Abstract
Objective
To evaluate the clinical efficacy and safety of baminercept, a lymphotoxin β receptor IgG fusion protein (LTβR-Ig), for the treatment of primary Sjögren’s syndrome (SS) and explore its possible mechanisms of action.
Methods
In this multicenter trial, 52 patients with primary SS were randomized in a 2:1 ratio to receive subcutaneous injections of 100 mg of baminercept every week for 24 weeks or matching placebo. The primary endpoint was the change between screening and week 24 in stimulated whole salivary flow (SWSF). Secondary endpoints included the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI), as well as measurements of select chemokines and cytokines and enumeration of peripheral blood B and T cell subsets.
Results
The change from baseline to week 24 in SWSF was not significantly different between the baminercept- and placebo-treatment groups (baseline-adjusted mean change: −0.01 vs. 0.07mL/min; p=0.332). The change in ESSDAI during treatment was also not significantly different between the treatment groups (baseline-adjusted mean change: −1.23 vs. −0.15; p=0.104). While the incidence of adverse events was similar between the treatment groups, baminercept therapy was associated with a higher incidence of liver toxicity, including two serious adverse events. Baminercept also produced a significant decrease in plasma levels of CXCL13 and significant changes in circulating B and T cells, consistent with its known inhibitory effects on LTβR signaling.
Conclusion
In this trial, baminercept treatment failed to significantly improve glandular and extraglandular disease in patients with primary SS despite evidence from mechanistic studies that it blocked LTβR signaling.
Primary Sjögren’s syndrome (SS) is characterized by lacrimal and salivary gland dysfunction, systemic manifestations affecting various organ systems, serum Ro/SSA autoantibodies, and an increased risk of B cell lymphoma (1, 2). Treatment of primary SS has been hampered by the lack of approved disease-modifying therapy. Ideally, therapies for primary SS would target pathways involved in driving disease pathogenesis. In the target tissue, T helper 1 (Th1), Th17, and T follicular helper (Tfh) cells, B cells, and dendritic cells, as well as increased expression of type 1 and 2 interferon (IFN) and B cell activating factor (BAFF) appear to be major contributing factors in these mechanisms (3). Despite evidence implicating B cells in this disease process (4–7), treatment of primary SS with rituximab, a CD20 B cell depleting antibody, has failed in two randomized, controlled trials to show significant clinical benefit (8, 9).
In primary SS, lymphoid infiltrates in the salivary glands often form ectopic lymphoid organs structures comprised of distinct B and T cell compartments, high endothelial venules (HEV), and follicular dendritic cell (FDC) networks that depend on lymphotoxin β receptor (LTβR) signaling for their development and maintenance (10, 11). LTβR binds to LT α/β heterotrimers and LIGHT (also known as tumor necrosis factor superfamily member 14, TNFSF14). LTα/β heterotrimers are expressed on the surface of hematopoietic cells, including mature B, T, and NK cells (12). In mice, blockade of the LTβR system reduces HEV addressin expression and inhibits entry of lymphocytes into lymph nodes and mucosal environments, resulting in blood lymphocytosis (13). LTβR-Ig treatment in male NOD mice, an experimental model of SS, inhibits glandular inflammation, blocks HEV formation, and partially restores salivary flow (14). Baminercept, an LTβR-Ig fusion protein, and pacteclizumab, an anti-LTα antibody, have been investigated as treatments for rheumatoid arthritis (RA), but they failed in both instances to produce significant clinical efficacy (15, 16). Treatment with baminercept nevertheless altered lymphocyte trafficking and inhibited the whole blood IFN signature in accordance with its predicted mechanisms of action (15). Based on a strong scientific rationale, we evaluated baminercept therapy in a randomized, placebo-controlled trial of baminercept therapy for primary SS and explored its impact on LT/LIGHT-dependent pathways.
Patients and Methods
Study population
Eligible subjects were 18–75 years of age, met the American-European Classification Criteria for SS (17), had a stimulated whole salivary flow (SWSF) ≥ 0.1 ml/min, and had either severe parotid gland swelling or one or more systemic disease manifestations: fatigue (> 50 mm on a 100 mm VAS), joint pain (> 50 mm on a 100 mm VAS), peripheral neuropathy (documented by nerve conduction velocity study), interstitial lung disease (documented by radiography and/or altered pulmonary function tests [PFTs]), leukocytoclastic vasculitis, renal tubular acidosis, interstitial nephritis, or other extraglandular manifestations causing organ system dysfunction. Participants were allowed to take prednisone if the dose was ≤ 10 mg/day and maintained at the entry dose during the treatment period. If taking hydroxychloroquine, it was maintained at that dose during the treatment period. Subjects taking a cholinergic stimulant (e.g., pilocarpine, cevimeline) were allowed to continue these medications. To be included in the study, women of childbearing age agreed to use acceptable means of birth control.
Key exclusion criteria included a diagnosis of another connective tissue disease, severe pulmonary disease (resting oxygen saturation < 92%, force vital capacity < 50% predicted, or diffusion lung capacity for carbon monoxide < 50%), active or recurrent infection, a history of malignancy within the last 5 years (except for a resected basal or squamous cell skin carcinoma, cervical dysplasia, or in situ cervical cancer Grade I), a positive pregnancy test, or abnormal laboratory result (hemoglobin < 9.0 g/dL, neutrophil count < 1500/mm3, platelet count < 100,000/mm3, serum creatinine ≥ 2.0 mg/dL, AST/ALT > 1.5 times the upper limit of normal). Subjects were also excluded if taking medications with potent anticholinergic side effects (see supplementary material) or had received biologics or an immunosuppressive drug within a pre-specified washout period.
Study oversight
The study was designed by investigators in the Autoimmunity Centers of Excellence in collaboration with the sponsor, the National Institutes of Allergy and Infectious Diseases, the Statistical and Clinical Coordinating Center (SACCC), Rho, Inc., and Biogen. The study was approved at each site by an Institutional Review Board. The clinical data were collected according to Good Clinical Practice guidelines. The statistical analyses were performed by statisticians at the SACCC and reviewed with the authors. All of the authors contributed to the interpretation of the data and had full access to the data sets. Biogen supplied the study drug (baminercept, matching placebo) and was not otherwise involved in the conduct of the study.
Study design
The study was a phase 2, randomized, double-blind, placebo-controlled multi-center trial of baminercept in patients with primary SS. Eligible subjects were randomly allocated in a 2:1 ratio to receive 24 weekly subcutaneous injections of 100 mg of study drug (baminercept or placebo) starting at the baseline visit and ending at week 23. Randomization was stratified by site. The dose was chosen based on pharmacokinetic, pharmacodynamic, and safety data from healthy volunteers and patients with RA enrolled in previous trials of baminercept. Since elevated lymphocyte counts could unmask site personnel to the treatment assignment, these results were only available to the SACCC, the NIAID safety officer, and a physician sub-investigator at each site not involved in the subject assessments. Injections were self-administered on the same day each week plus or minus one day. The first three injections were self-administered in the clinic under direct observation at baseline, week 1, and week 2, while subsequent injections were given at home. Subjects returned at weeks 4, 8, 12, 18, 24, 30, 36, and 48 for safety and efficacy assessments and blood sampling for mechanistic studies.
Clinical assessments
Salivary flow was measured using the whole saliva technique, as described (18). Participants were instructed to withhold their dose of any secretagogue (pilocarpine or cevimeline) for 48 hours before the visit and to take nothing by mouth for 60 minutes or longer before saliva collection. The unstimulated whole salivary flow (UWSF) was measured for 15 minutes. SWSF was next measured in a 15-minute collection 60 minutes following a single 5 mg dose of pilocarpine. Ophthalmologists measured tear flow by the unanesthetized Schirmer-I test (19) and assessed ocular dryness by lissamine green staining of the temporal and nasal conjunctiva of each eye using a 0–18 scoring system in which the conjunctival surface of each eye was divided into six areas, and a quantitative scale of 0–3 (0, no staining; 3 maximum staining) was used to grade each of the areas (19). Visual analog scales (0 – 100) (VAS) were used for self-assessment of symptoms, including fatigue, overall dryness, and joint pain, as well as patient and physician global assessments of disease severity. The EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) was determined at baseline and week 24 for assessment of systemic disease activity (20). Quality of life was evaluated using the 36-Item Short Form Survey (SF-36). We also included in the design an optional labial salivary gland biopsy at week 0 and 24; however, only 9 paired samples were available for this sub-study and were not further analyzed.
Immunizations with vaccines
To assess immunocompetency, keyhole limpet hemocyanin (KLH) (IMMUNOTHEL™, Biosyn Corporation, Carlsbad, CA) and PNEUMOVAX 23 (Merck and Co., Inc., West Point, PA) were administered at week 8. KLH was given as a single 1 mg subcutaneous injection reconstituted in 0.5 mL of excipient and then emulsified with Montanide ISA-51 VG (Seppic, Inc., Fairfield, NJ) as per the manufacturer. PNEUMOVAX 23 is an FDA-approved polyvalent vaccine containing highly purified capsular polysaccharides from S. pneumonia. Antibody responses to KLH were assessed using commercial assays from Alpha Diagnostics International, (San Antonio, TX). IgG and IgM anti-KLH levels were calculated in units/ml based on the assay’s standard curve using 1:10 or 1:100 dilutions. Values >160,000 Units/ml were not further titrated and were reported as the maximum measured value. Antibodies to the 23 serotypes in Pneumovax™ were measured by Quest Diagnostics, Madison, NJ.
Flow cytometry
Blood samples were collected from patients in CPT tubes (BD Biosciences, Franklin Lakes, NJ) at baseline, weeks 4, 12, 24 and 48 and were shipped overnight from participating sites to a centralized location (Oklahoma Medical Research Foundation; OMRF) for processing where they were banked in liquid nitrogen until use. For analysis of the B and T cell subsets, PBMCs were stained at 4°C for 30 minutes in FACS buffer (PBS with 0.5% BSA) with a cocktail of fluorochrome-conjugated antibodies (see supplementary materials for further details).
Measurement of serum levels of autoantibodies, chemokines, and cytokines
Serum autoantibodies to Ro/SSA composite (Ro 52 and Ro 60), and La/SSB were assayed using a bead-based, multiplex assay on a BioPlex 2200 platform (Bio-Rad Technologies, Hercules, California, USA) as described (21).
IP-10 levels were assessed using an xMAP Bioplex 200 array system (Bio-Rad Technologies, Hercules, CA) as described (22). Results were quantified as median fluorescence intensity normalized to a known serum control (nMFI). Levels of BAFF were measured by an enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN; eBioscience/Affymetrix, San Diego, CA) per the manufacturer’s protocol. CXCL13 and LIGHT were also measured by ELISA (Human CD258/LIGHT Ready-Set-Go and ProcartaPlex Human BLC/CXCL13 Simplex, Affymetrix/eBioscience, San Diego, CA).
Endpoints
The primary efficacy endpoint was the change in SWSF between screening and week 24. Key secondary efficacy endpoints included change in UWSF, ESSDAI, VAS scores for patient self-assessments of fatigue, overall dryness, and joint pain, VAS scores for patient and physician global assessments of disease activity, Schirmer I-test, ocular staining, and SF-36 scores. Salivary flow was calculated as the amount of saliva divided by the duration, in minutes, of collection. The proportion of subjects experiencing any Grade 3 or above adverse event (AE) was a safety endpoint of particular interest.
Statistical analyses
Subjects who received at least one dose of study treatment and had a screening SWSF assessment were included in the modified intent-to-treat (mITT) population. For the primary mITT analysis, missing data for week 24 SWSF was imputed by carrying forward the last observed post-baseline value. However, as pre-specified in the analysis plan, one subject without a post-baseline SWSF was excluded from the primary analysis. An analysis of covariance (ANCOVA) model was used for the primary analysis comparing the change between treatment groups in SWSF from screening to week 24, adjusted for the screening value.
ANCOVA models were also used to test secondary endpoints for changes from baseline for continuous outcomes (except for the analysis of B cell and T cell results and anti-KLH and anti-pneumococcal antibody responses); baseline/screening values were considered covariates. A Wilcoxon rank-sum test was used to compare the change from baseline in the frequency of B cell and T cell percentages across treatment groups and the change from baseline in the anti-KLH and anti-pneumococcal antibody responses. A Fisher’s exact test was used for categorical outcomes. Subjects in the mITT population with available data were used for secondary efficacy and mechanistic analyses. The safety population consisted of all subjects who received at least one dose of study drug. Secondary analyses to support the primary and secondary objectives were considered exploratory; p-values are presented without adjustment for multiple comparisons.
The study was powered for the primary analysis to test for a treatment group difference in change in SWSF from screening to week 24. Assumptions were informed using data from prior studies (18, 23). A change of 0.02 mL/min was assumed to be the maximum in the placebo arm. In the baminercept group, a change of 0.33 mL/min was considered to represent clinically meaningful improvement. The standard deviation was assumed to be 0.4 in both arms. For a two-sided t-test with α =0.05, 72 subjects, randomized at 2:1, and allowing for 10% dropout were needed for 80% power.
Results
Subject characteristics
Study enrollment was terminated early because of expiration of study drug. Of the 104 subjects screened, 52 subjects were eligible and randomized into the trial (Figure S1). All 33 subjects in the baminercept group and 19 subjects in the placebo group were included in the mITT and safety samples. However, one baminercept-treated subject was excluded from the primary endpoint analysis because of no post-treatment measurements of salivary flow. Demographic and baseline characteristics were similar between the treatment groups except the tear flow in the right eye was higher in the placebo group (Table 1). Subjects had low systemic disease activity, as indicated by the low mean ESSDAI score at entry.
Table 1.
Baseline characteristics (mITT)
| Baminercept N=33 |
Placebo N=19 |
|
|---|---|---|
| Age (years) mean (SD) | 50 (10.9) | 55 (11.0) |
| Gender (N, % female) | 31 (94) | 18 (95) |
| Race N (%) | ||
| White | 28 (85) | 16 (84) |
| Black | 3 (9) | 3 (16) |
| Other | 2 (6) | 0 (0) |
| Physician global disease activity (0–100 mm), mean (SD) | 48.6 (20.6) | 50.3 (20.5) |
| Subject global disease activity (0–100 mm), mean (SD) | 70.3 (16.5) | 73.5 (16.4) |
| Subject assessment of symptoms (0–100 mm), mean (SD) | ||
| Overall dryness | 71.3 (17.7) | 71.4 (16.9) |
| Fatigue | 70.0 (17.5) | 67.0 (18.5) |
| Joint pain | 46.5 (24.9) | 55.1 (25.1) |
| Unstimulated whole salivary flow (mL/min), mean (SD) | 0.11 (0.21) | 0.09 (0.11) |
| Stimulated whole salivary flow (mL/min), mean (SD) | 0.44 (0.49) | 0.49 (0.30) |
| Schirmer-I test (mm), mean (SD) | ||
| Left eye | 10.2 (11.4) | 10.5 (12.8) |
| Right eye | 9.8 (10.7) | 14.6 (14.7) |
| Total ocular staining score (0–36),* mean (SD) | 19.2 (11.9) | 15.1 (11.5) |
| ESSDAI score (0–123), mean (SD) | 2.7 (2.8) | 3.8 (4.2) |
| SF-36 (0–100), mean (SD) | ||
| Physical aggregate score | 39.2 (8.1) | 39.8 (10.8) |
| Mental aggregate score | 46.1 (10.9) | 45.0 (11.2) |
| Anti-SS-A (Ro) or anti-SS-B (La) positive, N (%) | 29 (88) | 15 (79) |
| Positive lip biopsy, N (%) | 9 (27) | 7 (37) |
| IgG (mg/dL), mean (SD) | 1431 (530) | 1196 (475) |
| Concomitant therapy, N (%) | ||
| Hydroxychloroquine | 19 (58) | 10 (53) |
| Prednisone ≤ 10 mg/d | 0 (0) | 1 (5) |
| Cholinergic stimulants | 12 (36) | 8 (42) |
Combined ocular staining score for both eyes.
ESSDAI = EULAR Sjögren’s syndrome disease activity index; SF-36 = 36-Item Short Form Health Survey
Clinical efficacy
The primary outcome, the change in SWSF between screening and week 24, was not significantly different between treatment groups (p = 0.33) (Table 2). The adjusted mean reduction in the ESSDAI for the baminercept group was numerically small and not statistically different from the placebo group (−1.23 vs. −0.15, p=0.104) (Tables 2 and S4). Treatment groups also did not significantly differ in change from baseline to week 24 in UWSF, physician global assessment, subject assessment of symptoms, ocular staining score, and SF-36 (Table 2). The adjusted change from baseline to week 24 in the right eye Schirmer-I test was statistically different between treatment groups (p-value = 0.036), but the difference was due to a fall in the placebo group.
Table 2.
Adjusted mean change (95% CI) from baseline to week 24 in clinical outcomes
| Baminercept | Placebo | P value | |
|---|---|---|---|
| Primary Endpoint (mITT with post-baseline SWSF) | |||
| Stimulated WSF (mL/min)1 | |||
| n | 32 | 19 | |
| Adjusted mean change (95% CI) | −0.01 (−0.10, 0.08) | 0.07 (−0.06, 0.19) | 0.332 |
| Secondary Endpoints (mITT with available data) | |||
| Unstimulated WSF (mL/min)2 | 0.06 (0.00, 0.12) | 0.07 (−0.01, 0.15) | 0.881 |
| ESSDAI score3 | −1.23 (−2.03, −0.43) | −0.15 (−1.18, 0.87) | 0.104 |
| Physician global assessment3 | −16.15 (−22.36, −9.94) | −13.80 (−21.91, −5.69) | 0.646 |
| Subject global assessment3 | −13.88 (−23.11, −4.65) | −9.75 (−21.81, 2.30) | 0.587 |
| Subject assessment of symptoms3 | |||
| Overall dryness | −8.27 (−17.50, 0.96) | −10.75 (−22.82, 1.32) | 0.744 |
| Fatigue | −8.02 (−16.41, 0.38) | −10.33 (−21.30, 0.64) | 0.737 |
| Joint pain | −5.62 (−14.75, 3.52) | −7.55 (−19.50, 4.41) | 0.797 |
| Schirmer-I test (mm)2 | |||
| Left eye | 0.75 (−2.73, 4.23) | 1.99 (−2.53, 6.51) | 0.662 |
| Right eye | 0.80 (−1.60, 3.21) | −3.48 (−6.63, −0.34) | 0.036 |
| Total ocular staining score2 | −0.10 (−2.97, 2.77) | −1.33 (−5.07, 2.41) | 0.603 |
| SF-363 | |||
| Physical aggregate score | 2.51 (−0.19, 5.22) | −0.63 (−4.18, 2.91) | 0.163 |
| Mental aggregate score | −1.04 (−4.82, 2.75) | −0.59 (−5.53, 4.35) | 0.885 |
Mean change and 95% CI adjusted for baseline results.
Primary endpoint statistics include all subjects in the mITT population who have a post-baseline stimulated salivary flow result. Results were consistent when assuming the baminercept subject without a post-baseline SWSF assessment had no change from baseline (LOCF).
N= Data from 27 and 16 subjects in the baminercept and placebo arms, respectively, were available for this analysis.
Data from 29 and 17 subjects in the baminercept and placebo arms, respectively, were available for this analysis.
Adverse events
The incidence of adverse events was similar between the two treatment groups. However, grade 3 or higher adverse events were more frequent in the baminercept than the placebo group (p=0.50) (Table 3). Treatment-emergent AEs are summarized in Table S1. Notable adverse events occurring in more than 5% of subjects and more common in the baminercept than the placebo group included but were not limited to injection site reactions, weight decrease, vomiting, upper abdominal pain, headache, paresthesia, cough, hepatobiliary disease, lymphopenia, anemia, and hypercholesterolemia. Upper respiratory infection was documented in 8 (24.2%) and 3 (15.8%) subjects in the baminercept and placebo groups, respectively.
Table 3.
Treatment-emergent adverse events
| Baminercept N=33 |
Placebo N=19 |
|||
|---|---|---|---|---|
| Subjects, N (%) | Events | Subjects, N (%) | Events | |
| Total SAEs | 5 (15) | 5 | 1 (5) | 1 |
| Total AEs | 33 (100) | 356 | 19 (100) | 204 |
| Related to study drug | 29 (88) | 177 | 18 (95) | 100 |
| Severity (total AEs)* | ||||
| Grade 1 | 33 (100) | 275 | 18 (95) | 164 |
| Grade 2 | 25 (76) | 70 | 13 (68) | 36 |
| Grade 3 | 8 (24) | 10 | 3 (16) | 4 |
| Grade 4 | 1 (3) | 1 | 0 (0) | 0 |
| Grade 5 | 0 (0) | 0 | 0 (0) | 0 |
Abbreviations: SAE=serious adverse event; AE=adverse event;
graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0
There were 5 serious adverse events (SAEs) in the baminercept group and 1 SAE in the placebo group during the treatment phase. Two of the SAEs in the baminercept group were categorized as hepatobiliary injury and characterized by reversible grade 3 elevations in liver enzyme values; one of the subjects had a reversible elevation in bilirubin (see supplementary material). Other SAEs in the baminercept group were breast cancer, pleurisy, and hospitalization for vasovagal syncope that were determined by the investigator to be unlikely to be related, possibly related, and unrelated to study drug, respectively. The SAE in the placebo group involved a subject who was hospitalized because of shortness of breath.
Elevated liver enzyme values occurred with greater frequency in the baminercept group than the placebo group, including the 2 subjects with grade 3 transaminase elevations mentioned above (Table S2). There were no other significant changes in serum chemistry values, hemoglobin concentration, total leukocyte count, or platelet count. However, a grade 4 neutropenia was observed in the baminercept group judged by the investigator to be unlikely related to study drug.
KLH and Pneumovax 23™ immunizations
To determine if baminercept therapy inhibited an antigen-specific response, subjects were immunized at week 8 with KLH/Montanide and Pneumovax 23™, with subsequent measurement of antibody responses at week 12. In this study, baminercept therapy did not suppress the antibody response to either KLH, a T cell-dependent response, or the different serotypes in the pneumococcal polysaccharide vaccine, T cell-independent responses (Supplementary Figures 2 and 3). The increase in IgM KLH titers upon baminercept treatment would be consistent with impaired class switch recombination as observed in rodent studies.
Serum levels of select autoantibodies, complement, cytokines, and chemokines
Serum levels of Ro/SSA and La/SSB autoantibodies, as well as serum IgG levels, did not change during treatment (Table S3). Serum C3 complement levels trended higher during treatment in the baminercept group, although the changes were not significantly different between groups (adjusted mean change 7.02 vs. −0.15 mg/dL; p=0.168) (Table S3). In parallel, serum levels of C4 complement also increased in the baminercept group relative to the placebo group (adjusted mean change 4.96 vs. −0.31 mg/dL; p= 0.003).
Baminercept and pateclizumab treatment have been shown to lower serum CXCL13 levels in patients with RA (16, JT Browning, unpublished data) and reduce CXCL13 expression in lacrimal gland homogenates isolated from a mouse model of SS (14). In our study, we found that baminercept therapy reduced serum CXCL13 levels compared with placebo (adjusted mean change: −104.7 vs. −12.7 pg/mL; p=0.038) (Table 4). In contrast, baminercept treatment failed to diminish serum levels of BAFF, LIGHT, or IP-10 (Table 4). The elevated serum levels of LIGHT detected in the previous baminercept study (15) were not observed herein and may have been due in our assay to interference from baminercept, which had been taken into account in the previous work.
Table 4.
Summary of cytokine and chemokine biomarkers (mITT)
| Baminercept | Placebo | P value | |
|---|---|---|---|
| BAFF (pg/mL) | |||
| N | 29 | 14 | |
| Week 0 Mean (SE) | 830 (51.7) | 670 (20.4) | |
| Week 24 Mean (SE) | 856 (66.3) | 660 (28.6) | |
| Adjusted Mean (CI) Change | 29.0 (−51.6, 109.6) | −17.6 (−135.7, 100.5) | 0.523 |
| LIGHT (pg/mL) | |||
| N | 26 | 13 | |
| Week 0 Mean (SE) | 513 (170.0) | 305 (129.2) | |
| Week 24 Mean (SE) | 410 (137.4) | 258 (108.0) | |
| Adjusted Mean (CI) Change | −88.0 (−150.2, −25.7) | −76.8 (−165.1, 11.5) | 0.840 |
| IP-10 (pg/mL) | |||
| N | 29 | 14 | |
| Week 0 Mean (SE) | 65 (21.3) | 21 (3.8) | |
| Week 24 Mean (SE) | 49 (12.0) | 24 (3.8) | |
| Adjusted Mean (CI) Change | −9.9 (−16.1, −3.7) | −10.5 (−19.5, −1.5) | 0.907 |
| CXCL13 (pg/mL) | |||
| N | 29 | 14 | |
| Week 0 Mean (SE) | 236 (43.7) | 193 (91.2) | |
| Week 24 Mean (SE) | 127 (26.4) | 191 (88.7) | |
| Adjusted Mean (CI) Change | −104.7 (−154.1, −55.3) | −12.7 (−83.8, 58.5) | 0.038 |
Only subjects with samples available at week 0 and 24 are included.
Abbreviations: BAFF=B cell activating factor; LIGHT=homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes; IP-10= Interferon gamma-induced protein 10 (CXCL10) Mean change and 95% CI adjusts for baseline results.
Analysis of circulating B and T cell subsets
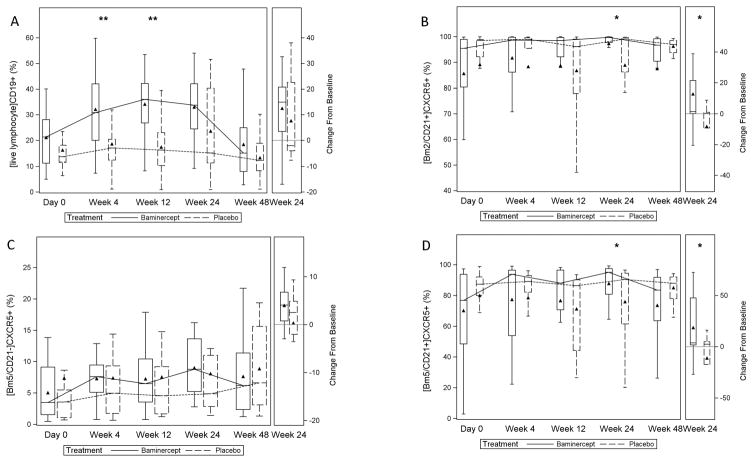
Baminercept therapy was associated with an increase in blood lymphocyte counts from baseline to week 24 relative to placebo (adjusted mean change: 0.75 x 109 cells/L vs. 0.05 x 109 cells/L; p=<0.001), with a recovery towards baseline values through week 48 (Figure S4). Likewise, the frequency of circulating CD19+ B cells trended higher during baminercept therapy; the adjusted mean change was significantly greater in the baminercept group than the placebo group at week 4 (p=0.006) and week 12 (p=0.005) (Figure 1A). In contrast, no significant changes were observed in the percentage of circulating CD3+, CD4+, and CD8+ T cells (Figure S5).
Figure 1. B cell subsets over time.
Frequency of subsets of B cells over time and the changes at week 24. Peripheral blood was obtained at day 0, week 4, week 12, week 24, and week 48 and analyzed by flow cytometry as described in Methods. Data are plotted as percent (subset analyzed indicated in brackets); ** P < 0.01, * P< 0.05, Wilcoxon rank-sum test for treatment group differences in the change from baseline. A-CD19+ B cells; week 4, p=0.006; week 12, p=0.005; week 24, p=0.320; B-[Bm2CD21+]CXCR5+ cells; week 4, p=0.244; week 12, p=0.122; week 24, p=0.033; C-[Bm5CD21−]CXCR5+ cells; week 4 p=0.357; week 12 p=0.820; week 24 p=0.162; D-[Bm5CD21+]CXCR5+ cells; week 4, p=0.159; week 12, p=0.066; week 24 p=0.027. Triangle = mean; line = median; bar = 25–75th percentile; whiskers = Q1 – 1.5*IQR, Q3 + 1.5*IQR.
To investigate the effects of baminercept therapy on circulating B cell subsets, we analyzed available samples from 27 and 13 subjects in the baminercept and placebo groups, respectively using the Bm1-Bm5 classification (24). Baseline values are shown in Figure S6. Most of the blood Bm2 cells were CD21+ resting naïve B cells (> 99%); whereas, the Bm5 compartment was comprised of CD21+ resting memory (~ 60%) and CD21− activated memory (~40%) cells (data not shown). CD21 is expressed on resting memory B cells and down-regulated upon activation (25). Median numbers of circulating CD21−/low B cells have been reported to be higher in primary SS than controls (26); in one study, CD21 expression was lower on both CD27+IgD+ unswitched and CD27+IgD− switched memory B cells (27). For this post-hoc analysis, changes in the percentage of a B cell subset were considered to be significant if the two groups were statistically different at week 24 with a p value < 0.05.
We hypothesized the reductions in CXCL13 observed with baminercept therapy would result in fewer CXCR5 expressing B cells migrating from the circulation into inflamed salivary glands and reactive lymphoid tissue. Consistent with this hypothesis, the changes in the frequencies of resting naïve (Bm2 CD21+) and memory (Bm5 CD21+) cells expressing CXCR5 were significantly greater at week 24 in the baminercept-treated subjects compared to the placebo group (Figure 1B and 1D). Alternatively, the loss of HEV portals could account for the observed B cell changes. In contrast, the percentages of (Bm5 CD21−) CXCR5+ memory B cells were not significantly altered by baminercept treatment (Figure 1C). No significant changes were observed in the other blood CD19+ B cell compartments, including the CXCR4+ and CXCR4− B cell subsets analyzed in this study (data not shown).
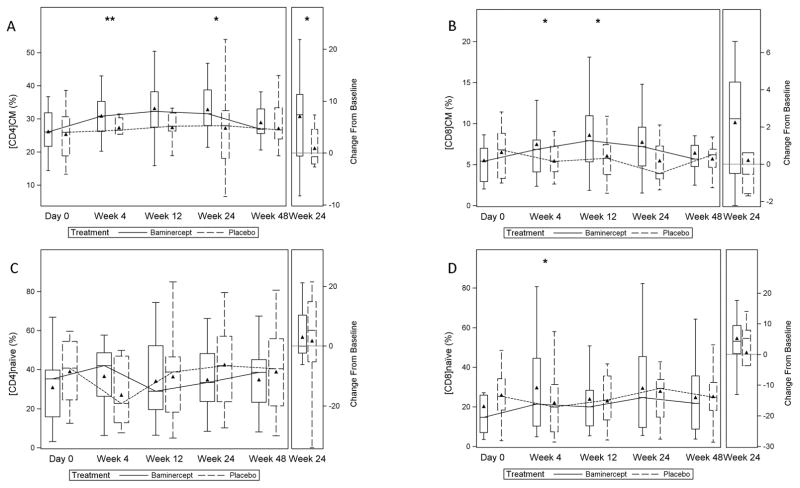
We also analyzed 144 samples from 34 patients (baminercept, n=23; placebo, n=11) for treatment-related changes in blood T cell subsets. Baseline values are shown in Figure S7. Blood T central memory (TCM) cells express CD62L (L-selectin) and CCR7, while T effector memory (TEM) cells are CD62L− and CCR7− (28). Both CCL19 and CCL21, the ligands for CCR7, are regulated by the LT/LIGHT pathway; they promote homing of T cells to lymphoid tissue (29) and are upregulated in salivary gland tissue from patients with primary SS (30, 31). In the previous RA study, baminercept therapy produced a decrease in serum levels of CCL19 and CCL21 (JT Browning, personal communication). At week 24, the baminercept-treated subjects showed a significant increase in the percentage of circulating CCR7+CD45RA− CD4+ TCM cells (Figure 2A), but no significant change in the percentage of CCR7+CD45RA−CD8+ TCM cells (Figure 2B). However, the percentage of CCR7+CD45RA−CD8+ TCM cells was numerically higher at week 24 in the baminercept group and by week 48 had trended towards baseline values. These results suggest baminercept therapy inhibited the migration of TCM cells through HEV into lymphoid and non-lymphoid tissue. No significant differences were noted between treatment groups in the changes in the frequency of CCR7+CD45RA+ naïve CD4+ and CD8+ T cells (Figure 2C and 2D), which also express CD62L and recirculate through secondary lymphoid tissue via HEV. No significant differences were found between treatment groups at week 24 in the frequencies of CCR7−CD45RA− CD4+ and CD8+ effector memory TEM cells and CCR7−CD45RA+ CD4+ and CD8+ effector memory TEMRA cells (Figures S8 and S9). Blood memory Tfh cells are a heterogeneous population that can be grouped into 3 major subsets, Tfh1, Tfh2, and Tfh3 subsets, and then further subdivided into quiescent and activated subsets based on their differential expression of PD-1, CCR7, and ICOS (32). We did not detect a change in circulating memory Tfh cells, which also express CXCR5 (32). Baminercept therapy did not appear to change the frequency of blood Th1, Th17, or T regulatory cells (Figure S10).
Figure 2. T cell subsets over time.
Frequency of subsets of T cells over time and changes at week 24. Peripheral blood was obtained at day 0, week 4, week 12, week 24, and week 48 and analyzed by flow cytometry as described in Methods. Data are plotted as percent; ** P < 0.01, * P< 0.05, Wilcoxon rank-sum test for treatment group differences in the change from baseline. A-CCR7+CD45RA−CD4+ TCM cells; week 4, p=0.005; week 12, p=0.121; week 24, p=0.043; B- CCR7+CD45RA−CD8+ TCM cells; week 4, p=0.010; week 12, p=0.020; week 24, p=0.096; C-CCR7+CD45RA+ naïve CD4+ T cells; week 4, p=0.138; week 12, p=0.180; week 24, p=0.832; D-CCR7+CD45RA+ naïve CD8+ T cells; week 4, p=0.020; week 12, p=0.067 week 24, p=0.785. Triangle = mean; line = median; bar = 25–75th percentile; whiskers = Q1 – 1.5*IQR, Q3 + 1.5*IQR.
Discussion
In this study, treatment with baminercept, an LTβR-Ig fusion protein, was not effective in improving the glandular signs and symptoms of primary SS. The study had several strengths, including its randomized, double-blinded, placebo-controlled design and the breadth of the clinical assessments. The low ESSDAI scores at entry hampered our ability to determine if baminercept reduced systemic disease activity. Physician estimates of low, moderate, and high disease activity have been correlated with ESSDAI scores of < 5, 5–13, and ≥ 14, respectively; and a decrease of 3 points or more has been defined as minimal clinically important improvement (33). Our study population had low disease activity by these criteria and the adjusted mean change (95% CI) in ESSDAI score of −1.23 (−2.03, −0.43) during baminercept treatment fell below the 3-point threshold. Although the small sample size was a limitation of this study, achieving our target sample size of 72 subjects would not have likely changed these outcomes.
Two cases of reversible hepatic injury were reported in the baminercept group. In nonclinical toxicology studies, maternal liver toxicity was observed at the highest dose tested (100 mg/kg) in some pregnant rats but not in other species (cynomolgous monkeys) or non-pregnant rats (Investigator Brochure, Biogen). Three SAEs involving changes in liver function tests have been reported in baminercept-treated patients with RA (Investigator Brochure, Biogen). In two phase II clinical trials involving patients with RA, elevations of AST and ALT levels were noted in 2% of baminercept-treated subjects compared to less than 1% of placebo-treated subjects (15). No liver-related SAEs or elevated liver enzymes were reported in patients with RA receiving pateclizumab, a monoclonal antibody against LTα (16).
Membrane LT is critical for regulating the expression of chemokines and homing of immune cells to lymphoid and non-lymphoid tissues (11, 34). Our finding that baminercept therapy reduced plasma levels of CXCL13 is consistent with the results of animal studies and previous clinical investigations (14, 35, 36) and may account for the corresponding increase in the frequency of circulating Bm2 CD21+ naïve and memory Bm5 CD21+ B cells expressing CXCR5, which is the receptor for CXCL13. On the other hand, baminercept therapy did not alter the percentage of CXCR5-expressing Bm5 CD21− activated memory B cells. The effects of LTβR signaling on the dynamics of recirculating B cell subsets are likely complex and incompletely understood.
Circulating memory T cells have been separated into distinct subsets based on their proliferative capacity, effector function, and migration properties. TCM cells are CD62L+, CCR7+, produce IL-2, show substantial proliferative capacity, and are prominent in secondary lymphoid organs, while TEM cells are CD62L−, CCR7−, produce IFN-γ, show less proliferative potential, and localize mostly in peripheral tissues (28, 37, 38). CCR7, which functions to coordinate trafficking of various T cell subsets through secondary lymphoid and non-lymphoid tissue, is highly expressed within immune cell aggregates and HEV-like structures of inflamed salivary gland tissue from patients with primary SS (30, 31). The finding that baminercept therapy increased the frequency of circulating TCM cells in our study and not the frequency of blood TEM cells supports the hypothesis that the HEV network was disrupted by inhibition of LTβR signaling. However, we interpret these results cautiously because of the limited number of samples available for this analysis and the lack of labial salivary gland biopsies to corroborate this interpretation. We did not observe a treatment-related change in circulating Tfh cells, which also express CXCR5. The heterogeneity of the blood Tfh compartment may have obscured our ability to detect changes in this subset. In an NOD mouse model of SS, LTβR-Ig treatment differentially impacted infiltrating CD4+ T cell subsets, markedly diminishing the PD1−ICOS− naïve subset while only slightly affecting the putatively pathogenic PD1highICOShigh and PD1lowICOSlow subsets, possibly explaining the lack of efficacy of baminercept in our current study (36).
In summary, 24 weeks of baminercept therapy was ineffective for improving the glandular dysfunction of primary SS. Despite this lack of treatment efficacy, our mechanistic analysis showed significant effects of baminercept therapy on LT/LIGHT-dependent pathways. These strong biological effects on pathways of known pathogenic significance raise the possibility that blocking LTβR signaling might be of therapeutic benefit at earlier stages of disease.
Supplementary Material
Acknowledgments
The authors would like to thank the patients who participated in this clinical trial and acknowledge the important contributions of the clinical coordinators at the different clinical sites. The authors thank Dr. Robert Kimberly (University of Alabama School of Medicine, Birmingham, AB for kindly providing the monoclonal antibody against CD32b-AF647 (4F5).
Financial support: Supported by a grant from National Institutes of Allergy and Infectious Diseases (NIAID), Autoimmunity Centers of Excellence 5U19-AI056363; Study drug and in-kind support for some of the mechanistic studies was provided by Biogen. Sample processing, storage and management was supported in part by ARP3053483. Rho, Inc was supported by funding from the National Institutes of Allergy and Infectious Diseases (1UMZAI117870 and HHSN272200900057C).
Footnotes
Financial interests: E. William St. Clair has received grant support from Genentech, Inc. and Bristol-Myers Squibb and consulting fees from Bristol-Myers Squibb; Alan N. Baer has received consulting fees from Glenmark Pharmaceuticals, Boston Pharmaceuticals, Novartis, and Bristol-Myers Squibb (less than $10,000); Jeffrey T. Browning was a full-time employee of Biogen during protocol development; Nathalie Franchimont is a full-time employee of Biogen, Inc.; Ghaith Noaiseh, Anne Parke, Andreea Coca, Tammy Utset, Mark C. Genovese, Daniel J. Wallace, James McNamara, Karen Boyle, Lynette Keyes-Elstein, Ignacio Sanz, Chungwen Wei, Kira Smith, Joel Guthridge, and Judith A. James have no competing financial interests.
References
- 1.Nocturne G, Mariette X. Sjögren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Hematol. 2015;168:317–327. doi: 10.1111/bjh.13192. [DOI] [PubMed] [Google Scholar]
- 2.Papageorgiou A, Voulgarelis M, Tzioufas AG. Clinical picture, outcome and predictive factors in Sjögren syndrome. Autoimmun Rev. 2015;14:641–649. doi: 10.1016/j.autrev.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Mavragani CP, Moutsopoulos HM. Sjögren’s syndrome. Annu Rev Pathol Mech Dis. 2014;9:273–285. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 4.Bohnhorst JØ, Bjørgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren’s syndrome. J Immunol. 2001;167:3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 5.Hansen A, Gosemann M, Pruss A, Reiter K, Ruzickova S, Lipsky PE, et al. Abnormalities in peripheral B cell memory of patients with primary Sjögren’s syndrome. Arthritis Rheum. 2004;50:1897–1908. doi: 10.1002/art.20276. [DOI] [PubMed] [Google Scholar]
- 6.D’Arbonneau F, Pers J-O, Devauchelle V, Pennec Y, Saraux A, Youinou P. BAFF-induced changes in B cell receptor antigen-containing lipid rafts in Sjögren’s syndrome. Arthritis Rheum. 2006;54:115–126. doi: 10.1002/art.21478. [DOI] [PubMed] [Google Scholar]
- 7.Lessard CJ, Li H, Adrianto I, Ice JA, Rasumssen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nature Genet. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot J-M, Perdriger A, Puéchal X, et al. Treatment of primary Sjögren syndrome with rituximab: A Randomized Trial. Ann Intern Med. 2014;160:233–242. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 9.Bowman SJ, Everett CC, O’Dwyer JL, Emery P, Pitzalis C, Ng W-F, et al. Randomized controlled trial of rituximab and cost-effectivenss analysis in treating fatigue and oral dryness in primary Sjögren’s syndrome. Arthritis Rheumatol. 2017;69:1440–1450. doi: 10.1002/art.40093. [DOI] [PubMed] [Google Scholar]
- 10.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nature Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 11.Lu TT, Browning JL. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front Immunol. 2014 Feb 11;5:47. doi: 10.3389/fimmu.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remouchamps C, Boutaffala L, Ganeff C, Dejardin E. Biology and signal transduction pathways of the lymphotoxin-αβ/LTβR system. Cytokine Growth Factor Rev. 2011;22:301–310. doi: 10.1016/j.cytogfr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev. 2008;223:202–220. doi: 10.1111/j.1600-065X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 14.Fava RA, Kennedy SM, Wood SG, Bolstad AI, Bienkowska J, Papandile A, et al. Lymphotoxin-beta receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjögren’s syndrome. Arthritis Res Ther. 2011;13:R182. doi: 10.1186/ar3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bienkowska J, Allaire N, Thai A, Goyal J, Plavina T, Nirula A, et al. Lymphotoxin-LIGHT pathway regulates the interferon signature in rheumatoid arthritis. PLoS ONE. 2014;9:e112545. doi: 10.1371/journal.pone.0112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emu B, Luca D, Offutt C, Grogan C, Rojkovich B, Williams MB, et al. Safety, pharmacokinetics, and biologic activity of pateclizumab, a novel monoclonal antibody targeting lymphotoxin α: results of a phase I randomized, placebo-controlled trial. Arthritis Res Ther. 2012;14:R6. doi: 10.1186/ar3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.StClair EW, Levesque MC, Luning Prak ET, Vivino FB, Alappatt CJ, Spychala ME, et al. Rituximab therapy for primary Sjögren’s syndrome: an open-label clinical trial and mechanistic analysis. Arthritis Rheum. 2013;65:1097–1106. doi: 10.1002/art.37850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO. 1995;4:221–232. [PubMed] [Google Scholar]
- 20.Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): user guide. RMD Open. 2015;1:e000022. doi: 10.1136/rmdopen-2014-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol. 2014;66:1888–99. doi: 10.1002/art.38573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods. 2009;350:125–32. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Rosas J, Ramos-Casals M, Ena J, García-Carrasco M, Verdu J, Cervera R, et al. Usefulness of basal and pilocarpine-stimulated salivary flow in primary Sjögren’s syndrome. Correlation with clinical, immunological and histological features. Rheumatol. 2002;41:670–675. doi: 10.1093/rheumatology/41.6.670. [DOI] [PubMed] [Google Scholar]
- 24.Bohnhorst JØ, Bjørgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren’s syndrome. J Immunol. 2001;167:3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 25.Adlowitz DG, Barnard J, Biear JN, Cistrone C, Owen T, Wang W, et al. Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS One. 2015 Jun;May;10(6):e0128269. doi: 10.1371/journal.pone.0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21−/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65:1085–1096. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts M, Kaminski D, Jenks SA, Maquire C, Ching K, Burbelo PD, et al. Primary Sjögren’s syndrome is characterized by distinct phenotypic and transcriptional profiles of IgD+ unswitched memory B cells. Arthritis Rheumatol. 2014;66:2558–2569. doi: 10.1002/art.38734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 29.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CL21 chemokine axis in the adaptive immune system. Cytokine Growth Fact Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, et al. Association of CXCL13 and CCL21 expression within the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 31.Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjögren’s syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–5140. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 32.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nature Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seror R, Bootsma H, Saraux A, Bowman SJ, Theander E, Brun JG, et al. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI) Ann Rheum Dis. 2016;75:382–389. doi: 10.1136/annrheumdis-2014-206008. [DOI] [PubMed] [Google Scholar]
- 34.Browning JL, Allaire N, Ngam-ek A, Notidis E, Hunt J, Perrin S, et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Gatmu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11:R24. doi: 10.1186/ar2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haskett S, Ding J, Zhang W, Thai A, Cullen P, Xu S, et al. Identification of novel CD4+ T cell subsets in the target tissue of Sjögren’s syndrome and their differential regulation by the lymphotoxin/LIGHT axis. J Immunol. 2016;197:3806–3819. doi: 10.4049/jimmunol.1600407. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;40:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 38.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.