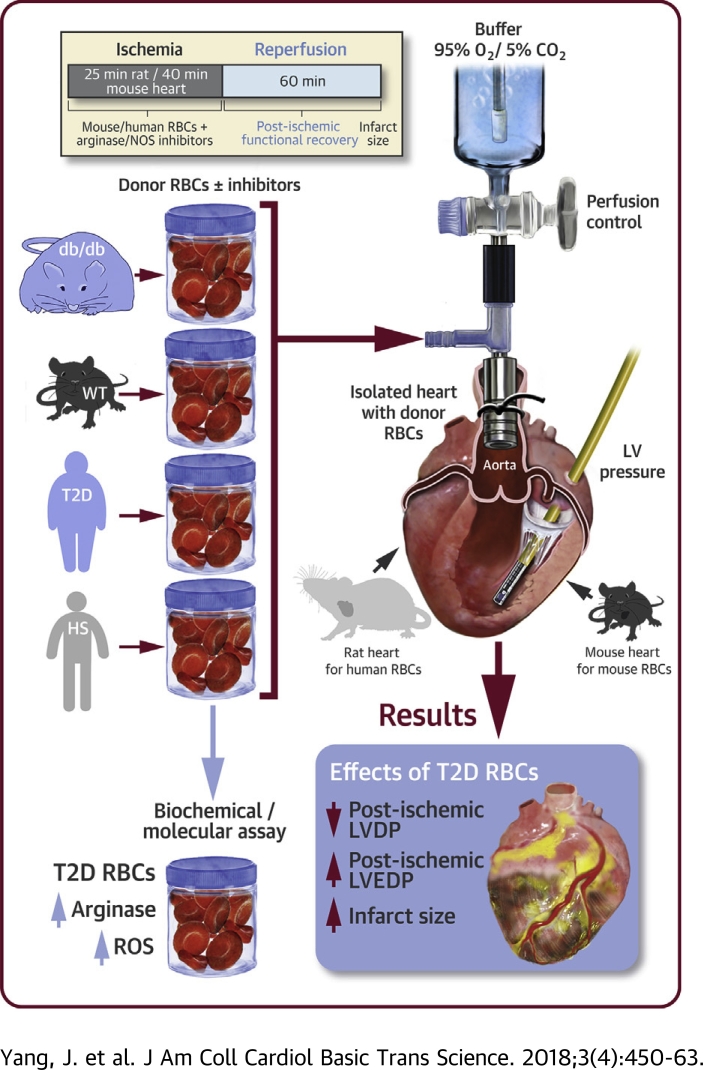
Visual Abstract
Key Words: arginase, nitric oxide synthase, reactive oxygen species, red blood cells, type 2 diabetes
Abbreviations and Acronyms: ABH, 2 (S)-amino-6-boronohexanoic acid; dP/dt, the first derivative of left ventricular pressure; eNOS, endothelial nitric oxide synthase; iNOS, inducible isoform of nitric oxide synthase; KH, Krebs-Henseleit; L-NAME, NG-nitro-L-arginine methyl ester; LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; NAC, N-acetylcysteine; NO, nitric oxide; nor-NOHA, Nω-hydroxy-nor-L-arginine; NOS, nitric oxide synthase; RBC, red blood cell; ROS, reactive oxygen species; WT, wild type
Highlights
-
•
RBCs from mice and patients with type 2 diabetes have increased arginase activity and production of reactive oxygen species.
-
•
RBCs from mice and patients with type 2 diabetes aggravate myocardial ischemia-reperfusion injury.
-
•
Inhibition of arginase in RBCs from mice and patients with type 2 diabetes improves post-ischemic myocardial recovery via reduced oxidative stress.
-
•
Inhibition of nitric oxide synthase in RBC reduces oxidative stress and restores post-ischemic myocardial functional recovery.
-
•
These data demonstrate a novel disease mechanism by which RBC drive post-ischemic cardiac dysfunction in type 2 diabetes.
Summary
This study tested the hypothesis that red blood cell (RBC) arginase represents a potential therapeutic target in ischemia-reperfusion in type 2 diabetes. Post-ischemic cardiac recovery was impaired in hearts from db/db mice compared with wild-type hearts. RBCs from mice and patients with type 2 diabetes attenuated post-ischemic cardiac recovery of nondiabetic hearts. This impaired cardiac recovery was reversed by inhibition of RBCs arginase or nitric oxide synthase. The results suggest that RBCs from type 2 diabetics impair cardiac tolerance to ischemia-reperfusion via a pathway involving arginase activity and nitric oxide synthase-dependent oxidative stress.
It is well established that type 2 diabetes is an important risk factor for development of myocardial infarction 1, 2 and for poor outcome following an acute coronary event (3). A key component contributing to cardiovascular complications including ischemic heart disease in type 2 diabetes is an altered vascular homeostasis involving reduced bioavailability of nitric oxide (NO) and increased oxidative stress 1, 2, 4, 5, 6, 7. The mechanisms behind reduced bioavailability of NO are complex and involve both reduced production by endothelial nitric oxide synthase (eNOS) and increased inactivation of NO by up-regulation of reactive oxygen species (ROS). Previously it has been demonstrated that an important regulator of NO production is arginase, which competes with eNOS for their common substrate L-arginine 8, 9. Arginase may also trigger formation of ROS by inducing uncoupling of NOS, a condition in which eNOS produces superoxide instead of NO 4, 9, 10, 11. By these mechanisms increased arginase activity is suggested to contribute to cardiovascular dysfunction.
The source of these actions of arginase in the cardiovascular system has until recently been considered to be the endothelium 8, 12. Of importance, we have recently demonstrated that arginase 1 expressed in red blood cells (RBCs) serves as a critical regulator of the formation and export of cardioprotective NO-like bioactivity produced by RBCs eNOS during ischemia-reperfusion (13). This novel source and effect of arginase was shown to be of importance for cardiac function. Thus, inhibition of RBCs arginase protects the heart from ischemia-reperfusion injury via an eNOS-dependent mechanism. Additionally, in vivo observations support a role of RBC eNOS during myocardial ischemia-reperfusion (14). However, the pathophysiological role of arginase as a regulator of NO bioavailability and ROS production in RBCs in the setting of myocardial ischemia-reperfusion under conditions with increased arginase activity remains unknown. Interestingly, increased arginase activity has emerged as an important regulator of NO formation and ROS production in diabetes 15, 16. Furthermore, a previous study has suggested that arginase is up-regulated in RBCs from patients with diabetes (17). Therefore, it is conceivable to assume that up-regulation of RBC arginase in diabetes is of functional importance for the susceptibility to myocardial ischemia-reperfusion injury. Based on these considerations, we tested the hypothesis that up-regulation of RBC arginase is a key mechanism driving reduced tolerance to myocardial ischemia-reperfusion via increased NOS-dependent ROS production in type 2 diabetes. This was tested using both RBCs from a mouse model of type 2 diabetes and RBCs from patients with type 2 diabetes in an ex vivo model of myocardial ischemia-reperfusion.
Methods
Expanded versions of the Methods are presented in the Supplemental Appendix.
Animals
The study was approved by the regional ethical committee for animal experiments (N192/12 and N108/14) and conforms to the Guide for Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Male db/db and wild-type (WT) mice and Wistar rats were purchased and housed until 15 weeks of age. Db/db mice were only included if they had a blood glucose level >15 mmol/l.
Human subjects
Blood samples were collected from 23 healthy subjects and 27 patients with type 2 diabetes (Supplemental Table 1). The investigation was conducted in accordance with the Declaration of Helsinki and was approved by the regional ethics committee (approval number 2014/463-31/3).
Heart isolation and perfusion
Following anesthesia, hearts were isolated from WT as well as db/db mice and Wistar rats and perfused in a Langendorff system 18, 19. After stabilization, the hearts were subjected to 25 min (rat hearts) or 40 min (mouse hearts) global ischemia followed by 60 min reperfusion (Supplemental Figure 1).
RBCs from mice were administered to isolated mouse hearts. Immediately following the onset of ischemia by clamping the inflow tube, 0.4 ml Krebs-Henseleit (KH) buffer or RBC suspension was injected into the coronary circulation via a side arm in the perfusion system (Supplemental Figure 1). This method allowed the RBC suspension to be present in the hearts during the ischemic period and was washed away by the reperfusion. The buffer and RBC suspension were incubated with vehicle, the arginase inhibitors Nω-hydroxy-nor-L-arginine (nor-NOHA, 1 and 3 mmol/l) and 2 (S)-amino-6-boronohexanoic acid ([ABH], 1 mmol/l), the NOS inhibitor NG-nitro-L-arginine methyl ester ([L-NAME], 0.1 mmol/l) or the combination of nor-NOHA and L-NAME for 20 min at 37°C before being administered to the hearts. When NOS and arginase inhibition were combined, L-NAME was added to the RBC suspension 5 min prior to nor-NOHA. Nor-NOHA and ABH are structurally different arginase inhibitors whereby nor-NOHA has a guanidinium chain, whereas ABH binds as a tetrahedral boronate anion (20). The concentrations were determined from pilot experiments using different concentrations of nor-NOHA demonstrating 1 mmol/l as an effective concentration and a previous publication (13).
RBCs from humans were administered in a volume of 3 ml to isolated rat hearts as described for mouse hearts. In addition, the role of the inducible isoform of NOS (iNOS) in human RBC was investigated using the specific iNOS inhibitor 1400W (0.1 mmol/l).
Determination of infarct size
Infarct size was measured by triphenyltetrazolium chloride staining in separate groups of experiments. The extent of myocardial necrosis was determined using Adobe Photoshop CS2 (Adobe Systems, Mountain View, California) by an investigator blinded to the group allocation.
Blood sampling and isolation of RBCs
Mouse whole blood was collected from the thoracic cavity after removal of the heart used in the experiment. Human whole blood was collected from the antecubital vein of healthy volunteers and patients with type 2 diabetes. RBCs were obtained by removing the plasma and buffy coat including top part of the RBC layer after centrifugation at +4°C and 1,000g for 10 min. To prepare RBC suspension, RBCs were washed 3× in KH buffer and then diluted 1:1 with KH buffer.
Effect of glucose incubation on RBC arginase expression and activity
Washed RBCs were diluted (hematocrit, 5%) with KH buffer containing either 5 mmol/l or 25 mmol/l glucose. After 24 h incubation, the RBCs were harvested and analyzed for arginase expression and activity.
Arginase protein expression
RBCs from mice were lysed and proteins were separated by 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and incubated overnight at 4°C using rabbit anti-arginase 1 antibody. Membranes were then incubated with IRDye 800-conjugated goat anti-rabbit immunoglobulin G and immunoreactive bands were visualized using infrared fluorescence.
Arginase activity
Arginase activity was determined by a colorimetric assay as previously described 21, 22. Each sample was incubated at 37°C for 1 h with L-arginine. The concentration of urea was determined using spectrophotometry.
Detection of ROS using flow cytometry
RBCs from WT or db/db mice were diluted to hematocrit 1% in phosphate-buffered saline. The RBCs were incubated with N-acetylcysteine (NAC) (1 mmol/l), ABH (0.1 mmol/l), L-NAME (0.1 mmol/l), L-arginine (3 mmol/l), or vehicle for 30 min. The fluorescent probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (10 μmol/l) was added to the RBCs and incubated for 1 h in the dark at room temperature for determination of intracellular ROS. The fluorescence (fluorescein isothiocyanate) intensity was analyzed using Beckman Coulter CyAn ADP Flow Cytometer (Brea, California). Autofluorescence induced by RBCs from WT and db/db mice was excluded by determining fluorescence in the absence of fluorescent probe.
Detection of reactive oxygen species using electron spin resonance
To further verify changes in ROS levels in RBCs, an additional approach—electron spin resonance—was used. Washed RBCs from mouse or human were diluted to hematocrit 1% with Krebs/N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid buffer. The RBCs were incubated with 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (200 μmol/l) for 30 min at 37°C and 20.9% O2 in the presence of nor-NOHA (1 mmol/l), L-NAME (0.1 mmol/l), 1400W (0.1 mmol/l), NAC (1 mmol/l), L-arginine (3 mmol/l), or vehicle before ROS formation was detected by electron spin resonance.
Statistics
The overall differences in left ventricular pressures during the reperfusion period between multiple groups were analyzed by 2-way analysis of variance with Bonferroni post hoc test. Differences between 2 groups were analyzed by unpaired or paired Student's t-test as appropriate. Shapiro-Wilk and Kolmogorov-Smirnov tests were employed for normality test before using analysis of variance or Student's t-test. All statistical analysis was calculated using GraphPad Prism version 6.05 (GraphPad, San Diego, California) or SPSS version 25 (IBM, Armonk, New York). Data are presented as mean ± SD.
Results
Mouse studies
Baseline characteristics
Body weight of db/db mice (n = 12) was higher than that of WT mice (n = 11) (51.4 ± 5.5 g vs. 28.8 ± 1.9 g; p < 0.001), whereas the mean heart weight was lower than that of WT mice (0.12 ± 0.01 g vs. 0.15 ± 0.02 g; p < 0.001). The heart weight–body weight ratio was significantly lower in db/db mice than in WT mice (0.0023 ± 0.0003 vs. 0.0054 ± 0.0007; p < 0.001). Baseline functional characteristics of the isolated hearts in the different study groups are shown in Supplemental Table 2.
Arginase expression and activity are increased in RBCs from mice with type 2 diabetes
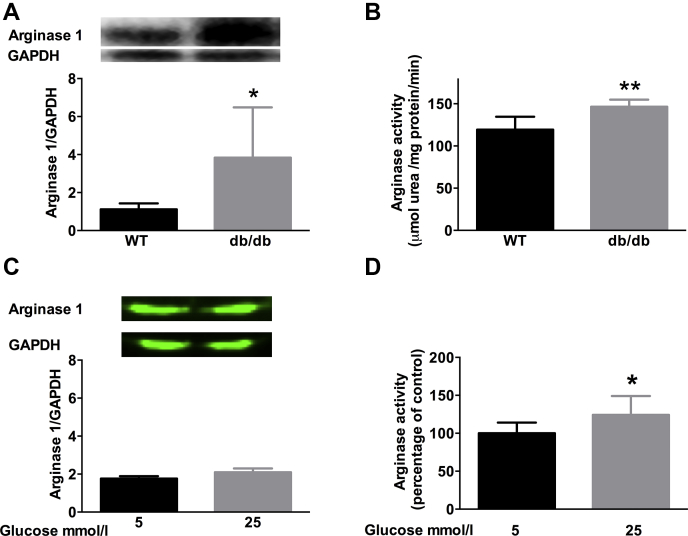
Expression of arginase 1 and arginase activity were increased in RBCs from db/db mice compared with RBCs from WT mice (Figures 1A and 1B). We have previously shown that arginase 2 is not expressed in rodent RBCs (13). Incubation with high glucose did not alter arginase expression in RBCs (Figure 1C) but it significantly increased arginase activity (Figure 1D).
Figure 1.
Arginase Expression and Activity Are Increased in RBC From Mice With Type 2 Diabetes
(A) Western blot of arginase 1 expression in red blood cells (RBCs) from wild type (WT) (n = 6) and db/db mice (n = 6). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. (B) Arginase activity in RBCs from WT (n = 4) and db/db (n = 5) mice. (C) Arginase 1 expression in RBCs from WT mice (n = 4) following incubation with 5 or 25 mmol/l glucose. (D) Arginase activity in RBCs from WT mice (n = 8) following incubation with 5 or 25 mmol/l glucose. Arginase activity in WT RBCs treated with 5 mmol/l glucose is used as standard. Significant differences between groups are shown; *p < 0.05, and **p < 0.01.
ROS production is increased in RBCs from mice with type 2 diabetes and is regulated by arginase and NOS
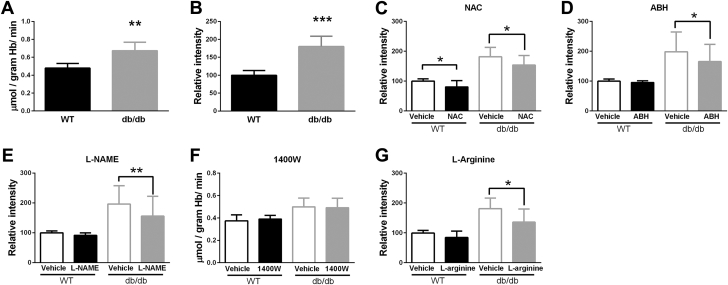
ROS production was significantly increased in RBCs from db/db mice compared with RBCs from WT mice (Figures 2A and 2B). NAC decreased ROS production in RBCs from both WT and type 2 diabetes mice (Figure 2C). Incubation of RBCs with the arginase inhibitor ABH reduced ROS production in RBCs from db/db mice but not in WT RBCs (Figure 2D), suggesting that the increased ROS production in RBCs from db/db mice involves increased arginase activity. Interestingly, the NOS inhibitor L-NAME also reduced ROS production in RBCs from db/db mice but not in WT RBCs (Figure 2E), suggesting NOS as a potential source of ROS production in RBCs from db/db mice. By contrast, the iNOS inhibitor 1400W did not alter ROS production in RBCs (Figure 2F). L-arginine also reduced ROS production in RBCs from db/db mice but not in WT RBCs (Figure 2G), suggesting the increased ROS production in diabetic RBCs is related to limited availability of L-arginine.
Figure 2.
ROS Production Is Increased in RBCs From Mice With Type 2 Diabetes and Is Regulated by Arginase and NOS
(A) Levels of reactive oxygen species (ROS) in RBCs from WT (n = 5) and db/db (n = 5) mice determined using electron spin resonance. (B to G) ROS levels in RBCs from WT and db/db mice determined using flow cytometry, (B) under basal condition (WT n = 6 and db/db n = 6), (C) following incubation with vehicle or the ROS scavenger N-acetylcysteine (NAC) (1 mmol/l, WT n = 6 and db/db n = 6), (D) following incubation with vehicle or the arginase inhibitor 2 (S)-amino-6-boronohexanoic acid (ABH) (0.1 mmol/l, WT n = 7 and db/db n = 7), (E) following incubation with vehicle or the nonselective nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (0.1 mmol/l, WT n = 8 and db/db n = 8), (F) following incubation with the inducible isoform of NOS inhibitor 1400W (0.1 mmol/l, WT n = 8 and db/db n = 8, using electron spin resonance), and (G) following incubation with vehicle or L-arginine (3 mmol/l, WT n = 5 and db/db n = 5). ROS generation in WT RBCs is used as standard in B to F. Significant differences are shown; *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviations as in Figure 1.
RBCs from db/db mice impair cardiac post-ischemic functional recovery
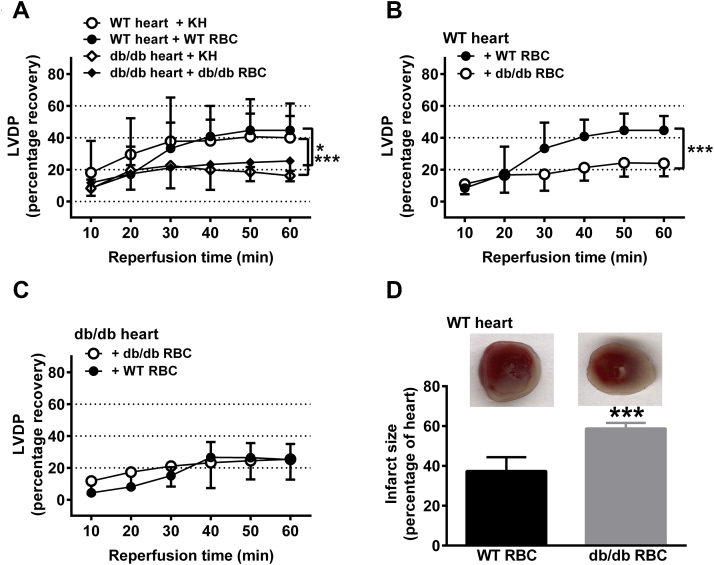
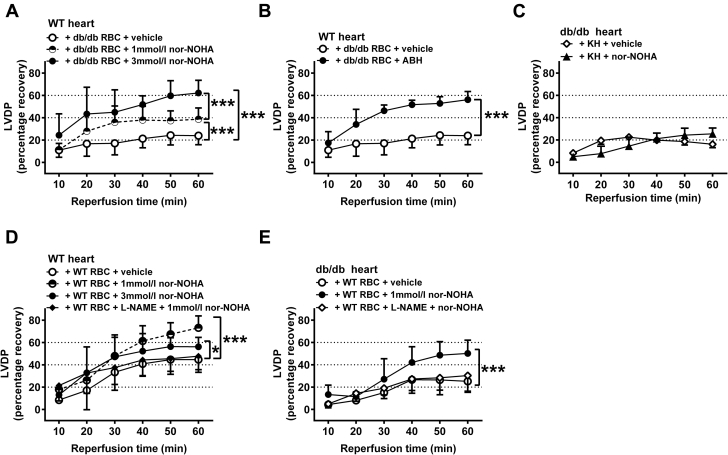
Next we investigated the functional impact of RBCs from db/db mice on cardiac post-ischemic recovery in the isolated perfused mouse heart subjected to global ischemia-reperfusion. The post-ischemic recovery of left ventricular developed pressure (LVDP) (Figure 3A), the first derivative of left ventricular pressure (dP/dt) (Supplemental Figure 2A) and left ventricular end-diastolic pressure (LVEDP) (Supplemental Figure 2D) in hearts from db/db mice were impaired in comparison with those of hearts from WT mice. Administration of RBCs from WT mice to WT hearts or administration of RBCs from db/db mice to db/db hearts did not affect post-ischemic recovery in comparison with administration of buffer only (Figure 3A, Supplemental Figures S2A and S2D). Importantly, administration of RBCs from db/db mice to WT hearts markedly reduced post-ischemic recovery of LVDP (Figure 3B) and dP/dt (Supplemental Figure 2B) to a level similar to that observed in hearts from db/db mice (Figure 3A, Supplemental Figure 2A). Further, post-ischemic LVEDP of WT hearts was elevated following administration of RBCs from db/db mice (Supplemental Figure 2E). These observations suggest that RBCs from db/db mice exert a detrimental effect on post-ischemic recovery of myocardial function. In contrast, administration of WT RBCs to db/db hearts did not affect post-ischemic recovery (Figure 3C, Supplemental Figures 2C and 2F). The supernatant from the last washing step of RBCs did not affect the post-ischemic functional recovery of isolated WT hearts (Supplemental Figure 3), excluding any effect of circulating contaminating factors including any remaining blood cells. In a separate set of experiments, the effect of RBCs from db/db mice on infarct size was determined. Infarct size was significantly larger in WT hearts given RBCs from db/db mice than in hearts given RBCs from WT mice (Figure 3D), supporting the theory that ischemia-reperfusion injury is aggravated by db/db RBCs.
Figure 3.
RBCs From db/db Mice Impair Cardiac Post-Ischemic Functional Recovery
(A) Hearts from WT mice were given Krebs-Henseleit (KH) buffer (WT heart + KH, n = 5) or RBCs from WT mice (WT heart + WT RBCs, n = 5), and hearts from db/db mice were given KH buffer (db/db heart + KH, n = 4) or RBCs from db/db mice (db/db heart + db/db RBCs, n = 9). (B) Hearts from WT mice were given RBCs from either WT (n = 5) or db/db (n = 5) mice. (C) Hearts from db/db mice were given RBCs from either WT (n = 5) or db/db (n = 6) mice. (D) Infarct size in WT hearts given RBCs from WT or db/db mice. Post-ischemic left ventricular developed pressure (LVDP) is expressed as percentage recovery from the pre-ischemic level. Significant differences between groups in A to C were analyzed using 2-way analysis of variance including all time points, whereas Student's t-test was performed in D; *p < 0.05 and ***p < 0.001. Abbreviations as in Figure 1.
RBCs from db/db mice impair cardiac post-ischemic recovery via an arginase-dependent pathway
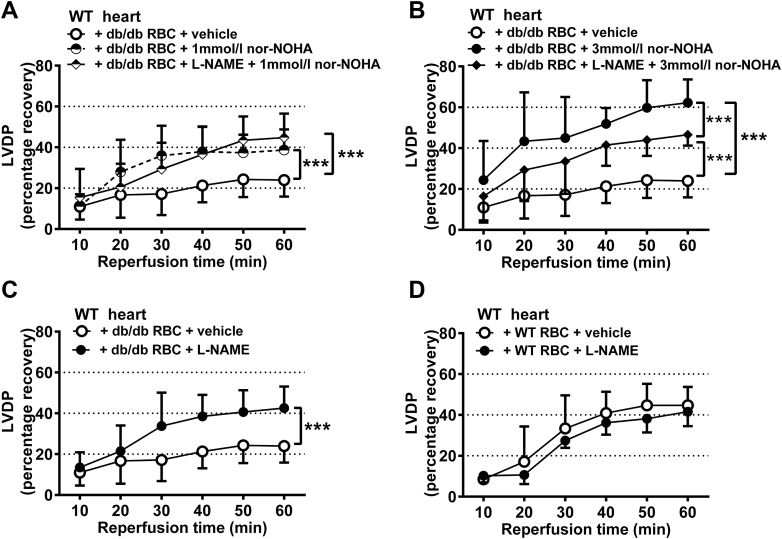
To identify the mechanisms by which RBCs from db/db mice impair cardiac post-ischemic recovery, RBCs were incubated with the arginase inhibitors nor-NOHA or ABH. Pre-treatment of RBCs from db/db mice with 1 mmol/l nor-NOHA reversed the impairment of post-ischemic myocardial recovery of LVDP (Figure 4A) as well as dP/dt (Supplemental Figure 4A) induced by db/db RBCs in WT hearts. Incubation of db/db RBCs with the higher concentration of nor-NOHA (3 mmol/l) induced a significant further improvement of post-ischemic function (Figure 4A, Supplemental Figures 4A and 5A). This concentration of nor-NOHA completely reversed the impairment induced by db/db RBCs, resulting in an LVDP that was comparable to that observed in the presence of WT RBCs. The structurally unrelated arginase inhibitor ABH also improved post-ischemic function when incubated with RBCs from db/db mice (Figures 4B and 5B, Supplemental Figures 4B). By contrast, administration of nor-NOHA to buffer-perfused db/db mouse hearts failed to alter the post-ischemic recovery (Figure 4C, Supplemental Figures 4C and 5C). Collectively, these data indicate that the increased arginase activity in RBCs contributes to the detrimental cardiac effect of RBCs from db/db mice.
Figure 4.
RBCs From db/db Mice Impair Cardiac Post-Ischemic Recovery via an Arginase-Dependent Pathway
(A) Hearts from WT mice were given RBCs from db/db mice incubated with vehicle (n = 5), Nω-hydroxy-nor-L-arginine (nor-NOHA) 1 mmol/l (n = 8) or 3 mmol/l (n = 4). (B) Hearts from WT mice were given RBCs from db/db mice incubated with vehicle (n = 5) or ABH (1 mmol/l, n = 4). (C) Hearts from db/db mice were given KH buffer with either vehicle (n = 8) or nor-NOHA (1 mmol/l, n = 5). (D) Hearts from WT mice were given RBCs from WT mice incubated with vehicle (n = 6), the arginase inhibitor nor-NOHA (1 mmol/l, n = 10), nor-NOHA (3 mmol/l, n = 8) or L-NAME (0.1 mmol/l) + nor-NOHA (1 mmol/l, n = 6). (E) Hearts from db/db mice were given RBCs from WT mice incubated with vehicle (n = 5), nor-NOHA (1 mmol/l, n = 5) or L-NAME (0.1 mmol/l) + nor-NOHA (1 mmol/l, n = 5). Post-ischemic LVDP is expressed as percentage recovery from the pre-ischemic level. Significant differences between treatments were analyzed using 2-way analysis of variance including all time points; *p < 0.05 and ***p < 0.001. Abbreviations as in Figures 1, 2, and 3.
Figure 5.
Inhibition of NOS Reverses the Impaired Cardiac Post-Ischemic Recovery Induced by db/db RBCs
(A) Hearts from WT mice were given RBCs from db/db mice incubated with vehicle (n = 5), the arginase inhibitor nor-NOHA (1 mmol/l, n = 8) or the NOS inhibitor L-NAME (0.1 mmol/l) + nor-NOHA (1 mmol/l, n = 5). (B) Hearts from WT mice were given RBCs from db/db mice incubated with vehicle (n = 5), nor-NOHA (3 mmol/l, n = 4), or L-NAME (0.1 mmol/l) + nor-NOHA (3 mmol/l, n = 6). (C) Hearts from WT mice were given RBCs from db/db mice incubated with vehicle (n = 5) or L-NAME (0.1 mmol/l, n = 5). (D) Hearts from WT mice were given RBCs from WT mice incubated with vehicle (n = 6) or L-NAME (0.1 mmol/l, n = 5). Post-ischemic LVDP is expressed as percentage recovery from the pre-ischemic level. Significant differences between treatments were analyzed using 2-way analysis of variance including all time points; ***p < 0.001. Abbreviations as in Figures 1, 2, 3, and 4.
Pre-treatment of WT RBCs with 1 mmol/l nor-NOHA improved the post-ischemic recovery of LVDP (Figure 4D), dP/dt, and LVEDP (Supplemental Figures 4D and 5D). However, in contrast to the observation using RBCs from db/db mice, pre-treatment of WT RBCs with 3 mmol/l nor-NOHA did not induce further protection (Figure 4D, Supplemental Figures 4D and 5D). Nor-NOHA also improved post-ischemic functional recovery when WT RBCs were given to db/db hearts (Figure 4E, Supplemental Figures 4E and 5E).
Inhibition of RBC arginase induces cardioprotection via a NOS-dependent mechanism
Given the findings that RBCs from db/db mice impair cardiac post-ischemic recovery via an arginase-dependent pathway, we next tested whether inhibition of RBC arginase induces cardioprotection via a NOS-dependent mechanism. To this end, RBCs were administered to isolated hearts following incubation with the NOS inhibitor L-NAME and arginase inhibition. The cardioprotective effects induced by inhibition of arginase in WT RBCs given to WT hearts and db/db hearts were completely abolished by inhibition of NOS (Figures 4D and 4E, Supplemental Figures 4D, 4E, 5D, and 5E). Interestingly, when db/db RBCs were given to WT hearts, NOS inhibition did not affect the improvement in recovery induced by 1 mmol/l nor-NOHA (Figure 5A, Supplemental Figures 6A and 6C), but partially blocked the protection induced by 3 mmol/l nor-NOHA (Figure 5B, Supplemental Figures 6B and 6D), suggesting a significant but partial role of NOS in signaling the cardioprotective effects of the higher dose of arginase inhibitor in the presence of RBCs from mice with type 2 diabetes.
Inhibition of NOS reverses the impaired cardiac post-ischemic recovery induced by db/db RBCs
To further determine the role of RBC NOS in the impairment of post-ischemic cardiac recovery in type 2 diabetes, RBCs from db/db mice were pre-treated with the NOS inhibitor L-NAME. L-NAME improved the cardiac post-ischemic recovery in the presence of RBCs from db/db mice (Figure 5C, Supplemental Figures 7A and 7C). However, L-NAME pre-treatment had no effect when given with WT RBCs (Figure 5D, Supplemental Figures 7B and 7D). These results indicate that RBC NOS per se contributes to the detrimental effect of RBCs from db/db mice on cardiac post-ischemic recovery.
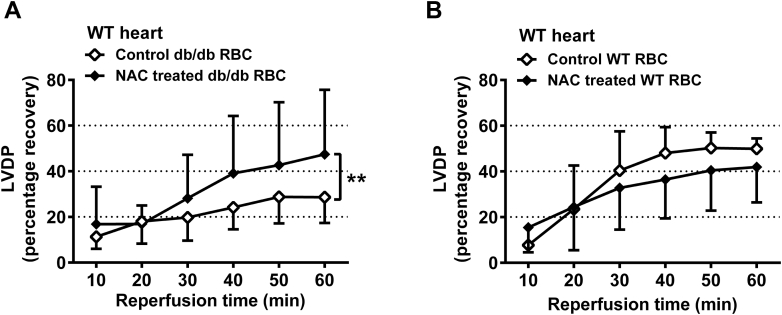
Antioxidant treatment reverses impaired cardiac post-ischemic recovery induced by db/db RBCs
To determine the functional role of increased oxidative stress in RBCs from type 2 diabetes mice, RBCs from NAC-treated WT or db/db mice were given to isolated WT hearts. NAC treatment abolished the impairment in cardiac post-ischemic recovery induced by RBCs from db/db mice (Figure 6A, Supplemental Figures 8A and 8C). By contrast, NAC treatment did not affect cardiac post-ischemic recovery in the presence of RBCs from WT mice (Figure 6B, Supplemental Figures 8B and 8D).
Figure 6.
Effects of Antioxidant on the Impaired Recovery of LVDP Induced by db/db RBCs
(A) Hearts from WT mice were given RBCs from db/db mice treated with vehicle (n = 7) or NAC (n = 7) for 4 weeks. (B) Hearts from WT mice were given RBCs from WT mice treated with vehicle (n = 6) or NAC (n = 7) for 4 weeks. LVDP is expressed as percentage recovery from the pre-ischemic level. Significant differences between treatments were analyzed using 2-way analysis of variance including all time points; **p < 0.01. Abbreviations as in Figures 1, 2, and 3.
Human study
Basal characteristics
Basal clinical characteristics of patients with type 2 diabetes and age-matched healthy subjects are shown in Supplemental Table 1. The patients with type 2 diabetes had higher body mass index, fasting glucose, glycated hemoglobin, and triglycerides, while they had lower total cholesterol, high-density lipoprotein, and low-density lipoprotein cholesterol in comparison with the healthy subjects. None of the healthy subjects was on medication.
ROS production is increased in RBCs from patients with type 2 diabetes and is regulated by arginase and NOS
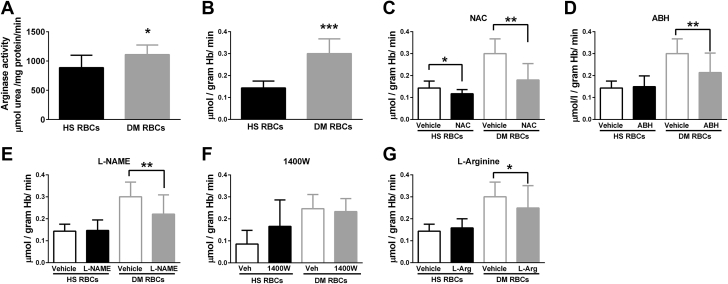
To make the important translation of the observations in mice to the clinical setting, we determined arginase activity, ROS production, and the influence of RBCs from patients with type 2 diabetes on cardiac function. Arginase activity and ROS production were increased in RBCs from patients with type 2 diabetes compared with RBCs from control subjects (Figures 7A and 7B). NAC reduced ROS production in RBCs from both healthy subjects and patients with type 2 diabetes (Figure 7C). Incubation of RBCs with the arginase inhibitor nor-NOHA reduced ROS production in RBCs from patients with type 2 diabetes but not in RBCs from healthy subjects (Figure 7D), suggesting that the increased ROS production in RBCs from patients with type 2 diabetes is arginase-dependent. Interestingly, the NOS inhibitor L-NAME significantly reduced ROS production in RBCs from patients with type 2 diabetes but not in RBCs from healthy subjects (Figure 7E), suggesting NOS as a source of ROS production in RBCs from patients with type 2 diabetes. The iNOS-specific inhibitor 1400W failed to alter ROS production in RBCs (Figure 7F). L-arginine also reduced ROS production in RBCs from patients with types 2 diabetes but not in healthy RBCs (Figure 7G).
Figure 7.
Arginase Activity and ROS Production Are Increased in RBCs From Patients With Type 2 Diabetes
(A) Arginase activity in RBCs from healthy subjects (HS, n = 9) and patients with type 2 diabetes (DM) (n = 9). (B to G) ROS production determined by electron spin resonance in RBCs from HS and DM (B) under basal condition (HS n = 6 and DM n = 6), (C) following incubation with vehicle (Veh) or the ROS scavenger NAC (1 mmol/l, HS n = 6 and DM n = 6), (D) following incubation with vehicle or the arginase inhibitor nor-NOHA (0.1 mmol/l, HS n = 6 and DM n = 6), (E) following incubation with vehicle or the nonselective NOS inhibitor L-NAME (0.1 mmol/l, HS n = 6 and DM n = 6), (F) following incubation with vehicle or the iNOS inhibitor 1400W (0.1 mmol/l, HS n = 5 and DM n = 6), and (G) following incubation with vehicle or L-arginine (L-Arg) (3 mmol/l, HS n = 6 and DM n = 6). Significant differences from vehicle or WT RBCs are shown; *p < 0.05, **p < 0.01 and ***p < 0.001. Abbreviations as in Figures 1, 2, and 4.
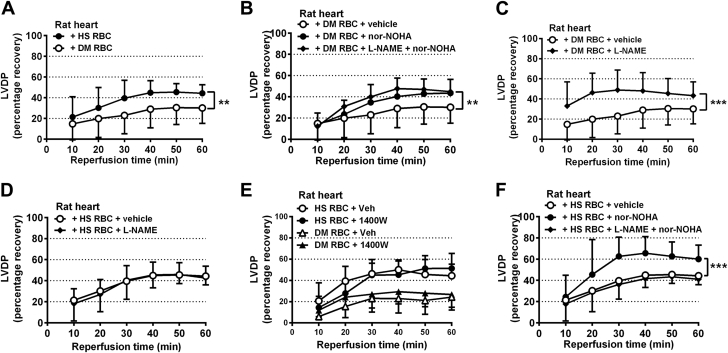
RBCs from patients with type 2 diabetes impair cardiac post-ischemic recovery via arginase and NOS
In line with the data obtained with RBCs from db/db mice, administration of RBCs from patients with type 2 diabetes markedly reduced post-ischemic recovery of LVDP (Figure 8A) and dP/dt (Supplemental Figure 9A) in isolated rat hearts. Further, RBCs from patients with type 2 diabetes elevated post-ischemic LVEDP (Supplemental Figure 10A). This observation suggests a detrimental effect of RBCs from patients with type 2 diabetes on post-ischemic myocardial functional recovery. Pre-treatment of RBCs from patients with type 2 diabetes with nor-NOHA completely reversed the impairment of post-ischemic myocardial recovery of LVDP (Figure 8B), dP/dt, and LVEDP (Supplemental Figures 9B and 10B). The NOS inhibitor L-NAME did not affect the protection induced by arginase inhibitor in RBCs from patients with type 2 diabetes. These data indicate that the increased arginase activity in RBCs is important for the detrimental cardiac effect of RBCs from patients with type 2 diabetes.
Figure 8.
RBCs From Patients With Type 2 Diabetes Impair Post-Ischemic Cardiac Function of Isolated Rat Hearts via an Arginase and NOS-Dependent Mechanism
Rat hearts were given (A) RBCs from either HS (n = 9) or patients with DM (n = 13), (B) RBCs from patients with DM and incubated with vehicle (n = 13), nor-NOHA (1 mmol/l, n = 12), or L-NAME (0.1 mmol/l) + nor-NOHA (1 mmol/l, n = 6), (C) RBCs from DM and incubated with vehicle (n = 13) or L-NAME (0.1 mmol/l, n = 5), (D) RBCs from HS and incubated with vehicle (n = 9) or L-NAME (0.1 mmol/l, n = 9), (E) RBCs from HS and incubated with vehicle (n = 6) or 1400W (0.1 mmol/l, n = 5) or RBCs from DM and incubated with vehicle (n = 5) or 1400W (n = 5), or (F) RBCs from HS incubated with vehicle (n = 9), nor-NOHA (1 mmol/l, n = 9), or L-NAME (0.1 mmol/l) + nor-NOHA (1 mmol/l, n = 9). Significant differences between treatments were analyzed using 2-way analysis of variance including all time points; *p < 0.05 and ***p < 0.001. Abbreviations as in Figures 1, 2, 3, and 4 and 7.
To further clarify the role of RBC NOS for the impairment of post-ischemic cardiac recovery in type 2 diabetes, RBCs from patients with type 2 diabetes were pre-treated with the nonselective NOS inhibitor L-NAME or the iNOS-selective inhibitor 1400W before being given to the isolated rat hearts. L-NAME significantly improved the cardiac post-ischemic recovery in the presence of RBCs from patients with type 2 diabetes (Figure 8C, Supplemental Figures 9C and 10C), but did not affect the cardiac post-ischemic recovery in the presence of RBCs from healthy subjects (Figure 8D, Supplemental Figure 9D and 10D). The iNOS inhibitor 1400W did not affect the cardiac post-ischemic recovery in the presence of RBCs from healthy subjects or patients with type 2 diabetes (Figure 8E, Supplemental Figures 9E and 10E).
Pre-treatment of RBCs from healthy subjects with nor-NOHA also improved the post-ischemic recovery of LVDP (Figure 8F), dP/dt, and LVEDP (Supplemental Figures 9F and 10F), an effect that was abolished by NOS inhibition (Figure 8F).
Discussion
The main findings of the present study are that RBCs from both mice and patients with type 2 diabetes have up-regulated arginase activity and ROS production, which aggravate myocardial ischemia-reperfusion injury. Furthermore, inhibition of arginase in RBCs from mice and patients with type 2 diabetes reduces ROS production and improves post-ischemic myocardial recovery. Finally, inhibition of NOS in RBCs reduces ROS production and restores post-ischemic myocardial functional recovery. These data demonstrate a novel disease mechanism in type 2 diabetes by which RBC impair post-ischemic cardiac function. This effect is driven by up-regulation of RBC arginase 1 that induces a NOS-dependent increase in ROS production.
Functional role of RBC arginase in ischemia-reperfusion and diabetes
The involvement of RBCs in the regulation of cardiovascular function including interactions between RBCs and the endothelium has been proposed previously 23, 24. It has long since been suggested that RBCs export bioactive NO (25) and that RBCs contain eNOS protein (26). However, it has been seriously questioned whether eNOS-derived NO is functionally active because it is expected to be rapidly scavenged by hemoglobin 27, 28. It is therefore of interest that RBCs were shown to protect the isolated heart from ischemia-reperfusion injury via a NOS-dependent mechanism (29). Furthermore, mice with blood cells lacking eNOS have lower circulating nitrite and nitrate levels and develop larger infarcts following ischemia-reperfusion than control mice, supporting a role of RBC eNOS also under in vivo conditions 14, 30. In a recent study, we demonstrated a novel function of RBC whereby these cells export cardioprotective NO bioactivity under tight regulation of arginase (13). However, the pathophysiological function of RBC arginase in the setting of diabetes and cardiovascular disease with elevated arginase activity has not been investigated. Endothelial cell arginase has been described to be up-regulated in various experimental models of diabetes via mechanisms related to hyperglycemia and reactive oxygen and nitrogen species 15, 16. Up-regulation of arginase activity results in reduced NO production by competing with eNOS for their common substrate L-arginine (8) and increased production of ROS by eNOS due to uncoupling of eNOS 11, 31. Given this, it was hypothesized that RBC arginase is up-regulated in type 2 diabetes and that this change contributes to impaired myocardial recovery following ischemia-reperfusion. In line with this hypothesis, we demonstrate that the expression and activity of RBC arginase are increased in type 2 diabetes, confirming a previous observation (17). Arginase activity was also stimulated by high glucose in WT RBCs, which is in line with observations in vitro and in vivo, but it remains to be established whether additional factors including ROS, dyslipidemia, and insulin 16, 32 in diabetes contribute to the increase in arginase expression and activity observed in the RBCs collected from diabetic animals and patients. Our findings suggest that this alteration of RBC in diabetes has important implications for cardiac dysfunction. Employing the isolated heart subjected to ischemia-reperfusion, we show that post-ischemic functional recovery is markedly impaired when RBCs from db/db mice or patients with type 2 diabetes were given to isolated WT hearts. Furthermore, RBCs from db/db mice increased infarct size in WT hearts. Inhibition of arginase using 2 structurally different inhibitors reversed this impairment in a concentration-dependent manner, suggesting that RBC arginase is a key mediator of the depressant effect of RBCs on post-ischemic cardiac function in db/db mice. Interestingly, arginase inhibitors given with buffer only (i.e., in the absence of RBCs) do not alter the post-ischemic cardiac recovery during perfusion, indicating that the cardioprotective effect of arginase inhibition is mediated via an effect on RBC arginase.
Role of RBC ROS
The present study shows that ROS production is increased in RBCs from both mice and patients with type 2 diabetes. Pre-treatment with the ROS scavenger NAC abolished the impairment of cardiac post-ischemic recovery induced by RBCs from db/db mice, suggesting that ROS plays a key role in the detrimental effect of diabetic RBCs on cardiac post-ischemic recovery. Generally, the sources of ROS include mitochondria, xanthine oxidase, and nicotinamide adenine dinucleotide phosphate oxidases (33). In RBCs, auto-oxidation of hemoglobin is also an important source of ROS generation (34). Because mature RBCs lack a nucleus and most organelles, mitochondria may not contribute to the overproduction of ROS. Our data suggest the increased ROS in RBCs from type 2 diabetes is dependent on arginase 1 and NOS, because both arginase and NOS inhibition diminished ROS production in diabetic RBCs. Of the 3 NOS isoforms, eNOS and iNOS, but not neuronal NOS, are present in RBCs (35). Because inhibition of iNOS did not alter ROS production in RBCs from either db/db mice or patients with diabetes, the present results suggest that RBC eNOS contributed to ROS production in these RBCs. Previous studies have demonstrated that arginase stimulates ROS production in endothelial cells by inducing uncoupling of eNOS 10, 16. The present data suggest a similar mechanism in RBC and that this mechanism is important for post-ischemic myocardial dysfunction in type 2 diabetes as demonstrated by the protective effect of both arginase and eNOS inhibition against cardiac post-ischemic dysfunction induced by diabetic RBCs.
Role of RBC NOS
The involvement of RBC NOS during ischemia-reperfusion and in the cardioprotective effect of RBC arginase inhibition was also investigated. Nonselective NOS inhibition with L-NAME, but not selective iNOS inhibition, improved post-ischemic cardiac recovery in the presence of diabetic RBCs, suggesting that eNOS activity contributes to RBC-induced cardiac dysfunction. This observation is in line with the inhibitory effect of L-NAME, but not 1400W, on ROS formation by diabetic RBCs. In line with our previous observations (13), the cardioprotective effects of arginase inhibition in RBCs from normoglycemic WT mice or healthy subjects were abolished following nonselective NOS inhibition indicating that the cardioprotective effect of arginase inhibition is mediated via increased NOS-dependent NO bioavailability. Importantly, the cardioprotective effect of the high concentration of the arginase inhibitor nor-NOHA (3 mmol/l) in db/db RBCs given to WT hearts was partly blocked by NOS inhibition. These observations, combined with the demonstration that arginase inhibition reduced ROS production in RBCs from db/db mice and patients with type 2 diabetes, suggest that arginase inhibition not only reduces NOS-dependent ROS production but also at high concentrations induces additional NOS-dependent protection via increased NO-like bioactivity.
Study limitations
Because this study was performed on isolated hearts ex vivo, it cannot, from the present data, be determined to what extent RBCs contribute to cardiovascular dysfunction in vivo. The relative contribution of RBC arginase to the protective effect induced by arginase inhibition on ischemia-reperfusion injury demonstrated in experimental animals in vivo 22, 36 and in patients with coronary artery disease (37) therefore remains to be established. Another limitation is that patients with type 2 diabetes had comorbidities and medication that affects oxidative stress and RBC function. However, similar effects were induced with RBCs from db/db mice, suggesting that these factors were of little influence. Further, several of the antidiabetic and cardiovascular drugs (metformin, glucagon-like peptide- 1 agonists, angiotensin-converting enzyme inhibitors, and receptor antagonist and statins) in the diabetes group are known to exert beneficial effects on endothelial function. It is therefore possible that these drugs may underestimate the negative effect of RBCs from the diabetes patients.
Conclusions
As schematically illustrated in Supplemental Figure 11, the present study demonstrates a novel disease mechanism by which RBCs from patients and mice with type 2 diabetes induce impaired cardiac tolerance to ischemia-reperfusion. This effect is mediated by increased arginase activity driving eNOS-derived ROS production by diabetic RBCs. Inhibition of RBC arginase in type 2 diabetes not only reduces NOS-dependent ROS production, but also at high concentrations induces NOS-dependent protection though increased NO-like bioactivity as in normoglycemic conditions. This novel disease mechanism is of importance for the development of impaired post-ischemic cardiac function and injury in type 2 diabetes, and the results suggest that RBC arginase is a potential therapeutic target for the treatment of cardiovascular complications in diabetes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The present study addresses the important clinical problem that patients with type 2 diabetes are at increased risk of suffering from a myocardial infarction and have a poor outcome following the infarction. Key mechanisms behind this are unclear. Our data demonstrate a novel disease mechanism by which RBCs impair post-ischemic cardiac function in type 2 diabetes by a mechanism driven by increased RBC arginase activity and increased production of ROS from NOS. Targeting arginase in RBCs may represent a novel therapeutic option for ischemic heart disease in type 2 diabetes.
TRANSLATIONAL OUTLOOK: The present study provides important translational aspects by the demonstration that not only RBCs from type 2 diabetes mice, but also RBCs from patients with type 2 diabetes exert a negative effect on post-ischemic cardiac recovery. This finding suggests that the observed functional changes of RBCs in type 2 diabetes are of significant relevance for human disease. We also show that administration of normal RBCs to an isolated heart does not alter cardiac post-ischemic recovery per se. Thus, the isolated heart model permits detailed analysis of the functional pathophysiological impact of RBC arginase and NOS by administration of RBCs from healthy individuals or patients with type 2 diabetes.
Acknowledgments
The authors gratefully acknowledge the superb technical assistance of Pellina Janson and Marita Wallin.
Footnotes
This work was supported by Swedish Research Council (2016-01284 to Dr. Pernow), the Swedish Heart and Lung Foundation (20160239 to Dr. Pernow), the Stockholm County Council (20160084 to Dr. Pernow), Karolinska Institutet/Stockholm County Council Strategic Cardiovascular Programme (20120741 to Dr. Pernow), Söderberg Foundation (M60/15 to Dr. Pernow), Family Erling-Persson Foundation (to Dr. Brismar), and the Diabetes Research and Wellness Foundation (720-1519-16 to Dr. Pernow), EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action (to Dr. Pernow) and Swedish research Council (2013-66-104153-33, to Dr. Catrina). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Jiangning Yang, Email: Jiangning.Yang@ki.se.
John Pernow, Email: John.Pernow@ki.se.
Appendix
References
- 1.Moreno P.R., Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004;44:2293–2300. doi: 10.1016/j.jacc.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 2.Paneni F., Beckman J.A., Creager M.A., Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryden L., Grant P.J., Anker S.D. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 4.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. 837a–d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S.P., Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Porcel M., Lerman L.O., Herrmann J., Sawamura T., Napoli C., Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003;23:885–891. doi: 10.1161/01.ATV.0000069209.26507.BF. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G., Boengler K., Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 8.Pernow J., Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98:334–343. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell R.B., Toque H.A., Narayanan S.P., Caldwell R.W. Arginase: an old enzyme with new tricks. Trends Pharmacol Sci. 2015;36:395–405. doi: 10.1016/j.tips.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.H., Bugaj L.J., Oh Y.J. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol (1985) 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuehr D., Pou S., Rosen G.M. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–14536. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 12.Bagi Z., Feher A., Dou H., Broskova Z. Selective up-regulation of arginase-1 in coronary arteries of diabetic patients. Front Immunol. 2013;4:293. doi: 10.3389/fimmu.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Gonon A.T., Sjoquist P.O., Lundberg J.O., Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci U S A. 2013;110:15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merx M.W., Gorressen S., van de Sandt A.M. Depletion of circulating blood NOS3 increases severity of myocardial infarction and left ventricular dysfunction. Basic Res Cardiol. 2014;109:398. doi: 10.1007/s00395-013-0398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronros J., Jung C., Lundberg J.O., Cerrato R., Ostenson C.G., Pernow J. Arginase inhibition restores in vivo coronary microvascular function in type 2 diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300:H1174–H1181. doi: 10.1152/ajpheart.00560.2010. [DOI] [PubMed] [Google Scholar]
- 16.Romero M.J., Platt D.H., Tawfik H.E. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang M., Ding Y., Su Y., Hu X., Li J., Zhang Z. Arginase-flotillin interaction brings arginase to red blood cell membrane. FEBS Lett. 2006;580:6561–6564. doi: 10.1016/j.febslet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Yang J.N., Tiselius C., Dare E., Johansson B., Valen G., Fredholm B.B. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine a1 receptors. Acta Physiol (Oxf) 2007;190:63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- 19.Tahepold P., Vaage J., Starkopf J., Valen G. Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg. 2003;125:650–660. doi: 10.1067/mtc.2003.36. [DOI] [PubMed] [Google Scholar]
- 20.Steppan J., Nyhan D., Berkowitz D.E. Development of novel arginase inhibitors for therapy of endothelial dysfunction. Front Immunol. 2013;4:278. doi: 10.3389/fimmu.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz D.E., White R., Li D. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 22.Gonon A.T., Jung C., Katz A. Local arginase inhibition during early reperfusion mediates cardioprotection via increased nitric oxide production. PLoS One. 2012;7:e42038. doi: 10.1371/journal.pone.0042038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortese-Krott M.M., Kelm M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2014;2:251–258. doi: 10.1016/j.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yedgar S., Koshkaryev A., Barshtein G. The red blood cell in vascular occlusion. Pathophysiol Haemost Thromb. 2002;32:263–268. doi: 10.1159/000073578. [DOI] [PubMed] [Google Scholar]
- 25.Jia L., Bonaventura C., Bonaventura J., Stamler J.S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbongard P., Schulz R., Rassaf T. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs A.J., Gladwin M.T., Patel R.P., Williams D.L., Butler A.R. Haemoglobin: no transporter, no inactivator or none of the above? Trends Pharmacol Sci. 2002;23:406–411. doi: 10.1016/s0165-6147(02)02067-9. [DOI] [PubMed] [Google Scholar]
- 28.Joshi M.S., Ferguson T.B., Jr., Han T.H. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B.C., Nichols W.W., Mehta J.L. Cardioprotective effects of red blood cells on ischemia and reperfusion injury in isolated rat heart: release of nitric oxide as a potential mechanism. J Cardiovasc Pharmacol Ther. 1996;1:297–306. doi: 10.1177/107424849600100405. [DOI] [PubMed] [Google Scholar]
- 30.Wood K.C., Cortese-Krott M.M., Kovacic J.C. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler Thromb Vasc Biol. 2013;33:1861–1871. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceylan-Isik A.F., Guo K.K., Carlson E.C. Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension. 2009;53:1023–1031. doi: 10.1161/HYPERTENSIONAHA.108.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashyap S.R., Lara A., Zhang R., Park Y.M., DeFronzo R.A. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care. 2008;31:134–139. doi: 10.2337/dc07-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J.M., Shah A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn V., Diederich L., Keller T.C.S. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal. 2017;26:718–742. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jubelin B.C., Gierman J.L. Erythrocytes may synthesize their own nitric oxide. Am J Hypertens. 1996;9:1214–1219. doi: 10.1016/S0895-7061(96)00257-9. [DOI] [PubMed] [Google Scholar]
- 36.Jung C., Gonon A.T., Sjoquist P.O., Lundberg J.O., Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res. 2010;85:147–154. doi: 10.1093/cvr/cvp303. [DOI] [PubMed] [Google Scholar]
- 37.Kovamees O., Shemyakin A., Pernow J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS One. 2014;9:e103260. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.