Corresponding Author

Key Words: atherosclerosis, Fenton reaction, heme oxygenase, iron
Contemporary advances in high-throughput screening of biological samples have added unbiased screens to the classical candidate approach for implicating mediators in various disease states. In this issue of JACC: Basic to Translational Science, Matic et al. (1) report transcriptomic and proteomic profiling of atherosclerotic plaques banked from carotid endarterectomies. In parallel, they looked at blood sampled near the lesion in vivo in comparison to peripheral blood. The investigators found a single molecule of many tested to be overexpressed in this collection of atherosclerotic lesions that received surgical remediation compared with nonatherosclerotic arterial specimens. They identified biliverdin reductase B (BLVRB) as significantly increased at a messenger RNA, local protein, and plasma protein levels, in company with parallel rises in heme oxygenase 1 (HMOX1). BLVRB participates in catabolism of heme.
These results that emerged from high-throughput screening technologies applied to human atherosclerosis draw our attention to pathways long implicated in atherogenesis, but remain worthy of renewed attention in light of new data including these from the Stockholm/Karolinska group. The strong regulation of heme-metabolizing enzymes in active human atheromata indicates a substantial local and/or systemic tissue response to heme, at least in carotid artery lesions sufficiently active to warrant surgical treatment.
From whence does heme that could evoke a clearance response within the plaque come? The microvessels that penetrate into plaques during their evolution (inward neo-angiogenesis) exhibit impaired barrier function and display characteristics of fragility that could lead to extravasation of erythrocytes and intraplaque hemorrhage (Figure 1). In the extreme, leaky microvessels in the plaque could give rise to rapid plaque expansion and promote hematoma formation that could embarrass blood flow. Such macroscopic episodes of intraplaque hemorrhage, although uncommon, likely represent the extreme case of much more common microscopic episodes of intraplaque hemorrhage. Matic et al. (1) found that exposure to ferric iron was associated with iron uptake by THP-1 human monocyte cell line–derived macrophage-like cells and stimulated HMOX1 and BLVRB expression, suggesting that local hemolysis and iron derived from heme drive the elevated concentrations of these enzymes in plaques. Intraplaque hemorrhage promotes erythrocyte sequestration and localized hemolysis, hemostasis, and coagulation, including platelet activation, fibrin formation, and trapping of leukocytes, mainly neutrophils, in human plaques (2). Foci of intraplaque hemorrhage colocalize with proteases and markers of oxidative stress, which are possible promoters of plaque rupture 3, 4. In contrast, products of local blood coagulation can lead to the release of platelet products, such as transforming growth factor-beta and platelet-derived growth factor, which can foster a local healing response that could augment smooth muscle production of extracellular matrix, smooth muscle proliferation, and thus, growth of the atherosclerotic lesion.
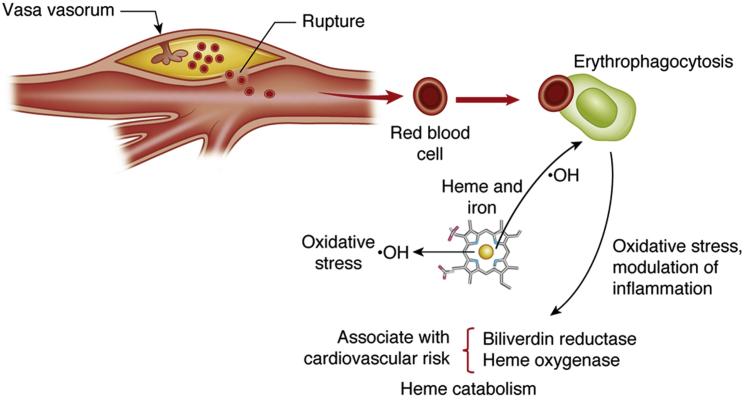
Figure 1.
Intraplaque Hemorrhage in Atherosclerosis
This figure depicts a plaque in a carotid artery bifurcation on top. Intraplaque hemorrhage can arise because of rupture of the fibrous, a breach in endothelial integrity, or disruption of the barrier function and leakage from plaque microvessels. Erythrocytes lyse readily in the plaque microenvironment, releasing iron and its prosthetic heme group. The heme-derived redox-active iron can drive oxidative stress through the Fenton reaction by generating hydroxyl radical (•OH). Iron can enter smooth muscle cells (not shown) and macrophages or smooth muscle cells by erythrophagocytosis. The phagocytes exposed to red blood cells and their products elaborate biliverdin reductase and heme oxygenase, enzymes involved in the catabolism of the heme moiety. The concentrations of these enzymes in plasma may be associated with cardiovascular risk. The phagocytes that have engulfed erythrocytes and their products can modulate inflammation both positively and negatively, with an uncertain net effect.
In addition to erythrocyte extravasation from fragile microvessels, entry of erythrocytes from the macrovascular compartment into the intima could arise from breaches in the integrity of the endothelial layer that lines the macrovascular lumen. Altered hydrodynamics at regions of flow disturbance could contribute to endothelial cell desquamation or impaired barrier function. Recent studies suggest accumulation of erythrocytes near the intimal interface with blood even in early atherogenesis (5). Not only do red blood cells (RBCs) accumulate locally within the intima, but hemoglobin, glycophorin A, and iron colocalize with regions of RBC collections that compose small intraplaque hematomas. The sites of local RBC accumulation in the intima also contain HMOX1, ferroportin, and members of the natural resistance-associated macrophage protein family of metal transporters. Furthermore, sites of these intimal hematomas contain markers of oxidation of lipids and proteins. Smooth muscle cells appear capable of erythrophagocytosis in addition to classical scavenging of modified or senescent RBC via scavenger receptors associated with mononuclear phagocytes including CD163 and CD36 (5).
Whether they are derived from microvascular leaks or from breaches in the macrovascular endothelium, redox-active ferrous iron (Fe++) derived from erythrocyte heme prosthetic groups can contribute to local oxidative damage through the Fenton reaction. The local plaque environment favors hemolysis of RBC, releasing heme and iron in the intimal lesions. Recent systems biology analyses point to hydroxyl radical derived through Fenton chemistry as an agonist in atherogenesis (6). In addition, genome-wide association studies have implicated iron metabolism and hepcidin as risk factors for biomarkers of atherosclerosis such as increased intima-media thickness (7). Experimental studies have implicated hepcidin in activating plaque macrophages following erythrophagocytosis. Some experimental data suggest that clearance of hemoglobin by CD163-positive cells and concomitant HMOX1 expression can provide an anti-inflammatory response that can defend against some of the potentially adverse effects of local heme metabolism and accumulation of iron (8). Beyond the role of RBC-derived heme and iron in potentiating atherosclerosis, the membranes of erythrocytes that enter the intima can provide a local source of cholesterol (9).
The finding of increased BLVRB in carotid atheromata shines a bright spotlight on these various pathogenic and potentially counter-regulatory pathways during human atherogenesis. Whereas intraplaque hemorrhage does not likely initiate the atherosclerotic process, operation of the iron-related pathways enumerated herein may sustain the disease and promote properties of plaques implicated in causing clinical complications (Figure 1).
The observations of Matic et al. (1) raise a number of interesting questions that merit further investigation. Is intraplaque hemorrhage a characteristic of carotid atheromata, or does it affect atherosclerotic plaques in other vascular beds? Do particular hemodynamic conditions near the carotid bifurcation predilect to the formation of intraplaque hemorrhage? In this regard, femoral artery atheromata rarely exhibit intraplaque hemorrhage (10). About one-third of acute coronary events arise from superficial erosion (11), a mechanism of plaque disruption that would not deposit RBC in the core of coronary atheromata (12). In contrast, because intraluminal thrombi characteristically contain abundant RBC (13) and abdominal aortic aneurysms usually associate with a burden of mural thrombosis, the heme pathways portrayed in the study by Matic et al. (1) might apply particularly to these manifestations of atherosclerosis.
The focus on heme metabolism stimulated by the results of Matic et al. (1) raises therapeutic questions as well. Does modulation of human atherosclerosis, for example, by aggressive lipid-lowering therapy, reduce RBC accumulation of plaque arising either from microvessels or intimal breaches? Substantial evidence suggests that contemporary preventive therapies including statins may change the character of human atherosclerotic plaques. “Stabilization” of microvessels and of the integrity of luminal endothelial cells might limit erythrocyte entry into the plaque. Microvascular permeability studies using magnetic resonance techniques could help to test this hypothesis (14).
Animal experiments could explore further mechanisms of intraplaque hemorrhage. Pigs and rodents have some limitations for probing plaque neovascularization (15). But, some preparations in rabbits (16) or mice (17) involve accumulation of RBC near neovessels, suggesting microvascular leakiness and intraplaque hemorrhage. Animal experiments that produce flow disturbance, for example, by the induction of a partial stenosis, might also help to define mechanisms of the entry of RBC from the microcirculation into the artery wall (18).
In sum, the results from the Karolinska group highlight the local responses in plaque to heme and provide support for the operation in human atherosclerosis of many pathways that involve erythrocyte extravasation and iron-mediated oxidative stress as a potential potentiator of human atherogenesis.
Footnotes
Dr. Libby was supported by grants from the National Heart, Lung, and Blood Institute (HL080472) and the RRM Charitable Fund. Dr. Franck was supported by the Lefoulon-Delalande Foundation and the UE PRESTIGE Fund. Dr. Michel has reported that he has no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Matic L.P., Iglesias M.J., Vesterlund M. Novel multiomics profiling of human carotid atherosclerotic plaques and plasma reveals biliverdin reductase B as a marker of intraplaque hemorrhage. J Am Coll Cardiol Basic Trans Science. 2018;3:464–480. doi: 10.1016/j.jacbts.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leclercq A., Houard X., Philippe M. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82:1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- 3.Michel J.B., Virmani R., Arbustini E., Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32:1977–1985a–c. doi: 10.1093/eurheartj/ehr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel J.B., Martin-Ventura J.L., Nicoletti A., Ho-Tin-Noe B. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis. 2014;234:311–319. doi: 10.1016/j.atherosclerosis.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Delbosc S., Bayles R.G., Laschet J. Erythrocyte efferocytosis by the arterial wall promotes oxidation in early-stage atheroma in humans. Front Cardiovasc Med. 2017;4:43. doi: 10.3389/fcvm.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formanowicz D., Radom M., Rybarczyk A., Formanowicz P. The role of Fenton reaction in ROS-induced toxicity underlying atherosclerosis—modeled and analyzed using a Petri net-based approach. Biosystems. 2018;165:71–87. doi: 10.1016/j.biosystems.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Galesloot T.E., Janss L.L., Burgess S. Iron and hepcidin as risk factors in atherosclerosis: what do the genes say? BMC Genet. 2015;16:79. doi: 10.1186/s12863-015-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaer C.A., Schoedon G., Imhof A., Kurrer M.O., Schaer D.J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006;99:943–950. doi: 10.1161/01.RES.0000247067.34173.1b. [DOI] [PubMed] [Google Scholar]
- 9.Tziakas D.N., Kaski J.C., Chalikias G.K. Total cholesterol content of erythrocyte membranes is increased in patients with acute coronary syndrome: a new marker of clinical instability? J Am Coll Cardiol. 2007;49:2081–2089. doi: 10.1016/j.jacc.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Herisson F., Heymann M.F., Chetiveaux M. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis. 2011;216:348–354. doi: 10.1016/j.atherosclerosis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Farb A., Burke A.P., Tang A.L. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 12.Partida R.A., Libby P., Crea F., Jang I.K. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J. 2018;39:2070–2076. doi: 10.1093/eurheartj/ehx786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Pinna R., Lindholt J.S., Madrigal-Matute J. From tissue iron retention to low systemic haemoglobin levels, new pathophysiological biomarkers of human abdominal aortic aneurysm. Thromb Haemost. 2014;112:87–95. doi: 10.1160/TH13-08-0721. [DOI] [PubMed] [Google Scholar]
- 14.Taqueti V.R., Di Carli M.F., Jerosch-Herold M. Increased microvascularization and vessel permeability associate with active inflammation in human atheromata. Circ Cardiovasc Imaging. 2014;7:920–929. doi: 10.1161/CIRCIMAGING.114.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Veken B., De Meyer G.R.Y., Martinet W. Intraplaque neovascularization as a novel therapeutic target in advanced atherosclerosis. Expert Opin Ther Targets. 2016;20:1247–1257. doi: 10.1080/14728222.2016.1186650. [DOI] [PubMed] [Google Scholar]
- 16.Giannarelli C., Ibanez B., Cimmino G. Contrast-enhanced ultrasound imaging detects intraplaque neovascularization in an experimental model of atherosclerosis. J Am Coll Cardiol Img. 2010;3:1256–1264. doi: 10.1016/j.jcmg.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 17.de Vries M.R., Niessen H.W., Lowik C.W., Hamming J.F., Jukema J.W., Quax P.H. Plaque rupture complications in murine atherosclerotic vein grafts can be prevented by TIMP-1 overexpression. PLoS One. 2012;7:e47134. doi: 10.1371/journal.pone.0047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franck G., Mawson T., Sausen G. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res. 2017;121:31–42. doi: 10.1161/CIRCRESAHA.117.310694. [DOI] [PMC free article] [PubMed] [Google Scholar]