Abstract
Background
There is strong evidence for the effectiveness of addressing tobacco use in health care settings. However, few smokers receive cessation advice when visiting a hospital. Implementing smoking cessation technology in outpatient waiting rooms could be an effective strategy for change, with the potential to expose almost all patients visiting a health care provider without preluding physician action needed.
Objective
The objective of this study was to develop an intervention for smoking cessation that would make use of the time patients spend in a waiting room by passively exposing them to a face-aging, public morphing, tablet-based app, to pilot the intervention in a waiting room of an HIV outpatient clinic, and to measure the perceptions of this intervention among smoking and nonsmoking HIV patients.
Methods
We developed a kiosk version of our 3-dimensional face-aging app Smokerface, which shows the user how their face would look with or without cigarette smoking 1 to 15 years in the future. We placed a tablet with the app running on a table in the middle of the waiting room of our HIV outpatient clinic, connected to a large monitor attached to the opposite wall. A researcher noted all the patients who were using the waiting room. If a patient did not initiate app use within 30 seconds of waiting time, the researcher encouraged him or her to do so. Those using the app were asked to complete a questionnaire.
Results
During a 19-day period, 464 patients visited the waiting room, of whom 187 (40.3%) tried the app and 179 (38.6%) completed the questionnaire. Of those who completed the questionnaire, 139 of 176 (79.0%) were men and 84 of 179 (46.9%) were smokers. Of the smokers, 55 of 81 (68%) said the intervention motivated them to quit (men: 45, 68%; women: 10, 67%); 41 (51%) said that it motivated them to discuss quitting with their doctor (men: 32, 49%; women: 9, 60%); and 72 (91%) perceived the intervention as fun (men: 57, 90%; women: 15, 94%). Of the nonsmokers, 92 (98%) said that it motivated them never to take up smoking (men: 72, 99%; women: 20, 95%). Among all patients, 102 (22.0%) watched another patient try the app without trying it themselves; thus, a total of 289 (62.3%) of the 464 patients were exposed to the intervention (average waiting time 21 minutes).
Conclusions
A face-aging app implemented in a waiting room provides a novel opportunity to motivate patients visiting a health care provider to quit smoking, to address quitting at their subsequent appointment and thereby encourage physician-delivered smoking cessation, or not to take up smoking.
Keywords: face aging, smoking cessation, HIV, mobile apps, HIV patients, HIV seropositivity, smoking, cessation, tobacco smoking, morphing
Introduction
There is strong evidence for the effectiveness of addressing tobacco use in health care settings [1-11]. However, few smokers receive cessation advice when visiting a hospital [12,13] which is caused by many different reasons [14] and is therefore difficult to change.
Face-aging interventions, in which a photograph of the user is altered to predict the user’s future appearance, have been shown to motivate healthier behavioral choices in adiposity prevention, skin cancer prevention, and smoking cessation settings [15-35]. These preliminary results can be explained by the high importance of appearance for a person’s self-concept, particularly during adolescence [36].
However, to the best of our knowledge, the only completed prospective randomized trial to investigate the effectiveness of a face-aging intervention on actual behavior (smoking) was that of Burford et al [37]. Burford and her team recruited 160 participants (80 allocated to the control group and 80 to the intervention group) from 8 metropolitan community pharmacies located around Perth city center in Western Australia. All the participants received standardized smoking cessation advice, but those in the intervention group were also digitally photoaged by the internet-based APRIL Face Aging software to show images of what they might eventually look like as a lifelong smoker and as a nonsmoker. At the 6-month follow-up, 5 (6%) of the 80 control group participants suggested they had quit smoking, although this was confirmed by carbon monoxide validation in only 1 of them. In contrast, 22 (27%) of the 80 intervention group participants reported quitting, with 11 confirmed by carbon monoxide testing, a statistically significant difference in confirmed quitting (χ21=9.0; P=.003; test power=80%). However, the study had several limitations: the photographs now appear technologically outdated, they were taken in an over-the-counter setting that always required the time of another person, they were not available for free, and the approach did nothing to address the poor initiation of smoking cessation by doctors as recommended by guidelines [38].
We have developed a 3-dimensional face-aging, tablet-based app, Smokerface, that alters a self-taken image of the user’s face to simulate what the user would look like in 1 to 15 years’ time as either a smoker or a nonsmoker [39]. In this study, we hypothesized that hospital waiting rooms provide an effective setting for encouraging smoking cessation via the app because this would allow most patients visiting a health care provider to be passively exposed without the need for preluding action by health care personnel. To the best of our knowledge, no previous interventions in the field have implemented new technology for behavioral change in waiting rooms. We chose an HIV outpatient clinic for piloting our intervention, because HIV-positive patients are approximately twice as likely to smoke as the general population [40-45], ensuring that a comparably high number of the sample exposed to the intervention would be current smokers.
Therefore, the aim of this study was to develop an intervention that would make use of the waiting time of patients for smoking cessation by exposing them to the face-aging app and to measure the perceptions of smokers and nonsmokers after using the app.
Methods
Ethical Considerations
We planned this study at the University Hospital of Essen in Germany in early 2017 and implemented it in October 2017. All the participants were adults, and participation in the intervention and questionnaire survey was voluntary. The questionnaire was anonymous, and no personal data were stored. All images were instantly deleted automatically by the kiosk version of the app. We considered oral consent to be sufficient for participation in the survey. Before participants could use the app, they were informed about the screen-mirroring procedure by an information board placed adjacent to the tablet. The ethics committee of the Essen University Hospital, Essen, Germany, approved the study.
Experimental Setup
We developed a kiosk version of the Smokerface app (Figure 1). We placed an Apple iPad (iOS) tablet (Apple Inc, Cupertino, CA, USA) on which this version of the app was running on a table in the middle of the waiting room of our HIV outpatient clinic and connected it to a large monitor attached to the opposite wall, which mirrored the screen of the iPad (Figure 2). An explanatory note was displayed on a board next to the tablet.
Figure 1.

Start screen of the kiosk version of our face-aging app Smokerface, running on an Apple iPad (iOS).
Figure 2.

Setup of the smoking cessation face-aging intervention in the waiting room of our HIV outpatient clinic. After the home screen, users were instructed to “Tap ‘Start’ to see how smoking affects your face!” The original setup was in German.
The tablet screen displayed the instruction “Tap ‘Start’ to see how smoking affects your face!” (written in German) and was in guided access mode to ensure that patients could not quit the app. The app then displayed images of the patient’s face simulating their appearance after 1 to 15 years of not smoking (Figure 3) or smoking (Figure 4).
Figure 3.

Example image of the user simulating how she might look after 9 years of aging without smoking. The screenshot was taken directly from the iPad.
Figure 4.

Example image of the user simulating how she might look after 9 years of aging with smoking a pack of cigarettes a day. The screenshot was taken directly from the iPad.
Procedure
A researcher counted all the patients who visited the waiting room, noted their sex, and timed their total time spent in the waiting room. If a patient did not try the app within 30 seconds of starting their wait, the researcher encouraged the patient to use the app, following a standardized protocol. Patients who used the app were then asked whether they were smokers or nonsmokers and were asked to complete, voluntarily, the appropriate one of 2 paper-and-pencil questionnaires. Both smoking status–specific questionnaires captured the age and sex of the participant, as well as the participant’s perceptions of using the app, on 4-point Likert scales (from “absolutely true” to “absolutely false”). In addition, the participant was asked about the number of other patients in the room during the use of the app, the reactions of the other patients, and how the participant perceived those reactions (on 4-point Likert scales).
The smokers’ questionnaire additionally included 2 standard Fagerström items to calculate the Heaviness of Smoking Index (HSI), a validated measure of smoking dependence that has also been shown to predict quit success [46]: “How many cigarettes do you smoke per day?” and “When do you smoke the first cigarette after waking up?” Prior to the study, we tested the questionnaires in a small subsample of 32 patients to ensure that the questions were understandable and to measure the time needed to complete them (approximately 4 minutes).
Data Analysis
We performed descriptive analysis of data with IBM SPSS Statistics version 25 (IBM Corporation). We undertook no tests for significance due to the explorative nature of the study.
Results
Sample Characteristics
The sample consisted of 464 patients (male: 355/464, 78.7%), of whom 179 filled out a questionnaire (male: 79.0%; median age 42 years; range 23-76 years). Of the 179 patients who completed the questionnaire, 84 (46.9%) smoked; of the smokers, 66/82 (80.5%) were men and 16/82 (19.5%) were women (2 smokers did not indicate their sex).
Among the 84 smokers, 25/83 (30%) had a low HSI, 43/83 (52%) had a medium HSI, and 15/83 (18%) had a high HSI. One participant did not answer both Fagerström items; therefore, we could not calculate the HSI for that person.
Participation
The intervention was implemented in our waiting room for 19 days, and Figure 5 illustrated study participation. The average waiting time for all patients was 21 minutes.
Figure 5.
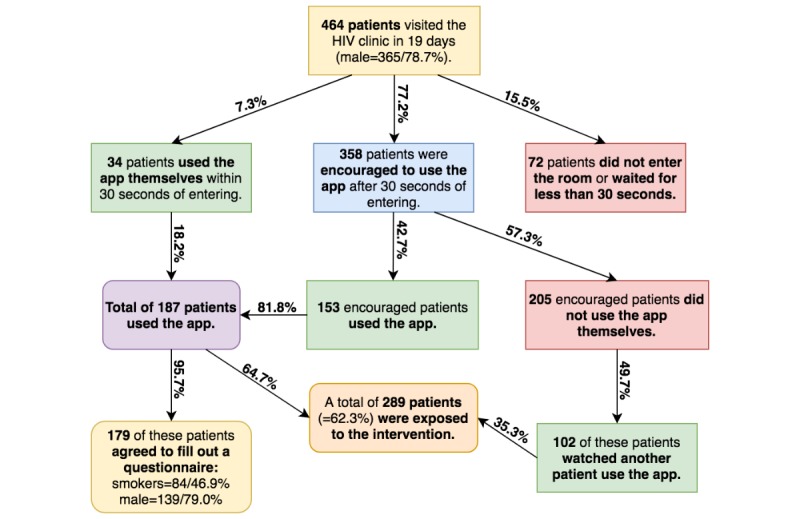
Levels of participation, sex, and smoking status of the waiting room visitors.
Perceptions About the Intervention
Among the 84 smokers, 55 of 81 (68%) reported that the intervention motivated them to quit (men: 45, 68%; women: 10, 67%, with 3 smokers not answering this question), 41 (51%) reported that it motivated them to discuss quitting with their doctor (men: 32, 49%; women: 9, 60%), and 72 (91%) perceived the intervention as fun (men: 57, 90%; women: 15, 94%). Of the nonsmokers, 92 of 94 (98%) reported that it motivated them to never take up smoking (men: 72, 99%; women: 20, 95%).
Other Patients in the Waiting Room
The numbers of other patients in the waiting room at the time a participant tried the app were as follows: no other patients, 30 (17%) cases; 1 to 3 other patients, 86 (49%) cases; 4 to 6 other patients, 56 (32%) cases; 7 to 10 other patients, 3 (2%) cases; 11 or more patients, 1 (0.6%) case.
Table 1 summarizes the participants’ descriptions of the reactions of the other patients in the waiting room (in answer to the question “How did the other people in the room react to your public selfie?”). In a considerable proportion of cases (48/132, 36.4%), 1 or more of the other patients reacted by trying the app themselves.
Table 1.
Reactions of other patients in the waiting room when a participant tried the app (only the cases where there was at least one other patient in the waiting room).
| Patient group | Others tried the app themselves, n (%) |
Quitting was a topic of discussion afterward, n (%) |
They encouraged me to quit or stay a nonsmoker, n (%) |
They were astonished, n (%) |
Their reactions were very strong, n (%) |
||||||||
| All patients | N=132 | N=138 | N=126 | N=132 | N=131 | ||||||||
| False/absolutely false | 84 (63.6) | 61 (44.2) | 70 (55.6) | 81 (61.4) | 90 (68.7) | ||||||||
| True/absolutely true | 48 (36.3) | 77 (55.8) | 56 (44.4) | 51 (38.6) | 41 (31.3) | ||||||||
| Smokers | N=63 | N=64 | N=77 | N=64 | N=60 | ||||||||
| False/absolutely false | 40 (63) | 23 (36) | 24 (41) | 39 (61) | 42 (70) | ||||||||
| True/absolutely true | 23 (36) | 41 (64) | 53 (59) | 25 (39) | 18 (30) | ||||||||
| Nonsmokers | N=69 | N=74 | N=67 | N=68 | N=71 | ||||||||
| False/absolutely false | 44 (64) | 38 (51) | 46 (69) | 42 (62) | 48 (68) | ||||||||
| True/absolutely true | 25 (36) | 36 (49) | 21 (31) | 26 (38) | 23 (32) | ||||||||
In most cases (77/138, 55.8%), the participant’s use of the app initiated a discussion on quitting in the waiting room; this was even more the case (41/64, 64%) when the participant was a smoker. In addition, 59% (53/77) of the participants were encouraged to quit by the other patients in the waiting room after using the app, which appeared to be often accompanied by quit advice (23/42, 55%; Table 2).
Table 2.
Participants’ perceptions of other patients’ reactions to the participant’s use of the app (only the cases where there was at least one other patient in the waiting room).
| Patient group | Motivated me to quit or remain a nonsmoker, n (%) |
Helpful, n (%) |
They gave me quitting advice, n (%) |
There were no reactions, n (%) |
|
| Smokers | N=49 | N=47 | N=42 | N=15 | |
| False/absolutely false | 19 (48) | 14 (30) | 19 (45) | N/Aa | |
| True/absolutely true | 30 (61) | 33 (70) | 23 (55) | 15 (100) | |
| Nonsmokers | N=51 | N=42 | — | N=19 | |
| False/absolutely false | 11 (22) | 15 (36) | N/A | N/A | |
| True/absolutely true | 40 (78) | 27 (64) | N/A | 19 (100) | |
aN/A: not applicable.
Table 2 summarizes how the participants perceived the reactions of the other patients, answering the question “How did you perceive those reactions?” The reactions were largely perceived as helpful (by 33/47, 70% of smokers and 27/42, 64% of nonsmokers). Indeed, the reactions provided motivation for 61% (30/49) of the smokers to quit, with advice on quitting offered to 55% (23/42) of the smokers.
Discussion
Principal Findings
The face-aging app setup was successful in exposing the majority of patients visiting our HIV outpatient clinic to a smoking cessation intervention. The face-aging procedure itself and the public nature of the face-aging procedure that triggered reactions of other waiting patients were perceived as motivating to quit smoking and helpful by the majority of smoking as well as nonsmoking patients.
To the best of our knowledge, this is the first study to implement new technology in a waiting room in order to use the patients’ waiting time to encourage smoking cessation. The results suggest a huge potential for the large-scale exposure of patients visiting health care providers to the technology. The effectiveness of this study remains subject to further study, and we aim to test the effectiveness in promoting smoking cessation in a randomized controlled trial. Further long-term studies should also examine the effects of group interactions and changes in the subjective norm because of setting the intervention in a waiting room.
Patients who are positive for HIV are approximately twice as likely to smoke as the general population (46.9% of our sample were smokers, compared with 23.9% in the general population) [40-45]. Effective interventions for this patient group remain scarce [4,47-55], and the population-attributable risk of death associated with smoking is double that of the general population [56]. In countries such as Germany where HIV care is well organized and antiretroviral therapy is free of charge, HIV-infected smokers lose more life-years to smoking than to HIV [56]. Hence, while our intervention is not specifically tailored to any patient group and could be applied in any patient waiting room, an HIV outpatient clinic provided an ideal setting for piloting the intervention.
The nonadherence of physicians to smoking cessation guidelines is a situation that is prevalent in many countries because of role incongruence and a lack of time, financial reimbursement, and appropriate training [14,38]. Being a physician can be an extremely stressful occupation in modern times, with increases in the level of bureaucracy required and the number of patients per doctor. Interventions that help a physician identify and motivate smokers willing to quit have the potential to increase population health and thereby reduce the workload for the medical profession, helping in the fight against tobacco-attributable diseases. Our approach shows promise as a possible simple solution to help physicians meet their smoking cessation obligations without placing too great a burden on them. However, our results also point to limitations and raise questions that need to be addressed in future research.
Initiation of the Use of the App
In this study, the researcher intervened by inviting patients to try the app if they did not do so spontaneously within 30 seconds of starting their wait in the waiting room. We decided on this approach for 3 reasons: (1) the HIV waiting room is particularly busy, with an average waiting time of only 21 minutes; (2) the number of patients waiting there tends to be low, with just 24 patients per day on average between 7:30 AM and 4:45 M; and (3) HIV patients tend to be rather shy in health care settings, as described by the experienced head of our HIV outpatient clinic. Nevertheless, 34 (7.3%) of the 464 patients tried the app within 30 seconds without prompting, indicating the likelihood of successful passive exposure with more time or in settings with higher patient density and longer waiting times. However, it is possible that other patient groups are even more reluctant to have their photograph transmitted to a publicly visible screen, and there might be barriers to the use of such a technology, especially with older patients. In this study, 48 of 132 (36%) participants reported that another patient tried the app straight after seeing them use it, which further strengthens the hypothesis that there would be less need of external prompting in fuller waiting rooms.
Short- and Longer-Term Implications
We received no complaints about patients feeling bullied, according to the physicians who worked in our outpatient clinic at the time of the study, and a great majority of the smokers perceived the intervention as fun. However, the question of feeling bullied could be addressed more explicitly in future research because of the nature of the intervention. We observed that the intervention resulted in interaction between patients where there had been none. Usually, patients waiting in this waiting room sit silently using their mobile phones, reading a newspaper, or just staring at the ground. When the intervention was implemented, patients began talking to each other about the intervention and smoking cessation, finding a common topic they could discuss. Our researcher reported that the overall atmosphere of these conversations was encouraging and positive, and this was reflected in the questionnaire data. Those who had already quit smoking shared their advice and even encouraged addressing the topic at the participant’s subsequent appointment; this was reported by 19 of 42 (45%) of the smokers in the questionnaire. In addition, 41 of 81 (51%) of the smokers reported that the intervention itself motivated them to address the topic at their upcoming appointment. Future studies should obtain information from the physicians treating those patients about whether smoking cessation was raised. Following this study, clinicians reported an increased rate of questions on how to quit, but this was not recorded in an objective fashion.
According to our data, 48 of 132 (36.4%) patients tried the app immediately after watching another person do it. In addition, it is reasonable to speculate that simply watching the intervention and perhaps engaging in a conversation arising from it in a full waiting room would motivate a patient to start quitting smoking due to a potential change in their subjective norm [57].
Projection of Potential Effects
During the 19 days of the study, a total of 289 patients in 1 waiting room used or were exposed to the smoking cessation app. This is equivalent to approximately 5500 patients per waiting room per year or to 176,000 patients per year if implemented in all 32 waiting rooms at our hospital. If we assume that the prospective effects measured by Burford et al (that 21.2% of smokers aged 18 to 30 years quit following the use of a similar method [37]) can be transferred to our intervention and that the prevalence of smoking among this hospital’s patients is approximately 30%, then approximately 11,000 smoking patients would quit per year. In our sample, the median age was 42 years, meaning that 9 life-years would be saved per patient on average, equivalent to 99,000 saved life-years in total each year [58]. The total cost was US $1500 for 1 waiting room, equivalent to US $0.48 per saved life-year. However, transferability has not been proven, and the prospective effects might be weaker for older patients. In our sample, just 22 (12.3%) of the 179 participants who completed the questionnaire were aged 18 to 30 years. Thus, if the intervention had no effect at all for any patient other than those aged 18 to 30 years, the cost per saved life-year would be US $3.90, 1.320 patients would quit, and 13,200 life-years would be saved per year of implementation.
As for any smoking cessation intervention that has not yet been evaluated in large randomized trials, health systems and insurance companies may be hesitant to reimburse clinics for implementation of this technology. Funding opportunities for health care providers will improve with prospective research on the technology’s influence on smoking behavior.
Study Limitations
This study had several limitations. Many of the participants were called by the nurse while still completing the questionnaire. We anticipated this problem and put the questions about individual perceptions of the intervention and important sociodemographic data and smoking status at the start of the questionnaire. The loss of data for these initial items was relatively low. Our study reported only cross-sectional data, and we could only estimate the influence on actual behavior. However, behavioral predictors, such as the behavioral intention to perform a certain behavior, indicate effectiveness in accordance with the theory of planned behavior [57]. In addition, although anonymity decreases social desirability bias, the participants completing the questionnaires may nevertheless have felt pressure to answer in a socially desirable way because the researcher was present in the room. To minimize this, the participants were left to themselves for completing the questionnaire, which they could then drop into a sealed box to further reduce the risk of bias.
Other Studies That Help Physicians Identify Unhealthy Behaviors
It should be noted that other eHealth interventions can be found in the literature that at least help physicians to identify unhealthy behaviors of patients while only indirectly influencing that behavior [59-76]. These mostly comprise digitized screening and early detection tools. The majority of this work focuses on mental health or the prediction of mental disease [59,64-66,68,69,74,77], and only a few publications have focused on predictors of chronic disease in general, including substance abuse [63,65,68,70,77]. However, helping physicians to identify smokers is only one aim of the intervention presented here; we think it is at least likewise important to investigate its direct effect on quitting behavior in future studies.
Conclusion
The use of a face-aging smoking cessation app in waiting rooms provides a new, enjoyable opportunity to motivate the majority of smokers visiting a health care provider to quit smoking or to address quitting at their subsequent appointment and nonsmokers to never take up smoking. It thereby facilitates physician-delivered smoking cessation. We plan a cluster-randomized trial of the app in 10 waiting rooms. This will focus on long-term smoking abstinence rates, analyzing the impact on different patient subgroups and the interplay of waiting times and modes of initiation. In addition, we plan to repeat the experiment using the Sunface skin cancer awareness app [25] to determine if it shows similar promise.
Abbreviations
- HSI
Heaviness of Smoking Index
Footnotes
Authors' Contributions: TJB initiated and designed the study, set up the intervention, instructed data collection, analyzed the data, and wrote the paper. SE, CMB, JK, CvK, AHE, RFS, WS, UM, AB, TR, CB, MvK, MVG, BBS and DS supported the design and conduct of the study, supported statistical analysis, and proofread the manuscript. All authors had full access to the data.
Conflicts of Interest: TJB is the owner of Smart Health Heidelberg GmbH (Handschuhsheimer Landstr. 9/1, 69120 Heidelberg), a technology company that develops and licenses health apps.
References
- 1.Rigotti NA, Chang Y, Rosenfeld LC, Japuntich SJ, Park ER, Tindle HA, Levy DE, Reid ZZ, Streck J, Gomperts T, Kelley JHK, Singer DE. Interactive voice response calls to promote smoking cessation after hospital discharge: pooled analysis of two randomized clinical trials. J Gen Intern Med. 2017 Sep;32(9):1005–1013. doi: 10.1007/s11606-017-4085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasser KE, Quintiliani LM, Truong V, Xuan Z, Murillo J, Jean C, Pbert L. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety-net hospital: a randomized clinical trial. JAMA Intern Med. 2017 Dec 01;177(12):1798–1807. doi: 10.1001/jamainternmed.2017.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava AB, Ramsey AT, McIntosh LD, Bailey TC, Fisher SL, Fox L, Castro M, Ma Y, Baker TB, Chen L, Bierut LJ. Tobacco use prevalence and smoking cessation pharmacotherapy prescription patterns among hospitalized patients by medical specialty. Nicotine Tob Res. 2018 Feb 22; doi: 10.1093/ntr/nty031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mussulman LM, Faseru B, Fitzgerald S, Nazir N, Patel V, Richter KP. A randomized, controlled pilot study of warm handoff versus fax referral for hospital-initiated smoking cessation among people living with HIV/AIDS. Addict Behav. 2018 Mar;78:205–208. doi: 10.1016/j.addbeh.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammett E, Veldheer S, Hrabovsky S, Yingst J, Berg A, Poole E, Stauffer D, Foulds J. TXT2STAYQUIT: pilot randomized trial of brief automated smoking cessation texting intervention for inpatient smokers discharged from the hospital. J Hosp Med. 2018 Dec 01;13(7):488–489. doi: 10.12788/jhm.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley A, Koshiaris C, Oke J, Ryan R, Szatkowski L, Stevens R, Aveyard P. Physician support of smoking cessation after diagnosis of lung, bladder, or upper aerodigestive tract cancer. Ann Fam Med. 2017 Dec;15(5):443–450. doi: 10.1370/afm.2100. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=28893814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice VH, Heath L, Livingstone-Banks J, Hartmann-Boyce J. Nursing interventions for smoking cessation. Cochrane Database Syst Rev. 2017 Dec 15;12:CD001188. doi: 10.1002/14651858.CD001188.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagidipati NJ, Hellkamp A, Thomas L, Gulati M, Peterson ED, Wang TY. Use of prescription smoking cessation medications after myocardial infarction among older patients in community practice. JAMA Cardiol. 2017 Sep 01;2(9):1040–1042. doi: 10.1001/jamacardio.2017.2369. http://europepmc.org/abstract/MED/28724116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigotti NA, Tindle HA, Regan S, Levy DE, Chang Y, Carpenter KM, Park ER, Kelley JHK, Streck JM, Reid ZZ, Ylioja T, Reyen M, Singer DE. A post-discharge smoking-cessation intervention for hospital patients: Helping Hand 2 randomized clinical trial. Am J Prev Med. 2016 Oct;51(4):597–608. doi: 10.1016/j.amepre.2016.04.005. http://europepmc.org/abstract/MED/27647060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas D, Abramson MJ, Bonevski B, George J. System change interventions for smoking cessation. Cochrane Database Syst Rev. 2017 Dec 10;2:CD010742. doi: 10.1002/14651858.CD010742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yudi MB, Farouque O, Andrianopoulos N, Ajani AE, Kalten K, Brennan AL, Lefkovits J, Hiew C, Oqueli E, Reid CM, Duffy SJ, Clark DJ, Melbourne Interventional Group The prognostic significance of smoking cessation after acute coronary syndromes: an observational, multicentre study from the Melbourne interventional group registry. BMJ Open. 2017 Oct 06;7(10):e016874. doi: 10.1136/bmjopen-2017-016874. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=28988174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raupach T, Falk J, Vangeli E, Schiekirka S, Rustler C, Grassi MC, Pipe A, West R. Structured smoking cessation training for health professionals on cardiology wards: a prospective study. Eur J Prev Cardiol. 2014 Jul;21(7):915–22. doi: 10.1177/2047487312462803. [DOI] [PubMed] [Google Scholar]
- 13.Keto J, Jokelainen J, Timonen M, Linden K, Ylisaukko-oja T. Physicians discuss the risks of smoking with their patients, but seldom offer practical cessation support. Subst Abuse Treat Prev Policy. 2015 Nov 02;10:43. doi: 10.1186/s13011-015-0039-9. https://substanceabusepolicy.biomedcentral.com/articles/10.1186/s13011-015-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balmford J, Leifert JA, Jaehne A. “Tobacco dependence treatment makes no sense because…”: rebuttal of commonly-heard arguments against providing tobacco dependence treatment in the hospital setting. BMC Public Health. 2014 Nov 19;14:1182. doi: 10.1186/1471-2458-14-1182. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-14-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinker TJ, Seeger W, Buslaff F. Photoaging mobile apps in school-based tobacco prevention: the mirroring approach. J Med Internet Res. 2016 Jun 28;18(6):e183. doi: 10.2196/jmir.6016. http://www.jmir.org/2016/6/e183/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flett K, Grogan S, Clark-Carter D, Gough B, Conner M. Male smokers' experiences of an appearance-focused facial-ageing intervention. J Health Psychol. 2017 Mar;22(4):422–433. doi: 10.1177/1359105315603477. [DOI] [PubMed] [Google Scholar]
- 17.Jiwa M, Burford O, Parsons R. Preliminary findings of how visual demonstrations of changes to physical appearance may enhance weight loss attempts. Eur J Public Health. 2015 Apr;25(2):283–5. doi: 10.1093/eurpub/cku249. [DOI] [PubMed] [Google Scholar]
- 18.Mahler HIM, Kulik JA, Butler HA, Gerrard M, Gibbons FX. Social norms information enhances the efficacy of an appearance-based sun protection intervention. Soc Sci Med. 2008 Jul;67(2):321–9. doi: 10.1016/j.socscimed.2008.03.037. http://europepmc.org/abstract/MED/18448221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson AL, Gaffney CA, Starr P, Dietrich AJ. The impact of an appearance-based educational intervention on adolescent intention to use sunscreen. Health Educ Res. 2008 Oct;23(5):763–9. doi: 10.1093/her/cym005. http://europepmc.org/abstract/MED/18039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen AL, Grogan S, Clark-Carter D, Dhealthpsy EB. Effects of an appearance-focussed versus a health-focussed intervention on men's attitudes towards UV exposure. Int J Mens Health. 2016;15:34. http://www.mensstudies.info/OJS/index.php/IJMH/article/view/749/pdf_311. [Google Scholar]
- 21.Stapleton J, Turrisi R, Hillhouse J, Robinson JK, Abar B. A comparison of the efficacy of an appearance-focused skin cancer intervention within indoor tanner subgroups identified by latent profile analysis. J Behav Med. 2010 Jun;33(3):181–90. doi: 10.1007/s10865-009-9246-z. [DOI] [PubMed] [Google Scholar]
- 22.Tuong W, Armstrong AW. Effect of appearance-based education compared with health-based education on sunscreen use and knowledge: a randomized controlled trial. J Am Acad Dermatol. 2014 Apr;70(4):665–9. doi: 10.1016/j.jaad.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Brinker TJ, Brieske CM, Schaefer CM, Buslaff F, Gatzka M, Petri MP, Sondermann W, Schadendorf D, Stoffels I, Klode J. Photoaging mobile apps in school-based melanoma prevention: pilot study. J Med Internet Res. 2017 Sep 08;19(9):e319. doi: 10.2196/jmir.8661. http://www.jmir.org/2017/9/e319/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinker TJ, Holzapfel J, Baudson TG, Sies K, Jakob L, Baumert HM, Heckl M, Cirac A, Suhre JL, Mathes V, Fries FN, Spielmann H, Rigotti N, Seeger W, Herth F, Groneberg DA, Raupach T, Gall H, Bauer C, Marek P, Batra A, Harrison CH, Taha L, Owczarek A, Hofmann FJ, Thomas R, Mons U, Kreuter M. Photoaging smartphone app promoting poster campaign to reduce smoking prevalence in secondary schools: the Smokerface Randomized Trial: design and baseline characteristics. BMJ Open. 2016 Nov 07;6(11):e014288. doi: 10.1136/bmjopen-2016-014288. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=27821601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinker TJ, Schadendorf D, Klode J, Cosgarea I, Rösch A, Jansen P, Stoffels I, Izar B. Photoaging mobile apps as a novel opportunity for melanoma prevention: pilot study. JMIR Mhealth Uhealth. 2017 Jul 26;5(7):e101. doi: 10.2196/mhealth.8231. http://mhealth.jmir.org/2017/7/e101/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinker TJ, Seeger W. Photoaging mobile apps: a novel opportunity for smoking cessation? J Med Internet Res. 2015 Jul 27;17(7):e186. doi: 10.2196/jmir.4792. http://www.jmir.org/2015/7/e186/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burford O, Smith M, Jiwa M, Carter O. Photoageing intervention (PAINT): a proposal for a randomised controlled trial in Australian primary care. Australas Med J. 2009;1(7):8–12. doi: 10.4066/AMJ.2009.108. [DOI] [Google Scholar]
- 28.Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females' perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018 Mar 25;25(1):23–32. doi: 10.1177/1757975916639871. [DOI] [PubMed] [Google Scholar]
- 29.Faria BL, Brieske CM, Cosgarea I, Omlor AJ, Fries FN, de Faria COM, Lino HA, Oliveira ACC, Lisboa OC, Klode J, Schadendorf D, Bernardes-Souza B, Brinker TJ. A smoking prevention photoageing intervention for secondary schools in Brazil delivered by medical students: protocol for a randomised trial. BMJ Open. 2017 Dec 10;7(12):e018589. doi: 10.1136/bmjopen-2017-018589. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=29229659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler HIM, Kulik JA, Gerrard M, Gibbons FX. Effects of photoaging information and UV photo on sun protection intentions and behaviours: a cross-regional comparison. Psychol Health. 2013;28(9):1009–31. doi: 10.1080/08870446.2013.777966. [DOI] [PubMed] [Google Scholar]
- 31.Lo Presti LL, Chang P, Taylor MF. Young Australian adults' reactions to viewing personalised UV photoaged photographs. Australas Med J. 2014;7(11):454–61. doi: 10.4066/AMJ.2014.2253. http://europepmc.org/abstract/MED/25550717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinker TJ, Owczarek AD, Seeger W, Groneberg DA, Brieske CM, Jansen P, Klode J, Stoffels I, Schadendorf D, Izar B, Fries FN, Hofmann FJ. A medical student-delivered smoking prevention program, education against tobacco, for secondary schools in Germany: randomized controlled trial. J Med Internet Res. 2017 Jun 06;19(6):e199. doi: 10.2196/jmir.7906. http://www.jmir.org/2017/6/e199/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinker TJ, Heckl M, Gatzka M, Heppt MV, Resende RH, Schneider S, Sondermann W, de Almeida e Silva C, Kirchberger MC, Klode J, Enk AH, Knispel S, von KC, Stoffels I, Schadendorf D, Nakamura Y, Esser S, Assis A, Bernardes-Souza B. A skin cancer prevention facial-aging mobile app for secondary schools in Brazil: appearance-focused interventional study. JMIR Mhealth Uhealth. 2018 Mar 09;6(3):e60. doi: 10.2196/mhealth.9794. http://mhealth.jmir.org/2018/3/e60/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinker Titus J, Alfitian Jonas, Seeger Werner, Groneberg David A, von Kalle Christof, Enk Alexander H, Herth Felix J F, Kreuter Michael, Bauer Claudia M, Gatzka Martina, Suhre Janina L. A Face-Aging Smoking Prevention/Cessation Intervention for Nursery School Students in Germany: An Appearance-Focused Interventional Study. Int J Environ Res Public Health. 2018 Aug 04;15(8) doi: 10.3390/ijerph15081656. http://www.mdpi.com/resolver?pii=ijerph15081656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinker Titus J, Klode Joachim, Esser Stefan, Schadendorf Dirk. Facial-Aging App Availability in Waiting Rooms as a Potential Opportunity for Skin Cancer Prevention. JAMA Dermatol. 2018 Jul 25; doi: 10.1001/jamadermatol.2018.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baudson TG, Weber KE, Freund PA. More than only skin deep: appearance self-concept predicts most of secondary school students’ self-esteem. Front Psychol. 2016;7:1568. doi: 10.3389/fpsyg.2016.01568. doi: 10.3389/fpsyg.2016.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burford O, Jiwa M, Carter O, Parsons R, Hendrie D. Internet-based photoaging within Australian pharmacies to promote smoking cessation: randomized controlled trial. J Med Internet Res. 2013 Mar 26;15(3):e64. doi: 10.2196/jmir.2337. http://www.jmir.org/2013/3/e64/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pipe A, Sorensen M, Reid R. Physician smoking status, attitudes toward smoking, and cessation advice to patients: an international survey. Patient Educ Couns. 2009 Jan;74(1):118–23. doi: 10.1016/j.pec.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Brinker TJ, Enk A, Gatzka M, Nakamura Y, Sondermann W, Omlor AJ, Petri MP, Karoglan A, Seeger W, Klode J, von Kalle C, Schadendorf D. A dermatologist’s ammunition in the war against smoking: a photoaging app. J Med Internet Res. 2017 Sep 21;19(9):e326. doi: 10.2196/jmir.8743. http://www.jmir.org/2017/9/e326/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeiher J, Kuntz B, Lange C. Smoking among adults in Germany. J Health Monit. 2017;2(2):2017. doi: 10.17886/RKI-GBE-2017-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009-2014. Prev Med. 2018 Jun;111:231–234. doi: 10.1016/j.ypmed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015 Mar 03;162(5):335–44. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 43.Reddy KP, Kong CY, Hyle EP, Baggett TP, Huang M, Parker RA, Paltiel AD, Losina E, Weinstein MC, Freedberg KA, Walensky RP. Lung cancer mortality associated with smoking and smoking cessation among people living with HIV in the United States. JAMA Intern Med. 2017 Nov 01;177(11):1613–1621. doi: 10.1001/jamainternmed.2017.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westreich D, Cates J, Cohen M, Weber KM, Seidman D, Cropsey K, Wright R, Milam J, Young MA, Mehta CC, Gustafson DR, Golub ET, Fischl MA, Adimora AA. Smoking, HIV, and risk of pregnancy loss. AIDS. 2017 Dec 20;31(4):553–560. doi: 10.1097/QAD.0000000000001342. http://europepmc.org/abstract/MED/27902507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson SM, Pacek LR, Dennis PA, Bastian LA, Beckham JC, Calhoun PS. Veterans living with HIV: a high-risk group for cigarette smoking. AIDS Behav. 2017 Jul;21(7):1950–1955. doi: 10.1007/s10461-017-1717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zawertailo L, Voci S, Selby P. The Cigarette Dependence Scale and Heaviness of Smoking Index as predictors of smoking cessation after 10weeks of nicotine replacement therapy and at 6-month follow-up. Addict Behav. 2018 Mar;78:223–227. doi: 10.1016/j.addbeh.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tamí-Maury I, Gritz ER. Mediators of a smoking cessation intervention for persons living with HIV/AIDS. Drug Alcohol Depend. 2015 Feb 01;147:76–80. doi: 10.1016/j.drugalcdep.2014.12.003. http://europepmc.org/abstract/MED/25542824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ledgerwood DM, Yskes R. Smoking cessation for people living with HIV/AIDS: a literature review and synthesis. Nicotine Tob Res. 2016 Dec;18(12):2177–2184. doi: 10.1093/ntr/ntw126. http://europepmc.org/abstract/MED/27245237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shuter J, Morales DA, Considine-Dunn SE, An LC, Stanton CA. Feasibility and preliminary efficacy of a web-based smoking cessation intervention for HIV-infected smokers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2014 Sep 01;67(1):59–66. doi: 10.1097/QAI.0000000000000226. http://europepmc.org/abstract/MED/25118794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aigner CJ, Gritz ER, Tamí-Maury I, Baum GP, Arduino RC, Vidrine DJ. The role of pain in quitting among human immunodeficiency virus (HIV)-positive smokers enrolled in a smoking cessation trial. Subst Abus. 2017;38(3):249–252. doi: 10.1080/08897077.2017.1291466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd-Richardson EE, de Dios MA, Morrow KM, Makgoeng SB, Tashima KT, Niaura RS. Outcomes of a tailored intervention for cigarette smoking cessation among Latinos living with HIV/AIDS. Nicotine Tob Res. 2015 Aug;17(8):975–82. doi: 10.1093/ntr/ntv014. http://europepmc.org/abstract/MED/26180222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidrine DJ, Frank SG, Savin MJ, Waters AJ, Li Y, Chen S, Fletcher FE, Arduino RC, Gritz ER. HIV care initiation: a teachable moment for Smoking Cessation? Nicotine Tob Res. 2017 Sep 25; doi: 10.1093/ntr/ntx218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vijayaraghavan M, Yuan P, Gregorich S, Lum P, Appelle N, Napoles AM, Kalkhoran S, Satterfield J. Disparities in receipt of 5As for smoking cessation in diverse primary care and HIV clinics. Prev Med Rep. 2017 Jun;6:80–87. doi: 10.1016/j.pmedr.2017.02.012. https://linkinghub.elsevier.com/retrieve/pii/S2211-3355(17)30028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz P, Ferketich A. Smoking and HIV: confronting the epidemic. Lancet HIV. 2018 Dec;5(3):e109–e110. doi: 10.1016/S2352-3018(18)30001-8. [DOI] [PubMed] [Google Scholar]
- 55.Grabovac I, Brath H, Schalk H, Degen O, Dorner TE. Clinical setting-based smoking cessation programme and the quality of life in people living with HIV in Austria and Germany. Qual Life Res. 2017 Dec;26(9):2387–2395. doi: 10.1007/s11136-017-1580-y. http://europepmc.org/abstract/MED/28429240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013 Mar;56(5):727–34. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 57.Montano D, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior: Theory, Research, and Practice. Fifth edition. San Francisco, CA: Jossey-Bass; 2015. pp. 95–124. [Google Scholar]
- 58.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004 Jun 26;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. http://europepmc.org/abstract/MED/15213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karasouli E, Adams A. Assessing the evidence for e-resources for mental health self-management: a systematic literature review. JMIR Ment Health. 2014;1(1):e3. doi: 10.2196/mental.3708. http://mental.jmir.org/2014/1/e3/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey M, Noble N, Mansfield E, Waller A, Henskens F, Sanson-Fisher R. The role of eHealth in optimizing preventive care in the primary care setting. J Med Internet Res. 2015 May 22;17(5):e126. doi: 10.2196/jmir.3817. http://www.jmir.org/2015/5/e126/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forjuoh SN, Ory MG, Wang S, des Bordes JK, Hong Y. Using the iPod touch for patient health behavior assessment and health promotion in primary care. JMIR Mhealth Uhealth. 2014;2(1):e14. doi: 10.2196/mhealth.2927. http://mhealth.jmir.org/2014/1/e14/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foraker RE, Kite B, Kelley MM, Lai AM, Roth C, Lopetegui MA, Shoben AB, Langan M, Rutledge NL, Payne PRO. EHR-based visualization tool: adoption rates, satisfaction, and patient outcomes. EGEMS (Wash DC) 2015;3(2):1159. doi: 10.13063/2327-9214.1159. http://europepmc.org/abstract/MED/26290891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose GL, Ferraro TA, Skelly JM, Badger GJ, MacLean CD, Fazzino TL, Helzer JE. Feasibility of automated pre-screening for lifestyle and behavioral health risk factors in primary care. BMC Fam Pract. 2015 Oct 23;16:150. doi: 10.1186/s12875-015-0368-9. https://bmcfampract.biomedcentral.com/articles/10.1186/s12875-015-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krist AH, Glasgow RE, Heurtin-Roberts S, Sabo RT, Roby DH, Gorin SNS, Balasubramanian BA, Estabrooks PA, Ory MG, Glenn BA, Phillips SM, Kessler R, Johnson SB, Rohweder CL, Fernandez ME. The impact of behavioral and mental health risk assessments on goal setting in primary care. Transl Behav Med. 2016 Jun;6(2):212–9. doi: 10.1007/s13142-015-0384-2. http://europepmc.org/abstract/MED/27356991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elley CR, Dawes D, Dawes M, Price M, Draper H, Goodyear-Smith F. Screening for lifestyle and mental health risk factors in the waiting room: feasibility study of the Case-finding Health Assessment Tool. Can Fam Physician. 2014 Nov;60(11):e527–34. http://www.cfp.ca/cgi/pmidlookup?view=long&pmid=25551137. [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrari M, Ahmad F, Shakya Y, Ledwos C, McKenzie K. Computer-assisted client assessment survey for mental health: patient and health provider perspectives. BMC Health Serv Res. 2016 Sep 23;16(1):516. doi: 10.1186/s12913-016-1756-0. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-016-1756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dempsey AF, Maertens J, Beaty B, O'Leary ST. Characteristics of users of a tailored, interactive website for parents and its impact on adolescent vaccination attitudes and uptake. BMC Res Notes. 2015 Dec 01;8:739. doi: 10.1186/s13104-015-1721-8. https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-015-1721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodyear-Smith F, Corter A, Suh H. Electronic screening for lifestyle issues and mental health in youth: a community-based participatory research approach. BMC Med Inform Decis Mak. 2016 Dec 08;16(1):140. doi: 10.1186/s12911-016-0379-z. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-016-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad F, Lou W, Shakya Y, Ginsburg L, Ng PT, Rashid M, Dinca-Panaitescu S, Ledwos C, McKenzie K. Preconsult interactive computer-assisted client assessment survey for common mental disorders in a community health centre: a randomized controlled trial. CMAJ Open. 2017;5(1):E190–E197. doi: 10.9778/cmajo.20160118. http://cmajopen.ca/cgi/pmidlookup?view=long&pmid=28401134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen-Silver J, Laher N, Freeman S, Mistry N, Sgro M. Family fIRST, an interactive risk screening tool for families in a school-based pediatric clinic: a look at feasibility and pilot data. Clin Pediatr (Phila) 2017 Mar;56(3):217–225. doi: 10.1177/0009922816657152. [DOI] [PubMed] [Google Scholar]
- 71.Foucher-Urcuyo J, Longworth D, Roizen M, Hu B, Rothberg MB. Patient-entered wellness data and tailored electronic recommendations increase preventive care. J Am Board Fam Med. 2017;30(3):350–361. doi: 10.3122/jabfm.2017.03.160231. http://www.jabfm.org/cgi/pmidlookup?view=long&pmid=28484067. [DOI] [PubMed] [Google Scholar]
- 72.Bruun LL, Soendergaard J, Halling A, Thilsing T, Thomsen JL. A novel approach to population-based risk stratification, comprising individualized lifestyle intervention in Danish general practice to prevent chronic diseases: results from a feasibility study. Health Informatics J. 2017 Dec;23(4):249–259. doi: 10.1177/1460458216645149. [DOI] [PubMed] [Google Scholar]
- 73.Staeheli M, Aseltine RH, Schilling E, Anderson D, Gould B. Using mHealth technologies to improve the identification of behavioral health problems in urban primary care settings. SAGE Open Med. 2017;5:2050312117712656. doi: 10.1177/2050312117712656. http://europepmc.org/abstract/MED/28634539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodyear-Smith F, Martel R, Darragh M, Warren J, Thabrew H, Clark TC. Screening for risky behaviour and mental health in young people: the YouthCHAT programme. Public Health Rev. 2017;38:20. doi: 10.1186/s40985-017-0068-1. https://publichealthreviews.biomedcentral.com/articles/10.1186/s40985-017-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thabrew H, Corter A, Goodyear-Smith F, Goldfinch M. Randomized trial comparing the electronic composite psychosocial screener YouthCHAT with a clinician-interview assessment for young people: a study protocol. JMIR Res Protoc. 2017 Jul 31;6(7):e135. doi: 10.2196/resprot.7995. http://www.researchprotocols.org/2017/7/e135/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wachtler C, Coe A, Davidson S, Fletcher S, Mendoza A, Sterling L, Gunn J. Development of a mobile clinical prediction tool to estimate future depression severity and guide treatment in primary care: user-centered design. JMIR Mhealth Uhealth. 2018 Apr 23;6(4):e95. doi: 10.2196/mhealth.9502. http://mhealth.jmir.org/2018/4/e95/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodyear-Smith F, Warren J, Bojic M, Chong A. eCHAT for lifestyle and mental health screening in primary care. Ann Fam Med. 2013;11(5):460–6. doi: 10.1370/afm.1512. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=24019278. [DOI] [PMC free article] [PubMed] [Google Scholar]


