 3 and (B) Generation 4 Pocket colposcopes. The probes are sealed to enable submersion in chemical disinfectants between patient uses, and are powered by a smart phone or laptop. The Generation 3 Pocket colposcope uses cross-polarization to reduce specular reflection and therefore requires an external voltage booster to increase illumination power, while the Generation 4 Pocket colposcope eliminated the voltage booster and cross-polarizer through an innovative reflector design at the tip of the probe that minimizes glare and maximizes illumination and collection efficiency. Scale bar 4 cm. Representative clinical images captured with the (C–F, K–N) standard-of-care and (G–J, O–R) Pocket colposcopes. Images of cervices stained with acetic acid and Lugol’s iodine are shown in C–J and K–R respectively. (CGKO) show normal cervical tissue, (DHLP) show LSIL/CIN1 from 2 to 7 o’clock, (EIMQ) show HSIL/CIN2+ from 3 to 12 o’clock, and (FJNR) show invasive cancer from 7 to 3 o’clock. Scale bar 1 cm.
Patient population
Images were collected with the Pocket and a standard-of-care colposcope (Goldway SLC-2000B) at la Liga Contra el Cáncer under Duke University Medical IRB approved protocol (Pro00052865). 200 Peruvian women with abnormal cytology and/or HPV positivity were enrolled in this study. Informed written consent was obtained from each patient. Patients were not involved in the development of the Pocket colposcope or study.
Imaging procedures
The speculum was placed in the vaginal canal, acetic acid was applied to the cervix, and then images were captured with the standard-of-care and Pocket colposcopes. Acetic acid was not reapplied immediately before imaging with the Pocket colposcope for the first 168 patients, but was reapplied for the last 32 patients to improve visualization of aceto-whitening. After acetic acid application and imaging, Lugol’s iodine was applied to the cervix and images were captured with both colposcopes. All clinical decisions were made with the standard-of-care colposcope, including guiding biopsy. To achieve high level disinfection between patient uses, the Pocket colposcope was submerged in 0.0675% bleach for 10 minutes at 25°C, as per established guidelines (25).
Image quality review
Cases were excluded from subsequent analysis if: 1) images and/or pathology were missing as statistics could not be calculated for incomplete cases; or 2) images were unreadable due to incorrectly focusing the device during image acquisition. To evaluate image quality, two reviewers separately viewed and scored each image as having low, medium, or high image quality, which were defined as follows: (high) image is in focus and all 4 quadrants of the cervix are visible, (medium) image is slightly out of focus and a majority of the 4 quadrants of the cervix are visible, and (low) image is not in focus and a majority of the 4 quadrants of the cervix are not visible. Discrepancies between the two reviewers were resolved by consensus review. Images with high or medium image quality were included in subsequent analysis.
Image interpretation
In order to remove bias, image pairs that passed image quality review were cropped to remove the view of the speculum and vaginal side walls, thereby blinding the physician to which colposcope was used to acquire the image. Image pairs were split, randomized, labeled with a random identifier, and placed into different documents that were sent electronically to four physicians. Evaluation of each image by the physicians was performed remotely using a REDCap electronic survey. The survey includes the randomized identifier code, technical questions about the image quality, and clinical questions evaluating the diagnosis of the cervical image. The physician responses to each question were automatically saved in our REDCap database. The data was exported to Microsoft Excel (Microsoft Office Professional Plus 2013, Redmond, WA) for further analysis.
The physicians provided a diagnosis of normal, cervical intraepithelial neoplasia (CIN) 1, CIN2, CIN3, or cancer for each cervix image – CIN 1 is considered a low-grade squamous intraepithelial lesion (LSIL) while CIN 2 and 3 are considered high-grade SIL (HSIL). The physician diagnoses of the cervical images were compared to the pathology confirmed diagnosis given as: normal, cervicitis, condyloma, no biopsy, CIN1, CIN2, CIN3, or invasive cancer. Pathological diagnosis was grouped as negative (normal, cervicitis, condyloma) versus pre-cancer or cancer (CIN1-3, cancer). Patients who were colposcopically normal did not undergo a biopsy procedure (i.e. no biopsy) according to the standard-of-care guidelines and were considered negative given that colposcopy has high sensitivity, but relatively low specificity.
Comparison to previous studies
Lugol’s iodine was used as a secondary contrast agent in this study. In order to assess if the addition of Lugol’s iodine improves the percent agreement between colposcopy and pathology, we analyzed data from two previous studies in which similar numbers of acetic acid images only were captured with the same versions of the Pocket colposcope (Generation 3 and 4) and reviewed by the same physician (22, 23).
Statistical analysis
All statistical analyses were performed in Stata Version 13.0 (College Station, TX). First, the percent agreement and kappa statistic between the Pocket and standard-of-care colposcope were computed for multiple raters. Specifically, Fleiss’ kappa statistics, which account for more than two possible ratings per image, were computed for both the Pocket and standard-of-care colposcopes to assess inter-observer variation across four physicians. Additionally, kappa statistics were computed for each physician to provide a measure of intra-observer agreement between the two colposcopes. Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by comparing physician diagnoses of the cervical images to pathology-confirmed diagnosis. The percent of incorrect diagnosis was calculated by tabulating cases where the physician interpretation from colposcopy did not match pathology. P values associated with percent agreement and kappa statistics are included in subsequent tables. A significance level of p<0.05 was considered to reject the null hypothesis for all analyses.
Results
Image quality review
A total of 129 image pairs met image quality criteria and were retained for further analysis. A flow chart illustrating the process for image exclusion is shown in Figure S1A, and representative examples of low, medium, and high quality images are shown in Figure S1B. The reasons for exclusion of patient images are summarized in Figure S1C. Briefly, approximately 36% of cases (71/200) were excluded because either images or pathology were missing in 17.5% of cases (35/200) or images were unreadable due to low image quality in 18% of cases (36/200). Approximately half of the unreadable images were excluded because the first Pocket colposcope that was used in this study had technical malfunctions and was replaced once the problem was noted. The percent of unreadable Pocket colposcope images decreased after the first 50 patients (Figure S1D) as providers became more familiar with operating and focusing the device. All other image exclusions were comparable between the Pocket and standard-of-care colposcopes.
Patient characteristics
Relevant patient information for the 129 patients included in our analysis is shown in Table S1. The mean age of women enrolled in our study was 37 years and ranged from 20–67 years. Previous Pap smear results were recorded for 27% of women in our study, two thirds of whom had abnormal results. The remaining 73% of women had unknown Pap smear results that were not recorded in la Liga Contra el Cáncer database. Of the 129 women included in our analysis, 48 were diagnosed as colposcopically normal at the time of the procedure and therefore had no biopsy as per established screening guidelines. Biopsy confirmed pathology from the remaining 81 women indicated that 20 women were negative for pre-cancer or cancer, 39 had LSIL lesions, 15 had HSIL lesions, and 7 had invasive cancer.
Comparison between Pocket and standard-of-care colposcopes
Representative image pairs captured with the Pocket colposcope and standard-of-care colposcope are shown in Figure 1C-R. Lesions in LSIL, HSIL, and invasive cancer images pairs are readily seen in images acquired with both colposcopes. Aceto-whitened lesions were confirmed and somewhat enhanced with the addition of Lugol’s iodine, which stain lesions a mustard yellow color.
The percent agreements across the four physicians for the Pocket compared to the standard-of-care colposcope is shown in Table 1. Physician interpretation of the colposcopy images acquired by the two different colposcopes was concordant for 83.1% of the images. The percent agreement increased from negative (82.2%) to LSIL (82.6%) to HSIL+ (86.4%) image pairs.
Table 1.
Percent agreement between the Pocket colposcope and standard-of-care colposcope stratified by pathology (all, negative, CIN+, LSIL, and HSIL+). The percent agreements for each physician, average percent agreement across physicians, and standard deviation of percent agreements are shown in each row.
| All (n=129) |
Negative (n=68) | CIN+ (n=61) |
LSIL (n=39) |
HSIL+ (n=22) |
|
|---|---|---|---|---|---|
| Physician 1 (P value) | 83.6% (p < 0.0001) | 83.6% (p < 0.0001) | 83.6% (p < 0.0001) | 79.0% (p = 0.0001) | 90.9% (p = 0.0002) |
| Physician 2 (P value) | 89.2% (p < 0.0001) | 85.3% (p < 0.0001) | 93.6% (p = 0.0001) | 92.3% (p = 0.011) | 95.5% (p = N.S.) |
| Physician 3 (P value) | 81.5% (p < 0.0001) | 79.4% (p < 0.0001) | 83.9% (p = 0.0005) | 82.1% (p = 0.0096) | 86.4% (p = N.S.) |
| Physician 4 (P value) | 78.1% (p < 0.0001) | 80.3% (p < 0.0001) | 75.8% (p < 0.0001) | 76.9% (p < 0.0001) | 72.7% (p = 0.023) |
| Average | 83.1% | 82.2% | 84.2% | 82.6% | 86.4% |
| Standard deviation | 4.7% | 2.8% | 7.3% | 6.8% | 9.8% |
P values associated with all percent agreement values are shown within the each row (N.S. = not significant).
Kappa statistics were calculated to assess inter and intra-observer agreement and are shown in Table 2. The Fleiss’ kappa statistic for the Pocket and standard-of-care colposcope was 0.29 and 0.46 respectively, indicating that raters achieve fair to moderate inter-observer agreement. For CIN+ images, the Fleiss’ kappa statistics for the Pocket and standard-of-care colposcopes were 0.26 and 0.30, respectively, indicating that raters achieve fair inter-observer agreement regardless of the colposcope used to capture the image for pre-cancerous and cancerous lesions. Additionally, individual physicians achieved an average kappa coefficient of 0.61, indicating that raters achieve substantial intra-observer agreement between colposcopes.
Table 2.
The Fleiss kappa statistic for multiple raters was computed for both the Pocket and standard-of-care colposcopes to assess inter-observer agreement. Kappa statistics were also computed for each physician to assess the intra-observer agreement achieved between the two colposcopes. Kappa statistics are stratified by pathology (all, negative, and CIN+). LSIL, HSIL, and cancer categories were not included due to small sample sizes.
| Measure | Device or physician |
All (n=129) |
Negative (n=68) |
CIN+ (n=61) |
|---|---|---|---|---|
| Inter-observer agreement (4 physicians, 1 device) | Pocket (P value) | 0.29 (p < 0.0001) | 0.22 (p < 0.0001) | 0.26 (p < 0.0001) |
| Standard-of-care (P value) | 0.46 (p < 0.0001) | 0.42 (p < 0.0001) | 0.30 (p < 0.0001) | |
| Intra-observer agreement (1 physician, 2 devices) | Physician 1 (P value) | 0.61 (p < 0.0001) | 0.58 (p < 0.0001) | 0.41 (p = 0.0005) |
| Physician 2 (P value) | 0.60 (p < 0.0001) | 0.61 (p < 0.0001) | 0.47 (p < 0.0001) | |
| Physician 3 (P value) | 0.56 (p < 0.0001) | 0.52 (p < 0.0001) | 0.53 (p < 0.0001) | |
| Physician 4 (P value) | 0.67 (p < 0.0001) | 0.59 (p < 0.0001) | 0.63 (p < 0.0001) | |
| Average | 0.61 | 0.58 | 0.51 | |
| Standard deviation | 0.045 | 0.039 | 0.094 |
P values associated with all kappa statistics are shown within the each row.
Comparison between each colposcope and pathology
The average classification accuracy, sensitivity, specificity, PPV, and NPV across physicians compared to pathology is shown in Figure 2A. For negative versus CIN+ patients, the average values across physicians were within 8.6% of each other for the two devices. The average accuracy, sensitivity, and specificity was similar for the Pocket (accuracy = 63.9%, sensitivity = 71.2%, specificity = 57.5%) and standard-of-care colposcopes (accuracy = 67.6%, sensitivity = 79.8%, specificity = 56.6%). For negative versus HSIL+ patients, the average values across physicians were within 1.6% of each other for the two devices. When compared to negative versus CIN+ patients, the overall accuracy and specificity remained approximately the same for negative versus HSIL+ patients; however, the sensitivity for negative versus HSIL+ patients increased compared to negative versus CIN+ patients for both colposcopes.
Figure 2.
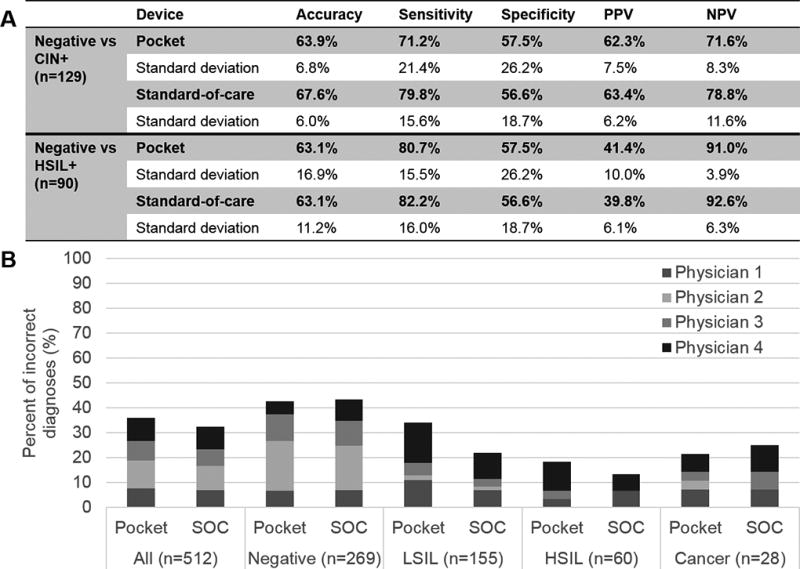
(A) Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the Pocket and standard-of-care colposcopes calculated using pathology as the gold standard. Average values and the standard deviation of values across physicians are shown for negative (no biopsy, normal, cervicitis, and condiloma) versus CIN+ patients (LSIL, HSIL, and cancer) and for negative versus HSIL+ patients (HSIL and cancer). (B) Percent of incorrect diagnosis by pathology for the Pocket and standard-of-care (SOC) colposcopes. Individual colors represent the percent of incorrect diagnoses for each physician.
Missed cases
Figure 2B shows the percent of incorrect diagnosis for both colposcopes by pathological category. The highest overall percent of incorrect diagnosis was for negative followed by LSIL image pairs. On average, physicians misdiagnosed 3 HSIL and 2 cancer images with the Pocket colposcope and 2 HSIL and 2 cancer images with the standard-of-care colposcope. A majority of misdiagnoses for cancer images acquired with both colposcopes were from a single patient, who had a cervix with atropic changes and consequently was difficult to evaluate due to poor staining with both contrast agents. Overall the standard-of-care and Pocket colposcope achieved similar percentages of incorrect diagnoses – specifically, they were within 6% of each other for all individual pathological categories for all physicians.
Summary of results and comparison to previous studies
Table S2 summarizes the percent agreement, accuracy, sensitivity, and specificity achieved in two previous studies in which acetic acid images only were reviewed compared to the current study in which both acetic acid and Lugol’s iodine images were reviewed by the same physician. The overall percent agreement between the Pocket and standard-of-care colposcopes increased from 75.2% to 81.5% when Lugol’s iodine was added. Furthermore, the percent agreement increased for all pathological categories, including negative (from 70.5% to 79.4%), CIN+ (from 79.0% to 83.9%), LSIL (from 75.0% to 82.1%), and HSIL+ (from 81.1% to 86.4%) when Lugol’s iodine was added. For negative versus CIN+ patients, the accuracy increased from 63.4% to 69.0% for the Pocket colposcope and from 64.4% to 73.6% for the standard-of-care colposcope when Lugol’s iodine was added. Similar increases were observed in sensitivity and specificity for negative versus CIN+ patients – sensitivity increased from 75.4% to 82.0% and specificity increased from 47.7% to 57.4% for the Pocket colposcope. Sensitivity increased from 82.5% to 88.5% and specificity increased from 40.9% to 60.3% for the standard-of-care colposcope when Lugol’s iodine was used as a secondary contrast agent. For negative versus HSIL+ patients, the overall accuracy and specificity remained approximately the same compared to negative versus CIN+ patients; however the sensitivity increased from 78.4% to 86.4% for the Pocket colposcope and from 81.1% to 90.9% for the standard-of-care colposcope when Lugol’s iodine was added.
Discussion
Main findings
The Pocket and standard-of-care colposcopes performed similarly when used to identify precancerous and cancerous lesions using acetic acid and Lugol’s iodine as contrast agents. Specifically, physician interpretation of images from the two colposcopes agreed 83.1% of the time. Analysis of inter- and intra-observer agreement with kappa statistics indicated that physicians achieved fair and moderate inter-observer agreement for the Pocket (0.29) and standard-of-care colposcopes (0.46), respectively, and achieved substantial intra-observer agreement between the two colposcopes (0.61). The average accuracy was 63.9% and 67.6% for the Pocket and standard-of-care colposcope, respectively. The average sensitivity and specificity of the Pocket colposcope was 71.2% and 57.5%, while the average sensitivity and specificity of the standard-of-care colposcope was 79.8% and 56.6%. Adding Lugol’s iodine as a secondary contrast agent improved the percent agreement between colposcopes for all pathological categories by up to 8.9% and the accuracy of physician interpretation compared to pathology by up to 9.2%.
Strengths and limitations
One of the strengths of this study is the randomization and blinding of the paired cervical images before being evaluated by the physicians. Specifically, physicians did not know which device was used to capture each image and could evaluate images without bias for or against one of the colposcopes. Additionally, histopathologic confirmation was obtained for all patients who received a biopsy in this study, which provided an independent clinical standard against which the physician’s interpretation from both colposcopes could be compared. Further, this study was completed in 129 patients in an international setting by health providers that were not directly involved in developing the device, which provides a better assessment of how the Pocket colposcope might perform in LMICs. Additionally, this study incorporated imaging of cervices stained with Lugol’s iodine, which had not previously been imaged or validated with the Pocket colposcope.
There were also several limitations or implementation challenges associated with this study, which are summarized in Table S3. First, this is a preliminary study of a new device with a relatively small sample in a referral setting in Perú. Future studies will assess the performance of the Pocket colposcope with a larger patient population in a primary care setting in an LMIC. Second, referring facilities only provided cytology to la Liga Contra el Cáncer for 28% of patients; thus, cytology was unknown for a majority of cases in our study. Third, acetic acid was not re-applied immediately prior to imaging with the Pocket colposcope until the last 32 patients (22, 26). Revising the study protocol in future studies to re-apply acetic acid between imaging with the two colposcopes could improve visualization of aceto-whitening. Fourth, confirmatory pathology was not acquired for all patients in our study. Specifically, patients who were colposcopically normal according to standard-of-care colposcopy did not receive a biopsy because it would be considered a deviation not only from the standard-of-care procedures at la Liga Contra el Cáncer but also from World Health Organization’s cervical cancer screening guidelines. Lastly, no relevant medical history was provided with the images, but physicians were aware that this study was conducted in a referral population. By providing relevant medical history, physicians may be able to more accurately risk stratify women for follow up.
Interpretation
We observed inter-observer and intra-observer variability among physician’s image evaluation and diagnosis. The Fleiss’ kappa statistic, which is a measure of inter-observer agreement, for the Pocket and was 0.29 overall, indicating that raters achieve fair inter-observer agreement (between 0.21–0.40) (27). In comparison, the Fleiss’ kappa statistic for the standard-of-care colposcope was 0.46 overall, indicating that raters achieve moderate inter-observer agreement (between 0.41–0.60) (27). The overall inter-observer agreement for the Pocket colposcope was lower than the standard-of-care colposcope used in this study likely because aceto-whitening was fading when the Pocket images were acquired. For CIN+ images, the Fleiss’ kappa statistics for the Pocket and standard-of-care colposcopes were 0.26 and 0.30, respectively, indicating that raters achieve fair inter-observer agreement (between 0.21–0.40) regardless of the colposcope used to capture the image. Thus, kappa statistics indicated that the Pocket colposcope performed similarly to a standard-of-care colposcope when used to identify precancerous and cancerous lesions; however neither colposcope achieves substantial or perfect inter-observer agreement. A previous study of 540 cervical images where acetic acid was used to stain features found that the inter-observer agreement was mostly fair to moderate between providers with kappa statistics ranging from 0.33 to 0.54 (28), which is similar to the range of Fleiss’ kappa statistics observed in this study. While inter-observer agreement in this study was fair to moderate, intra-observer agreement was substantial (between 0.61–0.80). Specifically, the average kappa statistic for the two colposcopes was 0.61. A previous study found that intra-observer agreement was substantial with kappa statistics ranging from 0.60 to 0.86 for the evaluation of 100 cervical images where acetic acid was used to stain features (29). Thus, our observation that intra-observer agreement is higher than inter-observer agreement is consistent with previous studies. Our study along with other studies reflect a need for improvement in colposcopist training in order to increase inter-observer agreement and mitigate the risk of misdiagnosis.
We found that the addition of Lugol’s iodine as a secondary contrast agent improved the agreement between the two colposcopes for all pathological categories by 8.9% and the accuracy of physician interpretation compared to pathology by 5.6% and 9.2% for the Pocket and standard-of-care colposcopes, respectively. Previous studies comparing the performance of VIA to visual inspection with Lugol’s iodine (VILI) found similar diagnostic accuracy and rates of CIN2+ detection (30, 31); however, no studies have been conducted that assess the additive diagnostic accuracy of VIA + VILI.
Incorporating vascular imaging into colposcopy exams has the potential to further improve accuracy as neovascularization is reflective of high-grade lesions (32, 33). In traditional colposcopy, a green filter is placed in front of the camera in order to enhance the visualization of vascularization, which due to hemoglobin’s strong absorption of green light makes vasculature appear black (34). In the United States, standard colposcopy includes imaging the cervix with both white and green light. At la Liga Contra el Cáncer, green light colposcopy is available but rarely used by the physicians to make a diagnosis and therefore was not implemented in this study. A study that systematically compares the benefits of using Lugol’s iodine and acetic acid imaging with both white and green light may provide the justification for leveraging all three sources of contrast.
Conclusion
Our goal is to incorporate the Pocket colposcope into a tele-colposcopy platform that could be used at multiple points in the care pathway in LMICs, including primary care clinics where is could replace VIA as a screening tool and referral centers where it could be used to guide biopsy (if available) and identify lesion location prior to treatment. The Pocket colposcope performed similarly to a standard-of-care colposcope when used to identify precancerous and cancerous lesions using acetic acid and Lugol’s iodine during colposcopy exams in Perú. This type of point-of-care diagnostic combined with tele-colposcopy for expert physician review of images could be used a means of reducing the morbidity and mortality of cervical cancer in Perú and elsewhere.
Supplementary Material
Acknowledgments
We acknowledge financial support from the National Institutes of Health Quick Trials Grant 1R01CA195500 and Academic Industry Partnership Grant 1R01CA19338001.
CTL and NR have applied for a provisional patent related to this manuscript: PCT/US2014/067038 “Colposcopes having light emitters and image capture devices and associated methods” and JM, CTL, MA, and NR have applied for provisional patent: PCT/US2017/025197 “Colposcopes and mammoscopes having curved ends and flat ends, associated methods, and speculum-free imaging methods”.
Funding: This study was funded by National Institutes of Health Quick Trials Grant 1R01CA195500 and Academic Industry Partnership Grant 1R01CA19338001.
Footnotes
Disclosure of interests: The other authors declare that they have no conflicts of interest. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship: JLM, CTL, MK, and NR designed and built the Pocket colposcope used in this study. MA, MK, and NR designed and built the software interface for the Pocket colposcope used in this study. MSK, YBF, GV, and NR designed the study. MSK and YBF obtained IRB approval. YBF and GV collected patient images in Perú. MA, MSK, and EJO trouble-shooted image collection in Perú. DD, MK, and JP conducted the image quality review and created the image panels. DD, YBF, and JP collected pathology reports and entered them into the database. LCM, PTT, JWS, and GV reviewed the images and provided a diagnosis. JLM, CTL, and DD cleaned and analyzed the data. JLM, CTL, AE, and NR contributed to the statistical analysis. JLM and NR created the figures and tables. JLM, DD, and NR wrote the paper. All authors critically reviewed the paper.
IRB status: Cervical images were collected under Duke University Medical IRB approved protocol (Pro00052865), which was approved on June 10, 2015.
References
- 1.Group FIS. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. PubMed PMID: 17494925. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. PubMed PMID: 19586656. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–72. doi: 10.3322/caac.21139. PubMed PMID: 22422631; PubMed Central PMCID: PMC3801360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee on Practice B-G. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–38. doi: 10.1097/aog.0b013e318277c92a. doi: http://10.1097/AOG.0b013e318277c92a. PubMed PMID: 23090560. [DOI] [PubMed] [Google Scholar]
- 5.Papanicolaou GN. A New Procedure for Staining Vaginal Smears. Science. 1942;95(2469):438–9. doi: 10.1126/science.95.2469.438. PubMed PMID: 17842594. [DOI] [PubMed] [Google Scholar]
- 6.Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, Bell MC, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116(11):2531–42. doi: 10.1002/cncr.25065. PubMed PMID: 20310056; PubMed Central PMCID: PMC2876205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman HPaW, BK Excess cervical cancer mortality: A marker for low access to health care in poor communities. National Institutes of Health. 2007 Report No.: 05-5282. [Google Scholar]
- 8.Jeronimo J, Morales O, Horna J, Pariona J, Manrique J, Rubiños J, et al. Visual inspection with acetic acid for cervical cancer screening outside of low-resource settings. Rev Panam Salud Publica. 2005;17(1):1–5. doi: 10.1590/s1020-49892005000100001. PubMed PMID: 15720875. [DOI] [PubMed] [Google Scholar]
- 9.Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga lT, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(Suppl):6–12. doi: 10.1634/theoncologist.18-S2-6. PubMed PMID: 24334477. [DOI] [PubMed] [Google Scholar]
- 10.Jeronimo J, Castle PE, Temin S, Denny L, Gupta V, Kim JJ, et al. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J Glob Oncol. 2017;3(5):635–57. doi: 10.1200/JGO.2016.006577. Epub 2016/10/12. PubMed PMID: 29094101; PubMed Central PMCID: PMCPMC5646891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abotchie PN, Shokar NK. Cervical cancer screening among college students in Ghana: knowledge and health beliefs. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2009;19(3):412. doi: 10.1111/IGC.0b013e3181a1d6de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agurto I, Bishop A, Sanchez G, Betancourt Z, Robles S. Perceived barriers and benefits to cervical cancer screening in Latin America. Preventive medicine. 2004;39(1):91–8. doi: 10.1016/j.ypmed.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reproductive health matters. 2008;16(32):41–9. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 14.Bingham A, Bishop A, Coffey P, Winkler J, Bradley J, Dzuba I, et al. Factors affecting utilization of cervical cancer prevention services in low-resource settings. Salud publica de Mexico. 2003;45:408–16. doi: 10.1590/s0036-36342003000900015. [DOI] [PubMed] [Google Scholar]
- 15.Fort VK, Makin MS, Siegler AJ, Ault K, Rochat R. Barriers to cervical cancer screening in Mulanje, Malawi: a qualitative study. Patient preference and adherence. 2011;5:125. doi: 10.2147/PPA.S17317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mupepi SC, Sampselle CM, Johnson TR. Knowledge, attitudes, and demographic factors influencing cervical cancer screening behavior of Zimbabwean women. Journal of Women's Health. 2011;20(6):943–52. doi: 10.1089/jwh.2010.2062. [DOI] [PubMed] [Google Scholar]
- 17.Nwankwo K, Aniebue U, Aguwa E, Anarado A, Agunwah E. Knowledge attitudes and practices of cervical cancer screening among urban and rural Nigerian women: a call for education and mass screening. European journal of cancer care. 2011;20(3):362–7. doi: 10.1111/j.1365-2354.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 18.Watkins M, Gabali C, Winkleby M, Gaona E, Lebaron S. Barriers to cervical cancer screening in rural Mexico. Int J Gynecol Cancer. 2002;12(5):475–9. doi: 10.1046/j.1525-1438.2002.01170.x. [DOI] [PubMed] [Google Scholar]
- 19.Were E, Nyaberi Z, Buziba N. Perceptions of risk and barriers to cervical cancer screening at Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya. African health sciences. 2011;11(1) [PMC free article] [PubMed] [Google Scholar]
- 20.Raifu AO, El-Zein M, Sangwa-Lugoma G, Ramanakumar A, Walter SD, Franco EL, et al. Determinants of Cervical Cancer Screening Accuracy for Visual Inspection with Acetic Acid (VIA) and Lugol's Iodine (VILI) Performed by Nurse and Physician. PLoS One. 2017;12(1):e0170631. doi: 10.1371/journal.pone.0170631. Epub 2017/01/20. PubMed PMID: 28107486; PubMed Central PMCID: PMCPMC5249231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam CT, Krieger MS, Gallagher JE, Asma B, Muasher LC, Schmitt JW, et al. Design of a Novel Low Cost Point of Care Tampon (POCkeT) Colposcope for Use in Resource Limited Settings. PLoS One. 2015;10(9):e0135869. doi: 10.1371/journal.pone.0135869. PubMed PMID: 26332673; PubMed Central PMCID: PMCPMC4557989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller JL, Asma E, Lam CT, Krieger MS, Gallagher JE, Erkanli A, et al. International Image Concordance Study to Compare a Point-of-Care Tampon Colposcope With a Standard-of-Care Colposcope. J Low Genit Tract Dis. 2017;21(2):112–9. doi: 10.1097/LGT.0000000000000306. PubMed PMID: 28263237; PubMed Central PMCID: PMCPMC5365351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam CT, Mueller J, Asma B, Asiedu M, Krieger MS, Chitalia R, et al. An integrated strategy for improving contrast, durability, and portability of a Pocket Colposcope for cervical cancer screening and diagnosis. PLoS One. 2018;13(2):e0192530. doi: 10.1371/journal.pone.0192530. Epub 2018/02/09. PubMed PMID: 29425225; PubMed Central PMCID: PMCPMC5806883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asiedu MN, Agudogo J, Krieger MS, Miros R, Proeschold-Bell RJ, Schmitt JW, et al. Design and preliminary analysis of a vaginal inserter for speculum-free cervical cancer screening. PLoS One. 2017;12(5):e0177782. doi: 10.1371/journal.pone.0177782. Epub 2017/05/31. PubMed PMID: 28562669; PubMed Central PMCID: PMCPMC5451045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health CfD, Radiological. Reprocessing of Reusable Medical Devices: Information for Manufacturers - FDA-Cleared Sterilants and High Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices - March 2015. 2017 [Google Scholar]
- 26.Pogue BW, Kaufman HB, Zelenchuk A, Harper W, Burke GC, Burke EE, et al. Analysis of acetic acid-induced whitening of high-grade squamous intraepithelial lesions. J Biomed Opt. 2001;6(4):397–403. doi: 10.1117/1.1412850. PubMed PMID: 11728197. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. PubMed PMID: 843571. [PubMed] [Google Scholar]
- 28.Manga S, Parham G, Benjamin N, Nulah K, Sheldon LK, Welty E, et al. Cervical Cancer Screening in Cameroon: Interobserver Agreement on the Interpretation of Digital Cervicography Results. J Low Genit Tract Dis. 2015;19(4):288–94. doi: 10.1097/LGT.0000000000000133. PubMed PMID: 26164295. [DOI] [PubMed] [Google Scholar]
- 29.Ferris DG, Cox JT, Burke L, Litaker MS, Harper DM, Campion MJ, et al. Colposcopy Quality Control: Establishing Colposcopy Criterion Standards for the National Cancer Institute ALTS Trial Using Cervigrams. J Low Genit Tract Dis. 1998;2(4):195–203. PubMed PMID: 25950212. [PubMed] [Google Scholar]
- 30.Huchko MJ, Sneden J, Zakaras JM, Smith-McCune K, Sawaya G, Maloba M, et al. A randomized trial comparing the diagnostic accuracy of visual inspection with acetic acid to Visual Inspection with Lugol's Iodine for cervical cancer screening in HIV-infected women. PloS one. 2015;10(4):e0118568. doi: 10.1371/journal.pone.0118568. PubMed PMID: 25849627; PubMed Central PMCID: PMCPMC4388564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huchko MJ, Sneden J, Leslie HH, Abdulrahim N, Maloba M, Bukusi E, et al. A comparison of two visual inspection methods for cervical cancer screening among HIV-infected women in Kenya. Bull World Health Organ. 2014;92(3):195–203. doi: 10.2471/BLT.13.122051. PubMed PMID: 24700979; PubMed Central PMCID: PMCPMC3949589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellas A, Moch H, Schultheiss E, Feichter G, Almendral AC, Gudat F, et al. Angiogenesis in cervical neoplasia: microvessel quantitation in precancerous lesions and invasive carcinomas with clinicopathological correlations. Gynecol Oncol. 1997;67(1):27–33. doi: 10.1006/gyno.1997.4835. PubMed PMID: 9345352. [DOI] [PubMed] [Google Scholar]
- 33.Smith-McCune K. Angiogenesis in squamous cell carcinoma in situ and microinvasive carcinoma of the uterine cervix. Obstet Gynecol. 1997;89(3):482–3. PubMed PMID: 9052610. [PubMed] [Google Scholar]
- 34.Ferris DG, Willner W, Ho J. Colposcopes: a critical review. The Journal of family practice. 1991;33(5):506–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


