Summary
Intracellular malate–starch interconversion plays an important role in stomatal movements. We investigated whether malate or oxaloacetate from the cytosolic membrane side regulate anion channels in the plasma membrane of Arabidopsis thaliana guard cells. Physiological concentrations of cytosolic malate have been reported in the range of 0.4–3 mM in leaf cells.
Guard cell patch clamp and two-electrode oocyte voltage-clamp experiments were pursued.
We show that a concentration of 1 mM cytosolic malate greatly activates S-type anion channels in Arabidopsis thaliana guard cells. Interestingly, 1 mM cytosolic oxaloacetate also activates S-type anion channels. Malate activation was abrogated at 10 mM malate and in SLAC1 anion channel mutant alleles Interestingly, malate activation of S-type anion currents was disrupted at below resting cytosolic free calcium concentrations ([Ca2+]cyt), suggesting a key role for basal [Ca2+]cyt signaling. Cytosolic malate was not able to directly activate or enhance SLAC1-mediated anion currents in Xenopus oocytes, whereas in positive controls cytosolic NaHCO3 enhanced SLAC1 activity, suggesting that malate may not directly modulate SLAC1. Cytosolic malate activation of S-type anion currents was impaired in ost1 and in cpk5/6/11/23 quadruple mutant guard cells.
Together these findings show that these cytosolic organic anions function in guard cell ion channel regulation.
Keywords: Arabidopsis, Cl− channel, ion channel regulation, malic acid, stomata
Introduction
Stomatal pores, which are formed by pairs of guard cells in the epidermis of aerial tissues, control gas exchange and account for loss of water, including during drought stress. Stomatal movements are regulated by several signals, including the phytohormone abscisic acid (ABA), CO2, humidity, reactive oxygen species, light, and pathogens (Hetherington & Woodward, 2003; Roelfsema et al., 2012; Murata et al., 2015; Ye et al., 2015). Stomatal movements are regulated by controlled transport of osmoregulatory ions through several types of ion channels. Blue light promotes stomatal opening. H+ efflux from the cytosol of stomatal guard cells is mediated by the H+-ATPase that hyperpolarizes the membrane potential, which consequently activates voltage-gated inward K+ channels, causing stomatal opening (Shimazaki et al., 1986, 2007; Schroeder et al., 1987; Kinoshita & Hayashi, 2011). By contrast, the plant hormone abscisic acid (ABA), elevated CO2 concentrations and ozone induce stomatal closure. These stimuli activate anion channels among regulation of several ion channels and transporters and the efflux of anions induces plasma membrane depolarization that activates outward K+ channels, causing stomatal closure (Pandey et al., 2007; Negi et al., 2014; Munemasa et al., 2015).
The SLAC1 gene was genetically mapped and isolated from EMS mutant screens and plays a central role in stomatal movements (Negi et al., 2008; Vahisalu et al., 2008). The SLAC1 (SLOW ANION CHANNEL-ASSOCIATED1) gene, is required for slow anion channel activity in Arabidopsis guard cells and stomatal closing mediated by multiple stimuli, including abscisic acid, CO2, ozone, H2O2 and Ca2+ (Negi et al., 2008; Vahisalu et al., 2008). Several protein kinases including OST1 (OPEN STOMATA1), and CPKs (Ca2+-dependent protein kinases), and GHR1 (GUARD CELL HYDROGEN PEROXIDE-RESISTANT1) can cause phosphorylation and activation of SLAC1 anion channels in Xenopus oocytes (Geiger et al., 2009, 2010; Lee et al., 2009; Brandt et al., 2012; Hua et al., 2012; Brandt et al., 2015).
S-type anion channels in guard cells and SLAC1 expressed in Xenopus oocytes are permeable to Cl− and NO3− (Schmidt & Schroeder, 1994; Geiger et al., 2011). However, SLAC1 and S-type anion channels in Arabidopsis guard cells are not permeable to HCO3− and malate (Geiger et al., 2009; Xue et al., 2011; Laanemets et al., 2013). Intracellular bicarbonate generated by carbonic anhydrases can act as a second messenger and activate S-type anion channels in guard cells (Hu et al., 2010; Xue et al., 2011; Tian et al., 2015; Wang et al., 2016).
R-type anion channels form a distinct type of anion channel in the plasma membrane of guard cells (Keller et al., 1989; Hedrich et al., 1990; Schroeder & Keller, 1992). R-type anion channels are encoded by the aluminium-activated malate transporter (ALMT) gene ALMT12 (Meyer et al., 2010b). ALMTs form a unique family of passive anion transport systems in plants. ALMTs are involved in dicarboxylic acid excretion required for aluminium tolerance (Hoekenga et al., 2006) and in the efflux of inorganic and organic anions including malate during stomatal closure (Gerhardt et al., 1987; Meyer et al., 2010b; Sasaki et al., 2010). AtALMT12 is mainly expressed in guard cells and targeted to the plasma membrane encoding for R-type anion channels (Meyer et al., 2010b). Loss of AtALMT12 impaired stomatal closure in response to ABA, darkness and high levels of CO2 (Meyer et al., 2010b; Sasaki et al., 2010).
Malate exists in several cellular compartments (i.e. vacuoles, cytosol, chloroplasts and mitochondria). Malate is transported among compartments and this malate transport is important for regulation of subcellular malate concentrations (Van Kirk & Raschke, 1978; Martinoia et al., 1985; Martinoia & Rentsch, 1994; Emmerlich et al., 2003; Meyer et al., 2010a; Hills et al., 2012). The uptake of apoplastic malate is mediated by the plasma membrane AtABCB14 (ATP BINDING CASSETTE TRANSPORTER) in Arabidopsis guard cells (Lee et al., 2008).
Plants exhibit CAM metabolism by using malic acid as a store of available CO2 during the night; as a result malate accumulates to high levels of up to 350 mM in vacuoles (Luttge, 1987; Martinoia & Rentsch, 1994). Decarboxylation decreases the malic acid level by 200 mM during the day. C3 and C4 plants accumulate malic acid as salts (i.e. K-malate) at concentrations of up to 100–200 mM in vacuoles (Winter et al., 1982). However, the cytosolic malate concentration is tightly controlled and its level is kept in the range from c. 0.4 to 3 mM in the dark and c. 2 to 5 mM in the light, since malate is at a central point of metabolic pathways affecting osmotic balance and pH homeostasis (Gerhardt et al., 1987; Martinoia & Rentsch, 1994; Winter et al., 1994; De Angeli et al., 2013).
Previous studies have shown that extracellular malate can activate R-type anion channels in isolated protoplasts and intact Vicia faba guard cells (Marten et al., 1992; Hedrich & Marten, 1993; Hedrich et al., 1994). These observations suggest a ‘feedforward’ mechanism for control of R-type anion channels (Hedrich & Marten, 1993; Wang & Blatt, 2011), such that malate released from guard cells during stomatal closing (Van Kirk & Raschke, 1978), can further enhance stomatal closing. However, analysis of cytosolic malate concentrations on guard cell plasma membrane ion channels has thus far shown that high malate concentrations ≥ 10 mM can inhibit S-type anion channels in Vicia faba guard cells (Schmidt & Schroeder, 1994; Wang & Blatt, 2011). Enhancement of guard cell ion currents by millimolar malate was also observed (Wang & Blatt, 2011). Moreover, 1 mM oxaloacetic acid (OAA) inhibits anion currents in Vicia faba guard cells (Wang & Blatt, 2011).
In this study, we investigated whether cytosolic malate and OAA can regulate anion channels in Arabidopsis guard cells. Interestingly, we have found that malate and OAA cause a clear activation of S-type anion channels in Arabidopsis guard cells. We have found that 1 mM malate and 1 mM OAA activate S-type anion channel Cl− currents in wild-type guard cells. Malate activation occurs at both resting and elevated cytosolic Ca2+ concentrations, but interestingly, physiological baseline cytosolic free Ca2+ concentrations are required for malate activation of S-type channels in guard cells. Furthermore, high cytosolic malate (10 mM) did not activate these channels, presumably due to the previously reported channel inhibition at high malate (Schmidt & Schroeder, 1994; Wang & Blatt, 2011). We further show that loss of slac1, ost1 and cpk5/6/11/23 impair 1 mM malate activation of S-type anion channel currents in guard cells. We also investigate reconstitution of malate regulation of the SLAC1 anion channel in Xenopus laevis oocytes. These experiments suggest that malate does not directly increase SLAC1-mediated anion channel activity, which in positive controls is found to be distinct from bicarbonate regulation of SLAC1.
Materials and Methods
Plant growth conditions
Arabidopsis thaliana L. Heynh. [Author, please confirm inserted text ‘L. Heynh.’ is correct] seedlings were grown on Murashige and Skoog (MS) medium (Sigma-Aldrich) containing 1% (w/v) sucrose and 0.8% (w/v) agar for 7 d and were transplanted into soil (Sunshine Professional Blend). The potted plants were kept in a growth chamber (white light of 100 μmol m−2 s1 at 22°C, 70% relative humidity) for 4–5 wk.
Patch clamp analyses
Arabidopsis thaliana guard cell protoplasts were isolated as described previously (Yamamoto et al., 2016). During patch clamp recordings, the membrane voltage was stepped to potentials starting from +35 to −145 mV for 5 s with −30 mV decrements with a holding potential at +30 mV. All assays were conducted at room temperature (22°C) under dim light.
The bath solution contained 30 mM CsCl, 2 mM MgCl2, 10 mM MES-Tris (pH 5.6), and 1 mM CaCl2, with D-sorbitol added to an osmolality of 485 mmol/kg. The pipette solution contained 3.35 mM CaCl2, 6.7 mM EGTA, 2 mM MgCl2, 10 mM HEPES-Tris (pH 7.1), and 150 mM CsCl, with an osmolality of 500 mmol kg−1. The final free Ca2+ concentration in the pipette solution was 0.2 μM when indicated as calculated using Max Chelator software version 5.60 developed by Dr. Chris Patton at Stanford University. The free calcium concentration was buffered to 2 μM (5.86 mM CaCl2 in the pipette solution) or to 0.01 μM free Ca2+ (0.3 mM CaCl2 in the pipette solution) when indicated in the figures. Final osmolalities were adjusted with D-sorbitol. For analysis of malate and oxaloacetate activation of S-type anion channels, the indicated malate and oxaloacetate concentrations and 5 mM Mg-ATP were freshly added to the pipette solution and the pH was adjusted with Tris before patch clamp experiments. As reported in previous research, the time- and voltage-dependent kinetics of deactivation of S-type anion channels in guard cells and SLAC1-mediated currents in Xenopus oocytes (Schmidt & Schroeder, 1994; Brandt et al., 2015) show variability that may reflect distinct post-translational protein modification states that remain to be characterized. Intracellular malate and OAA activated anion currents independent of these channel states.
Two-electrode voltage-clamp recordings in Xenopus laevis oocytes
All constructs were cloned into the pNB1 oocyte expression vector using the USER (Uracil-Specific Excision Reagent) method (Nour-Eldin et al., 2006). To investigate intracellular malate effects on anion channel activity in oocytes, SLAC1yc and OST1yn cRNA (Geiger et al., 2009) or SLAC1 and CPK6 cRNA or SLAH3 and CPK21 cRNA were co-injected into oocytes and incubated in ND96 buffer at 16°C for 2 d before voltage-clamp recordings (Wang et al., 2017). The extracellular recording solution contained 10 mM MES/Tris (pH 7.4), 1 mM MgCl2, 1 mM CaCl2, 2 mM KCl, 24 mM NaCl, and 70 mM Na-gluconate. Osmolality was adjusted to 220 mM using D-sorbitol. To investigate effects of malate, 11.5 mM bicarbonate, 1 mM, 10 mM or 20 mM malate were injected into each oocyte as final calculated concentrations based on oocyte volume calculations (final concentration = 25 nl [injected volume] / 500 nl [oocyte volume] × injected concentration) (Wang et al., 2016). To maintain the same injection volume of 25 nl in all experiments, 20 mM, 200 mM, 400 mM malate and 230 mM NaHCO3 solutions were prepared to achieve final concentrations of 1 mM, 10 mM and 20 mM malate and 11.5 mM bicarbonate in oocytes. For malate and bicarbonate injections, oocytes were recorded consistently 10 min after injections (Wang et al., 2016). Steady state currents were recorded starting from a holding potential of 0 mV and ranging from +40 to −160 mV in −20 mV decrements, followed by a −120 mV voltage ‘tail’ pulse (Geiger et al., 2009; Wang et al., 2016).
Results
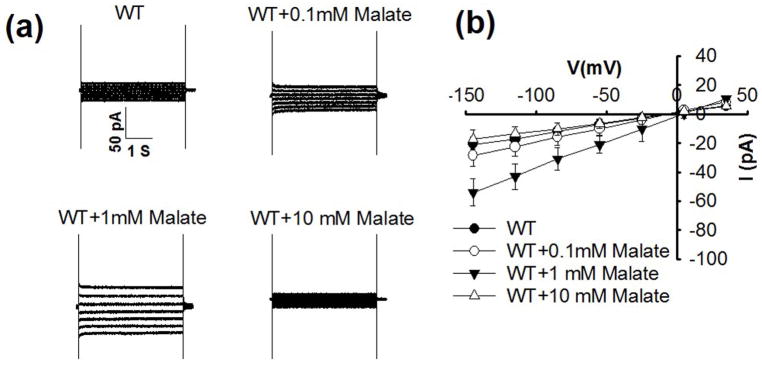
We investigated whether physiological cytosolic malate affects the activity of S-type anion channels in the plasma membrane of Arabidopsis guard cells. Interestingly, adding 1 mM malate to the patch clamp pipette solution that dialyzes the cytoplasm of guard cells caused enhancement of whole guard cell ion currents (Fig. 1, Supporting Information Fig. S1), similar to Vicia faba guard cells (Wang & Blatt, 2011). Addition of 0.1 mM malate to the cytosol was not sufficient to cause a robust enhancement in ion currents (Fig. 1). In one of the experimental data sets, 0.1 mM cytosolic malate caused a significant but small enhancement of ion currents in guard cells (Fig. S1; P < 0.02 at −145 mV, n = 8 guard cells). All experiments were performed in the presence of 165.6 mM chloride ions in the pipette solution that dialyzes the cytosol, suggesting that the effect of the malate anion is unique relative to chloride ions.
Fig. 1.
Cytosolic malate at 1 mM activates ionic currents in Arabidopsis thaliana wild-type (WT) guard cells, whereas 0.1 mM did not significantly enhance ion currents in these experiments and 10 mM malate showed no activation of currents. (a) Typical whole-cell recordings of ionic currents in guard cell protoplasts of wild type plants without malate or with 0.1 mM, 1 mM and 10 mM malate added to the pipette solution that dialyzes the cytosol of guard cells. (b) Steady state current-voltage relationships recorded as in (a). The number of guard cells was n = 5–8 for each condition. Data are mean ± SE (One-way ANOVA and Tukey’s test: P < 0.01 for WT vs WT+1 mM malate; P < 0.01 for WT+10 mM malate vs WT+1 mM malate; P < 0.05 for WT+0.1 mM malate vs WT+1 mM malate at −145 mV).
Previous studies have however shown that higher cytosolic malate concentrations (i.e. ≥10 mM) can inhibit or block S-type anion channels in Vicia faba guard cells (Schmidt & Schroeder, 1994; Wang & Blatt, 2011) and that S-type anion channels in Arabidopsis guard cells are largely impermeable to malate anions (Laanemets et al., 2013). We therefore tested the effect of adding 10 mM malate to the cytosol. Interestingly, at 10 mM malate, the activation of guard cell ion currents was not observed (Figs 1, S1).
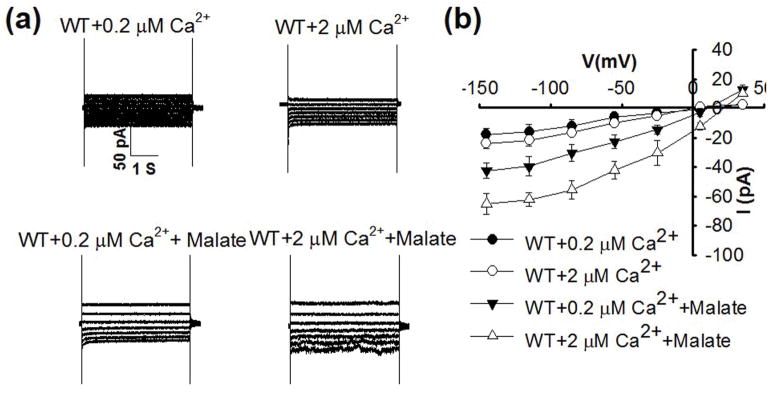
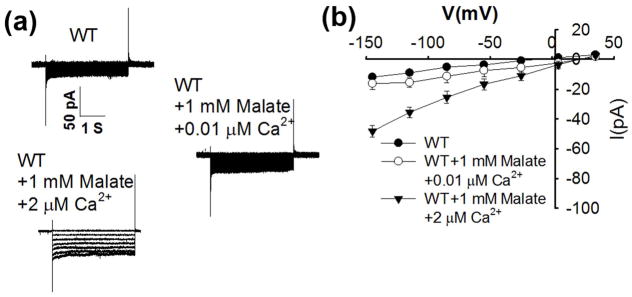
Robust abscisic acid activation of S-type anion channels in Arabidopsis guard cells is mediated by simultaneously elevating cytosolic Ca2+ (Siegel et al., 2009; Brandt et al., 2015). Addition of 1 mM malate to the cytosol in guard cells enabled activation of ion currents when the cytosolic free Ca2+ concentration ([Ca2+]cyt) was buffered to 0.2 μM (Fig. 2). When [Ca2+]cyt was buffered to an elevated level of 2 μM, anion currents were not activated in the absence of malate (Figs 2, 3), consistent with previous studies (Allen et al., 2002; Brandt et al., 2015). Addition of 1 mM malate to the pipette solution with [Ca2+]cyt buffered to 2 μM led to even stronger enhancement of guard cell anion currents than at 0.2 μM [Ca2+]cyt (Fig. 2). However, when the cytosolic free Ca2+ was buffered to a slightly below resting concentration of 0.08 μM and a sub-resting level of 0.01 μM, 1 mM malate did not significantly enhance anion channel currents (Figs 3, S2).
Fig. 2.
Malate activation of anion currents in Arabidopsis thaliana wild-type (WT) guard cells occurs at a buffered resting cytosolic free Ca2+ concentration of 0.2 μM, and malate activation of S-type anion channel currents is enhanced by 2 μM free Ca2+ compared with 0.2 μM free Ca2+ in the pipette solution. (a) Typical whole-cell recordings of S-type anion channel currents in guard cell protoplasts of wild type plants with 0.2 μM free Ca2+, 2 μM free Ca2+, 0.2 μM free Ca2+ + 1 mM malate and 2 μM free Ca2++1 mM malate in the pipette solution. (b) Steady state current-voltage relationships recorded as in (a). The number of guard cells was n = 5–7 for each condition. Data are mean ± SE (P < 0.01 for 0.2 μM free Ca2+ vs 0.2 μM free Ca2+ + 1 mM malate; P < 0.01 for 2 μM free Ca2+ vs 2 μM free Ca2+ + 1 mM malate at −145 mV).
Fig. 3.
Cytosolic malate (1 mM) does not activate S-type anion channel currents in Arabidopsis thaliana wild-type (WT) guard cells at a low free cytosolic Ca2+ concentration of 0.01 μM. (a) Typical whole-cell recordings of S-type anion channel currents in guard cell protoplasts of wild type plants with or without 1 mM malate at 0.01μM and 2 μM free Ca2+. Note 2 μM free Ca2+ was buffered with 5.7 mM CaCl2 + 6.7 mM EGTA; 0.01 μM free Ca2+ used 0.3 mM CaCl2 + 6.7 mM EGTA. (b) Steady-state current-voltage relationships of recordings as in (a). The number of guard cells was from seven to nine for each condition. Data are mean ± SE (P < 0.01 for WT vs WT+1 mM malate+2 μM free Ca2+; P < 0.01 for WT+1 mM malate+0.01 μM free Ca2+ vs WT+1 mM malate+2 μM free Ca2+).
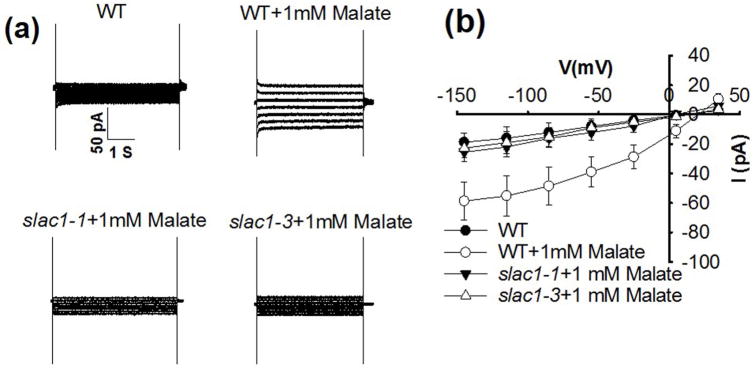
The SLAC1 gene is required for S-type anion channel activity in guard cells and in Xenopus oocytes (Negi et al., 2008; Vahisalu et al., 2008; Geiger et al., 2009; Lee et al., 2009). We investigated whether cytosolic malate activation is mediated by SLAC1-associated anion currents by using slac1 mutant plants, in which R-type anion channel currents are intact (Vahisalu et al., 2008). The malate activation of anion currents in guard cells was disrupted in two slac1 mutant alleles, showing that intracellular malate activates S-type anion channels in guard cells and that SLAC1 plays a key role in this response (Fig. 4).
Fig. 4.
slac1-1 and slac1-3 mutants impair 1 mM malate activation of S-type anion channel currents in Arabidopsis thaliana guard cells. (a) Typical whole-cell recordings of S-type anion channel currents in guard cell protoplasts of wild type (WT), slac1-1 and slac1-3 mutant plants with 1 mM malate and 2 μM free Ca2+ in the pipette solution that dialyzes the cytosol. (b) Steady-state current-voltage relationships recorded as in (a). The number of guard cells was n = 5–7. Data are mean ± SE (P < 0.01 for slac1-1 + 1 mM malate vs WT + 1 mM malate; P < 0.01 for slac1-3 + 1 mM malate vs WT + 1 mM malate at −145 mV).
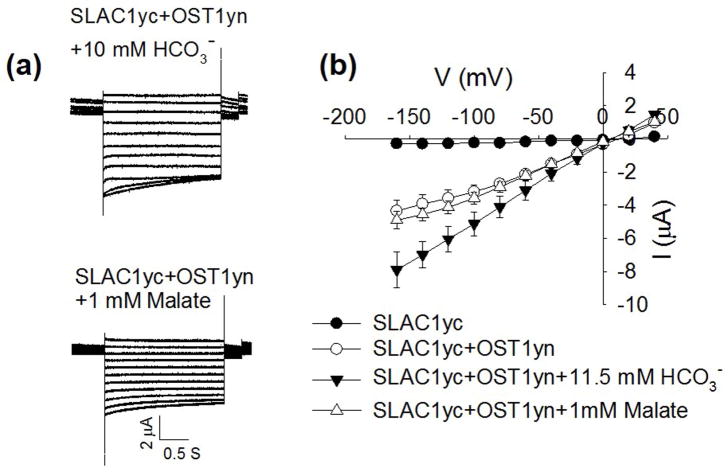
S-type anion currents have been shown to be activated by high intracellular concentrations of bicarbonate anions (HCO3−) in guard cells (Hu et al., 2010; Xue et al., 2011; Tian et al., 2015) and the same concentrations of intracellular bicarbonate enhances the activity of SLAC1 channels activated by the protein kinases OST1, CPK6 and CPK23 in Xenopus oocytes (Wang et al., 2016). We investigated whether SLAC1-mediated currents in Xenopus oocytes are also directly enhanced by intracellular malate. However, micro-injection of malate at a final concentration of 1 mM in the cytoplasm of oocytes did not enhance OST1- or CPK6-mediated SLAC1 channel activity in oocytes (Figs 5, S3a). In positive control experiments, injection of HCO3− enhanced SLAC1-mediated currents in the same batches of oocytes (Fig. 5), consistent with previous findings (Wang et al., 2016). These data suggest that the mechanism of HCO3− enhancement of SLAC1 activity differs from the malate activation of S-type anion channels in guard cells found here. We also tested the SLAH3 channel that contributes to S-type anion channel function in guard cells (Geiger et al., 2011). Cytosolic malate (1 mM) also did not enhance SLAH3 anion channel activity in Xenopus oocytes (Fig. S4). Additional experiments were conducted by injecting malate at final concentrations of 10 mM or 20 mM into oocytes expressing SLAC1 and OST1. However, these treatments did not clearly enhance or inhibit SLAC1-mediated anion currents (Fig. S3b) (n > 3 oocyte batches tested).
Fig. 5.
Cytosolic malate at 1 mM did not enhance SLAC1yc-OST1yn–mediated anion channel currents in Xenopus oocytes. (a) Whole-cell currents were recorded from oocytes expressing SLAC1yc and OST1yn after injection of the indicated final NaHCO3 or malate concentrations. (b) Steady state current-voltage relationships from oocytes recorded as in (a). The number of oocytes was n = 9–14. Data are mean ± SE. Experiments shown here are from one batch of oocytes, with similar findings made in three independent oocyte batches. (P < 0.01 for SLACyc+OST1yn vs SLACyc+OST1yn +11.5 mM HCO3−; P < 0.01 for SLACyc+OST1yn +1 mM malate vs SLACyc+OST1yn +11.5 mM HCO3− at −160 mV.)
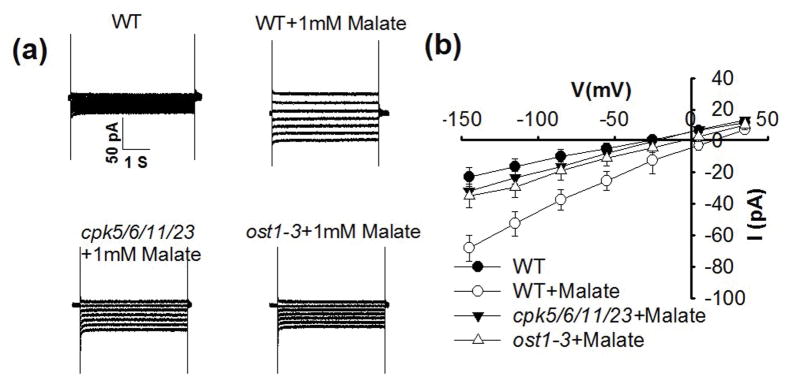
We further investigated whether the intracellular malate activation of S-type anion currents in guard cells (Figs 1, 2) depends on upstream protein kinases, using the ost1 and cpk5/6/11/23 mutants that impair abscisic acid activation of S-type anion channels in guard cells (Li et al., 2000; Geiger et al., 2009; Brandt et al., 2015). The ost1 and cpk5/6/11/23 mutants displayed no S-type anion currents without malate consistent with previous findings in these mutants (Geiger et al., 2009; Brandt et al., 2015) and also with zero malate controls in wild-type guard cells (Figs 1, 4, 6, S5). We found that malate activation of S-type anion currents was impaired in the ost1 and cpk5/6/11/23 mutants (Fig. 6).
Fig. 6.
cpk5/6/11/23 and ost1-3 mutant plants impair 1 mM malate activation of S-type anion channel currents in Arabidopsis thaliana guard cells. (a) Typical whole-cell recording of S-type anion channel currents in guard cell protoplasts of wild type (WT), cpk5/6/11/23 and ost1-3 mutant plants. (b) Steady state current-voltage relationships recorded as in (a). The number of guard cells was n = 4–7. Data are mean ± SE. The pipette solution contained 2 μM free Ca2+ (P < 0.01 for cpk5/6/11/23+1 mM malate vs WT+1 mM malate; P < 0.01for ost1-3+1 mM malate vs WT+1 mM malate at −145 mV).
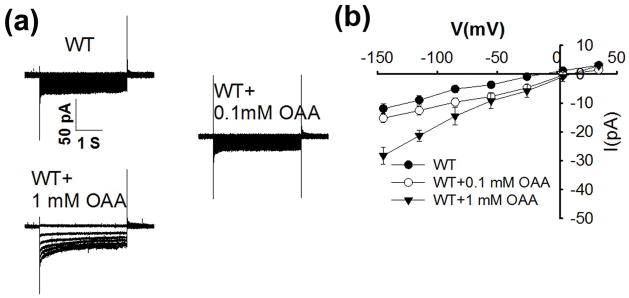
Oxaloacetic acid is a precursor in the malate synthesis pathway in guard cells (Martinoia & Rentsch, 1994). To test the effect of OAA on S-type anion channel currents, we added oxaloacetate (OAA) to the pipette solution. Surprisingly, 1 mM OAA was able to activate S-type anion channel currents, while this stimulation was not observed at 0.1 mM cytosolic OAA (Fig. 7). The unexpected activation of S-type anion currents by cytosolic OAA was found in independent experimental sets by J.W. and C.W.
Fig. 7.
Cytosolic oxaloacetate (OAA) at 1 mM activates S-type anion channel currents in wild-type (WT) Arabidopsis thaliana guard cells, whereas 0.1 mM OAA does not show activation. (a) Typical whole-cell recordings of S-type anion channel currents in guard cell protoplasts of wild type (WT) plants without OAA or with 0.1mM or 1 mM OAA added to the pipette solution. The pipette solutions contained 2 μM free Ca2+. (b) Steady state current-voltage relationships recorded as in (a). The number of guard cell is n = 6–9 for each condition. Data are mean ± SE. Note that Fig. 3 shares the same WT I–V curves, as data were obtained in the same experimental data sets (P < 0.01 for WT vs WT+1 mM OAA; P < 0.01 for WT+0.1 mM OAA vs WT+1 mM OAA at −145 mV).
Discussion
Previous studies have shown that extracellular malate enhances R-type anion channel activity but not S-type anion channel activity in guard cells (Marten et al., 1992; Hedrich & Marten, 1993; Hedrich et al., 1994). Moreover, intracellular malate at high concentrations ≥ 10 mM inhibits S-type anion channel activity in guard cells (Schmidt & Schroeder, 1994; Wang & Blatt, 2011) and at 1 mM malate an enhancement of anion currents occurs in Vicia faba guard cells (Wang & Blatt, 2011).
Cytosolic malate concentrations in the range from 0.4 to 3 mM have been reported in plant cells (Gerhardt & Heldt, 1984; Winter et al., 1994; Farre et al., 2001). In the present study we have found that cytosolic malate concentrations of 1 mM activate S-type anion channels in Arabidopsis guard cells, whereas lower malate concentrations of 0.1 mM showed either weak or no substantial activation of anion currents in guard cells (Figs 1, S1). Moreover, another cytosolic dicarboxylic acid OAA also stimulates S-type anion channels in the guard cell plasma membrane (Fig. 7). By contrast, in Vicia faba guard cells 1 mM intracellular oxaloacetate inhibited plasma membrane anion currents (Wang & Blatt, 2011).
The present study suggests that cytosolic OAA and malate at concentrations in the range of 1 mM, predicted to be physiological (Gerhardt & Heldt, 1984; Winter et al., 1994; Farre et al., 2001) can play an important role in anion channel up-regulation in Arabidopsis guard cells thus functioning in stomatal closing. During stomatal opening, guard cells synthesize malate from starch and transport malate into vacuoles, where malate is stored at high concentrations in the >100 mM range as osmotic counter ion to K+ ions (Winter et al., 1994). An increase in malate production in guard cells required for stomatal opening has been predicted to lead to an increase in the cytosolic malate concentration (Wang & Blatt, 2011). An increase in cytosolic malate to 10 mM would inhibit S-type anion channel activity (Figs 1, S1) (Schmidt & Schroeder, 1994; Wang & Blatt, 2011), which would favor stomatal opening. Thus high cytosolic malate concentrations may contribute to stomatal opening by inhibiting S-type anion channels. In line with this prediction, the stomatal opening signals blue light, red light and/or low CO2 cause down-regulation of S-type anion channel activity in guard cells (Roelfsema et al., 2002; Roelfsema et al., 2006).
Malate enhanced S-type anion currents in guard cells both at a resting cytosolic free Ca2+ concentration of 0.2 μM and at 2 μM free Ca2+ (Fig. 2). Interestingly however, when the free calcium was clamped to 0.08 μM, a concentration that is only slightly below resting levels, or to a sub-resting level of 0.01 μM free Ca2+, S-type anion channels were not activated by intracellular malate (Figs 3, S2). A previous study showed that buffering the free calcium concentration to sub-resting levels disrupts abscisic acid activation of anion channels in Vicia faba guard cells (Levchenko et al., 2005). A contribution of baseline resting Ca2+ levels to stimulus-induced stomatal closing has been observed (Gilroy et al., 1991; Grabov & Blatt, 1998; Levchenko et al., 2005; Siegel et al., 2009) but sub-resting levels have been less studied. Based on the present findings and previous research (Levchenko et al., 2005) further analyses of guard cell ion channel regulation at sub-baseline levels should be of interest for dissecting functions of resting calcium concentrations on ion channel regulation, in particular based on studies showing that abscisic acid and elevated CO2 increase the sensitivity of S-type anion channels and inward-rectifying K+ channels to cytosolic Ca2+ (Siegel et al., 2009; Chen et al., 2010; Xue et al., 2011; Brandt et al., 2015).
Predicted physiological cytosolic malate concentrations (0.4–3 mM) activate S-type anion channels in guard cells (Figs 1, 2, 4, 6) (Wang & Blatt, 2011). During stomatal closing, malate concentrations are reduced in guard cells through efflux and starch synthesis, (Van Kirk & Raschke, 1978; Schnabl, 1981; Schnabl et al., 1982), indicating that low millimolar cytosolic malate concentrations may occur. Thus the up-regulation of anion channel activity identified here could contribute to stomatal closing. Consistent with these findings, malate activation of S-type anion channels was disrupted in the ost1 and cpk5/6/11/23 mutants (Fig. 6) that impair stomatal closing (Mustilli et al., 2002; Yoshida et al., 2002; Brandt et al., 2015). The enhancement of S-type anion channel activity by intracellular malate depends on the SLAC1 gene, as slac1 mutants disrupted the cytosolic malate response (Fig. 4).
The ALMT9 and ALMT6 chloride channels are targeted to the tonoplast of guard cells. ALMT9 has been shown to mediate malate and fumarate currents directed into vacuoles of mesophyll cells (Kovermann et al., 2007; De Angeli et al., 2013; Zhang et al., 2013, 2014). AtALMT6 mediates Ca2+ and pH dependent malate currents into guard cell vacuoles (Meyer et al., 2011). Most recently, another tonoplast targeted ALMT channel, AtALMT4, was shown to mediate malate efflux from vacuoles, functioning in stomatal closure in response to ABA (Eisenach et al., 2017). Interestingly, malate is not only transported into vacuoles by ALMT9, but cytosolic malate and oxaloacetate also up-regulate the ALMT9 channels that reside in the vacuolar membrane of guard cells and other plant cells, with ALMT9 channel activation occurring at c. 0.3 mM cytosolic malate (De Angeli et al., 2013).
As ALMT6 & ALMT9 channels function in stomatal opening (Meyer et al., 2011; De Angeli et al., 2013) and S-type anion channels function in stomatal closing, these findings together suggest that an additional degree of regulation of these ion channels would be required to avoid futile simultaneous activation of counteracting ion channels in the guard cell vacuolar and plasma membranes. During stomatal opening S-type anion channels are directly down-regulated by type 2C protein phosphatases (Brandt et al., 2015). This tight down-regulation by PP2Cs may preclude malate activation of S-type anion channels during stomatal opening, if cytosolic malate concentrations are low. Based on the present findings suggesting that malate acts further upstream of S-type anion channels rather than as a direct channel activator, these data point to the hypothesis that malate facilitates stomatal closing under permissive conditions that trigger stomatal closing, when PP2C phosphatases are inhibited. This hypothesis will require further investigation.
Consistent with the hypothesis that malate regulation of S-type anion channels depends on additional coinciding signal transduction mechanisms, cytosolic malate activation of anion channels does not appear to occur via a direct interaction with and up-regulation of SLAC1; Cytosolic malate in oocytes did not affect SLAC1- and SLAH3-mediated anion channel activity (Figs 5, S3, S4), as found in experiments conducted independently by three of the authors (C.W., J.Z. and D.B.). In control experiments, SLAC1-mediated currents were upregulated by intracellular bicarbonate in the same oocyte batches showing typical SLAC1 properties (Fig. 5). This is consistent with a recent study, in which high intracellular HCO3− could enhance anion currents mediated by SLAC1, when protein kinases were co-expressed, including OST1yn, CPK6 or CPK23 in Xenopus laevis oocytes. By contrast, direct modulation of SLAC1 or SLAH3 activity by malate was not observed (Figs 5, S3, S4). These data, together with the requirement of protein kinases for malate activation of S-type channels in guard cells (Fig. 6) indicate that malate activation of S-type anion channels is likely to occur via modulation of signaling mechanisms upstream of S-type anion channels in guard cells.
In summary, the present study reveals a new mode of S-type anion channel regulation in guard cells by cytosolic malate and oxaloacetate. This newly recognized regulation mechanism could contribute to physiological stomatal closing, as well as to the well-known regulation of stomatal movements by malate. Further research will be needed to dissect which of the many known upstream stomatal regulation mechanisms are directly regulated by cytosolic malate concentrations.
Supplementary Material
Acknowledgments
This research was funded by a grant from the National Science Foundation (MCB-1616236) to J.I.S. and in part the NIH (GM060396), and in part by the National Natural Science Foundation of China (31770289 to C.W.) and Northwest A&F University (Z111021604 to C.W.).
Footnotes
Author contributions
This research was designed by J.I.S., J.W., J.Z. and C.W. C.W. and J.W. conducted patch clamp experiments with guard cells. J.Z., C.W. and D.B. conducted Xenopus oocyte experiments. J. I. S., J. Z. and C.W. wrote the manuscript with comments from all authors.
Additional Supporting Information may be found online in the Supporting Information tab for this article:
Fig. S1 Cytosolic malate at 1 mM activates ionic currents in Arabidopsis thaliana wild-type (WT) guard cells with 0.1 mM malate showing partial activation in the depicted experimental set, whereas 10 mM malate showed no activation of currents.
Fig. S2 Cytosolic malate (1 mM) does not activate S-type anion channel currents in Arabidopsis thaliana wild-type guard cells at 0.08 μM free cytosolic Ca2+.
Fig. S3 Cytosolic malate at 1, 10 and 20 mM does not significantly enhance or inhibit SLAC1-mediated ion currents in Xenopus oocytes co-expressing OST1 or CPK6.
Fig. S4 Cytosolic malate at 1 mM does not significantly enhance SLAH3-mediated ion currents in Xenopus oocytes.
Fig. S5 Average S-type anion channel currents recorded at −145 mV from different genotypes as shown in Figs 4 and 6 under control conditions (0 mM malate).
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell. 2002;14:1649–1662. doi: 10.1105/tpc.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjarvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci, USA. 2012;109(26):10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG, Poretsky E, Belknap TF, Waadt R, Aleman F, et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife. 2015;4:e03599. doi: 10.7554/eLife.03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant Journal. 2010;61(5):816–825. doi: 10.1111/j.1365-313X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- De Angeli A, Zhang J, Meyer S, Martinoia E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nature Communications. 2013;4:1804. doi: 10.1038/ncomms2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, Baetz U, Huck NV, Zhang J, De Angeli A, Beckers GJM, Martinoia E. ABA-induced stomatal closure involves ALMT4, a phosphorylation-dependent vacuolar anion channel of Arabidopsis. Plant Cell. 2017;29(10):2552–2569. doi: 10.1105/tpc.17.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE. The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proceedings of the National Academy of Sciences, USA. 2003;100(19):11122–11126. doi: 10.1073/pnas.1832002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L. Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiology. 2001;127(2):685–700. doi: 10.1104/pp.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling. 2011;4(173):ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases. Proc Natl Acad Sci, USA. 2010;107(17):8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci, USA. 2009;106(50):21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Heldt HW. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiology. 1984;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. Subcellular metabolite levels in spinach leaves – regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiology. 1987;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewayas AJ. Role of calcium in signal transduction of commelina guard-cells. Plant Cell. 1991;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proceedings of the National Academy of Sciences, USA. 1998;95(8):4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I. Malate-induced feedback-regulation of plasma-membrane anion channels could provide a CO2 sensor to guard-cells. Embo Journal. 1993;12(3):897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I, Lohse G, Dietrich P, Winter H, Lohaus G, Heldt HW. Malate-sensitive anion channels enable guard cells to sense changes in the ambient CO2 concentration. Plant J. 1994;6:741–748. [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;21:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiology. 2012;159(3):1026–1042. doi: 10.1104/pp.112.197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Pineros MA, Cancado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci, USA. 2006;103(25):9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Godoski J, Ries A, Kuhn J, Schroeder J. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nature Cell Biol. 2010;12(1):87–93. doi: 10.1038/ncb2009. sup pp 81–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell. 2012;24(6):2546–2561. doi: 10.1105/tpc.112.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BU, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature. 1989;341:450–453. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Hayashi Y. New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. International Review of Cell and Molecular Biology. 2011;289:89–115. doi: 10.1016/B978-0-12-386039-2.00003-1. [DOI] [PubMed] [Google Scholar]
- Kovermann P, Meyer S, Hortensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant Journal. 2007;52(6):1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- Laanemets K, Wang YF, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via increased intracellular Ca2+ sensitivity of K+ uptake channels. New Phytologist. 2013;197(1):88–98. doi: 10.1111/nph.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla B, Kim YY, Jeon B, Maeshima M, Yoo JY, Martinoia E, Lee Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol. 2008;10(10):1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci, USA. 2009;106(50):21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R. Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proceedings of the National Academy of Sciences, USA. 2005;102(11):4203–4208. doi: 10.1073/pnas.0500146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287(5451):300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- Luttge U. Carbon-dioxide and water demand - crassulacean acid metabolism (CAM), a versatile ecological adaptation exemplifying the need for integration in ecophysiological work. New Phytologist. 1987;106(4):593–629. doi: 10.1111/j.1469-8137.1987.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Marten I, Zeilinger C, Redhead C, Landry DW, Alawqati Q, Hedrich R. Identification and modulation of a voltage-dependent anion channel in the plasma-membrane of guard-cells by high-affinity ligands. Embo Journal. 1992;11(10):3569–3575. doi: 10.1002/j.1460-2075.1992.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Flugge UI, Kaiser G, Heber U, Heldt HW. Energy-dependent uptake of malate into vacuoles isolated from barley mesophyll protoplasts. Biochimica Et Biophysica Acta. 1985;806(2):311–319. [Google Scholar]
- Martinoia E, Rentsch D. Malate compartmentation – responses to a complex metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:447–467. [Google Scholar]
- Meyer S, De Angeli A, Fernie AR, Martinoia E. Intra- and extra-cellular excretion of carboxylates. Trends Plant Sci. 2010a;15(1):40–47. doi: 10.1016/j.tplants.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KA, Geiger D, Marten I, Martinoia E, Hedrich R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant Journal. 2010b;63(6):1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant Journal. 2011;67(2):247–257. doi: 10.1111/j.1365-313X.2011.04587.x. [DOI] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol. 2015;28:154–162. doi: 10.1016/j.pbi.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S. Diverse stomatal signaling and the signal integration mechanisms. Annual Review of Plant Biology. 2015;66:369–392. doi: 10.1146/annurev-arplant-043014-114707. [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Hashimoto-Sugimoto M, Kusumi K, Iba K. New approaches to the biology of stomatal guard cells. Plant and Cell Physiology. 2014;55(2):241–250. doi: 10.1093/pcp/pct145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452(7186):483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- Nour-Eldin HH, Norholm MHH, Halkier BA. Screening for plant transporter function by expressing a normalized Arabidopsis full-length cDNA library in Xenopus oocytes. Plant Methods. 2006;2:17. doi: 10.1186/1746-4811-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hanstein S, Felle HH, Hedrich R. CO2 provides an intermediate link in the red light response of guard cells. Plant J. 2002;32(1):65–75. doi: 10.1046/j.1365-313x.2002.01403.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hedrich R, Geiger D. Anion channels: master switches of stress responses. Trends in Plant Science. 2012;17(4):221–229. doi: 10.1016/j.tplants.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Konrad KR, Marten H, Psaras GK, Hartung W, Hedrich R. Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant, Cell & Environ. 2006;29(8):1595–1605. doi: 10.1111/j.1365-3040.2006.01536.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 2010;51(3):354–365. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability) Plant Physiology. 1994;106(1):383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl H. The compartmentation of carboxylating and decarboxylating enzymes in guard cell protoplasts. Planta. 1981;152:307–313. doi: 10.1007/BF00388254. [DOI] [PubMed] [Google Scholar]
- Schnabl H, Elbert C, Kramer G. The regulation of the starch-malate balances during volume changes of guard cell protoplasts. Journal of Experimental Botany. 1982;33:996–1003. [Google Scholar]
- Schroeder JI, Keller BU. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci, USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard cell protoplasts. Proc Natl Acad Sci, USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. Light regulation of stomatal movement. Annual Review of Plant Biology. 2007;58:219–247. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E. Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature. 1986;319(6051):324–326. [Google Scholar]
- Siegel RS, Xue SW, Murata Y, Yang YZ, Nishimura N, Wang A, Schroeder JI. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. Plant Journal. 2009;59(2):207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Pan Y, Jia J, Zhang H, Bai F, Zhang P, Zhu H, He Y, Luo S, Li L, Luan S. A molecular pathway for CO2 response in Arabidopsis guard cells. Nature Communications. 2015;6:6057. doi: 10.1038/ncomms7057. [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan W-Y, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk CA, Raschke K. Release of malate from epidermal strips during stomatal closure. Plant Physiol. 1978;61(3):474–475. doi: 10.1104/pp.61.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hu HH, Qin X, Zeise B, Xu DY, Rappel WJ, Boron WF, Schroeder JI. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell. 2016;28(2):568–582. doi: 10.1105/tpc.15.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang J, Schroeder JI. Two-electrode voltage-clamp recordings in Xenopus laevis oocytes: reconstitution of abscisic acid activation of SLAC1 anion channel via PYL9 ABA receptor. Bio Protoc. 2017;7(2):2114. doi: 10.21769/BioProtoc.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Blatt MR. Anion channel sensitivity to cytosolic organic acids implicates a central role for oxaloacetate in integrating ion flux with metabolism in stomatal guard cells. Biochem J. 2011;439(1):161–170. doi: 10.1042/BJ20110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193(4):530–535. [Google Scholar]
- Winter K, Usuda H, Tsuzuki M, Schmitt M, Edwards GE, Thomas RJ, Evert RF. Influence of nitrate and ammonia on photosynthetic characteristics and leaf anatomy of Moricandia arvensis. Plant Physiology. 1982;70(2):616–625. doi: 10.1104/pp.70.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011;30(8):1645–1658. doi: 10.1038/emboj.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Negi J, Wang C, Isogai Y, Schroeder JI, Iba K. The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis. Plant Cell. 2016;28(2):557–567. doi: 10.1105/tpc.15.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Adachi Y, Munemasa S, Nakamura Y, Mori IC, Murata Y. Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant and Cell Physiology. 2015;56(6):1239–1248. doi: 10.1093/pcp/pcv051. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant and Cell Physiology. 2002;43(12):1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- Zhang J, Baetz U, Krugel U, Martinoia E, De Angeli A. Identification of a probable pore forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiology. 2013;163(2):830–843. doi: 10.1104/pp.113.219832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Martinoia E, De Angeli A. Cytosolic nucleotides block and regulate the Arabidopsis vacuolar anion channel AtALMT9. Journal of Biological Chemistry. 2014;289(37):25581–25589. doi: 10.1074/jbc.M114.576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.