Abstract
Objective
Determine the frequency, time-to-flare, and predictors of disease flare upon withdrawal of anti-tumor necrosis factor (TNF) therapy in children with polyarticular forms of juvenile idiopathic arthritis (PF-JIA) who demonstrated ≥ 6 continuous months of clinical inactive disease (CID).
Methods
In 16 centers 137 patients with PF-JIA in CID on anti-TNF therapy (42% of whom were also on methotrexate [MTX]) were prospectively followed. If CID was maintained for the initial 6 months on study anti-TNF was stopped and patients assessed for flare at 1, 2, 3, 4, 6, and 8 months. Life-table, t-tests, Chi square, and Cox regression analyses were used to identify independent variables that could significantly predict flare by 8 months or time-to-flare.
Results
106/137 (77%) maintained CID on anti-TNF therapy for the initial 6 months and were included in the phase of the study in which anti-TNF therapy was stopped. Stopping anti-TNF resulted in disease flare in 39/106 (37%) patients by 8 months. The mean/median time-to-flare were 212/250 (SEM 9.77) days. Patients with shorter disease duration at enrollment, older age at onset and diagnosis, shorter disease duration prior to experiencing CID, and shorter time from onset of CID to enrollment were found to have significantly lower hazard ratios (p< 0.05) for likelihood of flare by 8 months.
Conclusion
Over one-third of patients with PF-JIA in sustained CID will flare by 8 months following discontinuation of anti-TNF therapy. Several predictors of lower likelihood of flare were identified.
Current treatment approaches for children with polyarticular forms of juvenile idiopathic arthritis (PF-JIA) (1) have resulted in up to 50% of the patients demonstrating clinical inactive disease (CID) (2) while on treatment (2–13). Much of the success in inducing CID is due to the introduction of anti-tumor necrosis factor alpha (anti-TNF) biologic therapies used early in the treatment of a large proportion of children with JIA (4–8, 12, 13, 15). Anti-TNF therapy has known short- and medium-term toxicities in children with JIA; the long-term toxicities (>15 years) are unknown, and the medication cost is substantial (5, 13, 16–24). Prediction of which children with JIA on anti-TNF therapies in CID can discontinue therapy without experiencing a recurrence of clinically active disease is presently limited to retrospective or small prospective cohort studies and studies of proposed biomarkers (25–29). Studies describing which patients may safely discontinue treatment once CID is achieved have not been fully validated (30, 31).
The objective of this research was to determine the time-based risk of disease flare in patients with polyarticular forms of JIA (PF-JIA) (rheumatoid factor [RF] positive or negative and extended oligoarticular) who had experienced sustained CID at the time anti-TNF therapy was withdrawn. Secondary objectives included (1) the identification of clinical and demographic variables that impact time to and risk of flare, and (2) the impact of background methotrexate (MTX) on time-to flare and risk of flare after withdrawal of anti-TNF therapy.
PATIENTS AND METHODS
Study Design and Patient Flow
This multi-center, prospective study had 2 phases conducted over a 14 month, on-protocol period. In the first 6-month phase patients who were in a state of CID were enrolled and continued an anti-TNF agent and background drugs (if applicable). If CID was maintained for the initial 6 months of the study, the patient became eligible for the second phase during which anti-TNF therapy was withdrawn while background medications were held stable. TNF withdrawal was done by protocol in all sites and in all instances anti-TNF therapy was discontinued completely at the month 6 study visit and not tapered. These ‘evaluable patients’ returned for study visits at 1, 2, 3, 4, 6 and 8 months to determine if their disease had flared according to pre-specified criteria (see below for definition). The protocol did not specify ‘study visit windows’ outside of which data would be discarded. While greater than 90% of visits occurred within 2 weeks of the expected study date, some were outside this span. These data were used in the analysis resulting in the maximal follow-up after stopping the anti-TNF agent being up to 8 months in 67 patients and >8 but ≤10 months in 39 patients.
The study was approved by the Institutional Review Board (IRB) at each participating site and was conducted to be in compliance with the Helsinki Declaration (http://www.wma.net/en/20activities/10ethics/10helsinki/index.html). Parent/patient consent (assent, if appropriate) and screening for eligibility were done at the participating site at which the patient was recruited. Each patient’s eligibility was validated via eligibility case report forms immediately sent to the Pediatric Rheumatology Collaborative Study Group (PRCSG) Coordinating Center (CC) at Cincinnati Children’s Hospital Medical Center. The CC served as the clinical research organization for this study and oversaw all matters related to regulatory, financial, and clinical affairs, as well as data management, quality assurance, and analysis.
An independent data safety and monitoring board (DSMB) appointed by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) monitored all aspects of the study including recruitment and retention rate, modifications to the protocol and/or changes to the case report forms, and any concerns regarding patients’ overall well-being and safety. An initial DSMB meeting was held prior to study initiation to resolve outstanding issues. Following study startup, and for the first 2 years of enrollment, the DSMB met with all CC personnel via teleconference every 3 months. The teleconferences were conducted using a standardized protocol for DSMB meetings. As the study progressed, teleconferences were less frequent unless problems arose. The only problem resulting in an ad hoc DSMB meeting was slow patient enrollment. The DSMB members played an active, collaborative role in addressing enrollment issues and successfully advocated for increased and extended funding for the study from the NIH.
Patients were recruited from tertiary pediatric rheumatology centers in the United States beginning in July 2009. Prior to the start of patient recruitment, a face-to-face investigators’ meeting was held with all original study sites in attendance. New sites were added as the study progressed to increase the rate of enrollment. Personnel at new sites underwent identical training to that presented at the investigators’ meeting during an on-site visit by the study principal investigator (DJL).
Outcomes
The primary outcome for this study was disease flare using a variation of the validated criteria defined as 30% worsening in 3 or more of any of the 6 JIA American College of Rheumatology (ACR) core set variables (32), with no more than 1 improving by >30% (33). The 6 core set variables include the physician’s global assessment of disease activity (PhGA)(VAS 0–10, 0 = inactive disease), parent/patient assessment of overall well-being (PaGA) VAS 0–10, 0 = best well-being), functional ability measured by the Childhood Health Assessment Questionnaire (CHAQ), the number of joints with active arthritis (using the ACR definition of “active joint) (32), the number of joints with limitation of motion, and an acute phase reactant (ESR was used in this study). Because enrolled subjects began the second phase in CID so that most of the core set variables were normal or 0, a 30% worsening could represent a less than clinically important change. Thus, in this study, to be considered to have flared on a particular parameter, the patient had to worsen by at least 30% and also demonstrate a minimum change of the following amounts: PhGA or PaGA increase by at least 2 units on a 21 circle 0–10 unit VAS (34), increase in the active joint count or joints with limited motion count by at least 2 joints, an increase by a minimum of +0.125 on the CHAQ (validated minimally clinically important difference) (35), and an increase in the ESR from normal to abnormal. If a patient demonstrated active uveitis they were considered to have “flared”.
CID was defined using the ACR Provisional Criteria (2) for CID in JIA and includes the following: no joints with active arthritis (using the ACR definition of “active joint”)(32), no fever, rash, serositis attributable to JIA, no active uveitis (as per most recent exam by an ophthalmologist), an ESR ≤ the upper limit of the normal range for the assay used at the site (ESR could be elevated and the patient still be considered in CID if the elevation was not attributable by the treating physician to JIA), PhGA ≤ to 0.5 on a 21 circle 0–10 unit VAS, and duration of morning stiffness ≤15 minutes. “Clinical remission on medications” also used the ACR Provisional Criteria and required CID to be met for 6 continuous months.
Inclusion/Exclusion criteria
To be eligible for enrollment into the initial 6-month phase, subjects had to be between 4 and 20 years of age with a diagnosis of polyarticular (both rheumatoid factor positive and negative) or extended oligoarticular JIA (1) and be in a state of CID while receiving etanercept, adalimumab, or infliximab. All Food and Drug Administration (FDA) label exclusions for anti-TNF therapy had to be absent, a history of regular slit-lamp exams as recommended by the American Academy of Pediatrics guidelines (36) had to be present, and the hemoglobin had to be ≥10 g/dL at baseline. Females of childbearing potential and males who had reached puberty had to agree to use adequate contraception or abstinence during the study. An IRB-approved consent (and assent if applicable) had to be signed. Exclusion criteria included diagnosis with other categories of JIA, previous treatment with rituximab, concurrent treatment with any other biologic agent, or corticosteroids >0.2 mg/kg/d or >10 mg/d (whichever is less). Patients with a diagnosis of any other inflammatory, acute or chronic illness that might affect laboratory results or ability to discontinue anti-TNF biologic therapy were excluded.
Allowable treatments
Anti-TNF therapies allowed during the first 6 months of study included etanercept (ETN), infliximab (IFX), and adalimumab (ADA). The choice of which anti-TNF was used was at the discretion of the examining physician. Dosing and method of administration followed recommended guidelines. Patients were permitted to receive stable background therapy with NSAIDs, MTX, other DMARDs (except rituximab) and low dose corticosteroids (prednisone dose ≤0.2 mg/kg/d or ≤10 mg/d, whichever was less). All background therapies for JIA were to remain unchanged from the time of enrollment until the final study visit or discontinuation from study.
Data Management, Sample Size and Statistical Methods
Case report forms were sent from participating sites, via fax, to the study coordinating center (CC) where forms underwent real-time quality control procedures as all data required for the determination of “flare” had to be collected, verified and analyzed before the end of the study visit before any treatment changes were made. Additional data requests were issued if necessary. All data were stored, managed, and analyzed using Red Cap, Microsoft Excel, and SAS (Version 9.3TS), respectively.
Sample size estimation accommodated the following possibilities: (a) background MTX therapy may affect frequency of disease flare, (b) not all subjects enrolled in the study will exhibit persistent CID for the initial 6 months (and thus will not be in the part of the withdrawal phase), and (c) some patients will become lost-to-follow-up due to reasons beyond the control of investigators. We anticipated 102 evaluable patients would be necessary to test the hypothesis that 60% of patients would experience disease flare within 8 months of discontinuation of anti-TNF therapy, assuming half of whom would be on MTX. To attain enough evaluable patients, with an anticipated drop-out/non-evaluable rate of 15%, we estimated that 118 patients would need to be enrolled to meet statistical power requirements of 80%. The sample size was calculated to address the specific aims of the grant, one of which included the diagnostic ability of biomarkers. The sample size was calculated using the method described in Obuchowski and McClish (37).
Descriptive statistics were used to summarize medications, demographic and disease characteristics at baseline and 6 months. Kaplan-Meier survival curves were generated to estimate rate of flare over time following anti-TNF withdrawal for the study population and stratified by covariates, including type of anti-TNF agent used (ADA, ENB, INF), JIA category (extended oligoarticular, RF+ polyarticular, RF− polyarticular) and background MTX use (yes/no). Likelihood ratios and Chi square statistics were used to compare the survivorship functions among groups. Multivariable logistic analysis was used to model risk of flare (yes/no). Cox regression analyses were employed to assess predictors of time-to-flare. Hazard ratios (HR) were calculated to identify potential independent predictors of flare by 8 months. Stepwise selection procedures were used where variables with P values <0.25 from univariable analysis were entered in the multivariable analysis and only those significant at P<0.05 were retained. Interaction terms that were clinically meaningful were also considered. The interactions we considered were: MTX by TNF, JIA subtype by gender, and JIA subtype by age. The final model only retains the MTX by TNF interaction.
Some variables, such as the age and time duration variables, are known to be collinear. Entering all these variables in the same regression equation would cause collinearity issues. We selectively entered variables and avoided those that were derived from an already entered variable. In addition, some nonderived variables may present strong correlations with other variables in the model. To address this issue, we used the ridge option in the logistic regression procedure (38), which helps stabilize the regression estimate. For some variables in the regression analysis, the model suffers from very small cell size. The Firth option was used in the analysis to address this issue (39).
Biospecimens
Blood was drawn on each subject at baseline and at various follow-up visits for purposes of genetic, gene expression and translational studies. The results of these studies are to be reported elsewhere.
RESULTS
Sixteen tertiary pediatric centers in the US enrolled a total of 137 patients who met the eligibility criteria. Review of the prescreening logs indicated that 137/147 (93.1%) eligible subjects screened were enrolled. Data were locked for this analysis in August 2014. Demographic, disease and pharmacological treatments of the enrolled population are given in Table 1. One subject received an additional non-biologic DMARD (hydroxychloroquine) and one other subject received corticosteroids. Seven of 137 subjects (5.1%) had been treated with another biologic in the 6 months prior to the baseline visit. Four of the 137 (2.9%) had a history of uveitis. Slit lamp exams, as per Academy of Pediatric guidelines (36), were required before and during the study. Uveitis status was part of the CID definition. Three of 137 (2.2%) lost CID status due to a flare of uveitis.
Table 1.
Demographic, Disease and Treatment Characteristics of the Study Population at Enrollment.
| All patients (N = 137) | N (%) | |
|---|---|---|
| Race | ||
| Caucasian | 129 (94) | |
| African American | 7 (5) | |
| Asian | 1 (1) | |
| Gender | ||
| Female | 102 (74) | |
| Male | 35 (26) | |
| ANA (3 unknown) | ||
| Positive | 65 (48) | |
| Negative | 69 (50) | |
| Rheumatoid Factor (RF) (3 unknown) | ||
| Positive | 17 (12) | |
| Negative | 117 (85) | |
| JIA Category | ||
| Extended oligo | 18 (13) | |
| RF positive poly | 17 (12) | |
| RF negative poly | 102 (75) | |
| Anti-TNF treatment | ||
| Etanercept | 106 (77) | |
| Adalimumab | 25 (18) | |
| Infliximab | 6 (5) | |
| Background MTX | ||
| Yes | 57 (42) | |
| No | 80 (58) | |
| Mean (median) | Range | |
| Age (yrs) | 11.3 (11.6) | 3.4 – 20.1 |
| Age at onset (yrs) | 6.2 (5.0) | 0.7– 16.9 |
| Age at diagnosis (yrs) | 6.7 (5.8) | 1.0 – 17.6 |
| Disease duration (yrs) | 5.0 (3.9) | 0.6 – 18.6 |
| Age, first occurrence of CID (yrs) | 10.1 (10.0) | 2.5 – 18.6 |
| Time from onset to first CID (yrs) | 3.9 (2.7) | 0.3 – 14.8 |
| Time from first CID to enrollment into study (yrs) | 1.1 (0.5) | 0.0 – 12.1 |
| MTX dose (mg/kg/wk) | 0.44 (0.40) | 0.1 – 1.02 |
Patient disposition
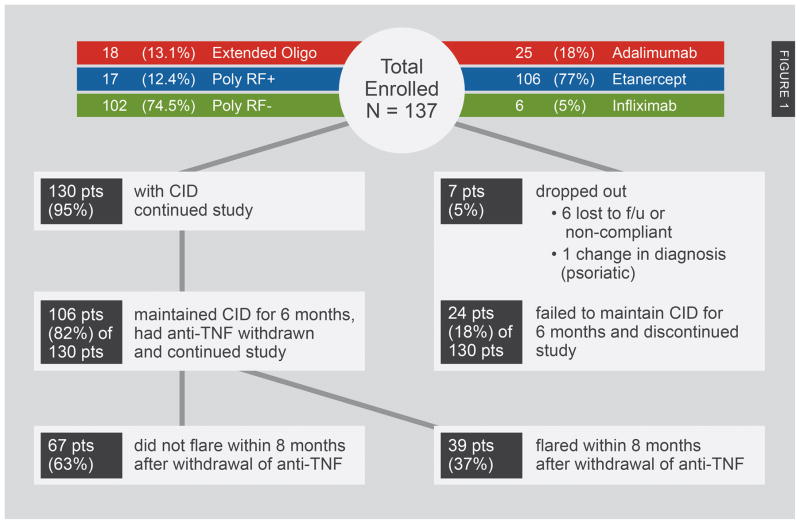
Figure 1 provides a graphical summary of outcome of all enrolled patients. Seven (5%) of the 137 enrolled patients dropped out during the first 6 months (6 due to being lost to follow-up or non-compliance, 1 due to a change in JIA category). Of the remaining 130, 24 (18.5%) did not remain in clinical remission on medications, i.e., maintain CID for the first 6 months, despite stable anti-TNF and background medications. Thus, a total of 106 (77.4% of the total enrolled) were considered ‘evaluable’ and entered the second phase of the study.
Figure 1.
Patient Disposition.
Demographic, disease and therapeutic characteristics of those unable to maintain CID for 6 months while on therapy
Overall, demographic, and disease characteristics of those not evaluable (not able to maintain CID for the first 6 months) were not significantly different than the evaluable (able to maintain CID for the first 6 months) group at study enrollment with the following exceptions (Table 2). Males (p = 0.04) were more likely and those with positive RF were less likely (p = 0.04) to experience clinical remission on medications for the first 6 months. Type of anti-TNF agent was significantly associated (p = 0.04) with those on etanercept the most likely to experience continuous CID for the first 6 months. Perhaps this is due to etanercept being more likely to be the initial anti-TNF agent used. In the extended oligoarticular group, 6% (1/18) were unable to maintain CID for 6 months, as were 18% (19/102) in the Poly RF– and 40% (7/17) in the Poly RF+ categories (Fisher’s exact, p= 0.04). ANA status and MTX use were not associated with the ability to maintain CID prior to treatment withdrawal (Chi-square p values; 0.48, 0.14, respectively).
Table 2.
Demographic and Disease Characteristics of All Enrolled Patients Comparing Patients who failed to maintain Clinical Inactive Disease in the First 6 months (“Not evaluable”) to those maintained CID for the First 6 Months (“Evaluable”).
| All Patients | Not evaluable | Evaluable | P value** | |
|---|---|---|---|---|
| No (%) | No. (%) * | No. (%)* | ||
| Total | 130 (100%) | 24 (18%) | 106 (82%) | -- |
| Gender | 0.03 | |||
| Females | 97 (75%) | 22 (92%) | 75 (71%) | |
| Males | 33 (25%) | 2 (8%) | 31 (29%) | |
| Race | 0.38 | |||
| Caucasian | 122 (94%) | 24 (100%) | 98 (92%) | |
| African American | 7 (5%) | 0 | 7 (7%) | |
| Asian | 1 (1%) | 0 | 1 (1%) | |
| JIA Diagnosis | 0.04 | |||
| Extended oligo | 18 (14%) | 1 (4%) | 17 (16%) | |
| RF positive poly | 15 (11%) | 6 (25%) | 9 (8%) | |
| RF negative poly | 97 (75%) | 17 (71%) | 80 (75%) | |
| ANA status (N = 103) | 0.39 | |||
| Positive | 63 (50%) | 10 (42%) | 53 (51%) | |
| Negative | 64 (50%) | 14 (58%) | 50 (49%) | |
| RF status (N = 104) | 0.02 | |||
| Positive | 15 (12%) | 6 (26%) | 9 (9%) | |
| Negative | 112 (88%) | 17 (74%) | 95 (91%) | |
| Concurrent MTX | 0.16 | |||
| Yes | 54 (42%) | 13 (54%) | 41 (39%) | |
| No | 76 (58%) | 11 (46%) | 65 (61%) | |
| Anti-TNF agent | 0.04 | |||
| etanercept | 101 (78%) | 14 (58%) | 87 (82%) | |
| adalimumab | 22 (17%) | 8 (33%) | 14 (13%) | |
| infliximab | 7 (5%) | 2 (8%) | 5 (5%) |
| Mean (std) | Mean (std) | Mean (std) | P value*** | |
|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | Median (Q1, Q3) | ||
| Age at enrollment (yrs) | 11.16 (4.48) | 11.13 (4.39) | 11.16 (4.52) | 0.98 |
| 11.49 (6.79, 14.77) | 10.8 (7.69, 14.98) | 11.65 (6.68, 14.72) | ||
| Age at disease onset (yrs) | 6.05 (4.57) | 6.21 (4.50) | 6.01 (4.61) | 0.85 |
| 4.48 (2.01, 9.74) | 4.64 (2.25, 9.79) | 4.24 (2.00, 9.70) | ||
| Age at diagnosis (yrs) | 6.58 (4.65) | 6.92 (4.68) | 6.5 (4.67) | 0.69 |
| 5.4 (2.44, 10.21) | 5.78 (2.73, 10.93) | 5.33 (2.34, 10.21) | ||
| Disease duration at enrollment (yrs) | 5.11 (3.81) | 4.75 (3.84) | 5.18 (3.82) | 0.63 |
| 3.89 (2.28, 6.51) | 3.72 (2.06, 5.54) | 3.98 (2.39, 6.56) | ||
| Age, first occurrence of CID (yrs) | 10.02 (4.61) | 9.73 (4.42) | 10.09 (4.67) | 0.73 |
| 9.94 (6.27, 13.97) | 9.09 (6.28, 14.36) | 10.13 (6.27, 13.87) | ||
| Time from disease onset to first occurrence of CID (mos) | 3.94 (3.44) | 3.32 (3.37) | 4.08 (3.45) | 0.34 |
| 2.68 (1.48, 5.36) | 2.29 (1.49, 3.46) | 2.88 (1.47, 5.8) | ||
| Time from first occurrence of CID to baseline (yrs) | 1.15 (1.77) | 1.40 (1.91) | 1.10 (1.74) | 0.45 |
| 0.48 (0.18, 1.49) | 0.50 (0, 2.35) | 0.48 (0.19, 1.45) | ||
| Duration of anti- TNF (yrs) | 2.48 (1.81) | 2.12 (1.60) | 2.55 (1.84) | 0.33 |
| 1.87 (1.30, 3.09) | 1.70 (1.05, 2.74) | 1.9 (1.32, 3.09) | ||
| MTX dosage (mg/kg) | 0.44 (0.21) | 0.39 (0.24) | 0.46 (0.20) | 0.28 |
| 0.42 (0.27, 0.60) | 0.26 (0.24, 0.51) | 0.47 (0.30, 0.60) |
Patients “who failed” includes both those who were unable to maintain clinical inactive disease in first 6 months and those who flared within 8 months after stopping anti-TNF therapy; those who “did not fail” includes only those who maintained clinical inactive disease for first 6 months and did not flare for 8 months after stopping anti-TNF therapy.
P value based upon Chi square comparison of number who flared vs. not flared between strata of individual variables.
P value based upon Student T test comparison of group means between two columns
Note: 3 ANA results unknown; 2 RF results unknown (in order to be included in the analysis the 2 patients with unknown RF results were considered to be RF (−); One Age at first occurrence of CID value unknown.
Disease Flare/No Flare by 8 months
Sixty-seven of 106 patients (63%) completed the 8-month phase without experiencing flare. Although the definition of “flare” did not contain either “systemic manifestations” (related to systemic form of JIA) or uveitis these were tracked prospectively at each study visit. No patient demonstrated “systemic manifestations”, which is not surprising, as systemic JIA patients were excluded. Three patients demonstrated uveitis flare during the study and, if this occurred during the time after stopping anti-TNF therapy, they were declared “flared” and removed from the study. The mean/median time-to-flare was 212/250 days and the standard error of the mean (SEM) was 9.8 days. Table 3 presents demographic, disease and therapeutic characteristics for those who flared and those who did not by 8 months. Older age at disease onset, older age at diagnosis and shorter disease duration were significantly associated with a lower risk for disease flare.
Table 3.
Hazard Ratio Estimates for Univariable Predictor Variables from Cox Regression Analyses of Time to Flare (N = 106 unless otherwise indicated).
| Hazard Ratio (CI) | P Value | |
|---|---|---|
| Gender (Female vs. Male) | 1.24 (0.61, 2.55) | 0.55 |
| JIA Diagnosis (Extended Oligo vs. Poly RF−) | 1.33 (0.611, 2.91) | 0.47 |
| JIA Diagnosis (Poly RF+ vs. Poly RF−) | 0.25 (0.04, 1.86) | 0.17 |
| ANA (Negative vs. Positive) | 0.76(0.39, 1.46) | 0.4 |
| RF (Negative vs. Positive) | 1.96 (0.47, 8.15) | 0.86 |
| Methotrexate (no vs yes) | 1.25 (0.64, 2.44) | 0.51 |
| Anti-TNF (infliximab vs. adalimumab) | 0.39 (0.09, 1.78) | 0.23 |
| Anti-TNF (etanercept vs. adalimumab) | 0.52 (0.16, 1.14) | 0.29 |
| Age at enrollment (yrs) | 1.00 (0.93, 1.08) | 0.97 |
| Age at onset (yrs) (n =105) | 0.92 (0.85, 0.99) | 0.03 |
| Age at diagnosis (yrs) | 0.91 (0.84, 0.99) | 0.02 |
| Disease duration at enrollment (mos) (n = 105) | 1.12 (1.04, 1.21) | <0.01 |
| Age, first occur of CID (yrs) (n = 105) | 0.98 (0.91, 1.05) | 0.48 |
| Time from disease onset to first CID (mos)(n=105) | 1.10 (1.01, 1.20) | 0.03 |
| CID duration, first occurrence to enrollment (mos) | 1.16 (1.01, 1.33) | 0.04 |
Table 3 shows hazard ratios using univariable Cox regression time-to-event analyses and associated significance levels. Five variables were found to be significantly associated with likelihood of flare by 8 months. Later age of onset and diagnosis, shorter disease duration at enrollment in the study, and shorter time from disease onset until first occurrence of CID were significant predictors of decreased risk for flare. Longer duration of CID prior to anti-TNF agent discontinuation was a significant predictor of increased risk of flare. Thus, some variables may help to predict risk of flare based on Cox regression analysis. Supplemental Table 1 compares those who either failed to maintain CID in the first 6 months or flared within the 8 months off anti-TNF therapy to those who were able to both maintain CID in the first 6 months and did not flare within 8 months off anti-TNF therapy. No variables were statistically different in comparing these 2 groups.
Table 4 provides the number of patients who flared and rate of flare at various study visits for each stratum of independent variables. Because of logistical difficulties in returning children to clinic for study visits on an exact date, end-of-study visit occurred later than 8 months but ≤10-month follow-up period in 39 patients. Using life-table analyses, no significant differences were found in flare rates between any strata of any variable. However, stepwise logistic regression identified older age at diagnosis (OR=1.14., 95%CI 1.02–1.26) and use of MTX in those on adalimumab (OR 34.12, 95%CI 1.40–829.8)) as the only significant predictors of decreased risk of flare over the 8 months following discontinuation of TNF. The area under the receiver operating characteristic (ROC) curve was 0.696, indicating fair accuracy in predicting flare (37). The stepwise Cox regression found the same set of predictors: HR = 1.10 (1.01, 1.20) for older age HR =11.6 (1.20, 112.78) for use of MTX among those patients treated with adalimumab (note the large confidence interval for this last HR due to few patients in this group, this likely represents Type 2 error).
Table 4. Life Table Analyses of Time to Flare after withdrawal of anti-TNF.
Analysis was divided into two intervals (0–4 mos and 4–10 mos) to accommodate that some patients were seen up to 10 months after stopping anti-TNF therapy.
| (0–4) month Flare/Effect Sample Size (Rate of Flare±SE) |
(4–10) month Flare/Effect Sample Size (Rate of Flare±SE) |
P value |
|
|---|---|---|---|
| All | 25/106 (23.5±0.04) | 14/50.5 (27.7 ±0.06) | |
| Gender | 0.55 | ||
| Females | 20/75 (26.7±0.05) | 9/34.5 (26.1 ± 0.07) | |
| Males | 5/31 (16.1±0.07) | 5/16 (31.2 ± 0.12) | |
| Race | 0.30 | ||
| Caucasian | 24/98 (24.5±0.04) | 14/47 (29.8 ± 0.07) | |
| African American | 1/7 (14.3±0.13) | 0/3 (0 ±0) | |
| Asian | 0/1 (0±0) | 0/0.5 (0 ±0) | |
| JIA Diagnosis | 0.12 | ||
| Extended oligo | 6/17 (35.3±0.12) | 2/7 (28.6 ± 0.17) | |
| RF positive poly | 1/9 (11.1±0.10) | 0/5.5 (0 ±0) | |
| RF negative poly | 18/80 (22.5±0.05) | 12/38 (31.6 ± 0.08) | |
| ANA status (N = 103) | 0.37 | ||
| Positive | 12/53 (22.6±0.06) | 10/26 (38.5 ± 0.10) | |
| Negative | 12/50 (24±0.06) | 3/23 (13 ±0.07) | |
| RF status (N = 104) | 0.26 | ||
| Positive | 1/9 (11.1±0.10) | 1/5.5 (18.2 ± 0.16) | |
| Negative | 24/95 (25.3±0.04) | 12/43.5 (27.6 ± 0.07) | |
| Concurrent MTX | 0.47 | ||
| Yes | 10/41 (24.4±0.07) | 3/17.5 (17.1 ± 0.09) | |
| No | 15/65 (23.1±0.05) | 11/33 (33.3 ± 0.08) | |
| Anti-TNF agent | 0.36 | ||
| etanercept | 21/87 (24.1±0.05) | 11/41 (26.8 ± 0.07) | |
| adalimumab | 3/14 (21.4±0.11) | 1/6.5 (15.4 ± 0.14) | |
| infliximab | 1/5 (20.0±0.18) | 2/3 (66.7 ± 0.27) | |
| Age at enrollment (yrs): | 0.68 | ||
| ≤ median | 13/44 (29.6±0.07) | 4/18 (22.2 ± 0.10) | |
| > median | 12/62 (19.4±0.05) | 10/32.5 (30.8 ± 0.08) | |
| Age at onset (yrs) (N=105): | 0.03 | ||
| ≤ median | 17/54 (31.5±0.06) | 8/23.5 (34.0 ± 0.10) | |
| > median | 8/51 (15.7±0.05) | 6/26.5 (22.6 ± 0.08) | |
| Age at diagnosis (yrs): | 0.02 | ||
| ≤ median | 17/50 (34.0±0.07) | 7/21 (33.3 ± 0.10) | |
| > median | 8/56 (14.3±0.05) | 7/29.5 (23.7 ± 0.08) | |
| Disease duration at enrollment (yrs, N = 105) | 0.20 | ||
| ≤ median | 0/2 (−) | 0/1 (−) | |
| > median | 25/103 (24.3±0.04) | 14/49 (28.6 ± 0.06) | |
| Age, first occurrence of ID** (yrs, N = 105) | 0.14 | ||
| ≤ median | 6/21 (28.6±0.10) | 5/10 (50.0 ± 0.16) | |
| > median | 19/84 (22.6±0.05) | 9/40 (22.5 ± 0.07) | |
| Time from disease onset to first occurrence of ID (mos, N = 105) | 0.06 | ||
| ≤ median | 2/16 (12.5±0.08) | 1/8 (12.5 ± 0.12) | |
| > median | 23/89 (25.8±0.05) | 13/42 (31.0 ± 0.07) | |
| Time from first occurrence of ID to baseline (yrs, N=105) | 0.04 | ||
| ≤ median | 10/53 (18.8±0.05) | 5/26 (19.2 ± 0.08) | |
| > median | 15/52 (28.9±0.06) | 9/24 (37.5± 0.10) |
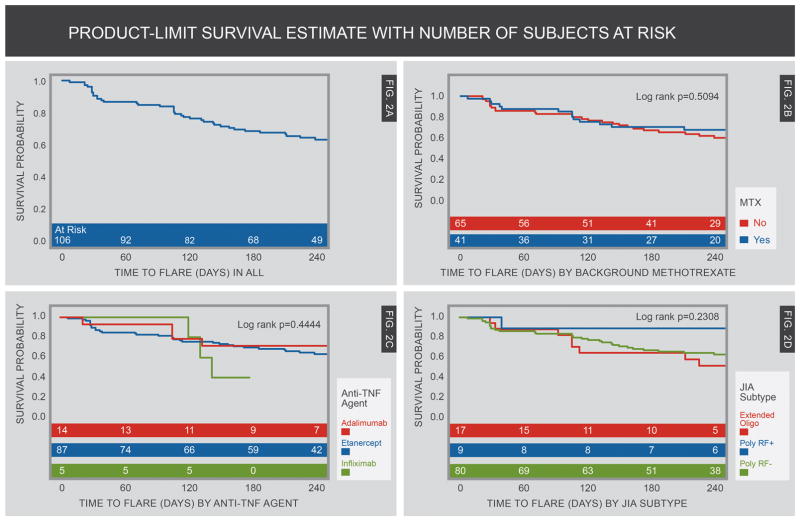
Figure 2A provides a Kaplan-Meier (K-M) curve showing the probability of flare among all evaluable patients throughout the 8-month phase. As mentioned above, because of logistical difficulties, one end-of-study visit occurred later than 8 months (10 months). Mean time to flare was 7.01 months (SEM 0.32) with a median of 8.26 (95%CI 7.80– 8.66) months. Figures 2B–2D present survival curves using 3 independent variables that had been hypothesized to predict flare rate and time-to-flare (± MTX, anti-TNF agent given prior to discontinuation of therapy, and subtypes of JIA). None of these variables proved to be statistically significant predictors of either likelihood of flare or time-to-flare.
Figure 2.
Kaplan-Meier Curves by 8 months of prospective observation in patients in JIA patients in clinical inactive disease (CID) who withdrew anti-TNF therapy. Patient visits were censored at 8 months follow-up even though some patients were followed longer than 8 months. Figure A shows the flare rate in the overall population who withdrew therapy. Figure B shows comparison of flare rates in those with and without background methotrexate therapy. Figure C shows comparison of flare rates of several types of anti-TNF therapy. Figure D shows flare rates of various JIA categories.
DISCUSSION
In this study, several new and clinically relevant observations were made about stopping anti-TNF therapy in patients with PF-JIA in CID. CID was an unstable state and 18.5% of the patients were unable to maintain CID for 6 continuous months of observation, even while continuing to receive the anti-TNF agent and stable doses of all background medications. This was especially true for the RF+ polyarthritis group. Unlike the studies of stopping MTX (in the absence of anti-TNF therapy), the duration of CID at the time of stopping the anti-TNF therapy was significantly related to likelihood of flare. In studies examining the effect of discontinuing MTX, CID durations of 6, 12 and 18 months all resulted in a similar flare rate of approximately 50% (40, 41). However, in this study there was an increased risk of disease flare for longer duration of CID. These data certainly do not support the existence of a protective effect of longer duration of CID before considering stopping anti-TNF therapy. In fact, the data suggest that CID, even in those who did demonstrate CID consistently for the first 6 months of the study, continued to be an unstable clinical state and prolonged observation of CID resulted in a significantly greater risk for flare.
Our results are in line with a growing literature suggesting that there is a “window of opportunity” early in the treatment of JIA that supports early introduction of aggressive therapy and rapid achievement of CID will result in better long-term control of JIA and improved outcomes (4, 42–44). In a secondary analysis of the TREAT JIA trial data (4), the duration of disease prior to initiation of aggressive therapy was significantly inversely associated with ability to realize CID on aggressive therapy. For each month less of disease duration prior to initiating aggressive therapy, the likelihood of reaching CID was increased by 1.7-fold. In this study, longer duration of disease prior to first achieving CID was significantly associated with a decreased ability to maintain CID after stopping the anti-TNF agent.
The major strength of this study is that the study subjects were followed prospectively by protocol and all assessments and determinations of disease status (CID, flare/no flare) were done with real time assessment centrally at the study coordinating center. In addition, 137 of the 147 eligible subjects screened (93.1%) were enrolled. However, this study also has limitations. Perhaps the largest short coming is that we do not capture the difficulty in regaining CID among those who flared. This shortcoming will be addressed in a paper describing the longer follow-up of this cohort. The study population was small and not population-based. The patients were all recruited from academic pediatric rheumatology centers, which may have introduced a selection bias for patients with more severe disease. If patients with milder disease were enrolled then, perhaps, the flare rate would have been lower. The proportion flaring was smaller than predicted in the preliminary power analysis further limiting power. The protocol did not specify ‘study visit windows’ outside of which data would be discarded. While greater than 90% of visits occurred within 2 weeks of the expected study date, some were outside this span. These data were used in the analysis. Estimation of the exact time-to-flare is challenging. Flares could only be determined by the examining physician and, therefore, could only occur at study visits. Thus, it is possible that some patients flared before their study visit. However, unscheduled study visits were performed and actual time of study visit was used in the data analysis for patients that were more symptomatic.
Despite the tremendous potential in JIA for the use of biomarker panels to determine exactly who is the best candidate for a therapy, and which therapy is more likely to induce remission, biomarker studies have yet to produce a validated assay (31). However, studies of serum levels of S100 A8/A9 have demonstrated good to excellent ability (sensitivity 100%, specificity 70%) to predict flares in JIA patients stopping methotrexate therapy (41). The predictive ability of S100 A8/A9 and S100 A12 has been studied in samples collected in this study and submitted in a companion manuscript to this journal. Increasingly, ultrasound and MRI are being used to assess JIA patients in CID. However, the few studies of the ability of ultrasound to predict flare in JIA patients in CID have provided conflicting results (45, 46). The predictive ability of MRI findings in JIA patients in CID has not been assessed.
A central issue in study design was which of several published definitions of flare to use. We considered but eventually choose to not use the definition used in the Foell manuscript (41) in which worsening in any one of the selected parameters was considered a disease “flare”. A multifactored definition of flare was selected to provide enough rigor in the degree of worsening to avoid the visit to visit minor variation in disease activity and to minimize the effect of the “noise” in the individual assessment measures which is especially influential in low disease activity states. We wanted the ‘flare” definition to represent enough worsening that it would likely result in a need for a change in systemic JIA therapy and not just a minor adjustment or injection of 1–2 joints. The definition chosen was also one that had been formally validated in a methodologic paper (33) and field tested in clinical trials (5, 9, 10, 16)).
In conclusion, we offer the following observations that may be informative for the clinical care of children with JIA. In this prospective study of children with PF-JIA who achieve CID while on anti-TNF therapy recruited from the population of patients routinely seen in pediatric rheumatology centers, CID is an unstable condition in about one-fifth of patients. Following discontinuation of the anti-TNF agent in those who did achieve clinical remission on medications, the mean time-to-flare was about 7.0 months, and by 8 months over a third will have flared. The data do not support the effectiveness in continuing background MTX in those who do stop anti-TNF therapy as a method to decrease the risk of flare. Longer duration of CID prior to stopping anti-TNF therapy was associated with an increased risk for flare and thus does not support the perception that longer maintenance of anti-TNF therapy as supportive of decreased risk for flare in those who have already achieved at least 6 months of CID.
Supplementary Material
Demographic and Disease Characteristics Comparing “Patients who failed” (combines those who failed to maintain CID in first 6 mos on anti-TNF and those who flared within 8 months after anti-TNF therapy withdrawn) to those “Patients who did not fail” (maintained CID for first 6 months on anti-TNF and did not flare for 8 months off anti-TNF therapy).
Acknowledgments
Grants, Financial Support: This work was sponsored by the NIH (NIAMS, Grant No. 2P60AR047784-06A2). The grant paid for all aspects of the study and supported the work of the Data Safety and Monitoring Board.
The authors wish to thank the patients and their parents/guardians who consented and participated in this study. The assistance of the health care professionals who performed duties related to this study and who are not named authors is greatly appreciated. The members of the DSMB served as strong advocates for this study. Their time spent in frequent and detailed review of the data and logistics and advice provided to us throughout the study is greatly appreciated.
Footnotes
ClinicalTrials.gov Identifier: NCT 00792233
AUTHOR CONTRIBUTIONS: All authors played a role in the development and execution of the protocol, reviewed and approved implementation of recommendations from the DSMB, and assisted in writing and approval of the finalized manuscript prior to its submission.
ROLE OF THE SPONSOR: This work was sponsored by the NIH (NIAMS, Grant No. 2P60AR047784-06A2). The grant paid for all aspects of the study and supported the work of the Data Safety and Monitoring Board.
References
- 1.Petty RE, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 2.Wallace CA, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63(7):929–36. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]
- 3.Wallace CA, et al. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(11):3554–62. doi: 10.1002/art.21389. [DOI] [PubMed] [Google Scholar]
- 4.Wallace CA, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol. 2014;41(6):1163–70. doi: 10.3899/jrheum.131503. [DOI] [PubMed] [Google Scholar]
- 5.Ruperto N, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–91. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 6.Ruperto N, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(6):1792–802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- 7.Visvanathan S, et al. The effect of infliximab plus methotrexate on the modulation of inflammatory disease markers in juvenile idiopathic arthritis: analyses from a randomized, placebo-controlled trial. Pediatr Rheumatol Online J. 2010;8:24. doi: 10.1186/1546-0096-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruperto N, Martini A. Emerging drugs to treat juvenile idiopathic arthritis. Expert Opin Emerg Drugs. 2011;16(3):493–505. doi: 10.1517/14728214.2011.581662. [DOI] [PubMed] [Google Scholar]
- 9.De Benedetti F, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385–95. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 10.Ruperto N, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 11.Lovell DJ, et al. Advances from clinical trials in juvenile idiopathic arthritis. Nat Rev Rheumatol. 2013;9(9):557–63. doi: 10.1038/nrrheum.2013.105. [DOI] [PubMed] [Google Scholar]
- 12.Solari N, et al. Factors associated with achievement of inactive disease in children with juvenile idiopathic arthritis treated with etanercept. J Rheumatol. 2013;40(2):192–200. doi: 10.3899/jrheum.120842. [DOI] [PubMed] [Google Scholar]
- 13.Horneff G, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;73(6):1114–22. doi: 10.1136/annrheumdis-2012-203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovell DJ, et al. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol. 2015;67(10):2759–70. doi: 10.1002/art.39234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace CA, et al. Extension study of participants from the trial of early aggressive therapy in juvenile idiopathic arthritis. J Rheumatol. 2014;41(12):2459–65. doi: 10.3899/jrheum.140347. [DOI] [PubMed] [Google Scholar]
- 16.Lovell DJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 17.Wargula JC, Lovell DJ. Use of etanercept in children. Bull Rheum Dis. 2000;49(12):1–4. [PubMed] [Google Scholar]
- 18.Lovell DJ, et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48(1):218–26. doi: 10.1002/art.10710. [DOI] [PubMed] [Google Scholar]
- 19.Lovell DJ, et al. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54(6):1987–94. doi: 10.1002/art.21885. [DOI] [PubMed] [Google Scholar]
- 20.Ruperto N, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096–106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 21.Lovell DJ, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58(5):1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 22.Lovell DJ, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810–20. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 23.Giannini EH, et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(9):2794–804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- 24.Giannini EH, et al. Effects of long-term etanercept treatment on growth in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(11):3259–64. doi: 10.1002/art.27682. [DOI] [PubMed] [Google Scholar]
- 25.Pratsidou-Gertsi P, et al. A follow-up study of patients with juvenile idiopathic arthritis who discontinued etanercept due to disease remission. Clin Exp Rheumatol. 2010;28(6):919–22. [PubMed] [Google Scholar]
- 26.Iglesias E, et al. Non-systemic juvenile idiopathic arthritis outcome after reaching clinical remission with anti-TNF-alpha therapy: a clinical practice observational study of patients who discontinued treatment. Rheumatol Int. 2014;34(8):1053–7. doi: 10.1007/s00296-013-2884-z. [DOI] [PubMed] [Google Scholar]
- 27.Chang CY, Meyer RM, Reiff AO. Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis Care Res (Hoboken) 2015;67(5):658–66. doi: 10.1002/acr.22477. [DOI] [PubMed] [Google Scholar]
- 28.Romano MPI, Gattinara M, Ardoino I, Donati C, Boracchi P, et al. Drug survival and reasons for discontinuation of the first course of biological therapy in 301 juvenile idiopathic arthritis patients. Reumatismo. 2013;65(6):278–85. doi: 10.4081/reumatismo.2013.682. [DOI] [PubMed] [Google Scholar]
- 29.Tynjala P, et al. Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis. 2009;68(4):552–7. doi: 10.1136/ard.2007.087130. [DOI] [PubMed] [Google Scholar]
- 30.Anink J, et al. MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis. Arthritis Res Ther. 2015;17:200. doi: 10.1186/s13075-015-0723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duurland CL, Wedderburn LR. Current developments in the use of biomarkers for juvenile idiopathic arthritis. Curr Rheumatol Rep. 2014;16(3):406. doi: 10.1007/s11926-013-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannini EHLD, Felson DT, Goldsmith CH. Preliminary core set of outcome variables for use in JRA clinical trials. Arthritis and Rheumatism. 1994;37(suppl) [Google Scholar]
- 33.Brunner HI, et al. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol. 2002;29(5):1058–64. [PubMed] [Google Scholar]
- 34.Filocamo G, et al. Evaluation of 21-numbered circle and 10-centimeter horizontal line visual analog scales for physician and parent subjective ratings in juvenile idiopathic arthritis. J Rheumatol. 2010;37(7):1534–41. doi: 10.3899/jrheum.091474. [DOI] [PubMed] [Google Scholar]
- 35.Brunner HI, et al. Minimal clinically important differences of the childhood health assessment questionnaire. J Rheumatol. 2005;32(1):150–61. [PubMed] [Google Scholar]
- 36.American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology. Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 1993;92(2):295–6. [PubMed] [Google Scholar]
- 37.Obuchowski NA, McClish DK. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med. 1997;16(13):1529–42. doi: 10.1002/(sici)1097-0258(19970715)16:13<1529::aid-sim565>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Cresse SHJ. Ridge Estimators in Logistic Regression. Appl Statist. 1982;41(1):191–201. [Google Scholar]
- 39.Firth D. Bias Reduction of Maximum Likelihood Estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 40.Gottlieb BS, et al. Discontinuation of methotrexate treatment in juvenile rheumatoid arthritis. Pediatrics. 1997;100(6):994–7. doi: 10.1542/peds.100.6.994. [DOI] [PubMed] [Google Scholar]
- 41.Foell D, et al. Methotrexate treatment in juvenile idiopathic arthritis: when is the right time to stop? Ann Rheum Dis. 2004;63(2):206–8. doi: 10.1136/ard.2003.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol. 2015;11(5):290–300. doi: 10.1038/nrrheum.2014.212. [DOI] [PubMed] [Google Scholar]
- 43.Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol Online J. 2014;12:13. doi: 10.1186/1546-0096-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Wallace C. Judicious use of biologicals in juvenile idiopathic arthritis. Curr Rheumatol Rep. 2014;16(11):454. doi: 10.1007/s11926-014-0454-3. [DOI] [PubMed] [Google Scholar]
- 45.Magni-Manzoni S, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72(2):223–8. doi: 10.1136/annrheumdis-2011-201264. [DOI] [PubMed] [Google Scholar]
- 46.Miotto E, SV, Mitraud SAV, Furtado RNV, Natour J, Len CA. Patients with juvenile idiopathic arthritis in clinical remission with postitive power Doppler signal in joint ultrasonography have an increased rate of clinical flare: a prospective study. Ped Rheumtol Online J. 2017;15(1):80. doi: 10.1186/s12969-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and Disease Characteristics Comparing “Patients who failed” (combines those who failed to maintain CID in first 6 mos on anti-TNF and those who flared within 8 months after anti-TNF therapy withdrawn) to those “Patients who did not fail” (maintained CID for first 6 months on anti-TNF and did not flare for 8 months off anti-TNF therapy).