Abstract
Objective
Inhibitory receptors (IRs) are essential to regulate effector immune responses and may play critical roles during autoimmune diseases. In Rheumatoid Arthritis (RA), we evaluated whether IR expression on T cells showed correlation with immune activation, disease activity and response to treatment as well as if IR-mediated pathways were functional.
Methods
By flow cytometry, we performed extensive phenotypic and functional evaluation of CD4+ and CD8+ T cells from blood and synovial fluid of RA patients ex vivo and after culture. All the parameters were correlated with disease activity score (DAS28) and response to treatment.
Results
In RA patients with low activation of T cells, IRs expression showed an inverse relationship with DAS28. Frequencies of T cells expressing multiple IRs were reduced in untreated RA patients but recovered normal levels in treated patients. RA patients that responded to treatment, showed augmented frequency of IRs-expressing T cells that correlated with reduced inflammatory cytokine production in comparison to non-responders. Synovial fluid was enriched in effector and memory T cells expressing multiple IRs. Remarkably, inhibitory pathways were operative in blood and synovial T cells from all RA patients, although cells from non-responder patients were less sensitive to inhibition.
Conclusion
IR expression on T cells from RA patients inversely correlated with effector T cell function and disease activity and may predict response to treatment. Furthermore, different inhibitory pathways are functional and cooperatively suppress synovial T cells, providing a rationale for new treatment strategies to regulate acute local inflammation.
Rheumatoid arthritis (RA) is a common chronic autoimmune disease (AID) characterized by persistent synovitis and systemic inflammation that frequently results in cartilage erosion and bone injury (1). Current consensus indicates that RA development is attributable to genetic and environmental factors, as well as abnormalities in innate and adaptive immunity (2). Within the multifactorial events and multiple immune mediators that participate in RA, T cells are linked to RA pathogenesis at different levels including initiation, progression and perpetuation (3, 4). Indeed, T cells expand and accumulate in the synovia, producing cytokines and mediators that sustain inflammation (5, 6).
Considering that AIDs such as RA are characterized by persistent activation of T cells, pathways that regulate T cell expansion and function may modulate disease pathogenesis. Indeed, co-inhibitory pathways have been shown to affect self-tolerance and autoimmunity (7). Lately, inhibitory receptors (IRs) including PD-1 (Programmed cell death protein 1), CTLA4 (cytotoxic T-lymphocyte-associated protein 4), CD160, BTLA (B- and T- lymphocyte attenuator), Tim-3 (T-cell immunoglobulin and mucin-domain containing-3), TIGIT (T cell immunoreceptor with Ig and ITIM domains) and others have emerged as key players in the control of T cell effector responses in chronic infections and cancer (8). IR expression is induced during the initial stages of T cell activation and is also associated to a terminal differentiation state termed “T cell exhaustion” characterized by the presence of multiple IRs and poor functionality (9). The role of IRs in RA and other AIDs is not well defined and the few existing reports focused at individual inhibitory pathways. In particular, the PD-1/PD-L1 pathway has been involved in the regulation of local and peripheral T cell effector function (10–13). Recently, transcriptome studies in T cells from patients with different AIDs (including RA) linked gene expression patterns of T cell exhaustion to a favorable long-term clinical outcome characterized by fewer relapses (14). Altogether, these evidences suggest that studying the expression and role of IRs on T cells from RA patients may provide useful information regarding the status of the ongoing inflammation and disease progression. Furthermore, these data will be helpful to establish whether manipulating inhibitory pathways could be beneficial to control the long term course of the disease.
In this study, we evaluated the expression of activation markers and multiple IRs in T cells from blood and synovial fluid of RA patients. Furthermore, we determined the correlation between these markers, the activity of the disease and the response to treatment. Finally, we established that the inhibitory pathways mediated by PD-1/PD-L1 and HVEM/CD160/BTLA are operative to regulate proliferation and cytokine production of T cells from RA patients.
Material and Methods
Patients
RA patients and healthy donors (HD) were recruited from the Rheumatology Service (Hospital Nacional de Clínicas, Córdoba, Argentina). RA patients were diagnosed according to the American College of Rheumatology and the European League Against Rheumatism (EULAR) classification criteria (15). Exclusion criteria included known or suspected ongoing infections or metabolic diseases for RA patients and any history of autoimmune disease or immunosupressive therapy for HD. RA disease activity score (DAS28-ESR) was assessed at the time of blood collection as described(16) and RA patients were divided into a remission group (DAS28<2,6) and an active disease group (DAS28≥2,6). RA patients were classified as untreated (treatment-naïve or without treatment in the last 6 months), DMARDs-treated (mainly methotrexate) and anti-TNF+/-DMARDs (any TNF blocking biological treatment plus methotrexate mainly). Response to treatment was defined according to EULAR criteria (1, 17): responders patients (rRA) showed a reduction in ΔDAS28 >1,2 while non-responders patients (nrRA) exhibited ΔDAS28 ≤1,2 after 3 month of treatment.
The study was approved by the Institutional Ethics Committee and performed according to the Declaration of Helsinki on studies with human subjects. All subjects provided written informed consent prior to any study procedure.
Specimens and cell separation
Peripheral blood (PB) was drawn from RA patients and HD. Information describing the study subjects is shown in Table 1. Serum samples were obtained for immunological laboratory determinations (Details in Supplementary Materials and Methods). Synovial fluid (SF) was collected from patients with active disease whenever possible. Mononuclear cells (MCs) from PB and SF were isolated by centrifugation in Ficoll-Hypaque density gradients (GE Healthcare Bio-Science AB) and cryopreserved for future determinations.
Table 1.
Demographic and clinical characteristics of RA patients and healthy donors
| Healthy Donors (HD) | Rheumatoid Arthritis Patients (RA) | ||||||
|---|---|---|---|---|---|---|---|
| Age range (years) | 30 – 66 | 22 – 78 | |||||
| Blood donors / SF donors | 26 / − | 51 / 10 | |||||
| Sex: Female / Male | 23 / 3 | 42 / 9 | |||||
|
| |||||||
| Disease activity (DAS28-ESR) | n | ESR Median±QD | CRP Median±QD | DAS28 Mean±DS | RF (+/−) | Anti-CCPs (+/−) | |
|
| |||||||
| Remission (DAS28<2,6) | 8 | 4 ± 2 | 5 ± 3 | 1.8±0.3 | 6/2 | 4/4 | |
| Active disease (DAS28≥2,6) | 43 | 15 ± 9 | 5 ± 6 | 4.4±1.0 | 28/15 | 26/17 | |
|
| |||||||
| Treatment | |||||||
|
| |||||||
| uTx | 25 | 19 ± 13 | 7 ± 13 | 4.3±1.1 | 15/10 | 14/11 | |
| DMARDs | 13 | 5 ± 5 | 5 ± 4 | 3.6±1.5 | 8/5 | 5/8 | |
| TNF inhibitors | 13 | 13 ± 15 | 5 ± 4 | 4.1±1.5 | 11/2 | 11/2 | |
ESR: erythrocyte sedimentation rate, DAS: disease activity score, CRP: C-reactive protein, uTx: untreated, DMARDs: Disease Modifying Anti-Rheumatic Drugs, RF: rheumatoid factor, Anti-CCPs: Anti- cyclic citrullinated proteins, QD: Quartile Deviation.
Flow cytometry and cell sorting
Flow cytometry for detection of surface molecules, intracellular cytokines and intranuclear transcription factors was performed according standards protocols described elsewhere (details in Supplementary Material and Methods).
In vitro cultures
Sorted CD4+ and CD8+ T cells (0,1×106) were resuspended in PBS-FBS 1%, stained with 1μM Carboxyfluorescein succinimidyl ester (CFSE) and stimulated in 96-well plates (Costar, flat bottom) pre-coated overnight with anti-CD3 1 μg/ml (OKT3, Biolegend) and anti-CD28 0,5 μg/ml (CD28.2, Biolegend) in presence or absence of soluble blocking anti-CD160 (CL1-R2, MBL, 2μg/ml) or anti-PD-1 (J116, eBiosciences, 10μg/ml), plate-bound agonistic HVEM:Fc (Biolegend, 5μg/ml) and/or PDL1:Fc (R&D Systems, 5μg/ml). At the end of the stimulation (3 days for neutralization and 6 days for receptor ligation), cells were stimulated with PMA/Ionomycin to determine cytokine production. Proliferation was determined as CFSE dilution or detection of the proliferation marker Ki-67 (anti-Ki67 eFluor660, SolA15, eBioscience). Fixable Viability Stain 780 was used for exclusion of dead cells.
Proliferation index was calculated as the ratio between the percentage cell proliferation in cells stimulated with anti-CD3/anti-CD28 plus anti-CD160, HVEM:Fc and/or PD-L1:Fc and the percentage of proliferation in cells stimulated only with the mitogen.
Statistics
Statistical analyses were performed with GraphPad Prism 7 software. P values <0.05 were considered significant. The D’Agostino-Pearson omnibus normality test was initially performed to determine the distribution of the datasets. The specific tests used are indicated in the legends for figures.
Results
CD8+ T cells from RA patients showed different levels of IR expression according to their activation status
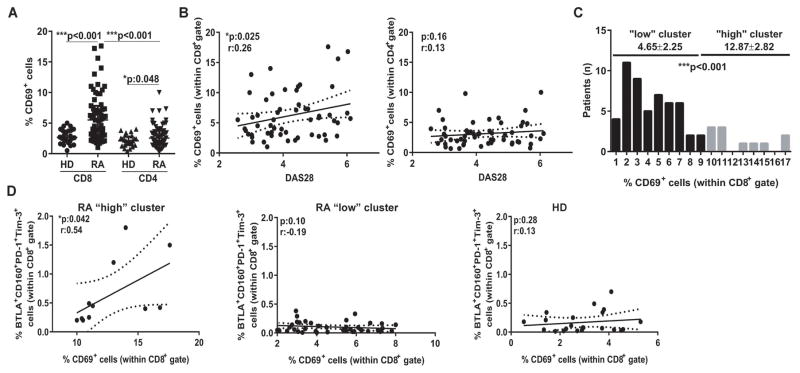
High expression of IRs has been described at different stages of T cell life. On one side, several IRs are upregulated in recently activated T cells that exhibit strong effector function (18) and may boost RA progression. On the other side, IRs expression is a hallmark of terminally differentiated exhausted cells (19), whose poor effector activity may be desirable in the context of RA. Therefore, the evaluation of the activation status is an essential first step to understand the significance of IR expression on T cells during RA. As measure of activation, we determined the expression of CD69 on T cells from RA patients with active disease and in HD. Although CD4+ and CD8+ T cells were present in similar frequencies in PB from RA patients and HD (data not shown), both T cell subsets showed an increased frequency of CD69+ cells in RA patients, indicating a status of high activation (Figure 1A). Of note, the highest frequencies of activated CD69+ cells were observed within the CD8+ T cells from RA patients, suggesting that upregulation of activation markers on this population may be more sensitive than on CD4+ T cells to denote immune activation. Indeed, the frequency of CD69+ within the CD8+, but not the CD4+, T cells showed a significant linear correlation with the activity of the disease determined by DAS28 (Figure 1B).
Figure 1. Evaluation of T cell activation status and IR expression.
A, Frequency of CD69+ cells within peripheral CD8+ and CD4+ T cells from HD and RA patients. Median±interquartile range is shown. B, Correlations of frequencies of CD69+ cells within CD8+ and CD4+ T cell gates with DAS28 from RA patients. C, Histogram of distribution of the frequencies of CD69+ cells within CD8+ T cell gate from RA patients. The cut-off value of 10 allowed to define two clusters of RA patients showing “low” and “high” T cell activation with significantly different means. D, Correlations between the frequencies of BTLA+CD160+PD-1+Tim-3+ cells and CD69+ cells within CD8+ T cell gate in RA patients from the “high” and “low” clusters and HD. Statistical analysis: p values calculated with Mann-Whitney Test (A and C) and with Pearson’s correlation coefficient (B and D).
According to the frequencies of CD69+CD8+ cells, two clusters of patients with significantly different means could be defined within the CD8+ population (Figure 1C). One cluster included patients with low frequencies of activated CD69+CD8+ T cells and therefore considered to exhibit a “low” T cell activation status. The other cluster comprised patients exhibiting more than 10% of CD69+CD8+ T cells that would denote a status of “high” T cell activation. Next, we evaluated expression of four IRs (CD160, PD-1, BTLA and Tim-3) on the CD8+ T cells from these “low” and “high” (T cell activation) clusters according to the gating strategy depicted in Supplementary Figure 1. Overall, CD8+ T cells of patients from the “high” cluster showed increased expression of IRs (particularly PD-1 and CD160) in comparison to counterparts of patients from the “low” cluster (Supplementary Figure 2). Remarkably, the frequency of CD8+ T cells co-expressing four IRs or different IR combinations correlated positively with the percentage of CD69+CD8+ T cells in patients from the “high” cluster, indicating that high CD8+ T cell activation is linked to IR upregulation during RA (Figure 1D and data not shown). In contrast, this correlation was not observed within the CD8+ T cells from patients from the “low” cluster or HD (Figure 1D), yet these cells exhibited variable IR expression that may be rather linked to a cell dysfunction stage as consequence of sustained chronic stimulation.
Frequency of IR-expressing T cells is reduced in untreated RA patients and increases upon successful treatment
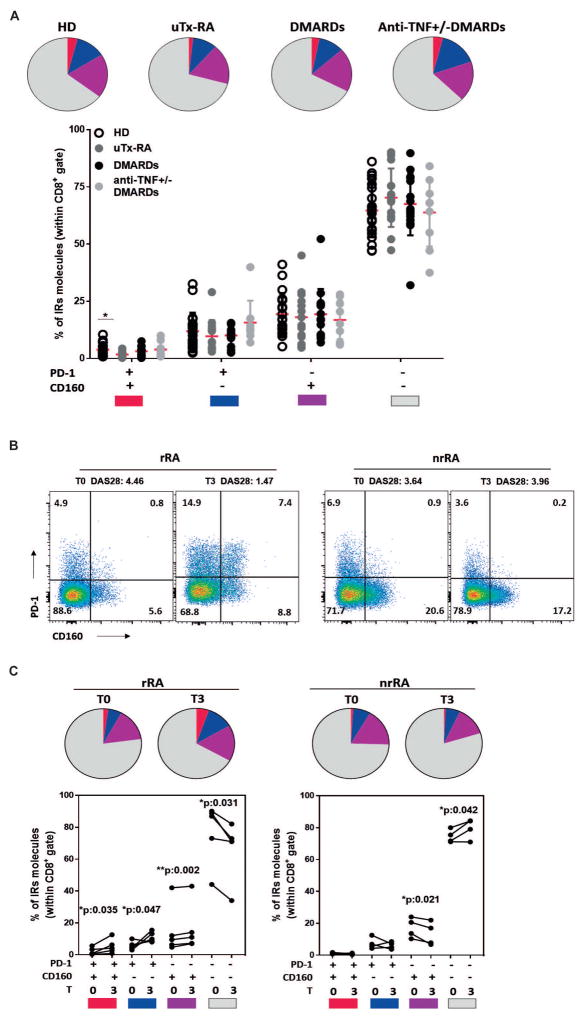
We next evaluated whether IR expression on T cells from RA patients with active disease but low T cell activation may evidence cell dysfunction. To this end, we first determined the frequencies of CD8+ and CD4+ T cells that expressed multiple IRs (PD-1, CD160, Tim-3 and BTLA) in HD and RA patients from the “low” cluster. RA patients were divided according to treatment in untreated (uTx), DMARDs and anti-TNF+/-DMARDs. As illustrated by the pie charts in (Supplementary Figure 3A), CD8+ T cells from uTx-RA patients show reduced frequencies of cells expressing multiple (two, three and four) IRs in comparison to counterparts from HD. Remarkably, the frequencies of IR-expressing CD8+ T cells tended to increase in DMARDs and anti-TNF+/-DMARDs treated patients. Statistical analysis confirmed that the percentage of CD8+ T cells expressing the four IRs evaluated (Supplementary Figure 3B) as well as those expressing particular combinations of three IRs (Supplementary Figure 3C) were significantly reduced in uTx-RA patients. Given that treated patients included patients showing good, moderate and bad response to treatment according to EULAR criteria, we performed a longitudinal analysis to determine the dynamic evolution of the IR expression patterns according to the clinical response after 3 months of treatment (t=3). Responder RA (rRA) patients exhibited an increase in the frequency of CD8+ T cells that expressed four and three IRs at t=3 in comparison to baseline (t=0). In contrast, no significant changes in the frequency of CD8+ T cells expressing different IRs combinations were observed in non-responder (nrRA) patients (Supplementary Figure 3D and 3E).
As the frequencies of T cells expressing four or three IRs were low and their study may be limiting at clinical level, we assessed whether changes in the expression of two or one IR could provide a more robust measure of therapy success. From the four IRs tested, we focused on CD160 and PD-1 that were the IRs expressed at higher frequencies. The percentage of CD8+ T cells expressing PD-1 and CD160, but not those expressing only one IR, were significantly decreased in uTX-RA patients in comparison to HD and tended to increase in treated RA individuals (Figure 2A). Importantly, the increase in the frequency of CD8+ T cells expressing one or two of these IRs correlated to treatment response (Figure 2B and 2C). Indeed, rRA patients exhibited significantly augmented frequency of PD-1+CD160+ double positive as well as of PD-1+CD160− and PD-1−CD160+ single positive CD8+ T cells while reduced double negative CD8+ T cells (Figure 2B and 2C). In contrast, nrRA patients showed a reduction or no changes in the CD8+ T cells expressing one or two IRs and a significant increase in the double negative cells (Figure 2B and 2C). We also identified some combinations of IRs expressed on CD4+ T cells (CD160+ and CD160+TIM3+) that correlated to treatment success in RA (data not shown).
Figure 2. Changes in the frequency of PD-1 and/or CD160 expressing T cells were linked to clinical response to treatment.
A, Pie charts showing the average values and symbol plot showing the individual values of the frequencies of peripheral CD8+ T cells expressing different color-coded combinations of PD-1 and CD160 in samples from HD, untreated (uTx-RA), and DMARDs and anti-TNF±DMARDs treated RA patients. B, Representative dot plots showing the frequency of peripheral CD8+ T cells expressing PD-1 and CD160 in one rRA and one nrRA patient in paired samples at baseline (T0) and 3 months post-treatment (T3). Changes in DAS28 are indicated above the plots. C, Pie charts showing the average values and symbol plots showing the individual values of the frequencies of peripheral CD8+ T cells expressing different color-coded combinations of PD-1 and CD160 in paired samples from rRA and nrRA patients at T0 and T3. Statistical analysis: p values calculated with One-way Anova followed by Bonferroni post-test (A) and Student’s paired T test (B and C).
Reduced IR-expressing CD8+ T cells are linked to increased effector function and activity of the disease in RA patients with low immune activation
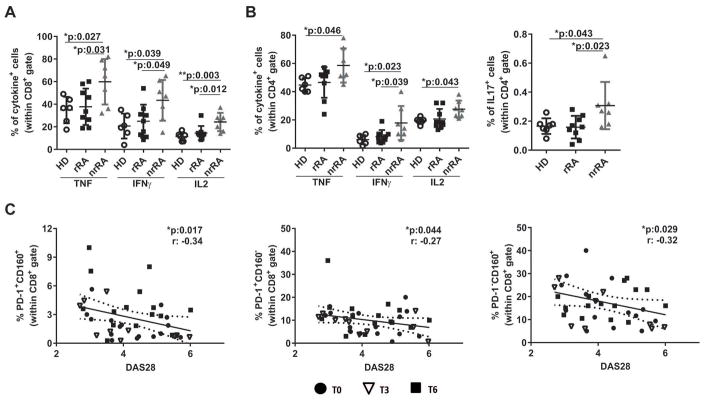
Next, we aimed at understanding how the changes in frequencies of IR-expressing CD8+ T cells observed in RA patients undergoing treatment influenced effector cytokines production upon activation. We determined that rRA patients had a frequency of CD8+ T cells secreting TNF, IFNγ and IL-2 as low as HD. In contrast, nrRA patients that showed no changes in IR expression upon treatment exhibited significantly increased percentages of CD8+ T cells producing effector cytokines in comparison to rRA patients and HD (Figure 3A). Also, CD4+ T cells from nrRA patients contained significantly augmented frequency of cells producing effector cytokines such as TNF, IFNγ, IL-2 and IL-17 when compared to counterparts from rRA patients and/or HD (Figure 3B).
Figure 3. Frequency of IR-expressing T cells inversely correlated to inflammatory cytokine production and activity of the disease.
A–B, Frequency of cells producing the indicated effector cytokines within peripheral CD8+ (A) and CD4+ (B) T cell gates in HD, rRA and nrRA patients. Mean±DS is shown. C, Correlations between the frequencies of peripheral CD8+ T cells expressing PD-1 and CD160 together, and PD-1 and CD160 individually and DAS28 from RA patients. Datasets include RA patients at the indicated times post-treatment. Statistical analysis: p values calculated with One-way Anova followed by Bonferroni post-test (A and B) and Pearson’s correlation coefficient (C).
Considering that the frequency of IR-expressing T cells in RA patients was inversely associated with production of effector cytokines, we speculated that IR expression on T cells could also correlate with a more comprehensive clinical parameter such as the activity of the disease. To test this, we evaluated whether the frequencies of CD8+ and CD4+ T cells expressing different IRs combinations correlated with DAS28 in the total cohort of patients involved in this study, including patients untreated (T=0) and treated during 3 (T=3) and 6 (T=6) months. This analysis showed a negative correlation between DAS28 and the frequencies of CD8+ T cells expressing PD-1 and CD160 together (Figure 3C, left graph) and individually (Figure 3C, middle and right graphs). Furthermore, similar negative correlations were observed among DAS28 and CD8+ T cells expressing four (Supplementary Figure 3E), and particular combinations of three (Supplementary Figure 3F) IRs. This negative correlation was also found for some patterns of IR expression on CD4+ T cells (Supplementary Table 1).
Altogether our data indicate that in patients exhibiting low T cell activation, reduced frequency of IR-expressing CD8+ and CD4+ T cells upon therapy significantly correlated with increased production of effector inflammatory cytokines and more severe activity of the disease.
Synovial fluid is enriched in effector CD8+ T cells that exhibit a potent cytokine producing function that inversely correlated with IR expression
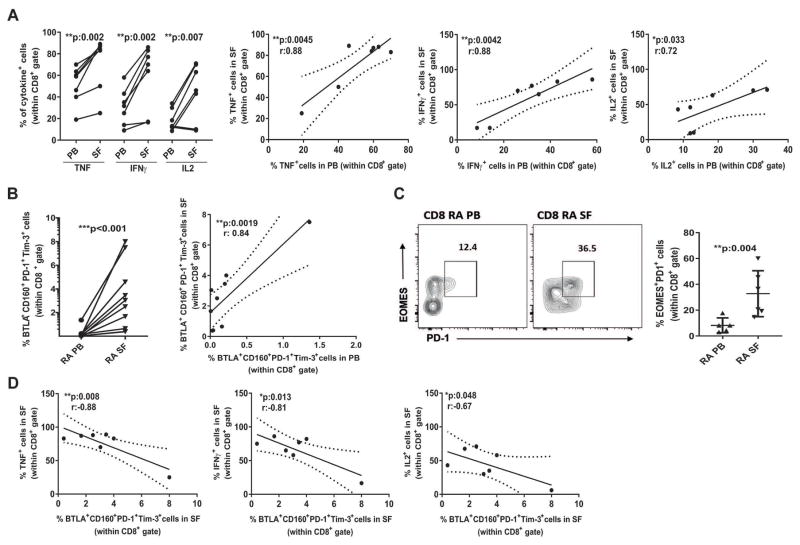
To establish the biological events undergoing at inflammatory sites during RA, we collected SF and evaluated the composition of the immune infiltrate in comparison to the paired PB samples. The CD45+ cell SF infiltrate was composed of around 20% lymphocytes, while the rest included mainly neutrophils and at minor extent monocytes (Supplementary Figure 4A). CD8+ and CD4+ T cells in SF showed similar frequencies and a strong positive correlation with the frequencies in the paired PB samples (Supplementary Figure 4B and C and data not shown). Similar to previous reports (20), we observed that central and effector memory subsets were significantly increased while naïve and terminal effector subsets were significantly reduced within the CD8+ and the CD4+ T cells from SF in comparison to the paired PB (Supplementary Figure 4D and 4F). Interestingly, after 3 months of treatment rRA, but not nrRA, patients showed significantly increased frequencies of CD8+ and CD4+ T cells with terminal effector phenotype (Supplementary Figure 4E and 4G). Next, we performed functional studies and determined that synovial CD8+ T cells produced higher levels of TNF, IFNγ and IL-2 than their peripheral counterparts and that there were significant positive correlations between the frequencies of cytokine-producing CD8+ T cells from SF and the paired PB (Figure 4A). Detailed phenotypic studies showed that the highly activated effector CD8+ T cells from SF were enriched in cells expressing BTLA, CD160, PD-1 and Tim-3 and that the percentage of synovial CD8+ T cells expressing four IRs showed a positive correlation with the paired PB (Figure 4B and Supplementary Figure 2). These results show that, somehow expected given its inflammatory nature, SF exhibit overall increased frequencies of IR-expressing and cytokine-producing T cells in comparison to the paired PB. Importantly, although the frequency of IR-expressing CD8+ T cells was low in PB, there was a significant positive correlation with those in the paired SF. We also determined that synovial CD8+ T cells showed high percentage of cells exhibiting a phenotype Eomes+PD1+ (Figure 4C), previously associated to poor effector function (19).
Figure 4. Synovial CD8+ T cells exhibited potent effector function that inversely correlated with IR expression.
A, Comparison and correlations between the frequencies of CD8+ T cells producing effector cytokines in paired SF and PB samples. B, Comparison and correlation between the frequencies of CD8+ T cells that express four IRs in paired SF and PB. C, Dot plot graph and cumulative data showing percentage of subpopulation Eomes+PD1+ expressing CD8+ T cells in paired SF and PB. D, Correlation between the frequencies of synovial CD8+ T cells that produce effector cytokines and express four IRs. Statistical analysis: Student’s paired T test and Pearson’s correlation coefficient (A and B), Mann-Whitney Test (C), and Pearson’s correlation coefficient (D).
We next, evaluated the correlation of IR-expression and effector cytokine production within SF and observed that, in complete agreement with the result obtained in PB for rRA versus nrRA patients (Fig. 3A), the frequency of IR-expressing synovial CD8+ T cells inversely correlated with the percentage of synovial cells producing effector cytokines (Figure 4D). Therefore, even in the complex environment of an inflammatory site such as synovia, a higher expression of IRs within the CD8+ T cell population is linked to poorer effector responses.
Within the CD4+ T cell population, only IFNγ and IL-17 producing cells were increased in SF versus the paired PB but there was no correlation between the frequencies of these cells in SF and PB (Supplementary Figure 5A and 5B). Furthermore, although a high percentage of synovial CD4+ T cells expressed multiple IRs, there was no correlation with the paired PB or the cytokine-producing population in SF (Supplementary Figure 5C–E).
Inhibitory pathways are operative to regulate proliferation and cytokine production by synovial and peripheral T cells from RA patients
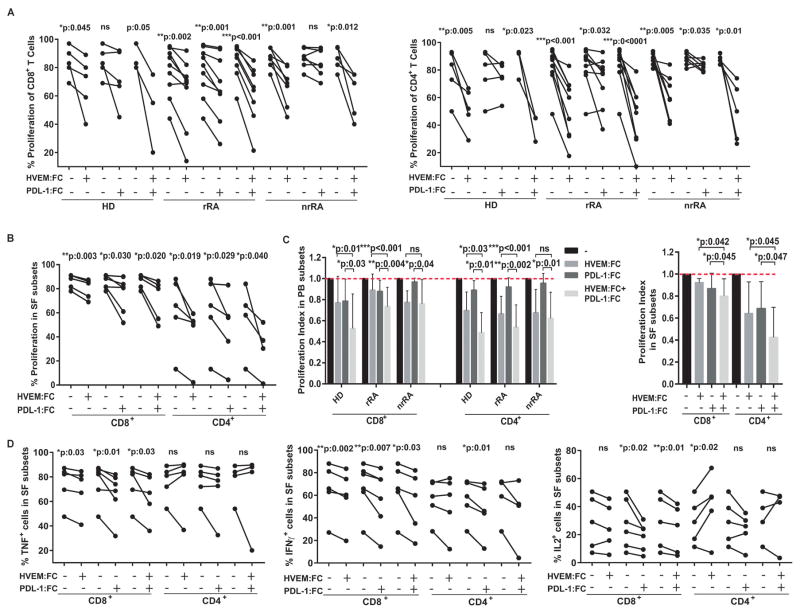
We finally investigated whether IRs are functional in T cells from RA patients and could be exploited to control ongoing inflammatory response. For this, we developed an assay to evaluate how T cell activation was affected by ligation of CD160/BTLA and/or PD-1 using HVEM:Fc and/or PD-L1:Fc proteins, respectively. HVEM:Fc significant reduced the proliferation of CD8+ and CD4+ T cells from HD as well as rRA and nrRA patients while PD-L1:Fc significantly decreased proliferation of CD8+ T cells only from rRA patients and CD4+ T cells from rRA and nrRA patients. Remarkably, the combination of HVEM:Fc and PD-L1:Fc inhibited proliferation of all samples assayed (Figure 5A). Next, we determined that CD160/BTLA and PD-1 pathways were also operative in the highly activated synovial CD8+ and CD4+ T cells (Figure 5B). Importantly, comparative analysis of the effect observed in Figures 5A and B, showed that the combination of HVEM:Fc and PD-L1:Fc could act synergistically to suppress T cell proliferation particularly in peripheral CD4+ and CD8+ T cells from rRA patients and in synovial T cells (Figure 5C). Agonism of CD160/BTLA and PD-1 pathways also decreased the frequency of synovial CD8+ but not CD4+ T cells able to produce effector cytokines (Figure 5D). Engagement of IRs expressed on peripheral T cells also inhibited effector cytokine production to a variable extent (Supplementary Table 2). Of note, stronger IRs ligation induced by higher agonist concentrations leaded to deletion of the activated T cells (data not shown).
Figure 5. Inhibitory pathways mediated by PD1/PD-L1 and HVEM/CD160/BTLA were operative in RA patients.
A–B, Proliferation of CD4+ and CD8+ T cells from PB (A) and SF (B) of HD, rRA and nrRA after stimulation in the absence or presence of HVEM:Fc, PD-L1:Fc and their combination. C, Proliferation index calculated according to data from A and B. D, Frequency of CD8+ and CD4+ T cells from SF producing the indicated effector cytokine. Statistical analysis: Student’s paired T test (A–C) and Wilcoxon T test (D). n.s.:not significant.
Next, we evaluated whether T cell:T cell interactions are able to trigger CD160- and PD-1-mediated pathways on T cells from HD and RA patients. We first determined that CD8+ and CD4+ T cells from HD and RA patients expressed HVEM. Of note, the frequencies of HVEM-expressing T cells were significantly reduced in uTx-RA patients in comparison to HD while increased to normal levels in treated patients (Supplementary Figure 6A). Blockade of CD160/HVEM engagement by an anti-CD160 Ab resulted in higher proliferation of all the samples assayed except for CD4+ T cells from nrRA patients (Supplementary Figure 6B). Inhibition of the CD160 pathway also increased the frequency of cytokine-producing cells within CD8+ and CD4+ T cells from HD and rRA patients, but showed a limited effect in T cells from nrRA patients (Supplementary Figures 6C–E).
In contrast, and despite the fact that a variable frequency of T cells from HD and RA patients expressed PD-L1 (unpublished observations), blockade of PD-1/PD-L1 engagement showed no effect in proliferation or cytokine production in cultures including only T cells (data not shown).
Discussion
Different reports showed that polymorphisms in the PD1, CTLA4 and CD244 genes are associated with a greater susceptibility to RA and other AIDs (10, 21, 22) while the CD160 gene is included within gene hubs significantly associated to RA (23). Furthermore, most of the patients receiving ‘checkpoint’ blockade as immunotherapy during cancer develop immune-related adverse events that involve most organ systems, including the joints (24, 25). Maybe more significant from a clinical point of view, recent data indicate that IR-mediated pathways, which negatively interfere with T cell activation and function, specifically protects against a relapsing course in multiple AIDs.(14) In this context, the regulation of the extent and duration of a pathological immune response by IR-mediated pathways may emerge as a relevant mechanism dictating the clinical course of the disease once it is established (26). We focused in RA and determined that patients showing a “high” T cell activation defined by high expression of CD69 on peripheral CD8+ T cells exhibited an increased frequency of IR-expressing T cells in concordance with the fact that IR expression is induced by activation (18). Recently, increased expression of TIGIT on the surface of CD4+ T cells was associated with a higher DAS28 and increased evidence of inflammation in RA patients (27). Given that T cell activation results in the transient upregulation of some IRs (18), it is likely that the TIGIT+ T cells identified by Luo and colleagues include activated T cells with increased potential to proliferate and produce effector cytokines. In contrast, our results indicated that the IR-expressing CD8+ T cells from RA patients with “low” T cells activation are more likely undergoing terminal differentiation (i.e. exhaustion), a T cell differentiation stage associated with multiple and sustained IR expression (28). Interestingly, in these patients, the frequency of CD8+ T cells expressing multiple IRs as well as those showing a phenotype compatible with terminal effector cells (TEMRA) increased upon successful treatment and inversely correlated with T cell effector function and the clinical activity of the disease. These data highlight that expression of activation/differentiation markers and multiple IRs in T cells could be useful markers of prognosis and treatment success in RA.
Recently, induction of T cell exhaustion has been suggested as a novel therapeutic approach for AIDs (26). In this line, some reports demonstrated that agonism of the PD-1 pathway regulated peripheral and synovial T cell responses during experimental and human RA (11, 12). However, many RA patients showed resistance to the PD-1 mediated inhibition likely as consequence of the presence of a decoy sPD-1 in the inflammatory environment (10, 13). Our results agree with these findings and further extend them as we showed that responsiveness to PD-1 ligation is conserved only in RA patients that exhibited good response to therapy. Furthermore, we determined that PD-1 inhibitory pathway is not triggered by T cell:T cell interactions and likely requires the presence of other PD-L1+ immune cells such as monocytes. We also addressed the role of additional IRs such as CD160 and BTLA as both belong to a bidirectional signaling network that critically modulates T cell biology. Within this network, HVEM (a member of the TNF receptor superfamily expressed on DC, regulatory cells or T cells) is able to ligate the both BTLA and CD160 on T cells to transduce inhibitory signals. These negative signals may be counterbalanced by costimulatory signals delivered after direct engagement of HVEM on T cells by LIGHT expressed on DC or other T cells (29). During different biological conditions (homeostasis, infection, autoimmunity or cancer) it may be a predominance of the negative over the positive signaling or vice versa as result of differences in ligand/receptor affinity and/or the differential expression pattern of these molecules on cell types at different stages of cell differentiation (30). In this regard, we determined that agonism of the IRs CD160 and BTLA suppressed proliferation and/or inflammatory cytokine production by peripheral and synovial CD4+ and CD8+ T cells from most RA patients. In addition, we also demonstrated that at least the CD160-mediated inhibitory signal can be triggered by T cell:T cell interactions. More importantly, we showed that combination of PD-1 and CD160/BTLA ligands exerted a synergistic inhibitory effect on T cells from all RA patients, including those that showed poor response to regular treatments.
Altogether, our and others data highlight that IRs play critical roles in the modulation of the ongoing inflammatory response and immunopathology during RA and put forward the notion that triggering these inhibitory pathways at local or systemic level may emerge as a rational approach for the treatment of refractory RA.
Supplementary Material
Acknowledgments
Grants and financial supporters:
Research reported in this publication was supported by the Agencia Nacional de Promoción Científica y Técnica (PID grant number 2012-0068) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI110340. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies
We express our sincere gratitude to all patients who participated in this study. We thank MP Abadie and MP Crespo for their excellent technical assistance in flow cytometry and cell sorting. We thank Sergio Alonso for his help in biochemical determinations.
Footnotes
Conflict of interests:
The authors declare no conflict of interests.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS, McInnes IB. Immunopathogenesis of Rheumatoid Arthritis. Immunity. 2017;46(2):183–96. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim K, Bang SY, Lee HS, Bae SC. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(1):13–24. doi: 10.1038/nrrheum.2016.176. [DOI] [PubMed] [Google Scholar]
- 4.James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66(7):1712–22. doi: 10.1002/art.38637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klarenbeek PL, de Hair MJ, Doorenspleet ME, van Schaik BD, Esveldt RE, van de Sande MG, et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann Rheum Dis. 2012;71(6):1088–93. doi: 10.1136/annrheumdis-2011-200612. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat Rev Rheumatol. 2016;12(1):63–8. doi: 10.1038/nrrheum.2015.171. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016;44(5):1034–51. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44(5):1052–68. doi: 10.1016/j.immuni.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuertes Marraco SA, Neubert NJ, Verdeil G, Speiser DE. Inhibitory Receptors Beyond T Cell Exhaustion. Front Immunol. 2015;6:310. doi: 10.3389/fimmu.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol. 2017;188(3):455–66. doi: 10.1111/cei.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moret FM, van der Wurff-Jacobs KM, Bijlsma JW, Lafeber FP, van Roon JA. Synovial T cell hyporesponsiveness to myeloid dendritic cells is reversed by preventing PD-1/PD-L1 interactions. Arthritis Res Ther. 2014;16(6):497. doi: 10.1186/s13075-014-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010;62(7):1870–80. doi: 10.1002/art.27500. [DOI] [PubMed] [Google Scholar]
- 13.Wan B, Nie H, Liu A, Feng G, He D, Xu R, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177(12):8844–50. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 14.McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–6. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 16.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 17.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57. vii–viii. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than “Exhaustion” of Human CD8 T Cells. Front Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalheiro H, Duarte C, Silva-Cardoso S, da Silva JA, Souto-Carneiro MM. CD8+ T cell profiles in patients with rheumatoid arthritis and their relationship to disease activity. Arthritis Rheumatol. 2015;67(2):363–71. doi: 10.1002/art.38941. [DOI] [PubMed] [Google Scholar]
- 21.Lei C, Dongqing Z, Yeqing S, Oaks MK, Lishan C, Jianzhong J, et al. Association of the CTLA-4 gene with rheumatoid arthritis in Chinese Han population. Eur J Hum Genet. 2005;13(7):823–8. doi: 10.1038/sj.ejhg.5201423. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Yamada R, Kochi Y, Sawada T, Okada Y, Matsuda K, et al. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat Genet. 2008;40(10):1224–9. doi: 10.1038/ng.205. [DOI] [PubMed] [Google Scholar]
- 23.Hua L, Lin H, Li D, Li L, Liu Z. Mining functional gene modules linked with rheumatoid arthritis using a SNP-SNP network. Genomics Proteomics Bioinformatics. 2012;10(1):23–34. doi: 10.1016/S1672-0229(11)60030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belkhir R, Burel SL, Dunogeant L, Marabelle A, Hollebecque A, Besse B, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. 2017;76(10):1747–50. doi: 10.1136/annrheumdis-2017-211216. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: Immune-Related Adverse Events With Use of Checkpoint Inhibitors for Immunotherapy of Cancer. Arthritis Rheumatol. 2017;69(4):687–99. doi: 10.1002/art.40043. [DOI] [PubMed] [Google Scholar]
- 26.McKinney EF, Smith KG. T cell exhaustion and immune-mediated disease-the potential for therapeutic exhaustion. Curr Opin Immunol. 2016;43:74–80. doi: 10.1016/j.coi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Luo Q, Deng Z, Xu C, Zeng L, Ye J, Li X, et al. Elevated Expression of Immunoreceptor Tyrosine-Based Inhibitory Motif (TIGIT) on T Lymphocytes is Correlated with Disease Activity in Rheumatoid Arthritis. Med Sci Monit. 2017;23:1232–41. doi: 10.12659/MSM.902454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ. T cell exhaustion. Nat Immunol. 2011:131. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 29.Ware CF, Sedy JR. TNF Superfamily Networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14) Curr Opin Immunol. 2011;23(5):627–31. doi: 10.1016/j.coi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87(2):223–35. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.