Abstract
Itching is a common clinical symptom of skin disease that significantly affects a patient's quality of life. Transient receptor potential vanilloid 1 (TRPV1) receptors of keratinocytes and peripheral nerve fibers in skin are involved in the regulation of itching as well as pain. In this study, we investigated whether curcumin, which acts on TRPV1 receptors, affects histamine-induced itching in mice, using behavioral tests and electrophysiological approaches. We found that histamine-induced itching was blocked by topical application of curcumin in a concentration-dependent manner. In ex-vivo recordings, histamine-induced discharges of peripheral nerves were reduced by the application of curcumin, indicating that curcumin acts directly on peripheral nerves. Additionally, curcumin blocked the histamine-induced inward current via activation of TRPV1 (curcumin IC50=523 nM). However, it did not alter chloroquine-induced itching behavior in mice, which is associated with transient receptor potential ankyrin 1 (TRPA1). Taken together, our results suggest that histamine-induced itching can be blocked by topical application of curcumin through the inhibitory action of curcumin on TRPV1 receptors in peripheral nerves.
Keywords: Curcumin, Histamine, Itching, TRPV1
INTRODUCTION
Curcumin is widely used as a food ingredient and health supplement for the treatment of various diseases [1,2,3]. Over the past decades, intensive research into the biological and pharmacological roles of curcumin has revealed that curcumin has a variety of biological properties. For example, curcumin inhibits protein kinase C and protein tyrosine kinase activity, cyclooxygenase, endonucleases, and platelet aggregation [4,5,6,7] and exhibits therapeutic potential as an anti-inflammatory, antioxidant, anti-cancer, antibiotic, and diabetic treatment [1,3]. Additionally, recent studies have reported that the interactions between curcumin and transient receptor potential vanilloid 1 (TRPV1) inhibit DNBS-induced colitis [8] and that curcumin has an analgesic effect by directly blocking TRPV1 in trigeminal ganglia (TG) and dorsal root ganglia (DRG) of mice [9,10].
In general, itching can be classified as histamine-dependent and histamine-independent itching, and histamine-dependent itching has a stronger relationship to TRPV1 than histamine-independent itching [11,12,13]. It is believed that histamine-dependent itching is associated with approximately 10% of total C-nerve fibers in human skin, which play a specific role in itching [14]. Histamine causes the surrounding skin to develop abnormal sensitivity toward other stimuli, thus the sensation of touch, pressure, or temperature can be perceived as itching. Diffuse, persistent itching caused by this process is termed “alloknesis” [15]. Histamine in the skin is mainly supplied by degranulation of mast cells, which are locally and functionally related to afferent C-nerve endings. Recently, it was reported that TRPV1 is expressed in most itch-specific C-nerve fibers expressing histamine receptors, and TRPV1 appears to play an important role in itching [16]. Additionally, histamine-induced signal transduction directly leads to the activation of TRPV1 by lipid metabolism following lipoxygenase activation, and histamine-induced itching is also significantly reduced in TRPV1 KO mice [17,18]. According to Shim et al., histamine released from mast cells binds to the H1 receptor located at the end of C-nerve fibers and produces arachidonic acid by activation of the phospholipase A2-lipoxygenase pathway [17]. Activation of TRPV1 via the increased intracellular concentration of arachidonic acid metabolites then induces itching. To examine the effect of curcumin on TRPV1-mediated histamine itching, we conducted behavioral experiments using model animals and electrophysiological studies of DRG neurons and skin-nerve preserved tissue. Our findings suggest that curcumin extract may inhibit histamine-induced itching through TRPV1.
METHODS
Animals
All experimental methods were approved by the Experimental Animal Ethics Committee of Hanyang University. Male C57BL/6 wild-type male mice (OrientBio, Sungnam, Korea) and TRPV1 knockout (KO) mice (The Jackson Laboratories, ME, USA) weighing 18–22 g were used in this study. All experimental animals were housed in a conventional facility with a 12:12 hour light cycle (lights on 8.00 am) and ad libitum access to water and chow. Temperature and humidity were 22℃ and 60%, respectively. All animals were allowed to adapt to the new environment for one week before the experiment.
Curcumin solvents
To investigate the optimal solvent for curcumin, which is a polar molecule, five polar solvents were evaluated: chloroform (CHCl3), ethanol, isopropanol, 1-butanol, and polyethylene glycerol 400 (PEG400). These representative polar solvents are harmless non-toxic at low concentrations. The solubility of curcumin was 10 mg/ml for ethanol and >11 mg/ml for DMSO. Although the solubility of curcumin in DMSO was high, DMSO itself has a variety of biological actions. Therefore, we used a mixture of 70% PEG400 and 30% ethanol as the solvent for curcumin. To determine the best conditions to dissolve curcumin, 1) 0.007 g of curcumin was dissolved in 1.0 g of ethanol, and then PEG400 was added or 2) 0.007 g of curcumin was dissolved in PEG400 and then 1.0 g of ethanol was added.
Itching test
Histamine (100 μg/50 μl, Sigma-Aldrich, MO, USA) was injected into wild-type (WT) mice and TRPV1 KO mice. One day before the experiment, nape hair was shaved to create an area with a diameter of 1.5 cm for intradermal injection. An experimental animal was placed in a small plastic cylinder (20 cm in diameter, 25 cm in height), and pads were placed in the cylinder to absorb excrement. Test animals were placed in a cylinder for 15 min to allow them to acclimate, and then histamine was injected intradermally into the nape of the neck of the experimental animal. To measure the number of direct scratching events mediated by the rear hind legs, experimental animals were monitored 15 min before intradermal injection and 30 min after intradermal injection. The definition of direct scratching was the animal lifting its hind paw to the shaven navel and scratching the affected area and then returning the paw to its original position. To assess which receptors in particular were affected by curcumin, histamine, compound 48/80 (100 μg/50 μl, Sigma-Aldrich), or chloroquine (100 μg/50 μl, Sigma-Aldrich) were injected in the same manner as described above in WT mice and TRPV1 KO mice and behavioral responses were observed.
Rota-rod performance test
To determine if curcumin affects the locomotor activity of mice, mice were placed on the horizontal bar rotating at a speed of 4 rpm using a rota-rod apparatus (ROTA-ROD, B.S Technolab INC, Seoul, Korea). Twenty-four hours before the actual rota-rod test, all mice were tested and those that were able to remain on the rod for at least 120 s were included in the study. Performance time on the bar (in sec) and number of falls were measured over a period of 2 min. Scores were then compared and analyzed 5 min before and 30 min after treatment with vehicle or curcumin. The test was repeated 3 times and the mean value for each animal was recorded.
Ex-vivo recordings
Mice were killed by CO2 inhalation, and the hairy skin of the hind paw innervated by the saphenous nerve was dissected after attached connective tissue, muscle, and/or tendon was removed. Organ bath consisted of two chambers (perfusion chamber and recording chamber) separated by an acrylic-based wall. The perfusion chamber was continuously superfused with SIF (in mM; 3.5 KCl, 107.8 NaCl, 0.69 MgSO4•7H2O, 1.53 CaCl2•2H2O, 1.67 NaH2PO4•2H2O, 26.2 NaHCO3, 9.64 C6H11NaO7, 7.6 sucrose, 5.55 glucose) that was saturated with a mixture of 95% O2 and 5% CO2 and maintained at 31±1℃. After dissection, the preparation was placed with the epidermal side down. Nerves attached to the skin were drawn to the recording chamber filled with paraffin oil. The nerve was placed on a fixed mirror, the sheath was removed, and nerve filaments were repeatedly teased to allow single fiber recordings to be made using two gold electrodes, one for recording and the other for reference. Spikes from single C-fibers were recorded extracellularly with a differential amplifier (DP311; Warner instruments, CT, USA), transferred to a computer by a data acquisition system (DAP5200a; Microstar Laboratories, Inc. WA, USA), and analyzed off-line using the window discrimination feature of the software (Dapsys 8; Bethel University, http://dapsys.net).
Primary DRG neuron culture
DRGs from all spinal levels of 6–8 week old mice were removed aseptically and incubated with collagenase (5 mg/ml, Roche, Swiss)/dispase-II (1 mg/ml, Roche) at 37℃ for 40 min, then digested with 2.5% trypsin (Invitrogen, MA, USA) for 7 min at 37℃, followed by 0.25% trypsin inhibitor (Sigma-Aldrich). Cells were mechanically dissociated with a flame-polished Pasteur pipette in the presence of 0.05% DNase I (Sigma-Aldrich). DRG cells were plated on glass cover slips and then plated onto glass coverslips previously coated with a solution of poly-D-Lysine. DRG cells were first plated on bare glass cover slips and then transferred to poly-D-Lysine-coated coverslips. DRG cells were incubated in a 5% CO2 atmosphere at 37℃ and were maintained for 24 h before use.
Electrophysiology
Whole-cell current-clamp recordings to measure currents were performed at room temperature using the HEKA EPC10 amplifier (HEKA Elektronik GmbH, Lambrecht/Pfalz, Germany). Patch pipettes were pulled from borosilicate capillaries (Chase Scientific Glass Inc., CA, USA). When filled with the pipette solution, the resistance of the pipettes was 4–6 MΩ. The recording chamber was continuously perfused (2 ml/min). Series resistance was compensated for (>80%), and leak subtraction was performed. Pulse v8.30 software (HEKA) was used during experiments and for analysis. The internal pipette solution was composed of (in mM): 140 KCl, 1 CaCl2, 2 MgCl2, 10 EGTA, 10 D-glucose, and 10 HEPES adjusted to pH 7.3 with NaOH, 295–300 mOsm. Extracellular solution contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 D-glucose, adjusted to pH 7.3 with NaOH, 300–310 mOsm. All drugs in this experiment were dissolved in extracellular solution. Voltage-clamp experiments were performed at a holding potential of −60 mV.
Data analysis
All data were analyzed with the SPSS Statistic 24 (IBM, New York, USA) package. Differences between two data sets were evaluated by paired or unpaired t-tests. The significance of differences among multiple groups were evaluated by one-way ANOVA followed by Tukey's post-hoc test. Differences were considered to be significant when the p value was less than 0.05 (p<0.05). All data are expressed as means±SEM (the standard error of the mean). Dose-response analysis was performed with Origin 6.1 software (MicroCal, MA, USA). Normalized histamine-induced scratch responses and histamine-induced current were plotted against the concentration of curcumin and fitted using a standard logistic equation, E=Emax[Cn/(Cn+IC50n)], where E is the measured effect of the drug, Emax is the maximum effect current, C is the drug concentration, IC50 is the drug concentration that yields 50% of the maximum effect, and n is the Hill coefficient of sigmoidicity.
RESULTS
Curcumin and capsaicin cream formulation
Solubility, transparency, color, and degree of precipitation in curcumin in various potential solvents were evaluated. Ethanol and PEG400 were selected as the most suitable solvents to dissolve curcumin, and a mixture of 1.0 g of ethanol and 1.0 g of PEG400 was finally used for the curcumin cream formulation as dissolution of curcumin was optimal in ethanol and PEG400. Because only 0.007 g of curcumin dissolved in ethanol at room temperature, it was dissolved at 40℃–50℃. When 1.0 g of PEG400 was dissolved, a transparent brown solution was obtained. In the second dissolution test condition, 0.007 g of curcumin dissolved completely in PEG400 (1.0 g) at room temperature and then ethanol (1.0 g) was added, resulting in the complete dissolution of curcumin. The latter method of curcumin dissolution was therefore used to prepare the cream for topical application; 0.001 weight% (wt.%), 0.1 wt.%, 1 wt.%, and 3 wt.% curcumin samples were made in white Vaseline [3]. For the capsaicin cream, 0.075 wt.% capsaicin cream (Darimbiotech. Korea) was diluted to 0.05 wt.% in white Vaseline.
Effect of curcumin on histamine-induced itching
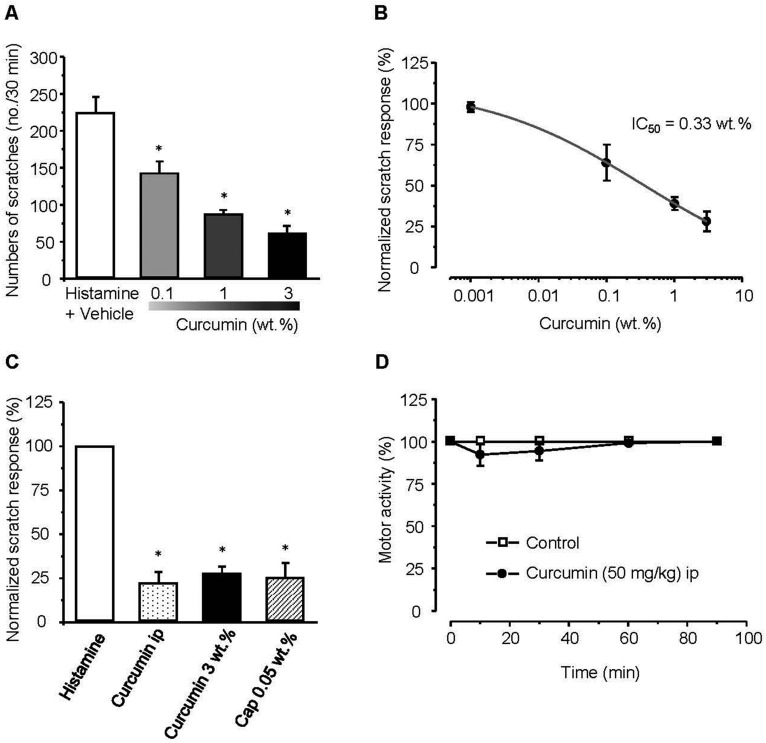
We investigated the anti-itching effect of curcumin on histamine-induced itching behavior in mice. Histamine was injected intradermally into the nape of the neck of C57BL/6 mice at a dose of 100 μg/site, after which scratching behavior was observed for 30 min. Various curcumin concentrations (0.001 wt.%, 0.1 wt.%, 1 wt.%, and 3 wt.%) were evaluated. Histamine-induced itching was not significantly reduced in mice treated with 0.001 wt.% curcumin compared with the vehicle-treated group, while itching decreased significantly in a dose-dependent manner in groups treated with 0.1 wt.%, 1 wt.%, and 3 wt.% curcumin cream (64.5±11.2%, n=6; 39.8±4.2%, n=7; and 27.8±6.3%, n=9, respectively; Fig. 1A). The IC50 of curcumin for inhibiting histamine-induced itching was approximately 0.33 wt.% (Fig. 1B).
Fig. 1. Inhibitory effect of curcumin on histamine-induced itching.
(A, B) After application of topical curcumin cream, there was a statistically significant decrease in the 0.1 wt.%, 1 wt.%, and 3 wt.% curcumin treatment groups and the IC50 of curcumin was 0.33 wt.%. (C) The effect of systemically administered curcumin or locally applied capsaicin on histamine-induced itching. Intraperitoneal injection of curcumin (50 mg/kg) and local treatment with 0.05 wt.% capsaicin cream decreased histamine-induced itching. Each bar graph shows a comparison of the total number of scratches in each group for a period of 30 min after administration of histamine versus the total number of scratches in the control group. (D) Rota-rod with motor function test after intraperitoneal injection of curcumin. The fall latency of curcumin-treated mice was similar to that of control mice. Results are presented as means±SEM and asterisks indicate p<0.05.
We next evaluated if curcumin had any systemic beneficial effects (Fig. 1C). When 50 mg/kg of curcumin was injected intraperitoneally 30 min before the injection of histamine, the total number of scratches was reduced by 22.3±6.3% (n=8). However, there was no significant difference between this group and the group that received local curcumin treatment. In addition, intraperitoneal administration of curcumin did not cause significant changes in the locomotor activity of mice in the rota-rod performance test (Fig. 1D), indicating that curcumin did not impair motor activity. In addition, we did not observed no changes in the mice applied with 0.05 wt.% capsaicin cream and 3 wt.% curcumin cream, the same as with the vehicle group. Overall, these results suggest that the anti-itching effects of curcumin are mediated locally rather than systemically.
TRPV1 is activated by capsaicin, and is eventually blocked by capsaicin after prolonged exposure due to desensitization of nociceptive afferents and depletion of neuropeptides such as substance P; thus, capsaicin cream has been used as an anti-itch agent. We compared the inhibitory effects of capsaicin and curcumin creams on histamine-induced itching (Fig. 1C). When 0.05 wt.% capsaicin cream was topically applied before inducing histamine-induced itching, the total number of scratches observed for 30 min was reduced (25.4±8.3%, n=6). The anti-itching effects of capsaicin and curcumin creams were not significantly different, indicating that curcumin has a similar anti-itch efficacy to that of capsaicin cream.
Inhibitory effect of curcumin on histamine-induced C-fiber activity and histamine-induced current in DRG neurons
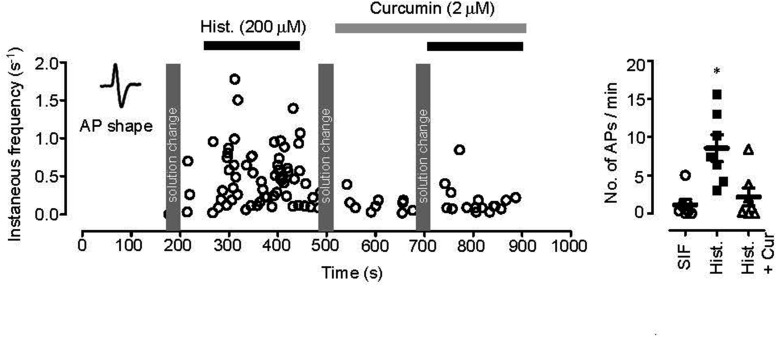
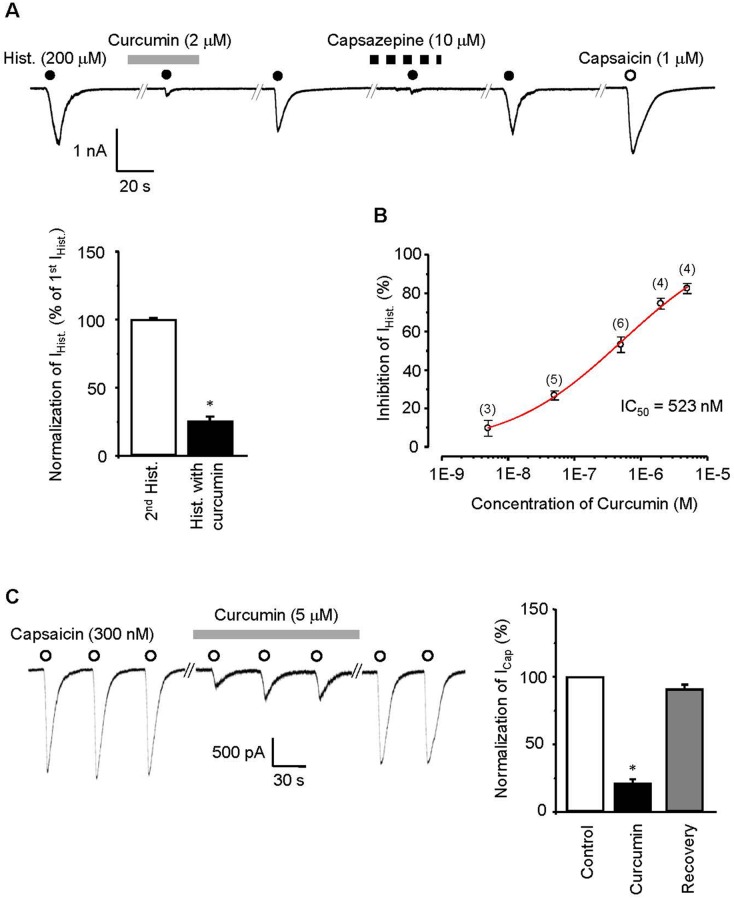
Curcumin can participate in excitatory regulation of peripheral nerve cells and produce anti-inflammatory action in the skin. Therefore, we examined whether curcumin acts on itching-responsible nerves to block histamine-induced itching using ex-vivo recordings of skin-nerve preserved tissue. The discharge response of a single saphenous nerve fiber was evoked by histamine (500 μM, firing rate of 8.54±1.74 action potentials/min; control discharge, firing rate of 1.14±0.67 action potentials/min), and then was inhibited by curcumin (5 μM, firing rate of 2.17±1.16 action potentials/min, p<0.05; Fig. 2). These results indicate that curcumin blocks histamine-induced discharges of peripheral nerves. Next, we tested the effect of curcumin on histamine-induced inward currents in capsaicin-sensitive DRG neurons, because histamine-induced currents are mediated by the activation of TRPV1 [17]. Histamine-induced current (200 μM) was significantly blocked by curcumin (2 μM, 25.4±3.2% of 1st histamine-induced current, p < 0.05; Fig. 3A) in a dose dependent manner with an IC50 value of 523 nM (Fig. 3B). In addition, histamine-induced current was blocked by capsazepine (10 μM, Sigma-Aldrich; Fig. 3A) and capsaicin-induced current under Ca2+ free condition decreased to 21.1±2.9% (n=6) after application of curcumin (5 μM), which recovered to control value (90.8±3.8%, n=6; Fig. 3C). These results indicate that the histamine-induced current mediated by the activation of TRPV1 is reduced by curcumin acting as a TRPV1 blocker.
Fig. 2. The effect of curcumin on histamine-induced firing rates in peripheral nerves.
The effect of curcumin was assessed using ex-vivo recordings. Application of histamine (200 μM) evoked discharges of single saphenous nerve fibers, which were inhibited by curcumin (2 μM). Black line indicates histamine treatment, gray line indicates curcumin treatment. In the right panel, each symbol represents the firing rate (number of action potentials/min), while horizontal lines indicate means±SEM. Asterisks indicates a significant difference at p<0.05 (paired t-test).
Fig. 3. Role of curcumin as a blocker for TRPV1.
(A) Under voltage clamp conditions at −60 mV, the inward current induced by histamine (200 μM) in DRG neurons was blocked by curcumin (2 μM). Black circle indicates histamine treatment, open circle indicates capsaicin treatment, gray line indicates curcumin treatment, and black dashed line indicates capsazepine (10 μM) treatment. The first histamine current was compared to the second histamine current or the histamine current after curcumin treatment. (B) The IC50 of curcumin was 523 nM based on the dose-response curve. (C) Curcumin pre-treatment (5 μM, 200 s) inhibited capsaicin-induced currents, which recovered to control value after washout of curcumin. Asterisks indicates a significant difference at p<0.05 (paired t-test).
Effect of curcumin on TRPA1-mediated itching behavior
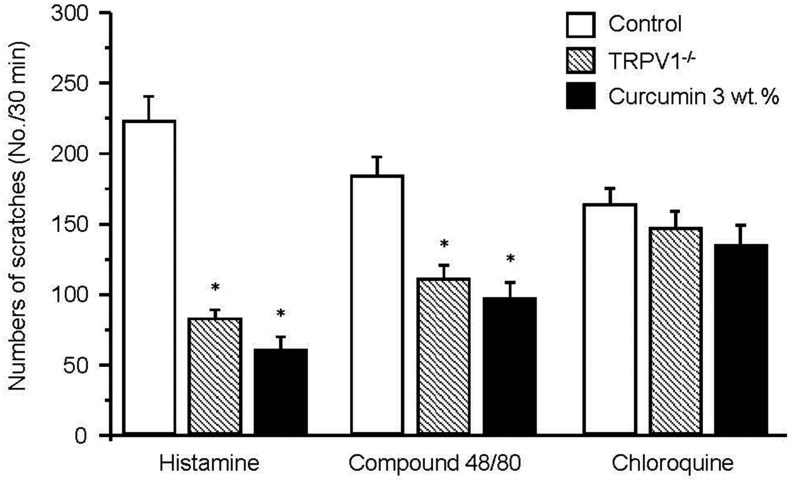
Because itching is distinguished by dependence on histamine, we examined the anti-itching effect of 3 wt.% curcumin on histamine-induced itching, compound 48/30-induced itching (histamine-associated itching), and chloroquine-inducted itching (histamine-independent itching via transient receptor potential ankyrin 1 (TRPA1) activation) in TRP1V KO mice compared to WT mice (Fig. 4). Histamine-induced itching behavior in TRPV1 KO mice injected with histamine (84±6 bouts, n=6) was 62% of that in WT mice (224±22 bouts, n=6). No significant decrease in histamine-induced itching in TRPV1 KO mice was observed after treatment with 3 wt.% curcumin (62±22 bouts, n=6). Compound 48/80 (100 μg/50 μl), which degranulates mast cells resulting in the release of histamine, evoked scratching behavior for 30 min (185±13 bouts, n=8) in WT mice. In contrast, compound 48/80-induced itching in TRPV1 KO mice (110±9 bouts, n=6) was reduced compared to that in WT mice (p<0.05, Fig. 4), and the former was not significantly reduced by curcumin (98±11 bouts, n=6). Itching behavior was observed after intradermal injection of chloroquine, which is known to act via a histamine-independent mechanism, in both WT and TRPV1 KO mice. WT mice treated with of chloroquine had 165±11 scratching bouts (n=6) over the course of 30 min while TRPV1 KO mice had 148±12 bouts (n=6), which is not a significant difference. The 3 wt.% curcumin did not reduce chloroquine-induced itching. The total number of scratching bouts in curcumin-treated TRPV1 KO mice was 136±14 (n=6). Taken together, these results suggest that the anti-itching effect of curcumin is mediated by the blocking of TRPV1, not of TRPA1.
Fig. 4. A comparison of the relationship between TRPV1 and TRPA1 in the blocking action of curcumin on an itch.
The total number of scratches were observed for 30 min after intradermal injection of histamine, compound 48/80, and chloroquine into WT, KO mice, and KO mice treated with 3 wt.% curcumin. A bar graph shows the total number of scratches as a mean±SEM. Asterisks indicate p<0.05.
DISCUSSION
Curcumin, a natural product with vanilloid rings, has anti-cancer, anti-inflammatory, and therapeutic effects in dementia, controls gastric acid secretion and diabetic symptoms, and functions as an analgesic [1,2,3,9,19,20,21]. However, to the best of our knowledge, its effectiveness as an anti-itching agent has not been evaluated. Our aim in this study was to determine if curcumin is effective at blocking histamine-induced itching, and if so, to determine its underlying mechanism of action. Our findings are as follows: 1) topical curcumin cream reduced histamine-induced pruritus in a concentration-dependent manner; 2) curcumin inhibited histamine-induced currents by blocking TRPV1 in itch-sensitive DRG neurons; and 3) topical curcumin cream was effective at treating histamine- and compound 48/80-induced pruritus, which are mediated by TRPV1, but not chloroquine-induced pruritus, which is mediated via TRPA1. These results suggest that natural curcumin extract can be used on peripheral sites as an anti-itching agent and may be a safe, long-term treatment for treating pruritic skin diseases.
The concentration of curcumin cream described in this study is wt.%, and curcumin cream is made by diluting curcumin dissolved in mixture of PEG400 and ethanol with white Vaseline. Thus, molar concentration of 3 wt.% curcumin cream used in itching behavioral test is about 285 μM. The previous study reported that the skin permeability of curcumin in rodents is about 10% when treated with curcumin for 24 h [22]. The skin permeability of curcumin when 3 wt.% curcumin cream treated to the skin for 1 h can be expected to be about 0.42% (1.19 μM of curcumin concentration), which is an appropriate concentration to reduce histamine-induced itching. Because the IC50 of curcumin for histamine-induced current is 523 nM and concentration of curcumin is 2 μM in ex-vivo recording, we believe that the permeated concentration of curcumin was sufficiently effective.
In clinical studies, it has been reported that topical and systemic curcumin can improve skin health [23] and curcumin has been used for symptomatic management of sulphur mustard-induced pruritus and itching in cancer patients [3,10]. However, the efficacy of curcumin in the management of itch has not been evaluated previously. Based on previous studies that reported that curcumin had an inhibitory effect on TRPV1 in the TG and DRG neurons of mice [9,10], we investigated the inhibitory effect of curcumin on histamine-induced itching and histamine-induced currents in DRG neurons. Consistent with our findings, previous studies reported that curcumin, curcuminoid, and curcumin derivatives directly inhibited capsaicin-induced currents in DRG-, TG-, and hTRPV1-expressing HEK293 cells and capsaicin-induced currents in mouse DRG neurons, which may explain the antinociceptive effects of these compounds on thermal pain [9,10,24,25]. This is consistent with clinical studies that have reported significant improvement in skin diseases such as atopic dermatitis and chronic pruritus upon curcumin treatment [3,10,26]. However, a previous study reported that curcumin was ineffective at directly modifying membrane currents in Xenopus oocytes expressing rat TRPV1 [8]. This discrepancy may be due to differences in the experimental approaches used in the various studies.
Histamine-independent itching is activated via a pathway other than TRPV1 and is typically associated with chloroquine and serotonin. In the case of chloroquine, this process involves the activation of TRPA1 [27]. MrgprA3, a member of the Mrgpr family of G-protein coupled receptors in TRPV1-expressing cells, is involved in chloroquine-induced itching, which is mediated by TRPA1, not TRPV1 [28]. Serotonin regulates the excitability of cells via PLCβ3 to induce itching [16]. Because histamine and compound 48/80 are likely to be associated with TRPV1 and chloroquine is not, we investigated if curcumin was effective at alleviating itching mediated by TRPA1 (chloroquine) as well as TRPV1-mediated pruritus (histamine and compound 48/80). Itching induced by histamine and compound 48/80 was significantly reduced in TRPV1 KO mice, but itching caused by chloroquine was not significantly different between WT and KO mice. Compound 48/80 induces an itching sensation through the activation of TRPV1 via histamine release from mast cells [29]. In addition, curcumin suppresses compound 48/80-induced mast cell degranulation and histamine release from mast cells [30,31]. Curcumin treatment had a significant inhibitory effect on compound 48/80-induced itching in WT mice, and compound 48/80-induced itching was significantly reduced in TRPV1 KO mice. In contrast, chloroquine treatment of TRPV1 KO mice resulted in a similar itching phenotype as observed in the WT group, and this itching was not inhibited by curcumin. Considering that chloroquine-induced itching, which is histamine-independent itching, is associated with TRPA1 [27], our results indicate that curcumin does not affect TRPA1-mediated itching.
In conclusion, curcumin relieves histamine-dependent itching by blocking TRPV1 but not TRPA1. These findings suggest that curcumin might be a potentially safe therapeutic option for treatment of pruritus that is both widely available and derived from natural products.
ACKNOWLEDGEMENTS
This work was supported by the research fund of Hanyang University (HY-2013-N).
Footnotes
Author contributions: H.K.L. performed patch clamp measurement. S.B.P. performed behavior test. S.Y.C. performed Ex-vivo recordings. J.S.J. supervised and coordinated the study. J.S.J. and H.K.L. wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 4.Chen HW, Huang HC. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br J Pharmacol. 1998;124:1029–1040. doi: 10.1038/sj.bjp.0701914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JY, Lin SJ, Lin JK. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- 6.Shah BH, Nawaz Z, Pertani SA, Roomi A, Mahmood H, Saeed SA, Gilani AH. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58:1167–1172. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I, Dal Maschio M, Palù G, Maschietto M, Scorzeto M, Vassanelli S, Brun P. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil. 2007;19:668–674. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 9.Yeon KY, Kim SA, Kim YH, Lee MK, Ahn DK, Kim HJ, Kim JS, Jung SJ, Oh SB. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. 2010;89:170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 10.Zhi L, Dong L, Kong D, Sun B, Sun Q, Grundy D, Zhang G, Rong W. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol Motil. 2013;25:e429–e440. doi: 10.1111/nmo.12145. [DOI] [PubMed] [Google Scholar]
- 11.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namer B, Hilliges M, Orstavik K, Schmidt R, Weidner C, Torebjörk E, Handwerker H, Schmelz M. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137:41–49. doi: 10.1016/j.pain.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Carstens E, Akiyama T. Itch: Mechanisms and treatment. Boca Raton, Florida: CRC Press/Taylor & Francis; 2014. [PubMed] [Google Scholar]
- 15.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 16.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ, Kim KT. Curcuma longa extract protects against gastric ulcers by blocking H2 histamine receptors. Biol Pharm Bull. 2005;28:2220–2224. doi: 10.1248/bpb.28.2220. [DOI] [PubMed] [Google Scholar]
- 20.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 21.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17:5972–5987. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res. 2016;30:1243–1264. doi: 10.1002/ptr.5640. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Shin TJ, Choi JM, Seo KS, Kim HJ, Yoon TG, Lee YS, Han H, Chung HJ, Oh Y, Jung SJ, Shin KJ. Antinociceptive curcuminoid, KMS4034, effects on inflammatory and neuropathic pain likely via modulating TRPV1 in mice. Br J Anaesth. 2013;111:667–672. [Google Scholar]
- 25.Ming-Tatt L, Khalivulla SI, Akhtar MN, Mohamad AS, Perimal EK, Khalid MH, Akira A, Lajis N, Israf DA, Sulaiman MR. Antinociceptive activity of a synthetic curcuminoid analogue, 2,6-bis-(4-hydroxy-3-methoxybenzylidene)cyclohexanone, on nociception-induced models in mice. Basic Clin Pharmacol Toxicol. 2012;110:275–282. doi: 10.1111/j.1742-7843.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 26.Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL, Kolivand M. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2012;108:1272–1279. doi: 10.1017/S0007114511006544. [DOI] [PubMed] [Google Scholar]
- 27.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Masrelated G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 30.Choi YH, Yan GH, Chai OH, Song CH. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat Cell Biol. 2010;43:36–43. doi: 10.5115/acb.2010.43.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugroho AE, Ikawati Z, Sardjiman, Maeyama K. Effects of benzylidenecyclopentanone analogues of curcumin on histamine release from mast cells. Biol Pharm Bull. 2009;32:842–849. doi: 10.1248/bpb.32.842. [DOI] [PubMed] [Google Scholar]