Visual Abstract
Key Words: atherosclerosis, biomarkers, intraplaque hemorrhage, omics analyses, translational studies
Abbreviations and Acronyms: BiKE, Biobank of Karolinska Endarterectomies; BLVR, biliverdin reductase; CAC, coronary artery calcium; CEA, carotid endarterectomy; Hb, hemoglobin; HMOX, heme oxygenase; Hp, haptoglobin; IPH, intraplaque hemorrhage; LC-MS/MS, liquid chromatography mass spectrometry/mass spectrometry; mRNA, messenger ribonucleic acid; TMT, tandem mass tags
Highlights
-
•
Multiomics profiling of gene and protein expression in carotid plaques, together with protein expression in peripheral and “local” plasma sampled around the lesion, was performed using a large cohort of patients from BiKE in discovery analyses.
-
•
BLVRB was identified as a biomarker of intraplaque hemorrhage and plaque instability in carotid atherosclerosis that was further validated in population samples.
-
•
BLVRB was characterized as a hitherto unappreciated key enzyme, functionally linked with HMOX1, in the hemoglobin catabolism under pathological conditions.
-
•
The novel translational approach applied for biomarker discovery in this study permits causal interpretation of candidates directly in relation to the underlying disease and paves the way for similar investigations in other vascular territories.
Summary
Clinical tools to identify individuals with unstable atherosclerotic lesions are required to improve prevention of myocardial infarction and ischemic stroke. Here, a systems-based analysis of atherosclerotic plaques and plasma from patients undergoing carotid endarterectomy for stroke prevention was used to identify molecular signatures with a causal relationship to disease. Local plasma collected in the lesion proximity following clamping prior to arteriotomy was profiled together with matched peripheral plasma. This translational workflow identified biliverdin reductase B as a novel marker of intraplaque hemorrhage and unstable carotid atherosclerosis, which should be investigated as a potential predictive biomarker for cardiovascular events in larger cohorts.
Atherosclerosis is a slowly progressing disease where an acute event, such as myocardial infarction or ischemic stroke, can be the first clinical manifestation. Cerebral thromboembolism from unstable atherosclerotic plaques in the carotid bifurcation is a common cause of stroke, and large clinical studies have shown that surgery for carotid stenosis, carotid endarterectomy (CEA), prevents stroke in patients with symptoms of cerebral embolism (1). Neither laboratory tests nor imaging are today clinically established for precise detection of vulnerable individuals or lesions, and selection of patients for intervention instead relies on surrogate variables with moderate predictive power, such as the degree of luminal narrowing by the stenosis (2). Identification of new molecular signatures for the unstable carotid atheroma can aid development of biomarkers to accurately phenotype patients and lesions and improve stroke prevention.
The unstable atheroma is characterized by enhanced inflammation, extracellular matrix degradation, cell apoptosis, and thinning of the fibrous cap, which lead to plaque rupture, atherothrombosis, and clinical manifestations (3). Another feature is vascularization from the adventitia, with immature and leaky neovessels. The mechanism of neoangiogenesis is slow, but can be observed early in atherosclerosis, regulated by lipid mediators leading to the smooth muscle overexpression of vascular endothelial growth factor, which then guides centripetal sprouting of adventitial vessels in the direction of the intimal lesion (4). Intraplaque hemorrhage (IPH) has been associated with the evolution of atherothrombosis in complex lesions and recognized as a consequence of plaque neovascularization, increased microvascular permeability, or extravasation of hemoglobin (Hb) due to microruptures (5).
A number of candidate biomarkers have been proposed for risk prediction of myocardial infarction and stroke; however, none of them have been firmly established in clinical practice, and new strategies are warranted 6, 7. Large-scale profiling technologies hold promise for discovery of new biomarkers, where liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS) and antibody-based technologies together provide quantification of abundant proteins as well as detection of less abundant ones, without the need for depletion of predominant proteins before analyses 8, 9. So far, biomarker studies have largely relied on analysis of proteins in peripheral blood; nevertheless, this traditional approach may be suboptimal for the discovery of disease-relevant targets because protein levels in peripheral blood can be at the borderline of detection and dependent on the accuracy of analytical methods, in addition to being less specific for the disease of origin (10).
Here, we developed a novel pipeline for identification of biomarkers for carotid atheroma by combining untargeted transcriptomic and proteomic profiling of plaques and plasma from patients with carotid stenosis undergoing stroke-preventive CEA, utilizing our Biobank of Karolinska Endarterectomies (BiKE) (11). Differentially regulated candidates were examined in plaques by gene expression microarrays and LC-MS/MS, and in plasma employing highly multiplexed affinity proteomic assays. We utilized peripheral as well as “local” plasma, retrieved in the proximity of the lesion during vessel clamping prior to arteriotomy, with the hypothesis that local samples are enriched with lesion-derived proteins. Our analysis revealed enrichment of biliverdin reductase (BLVR) B in plaques and plasma from patients with carotid atherosclerosis, which was associated with IPH and previously never described in the disease. This study illustrates a novel translational research platform for the identification of molecular signatures of carotid atherosclerosis and identifies BVLRB as a plausible plasma biomarker of end-stage vulnerable plaques, which should be explored in large independent cohorts.
Methods
The BiKE cohort and human tissue material
Patients undergoing surgery for symptomatic or asymptomatic high-grade (>50% NASCET [North American Symptomatic Carotid Endarterectomy Trial]) (12) carotid stenosis at the Department of Vascular Surgery, Karolinska University Hospital, and the Section for Vascular Surgery at the Department of Surgery, Södersjukhuset Hospital, Stockholm, Sweden, were consecutively enrolled in the study and clinical data were recorded on admission. Symptoms of plaque instability were defined as transient ischemic attack, minor stroke, and amaurosis fugax (retinal transient ischemic attack). Patients without qualifying symptoms within 6 months prior to surgery were categorized as asymptomatic, and the indication for CEA was based on results from the ACST (Asymptomatic Carotid Surgery Trial) (13). CEA (carotid plaques) and blood samples were collected at surgery and retained within BiKE. Patients with severe disability after major stroke are excluded from this cohort, because the remaining cost-benefit of stroke prevention is limited and does not outweigh the risk of surgery. In the study group, patients with atrial fibrillation were also excluded to minimize analysis of carotid lesions in patients with symptoms from cardioembolic rather than atheroembolic origin. For microarray analyses, only ribonucleic acid material of high quality with respect to purity and integrity (RNA Integrity Number [RIN] 7 to 10, A260 to 280 1.7 to 2.0, and Absorbance [A]260/230 0.7 to 1.5) isolated from carotid endarterectomies, was used. Nonatherosclerotic, further referred to as normal artery, control samples (n = 15 in total) were obtained from macroscopically disease-free iliac arteries and 1 aorta from organ donors without history of cardiovascular disease. For immunohistochemistry, 1 control internal carotid artery from a 61-year-old man undergoing surgical excision of a neck tumor was used. The BiKE study cohort demographics, details of sample processing, and full microarray analyses have been previously described 11, 14. The microarray expression dataset is available from Gene Expression Omnibus (GSE21545). The BiKE database was merged with the Swedish Hospital Discharge Register and the Swedish Cause of Death Register for follow-up of major adverse cardiovascular and cerebrovascular events, as previously reported (15). All human samples and patient data were collected with informed consent from patients, organ donors, or their guardians. All human studies were approved by the regional ethical committee.
Sampling of local and peripheral blood and plasma processing
CEA was performed in local anesthesia with sedation to permit periprocedural monitoring of neurology according to established clinical practice (16). After dissection of the superior thyroid artery and the common, internal, and external carotid arteries, the vessels were clamped for 5 min to assess cerebral collateral circulation and possible need for carotid shunting. Local blood samples were only retrieved from patients tolerating test occlusion without signs of neurological deficit, by evacuating blood (1 to 2 ml) assembled within the arterial compartment in between the vessel clamps with a 5-ml syringe and a 23-gauge needle (Figure 1). Concomitantly, 10 ml of blood was retrieved from peripheral arterial line. All samples were collected into ethylenediaminetetraacetic acid–coated Vacutainer tubes (Becton-Dickenson, San Jose, California) and centrifuged at 2,400 g for 10 min at 4°C directly after sampling. Supernatants were transferred to cryotubes and stored at −80°C until analyzed. The surgical procedure was continued with arteriotomy and CEA, and the plaque was retrieved for analysis as previously described 14, 15, 17.
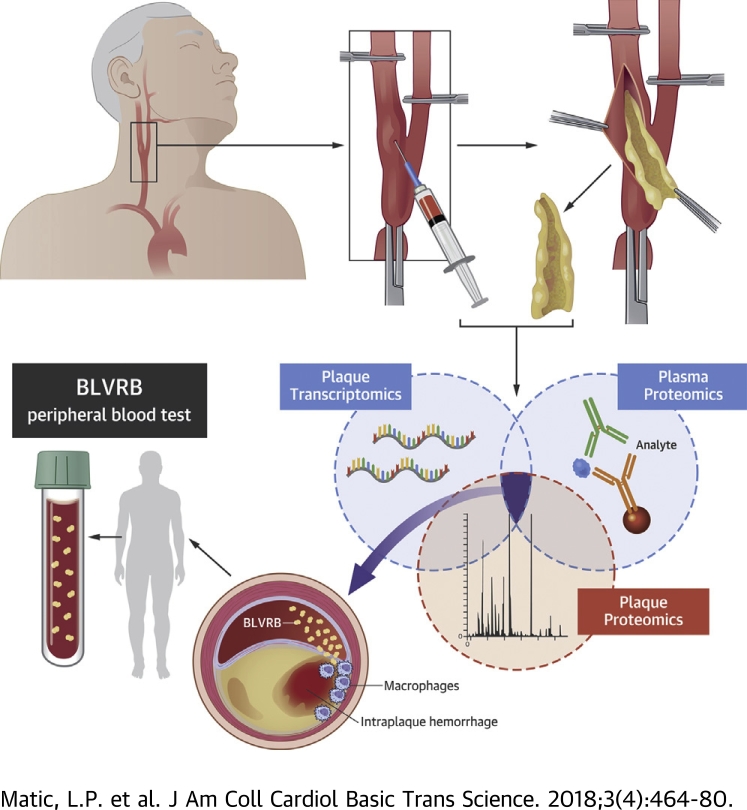
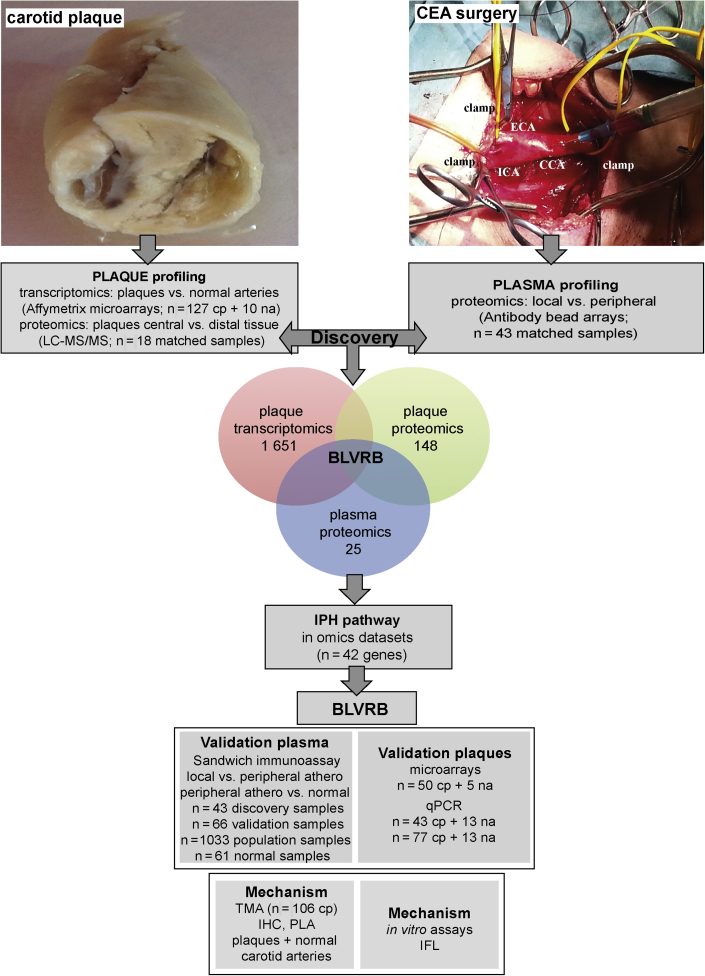
Figure 1.
Sampling at Surgery and Workflow of the Study
Carotid plaques and local and peripheral blood were collected at carotid endarterectomy (CEA) surgery. Sampling of local blood during test occlusion, after 5 min of vessel clamping and prior to arteriotomy, is shown in the photograph. The internal carotid artery (ICA), external carotid artery (ECA), common carotid artery (CCA), and positions of vessel clamps are indicated. Discovery analyses comprised carotid plaques (cp) from 127 patients and 10 normal arteries (na) used for microarray profiling; plaques and adjacent peripheral tissue from 18 patients for liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS); and matched local and peripheral plasma samples from 43 patients for screening by antibody bead arrays. Results from these 3 analyses were overlapped and revealed biliverdin reductase B (BLVRB) as the only significant candidate. Venn diagram shows numbers of significantly dysregulated candidates in each analysis. Both BLVRB and the intraplaque hemorrhage (IPH) pathway to which it functionally belongs were explored further in omics datasets. BLVRB was validated in plasma by sandwich immunoassays and in plaques utilizing additional microarrays and quantitative polymerase chain reaction (qPCR) from both same and nonoverlapping subcohorts of Biobank of Karolinska Endarterectomies patients and normal individuals. Functional investigations were conducted in plaques in situ and in vitro. IHC = immunohistochemistry; IFL = immunofluorescence; PLA =proximity ligation assay; TMA = tissue microarray.
Peripheral plasma from patients with carotid atherosclerosis was compared with peripheral blood samples from healthy individuals (n = 61) recruited through the Stockholm County regional screening program for abdominal aortic aneurysms. Control subjects were all male, were age 65 years, and had no medical history of cancer, acute myocardial infarction, angina pectoris, peripheral arterial occlusive disease, hypertension, or ongoing therapy with platelet aggregation inhibitors or statins.
For validation purposes, aliquots of ethylenediaminetetraacetic acid plasma were also obtained from participants in the SCAPIS (Swedish CArdioPulmonarybioImage Study) pilot study (18). The SCAPIS pilot cohort is a population sample (age 50 to 65 years) randomly recruited from the census register from low- and high-socioeconomic status geographical areas in Gothenburg, Sweden. Of a total 2,243 individuals that were invited to participate, a final number of 1,033 individuals were analyzed and underwent 2 days of testing including blood tests, anthropometrics, extensive imaging, and functional studies of the heart, lungs and metabolism. Coronary artery calcium (CAC) score was assessed using a state-of-the-art multislice computed tomography scanner (Somatom Definition Flash, Siemens Medical Solution, Forchheim, Germany). Image analyses were performed using a calcium-scoring protocol according to Agatston et al. (19).
LC-MS/MS proteomics sample preparation
CEA tissue specimens from 18 patients matched for male sex, age, and statin medication were analyzed using LC-MS/MS as previously described 20, 21. A central portion of the atherosclerotic plaque corresponding to the maximum stenosis was separated from the respective downstream peripheral end of the CEA sample and used for comparisons 22, 23, 24. Samples were crushed while frozen using a tissue pulverizer (Cellcrusher, Cork, Ireland) and lysed and sonicated in a buffer containing 4% sodium dodecyl sulfate, 25 mmol/l N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.6. Lysates were centrifuged and supernatants collected for digestion. Digestion was performed following a modified filter-aided sample preparation protocol (25), first with Lys-C (1:50 ratio) overnight and then with another round of trypsin digestion (1:50 ratio) again overnight. The resulting peptide mixtures were labelled with isobaric Tandem mass tags (TMT) (TMT10, Thermo Fisher Scientific, Waltham, Massachusetts). Samples were divided into 4 TMT10 sets, 2 containing the central samples and 2 containing the peripheral samples. Each set contained 9 samples, and the last channel contained an internal standard composed of peptides from both peripheral and central samples. After sample clean-up by solid-phase extraction (SPE strata-X-C, Phenomenex, Torrance, California), the sample pools were pre-fractionated by high resolution isoelectric focusing and the resulting fractions analyzed by LC-MS/MS.
A detailed description of all materials and methods is given in the Supplemental Appendix.
Results
Study design
Study samples were collected at carotid surgery from consecutively enrolled patients. Plaques were profiled by microarrays (n = 127; 10 normal arteries, discovery dataset) and LC-MS/MS (18 plaques, matched central vs. peripheral adjacent tissue). Matched local and peripheral plasma samples (n = 43, discovery cohort) were screened by multiplexed antibody bead array assays using 10,260 antibodies (26). Differentially regulated candidates from all 3 omics analyses were overlapped in the discovery step and prioritized for further studies based on significance levels adjusted for multiple testing and the direction of effect in plaques and local plasma (up-regulation). Validation was performed in the same as well as other sets of BiKE patients by the following: 1) analysis of gene expression in plaques utilizing additional microarrays (50 plaques and 5 normal arteries, validation dataset); 2) quantitative polymerase chain reaction (plaques from 43 patients in discovery cohort and additional 77 nonoverlapping patients and 13 normal arteries); 3) antibody bead arrays in plasma (33 additional patients, validation cohort); 4) sandwich immunoassay in plasma from atherosclerotic and healthy individuals (43 samples from the discovery cohort; 66 additional nonoverlapping matched local and peripheral samples referred to as the validation cohort; and peripheral plasma from 61 healthy individuals); 5) sandwich immunoassay in an independent population cohort of individuals stratified by CAC score (1,033 peripheral plasma samples); and finally 6) functional and mechanistic studies conducted in situ and in vitro (Figure 1). The demographics of the study cohorts are given in Supplemental Table 1 and were also described in detail previously 11, 18.
Combined omics profiling reveals a novel candidate marker of carotid atherosclerosis
Following Bonferroni corrections, transcriptomic profiling of plaques versus normal arteries yielded 1,651 significantly dysregulated genes, whereas proteomic profiles comparing plaques versus adjacent arterial tissue and local versus peripheral plasma revealed 148 and 25 differentially dysregulated proteins, respectively (Figure 1). Pathway analyses of all significantly dysregulated molecules were first performed to validate the 3 omics discovery datasets with respect to the published data (Supplemental Figures 1A to 1C). Results confirmed that cell proliferation, nitric oxide signaling, lipoprotein and apoptotic particle clearance, immune cell activation, chemokine secretion, blood coagulation, and extracellular matrix disassembly were dominant in plaques by transcriptomics; similarly, extracellular matrix, heme-binding, and platelet-derived growth factor binding were also the most enriched functional categories by plaque proteomics, whereas extracellular matrix degradation, lipid and ion-channel transport, as well as matrix metalloproteinase activation signatures were the most enriched in local plasma.
After overlapping these 3 datasets, BLVRB emerged as the only significant candidate that was enriched both in plaques and in local plasma (Bonferroni cutoff: p = 4.8 × 10−6) (Supplemental Figure 1D). Of note, lipoprotein metabolism- associated apolipoprotein C1, also up-regulated in plaques and local plasma, was below the multiple testing threshold in plasma, and several other candidates, that is, vascular endothelial injury–linked melanoma cell adhesion molecule and structural proteins myosin 10 and calponin 3 acidic, were not suitable according to the selection criteria.
Because BLVRB has been associated with Hb degradation and iron metabolism (27), we next hypothesized that it may have a role in IPH and extended our molecular analyses to explore the potential of this pathway as a source of signature candidates in carotid atherosclerosis. All genes belonging to the gene ontology categories “Hb degradation” and “iron metabolism” were examined in the 3 different omics datasets together with their functionally related genes based on coexpression, cointeraction, and shared biological function across tissues (42 candidates) (Supplemental Figure 2, Supplemental Table 2). Of all 42 candidates, 18 had significantly altered expression in plaque microarrays, 13 had altered plaque protein levels and the majority of these candidates were up-regulated (Supplemental Table 3). BLVRB was confirmed as being among the most significant candidates in these analyses and the only one enriched also in local plasma; thus, it was selected for further validation studies.
BLVRB is enriched in plaques and plasma from patients with carotid atherosclerosis
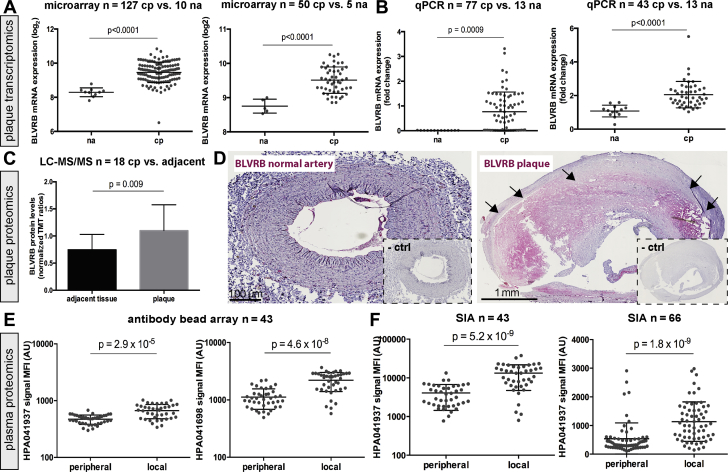
Strong up-regulation of the BLVRB transcript was confirmed in discovery and validation microarray datasets comparing plaques to normal arteries (Figure 2A) and by quantitative polymerase chain reaction from nonoverlapping validation samples (Figure 2B). BLVRB protein was also enriched in plaques as analyzed by LC-MS/MS (Figure 2C). Abundant BLVRB protein was observed in plaques by immunohistochemistry, whereas only weak signal was present in the adventitia of normal arteries (Figure 2D).
Figure 2.
BLVRB Is Enriched in Plaques and Plasma From Carotid Atherosclerosis Patients
BLVRB messenger ribonucleic acid (mRNA) was highly up-regulated in comparison between plaques and normal arteries, both in the discovery (n = 127 cp + 10 na) and validation microarray dataset (n = 50 cp + 5 na) (A), and these results were confirmed by qPCR from 2 sets of plaques compared with normal arteries (B). Plots show log2 mean ± SD for microarrays and mean fold change for qPCR. By proteomic analysis of plaques compared with matched adjacent arterial tissue (n = 18), BLVRB was also strongly up-regulated (C). Immunohistochemistry confirms the lack of signal for BLVRB in normal artery, but strong widespread staining in carotid plaque (red signal, negative control [ctrl] subjects shown in insets) (D). BLVRB was detected with 2 antibodies in the bead array–based plasma screening as strongly up-regulated in local versus peripheral plasma (E). This enrichment was confirmed by sandwich immunoassay (SIA) in the same samples as well as in another set of 66 patients (F). Plots show median of fluorescence intensity (MFI) with mean ± SD. Bonferroni corrected p values shown in A and C, nominal values in E (Bonferroni cutoff in plasma screening p = 4.8 × 10−6). AU = arbitrary unit(s); TMT = technology, media, telecommunications; other abbreviations as in Figure 1.
In plasma, BLVRB protein was enriched in local versus peripheral discovery samples as detected with 2 antibodies independently (HPA041698, p = 4.57 × 10−8; HPA041937, p = 2.86 × 10−5) (Figure 2E). The enrichment was confirmed by antibody bead array in a validation set of 33 additional patients (Supplemental Figure 3A) and by sandwich immunoassay in both discovery and validation plasma samples (Figure 2F). Immunocapture–mass spectrometry verified that HPA041937 specifically detects BLVRB from plasma (Supplemental Figure 4A). Of note, enrichment of BLVRB was not related to any possible sampling-associated hemolysis (Supplemental Figure 4B).
BLVRB is up-regulated in association with carotid plaque instability
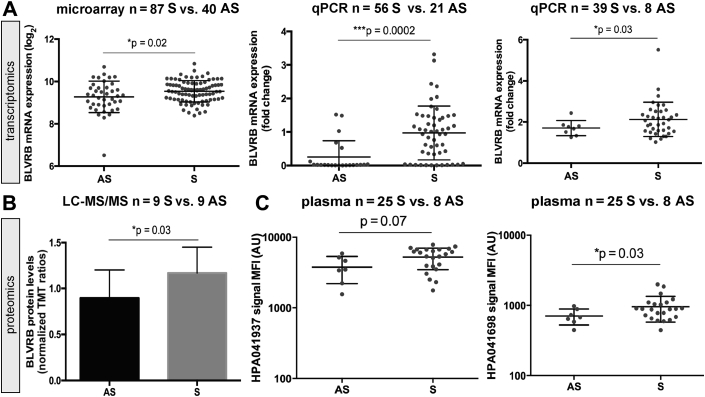
Because IPH has been associated with vulnerable lesions (5), we next examined whether BLVRB expression could be linked to clinical symptoms of plaque instability (Figure 3). BLVRB transcript was up-regulated in plaques from symptomatic versus asymptomatic patients by microarrays (Figure 3A) and by quantitative polymerase chain reaction from 2 validation sets of patients. BLVRB protein levels were also significantly higher in plaques from symptomatic patients by LC-MS/MS (Figure 3B). The same trend was observed in local plasma assayed with antibody bead arrays for both BLVRB antibodies (Figure 3C) and in sandwich immunoassay (Supplemental Figure 3B).
Figure 3.
BLVRB Is Up-Regulated in Patients With Symptomatic Carotid Atherosclerosis
Stratification of patients based on clinical symptoms of atherosclerosis showed that BLVRB mRNA (A) and protein (B) expression was induced in comparison between symptomatic (S) and asymptomatic (AS) patients in plaques by microarrays, qPCR, and LC-MS/MS, as well as in local plasma by antibody bead arrays using both human protein atlas antibodies targeting BLVRB (HPA041937 and HPA041698) (C). Abbreviations as in Figures 1 and 2.
BLVRB localizes to CD68+ cells in plaques
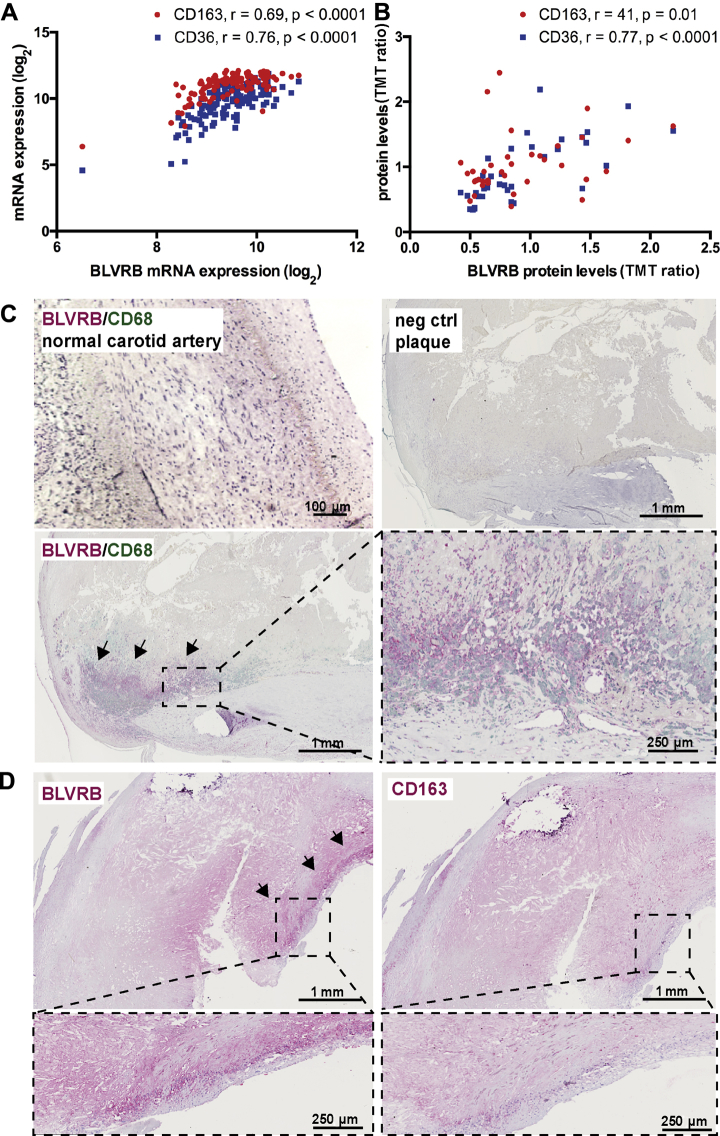
To understand which cell types in plaques express BLVRB, we next correlated BLVRB messenger ribonucleic acid (mRNA) and protein levels from microarrays and LC-MS/MS data with the expression of various cell markers (Figure 4A, Supplemental Figure 5A). BLVRB showed strong correlations with macrophage markers CD163 and CD36 both on the transcriptomic and proteomic level (Figures 4A and 4B). Correlations with markers of lymphocytes and endothelial cells were weak to moderate, whereas with markers of smooth muscle cells they were negative (Supplemental Figure 5A). Next, these correlations were confirmed by immunohistochemistry where BLVRB protein was found in the plaque necrotic core overlapping with CD68+ cells (Figure 4C), but not with lymphocytes, endothelial cells, or smooth muscle cells (Supplemental Figure 5B). BLVRB was also detected in plaque regions positive for CD163, although showing a broader staining pattern (Figure 4D).
Figure 4.
BLVRB Localizes to CD68+ Cells in Plaques
In plaque transcriptomic (A) and proteomic (B) datasets, BLVRB showed strong significant correlations to markers of macrophages (CD163, CD36). By double immunohistochemistry, BLVRB (red signal) localized primarily to CD68+ cells in the necrotic core of plaques (green signal, enlarged insets). No signal was observed in adventitia of normal arteries (C). By single immunohistochemistry, BLVRB was also detected in necrotic core regions positive for CD163 (D). neg = negative; other abbreviations as in Figures 1 and 2.
BLVRB may be associated with heme-metabolism via HMOX1 in regions of intraplaque hemorrhage
Whereas the role of BLVRA in Hb metabolism via heme oxygenase (HMOX) has been well described (28), less is known about the implication of BLVRB in the same process, and so far there are no reports addressing this question in relation to atherosclerosis. We constructed a functional coupling network from extended BLVRB-HMOX protein-protein interactions and found that BLVRB may be linked with HMOX1 indirectly via several proteins involved in lipid metabolism (5-lipoxygenase-activating protein), activated oxygen species (superoxide dismutase 2, mitochondrial), and matrix degradation (cathepsin B) in plaques (Supplemental Figure 6A).
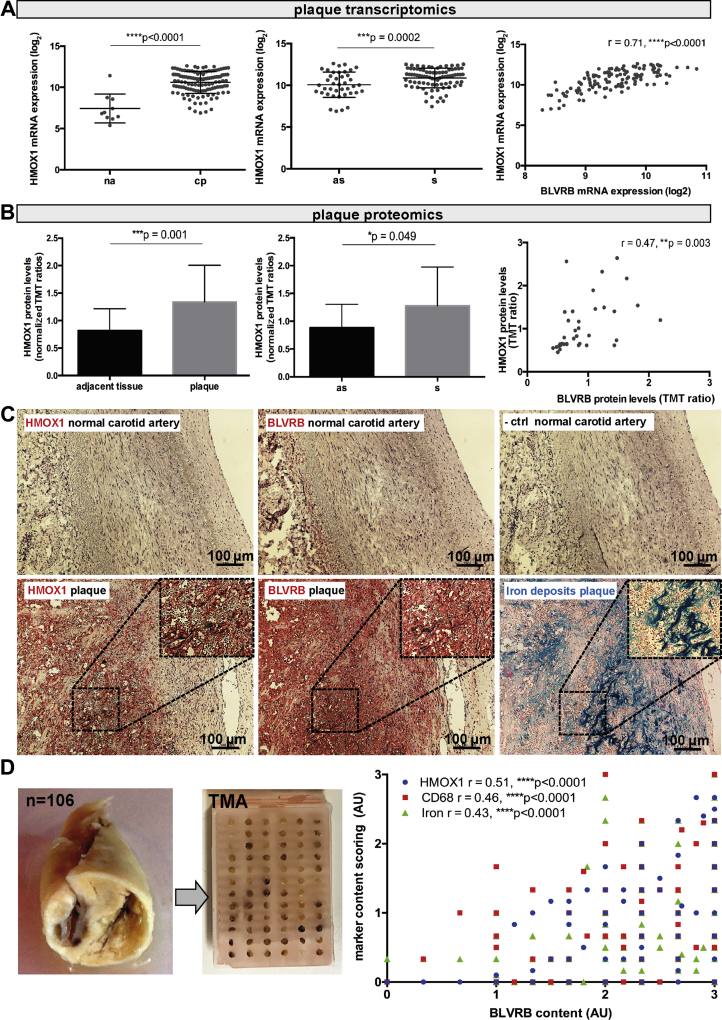
Interestingly, HMOX1 mRNA was also up-regulated in microarrays comparing plaques with normal arteries (Figure 5A), as well as in plaques from symptomatic versus asymptomatic patients, and strongly correlated with the expression of BLVRB. On a protein level, the same results were observed for HMOX1 and BLVRB (Figure 5B); however, neither BLVRA nor HMOX2 isozymes were significantly deregulated in this analysis (Supplemental Table 3). This strong enrichment of HMOX1 and BLVRB in carotid atherosclerosis was further verified in a publicly available dataset (GEO Gene Expression Omnibus accession number GSE43292), comparing plaques with adjacent arterial tissue (32 matched patient samples) (Supplemental Figure 3C).
Figure 5.
BLVRB May Be Associated With Heme-Catabolism in Plaques via HMOX1
Heme oxygenase 1 (HMOX1) mRNA was highly up-regulated in Biobank of Karolinska Endarterectomies microarrays comparing plaques and normal arteries and strongly correlated to that of BLVRB. Plots showing log2 mean ± SD and Spearman correlation (A). Induction of HMOX1 and correlation with BLVRB could be verified on the protein level by LC-MS/MS from plaques compared with adjacent tissue (B). No staining for HMOX1 was observed in the normal carotid artery, whereas weak signal for BLVRB could occasionally be seen in adventitia. Using consecutive sections of plaques, HMOX1 was localized to the sites of hemorrhage with infiltrated erythrocytes, where it overlapped with BLVRB and Perls blue staining for iron deposits (C, enlarged insets). The correlation between BLVRB and HMOX1 content and intraplaque hemorrhage was also assessed quantitatively, using histological staining on TMA constructed from 106 plaques (D, plot to the right). Abbreviations as in Figures 1, 2, and 3.
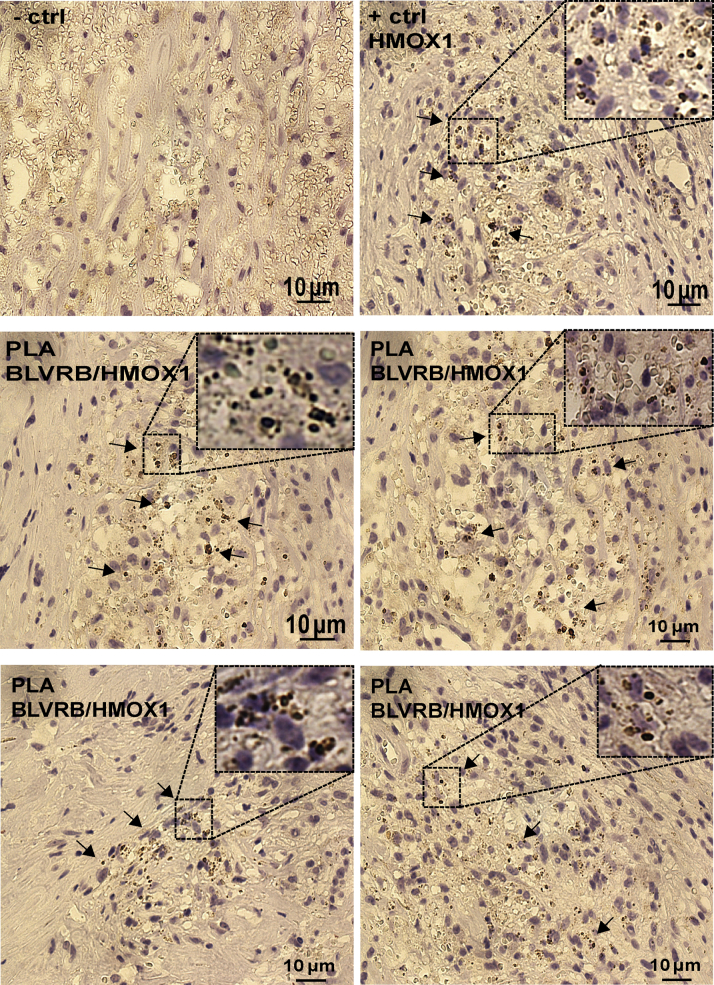
Next, the association of BLVRB and HMOX1 was confirmed in situ by immunohistochemistry, iron deposits staining, and proximity ligation assay (Figures 5C, 5D, and 6, Supplemental Figures 6B and 6C). In plaques, HMOX1 signal was restricted to IPH sites with Perl blue reactivity indicating iron deposits, where it overlapped with BLVRB (Figure 5C, enlarged insets) that otherwise showed a wider immunoreactivity in the necrotic core. The strong positive correlation between BLVRB and HMOX1 content and iron deposits and CD68 content could also be confirmed using tissue microarrays constructed from 106 plaques (Figure 5D). Moreover, in plaque regions with IPH, proximity ligation assay demonstrated cointeraction between BLVRB and HMOX1 proteins (Figure 6, insets).
Figure 6.
BLVRB and HMOX1 Colocalize and Cointeract in Plaques
By PLA, BLVRB and HMOX1 proteins cointeracted directly in plaques in regions with hemorrhage (arrows and enlarged insets). PLA probe detecting HMOX1 was used as a positive control. Abbreviations as in Figures 1, 2, and 5.
BLVRB and HMOX1 are induced in macrophages on stimulation with iron
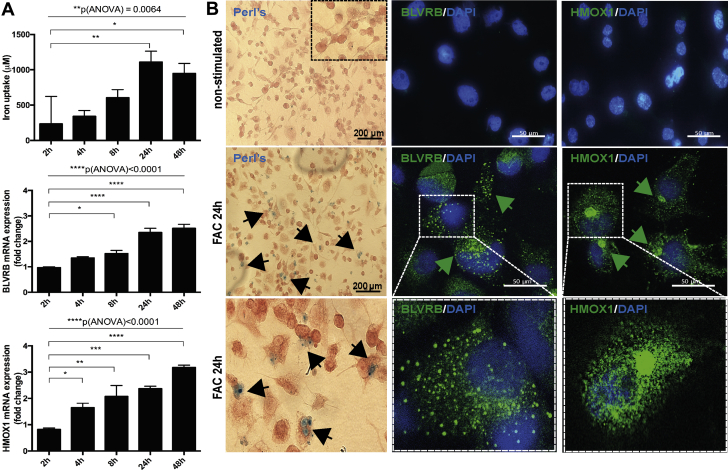
The association between IPH and HMOX1/BLVRB was next examined in vitro, where a rapid increase of HMOX1 mRNA in THP-1 human monocytic cell line–derived human macrophages was detected at 8 h on stimulation with an iron source (ferric ammonium citrate), whereas BLVRB mRNA was strongly up-regulated after 24 h, coinciding with the highest iron uptake (Figure 7A). By immunofluorescence, induction with iron caused abundant BLVRB protein accumulation in subcellular vesicles, whereas HMOX1 was observed in perinuclear regions and in the cytoplasm (Figure 7B, enlarged images).
Figure 7.
BLVRB and HMOX1 Are Induced in Macrophages on Iron Exposure
THP-1 human monocytic cell line–derived human macrophages were stimulated with ferric ammonium citrate (FAC) and cellular iron uptake quantified over time using the ferrozine assay (top panel). qPCR shows a rapid induction of HMOX1 expression already after 4 h of stimulation, whereas BLVRB was up-regulated after 24 h. Plots show mean fold change ± SEM of 3 independent experiments. p values calculated with analysis of variance (ANOVA) across all groups and Student's t-test in comparison to 2 h time point. All results normalized to the nonstimulated control cells (A). Perls staining confirms presence of cellular iron deposits after 24 h of stimulation. By immunofluorescence, BLVRB protein is induced and localized to subcellular vesicles in FAC-treated cells, whereas HMOX1 shows perinuclear and diffused cytoplasmic localization (arrows). Bottom panels show higher magnification (B). DAPI = 4,6-diamino-phenylindole; other abbreviations as in Figures 1, 2, and 5.
BLVRB and HMOX1 levels correlate with adverse cardiovascular and cerebrovascular events
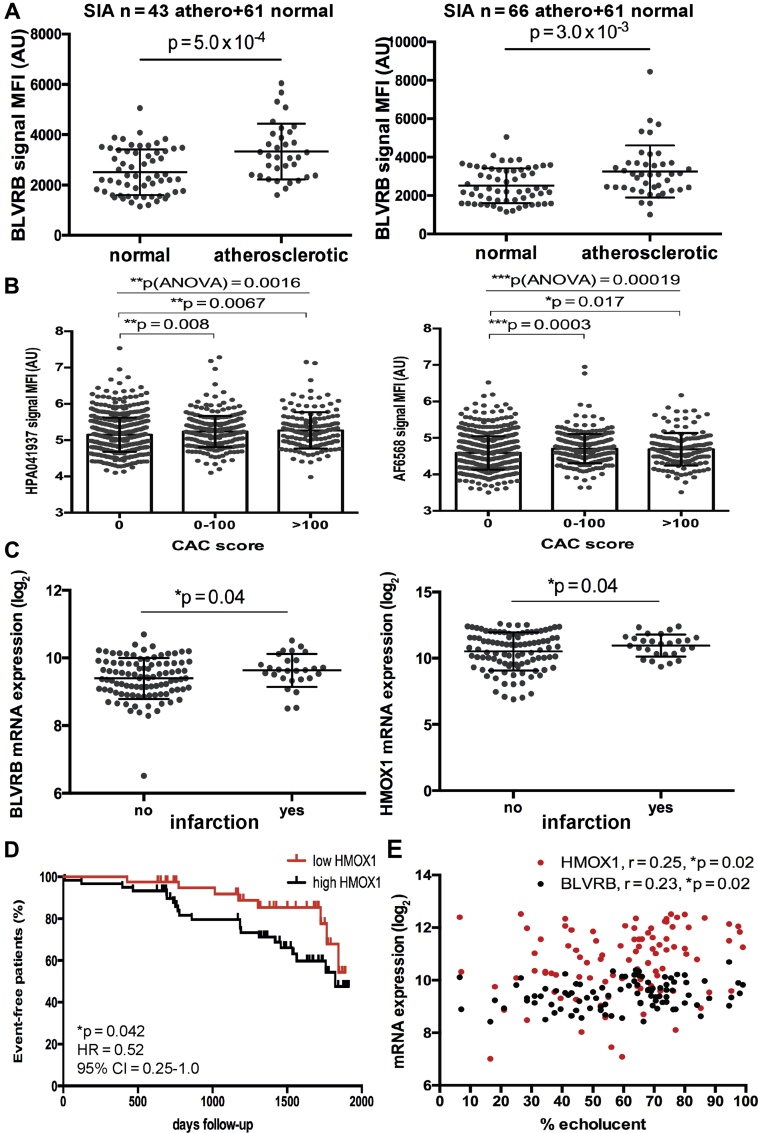
With respect to clinical importance, significant enrichment of BLVRB could be confirmed also in peripheral plasma from patients with carotid atherosclerosis versus healthy control subjects, both in the discovery and in the validation set of samples from BiKE (Figure 8A). Furthermore, elevated BLVRB levels could be detected with 2 antibodies in an independent population cohort where individuals were stratified by CAC score as a surrogate marker of coronary artery disease (1,033 individuals in total) (Figure 8B). In association with clinical parameters, we also found that BLVRB and HMOX1 expression were increased in plaques from patients with history of myocardial infarction (Figure 8C), and patients with above-median plaque expression levels of HMOX1 had a higher risk of future adverse cardiovascular and cerebrovascular events (Figure 8D). A similar trend was noted for BLVRB, although it did not reach statistical significance (Supplemental Figure 7). Last, expression of BLVRB and HMOX1 in plaques was positively correlated to carotid plaque echolucency as evaluated by pre-operative ultrasound (Figure 8E).
Figure 8.
Increased BLVRB and HMOX1 Levels Associate With Adverse Cardiovascular Events
BLVRB showed significant enrichment by SIA comparing peripheral plasma from both discovery (n = 43) and validation (n = 66) subsets of patients with carotid atherosclerosis compared with peripheral plasma from normal individuals (n = 61) (A). BLVRB was also enriched in an independent population cohort stratified by coronary artery calcium (CAC) score (n = 599 individuals with score 0, n = 281 score 0 to 100, n = 151 score >100), as shown with 2 different antibodies. p values calculated with ANOVA across all groups and Student t-test in comparison to score 0 (B). Plots show MFI with SD. BLVRB and HMOX1 transcripts were also significantly up-regulated in plaques from patients that experienced a previous infarction (n = 27) compared with those without previous events (n = 98). Plots showing mRNA expression log2 mean ± SD (C). Plot illustrating major adverse cardiovascular and cerebrovascular events–free survival of patients during the 2,000-day follow-up period after surgery, based on HMOX1 expression in Biobank of Karolinska Endarterectomies plaques above (black) and below (red) the median values (x-axis, days event-free survival). Hazard ratio (HR) refers to HMOX1 high/HMOX1 low. Both symptomatic (n = 86) and asymptomatic (n = 40) patients were included in the analysis. Each mark along the lines indicates an event. Total number of events in the cohort was 58 (D). Expression of BLVRB and HMOX1 in plaques positively associated with plaque echolucency estimated by duplex ultrasound (E). Abbreviations as in Figures 1, 2, 5, and 7.
Discussion
Here, we integrated data from 3 different omics-technology platforms for unbiased discovery of molecular signatures in carotid atherosclerosis, with potential for future biomarker development to identify individuals who are at high risk of stroke. We applied a novel translational workflow, where differentially regulated candidates were interrogated in transcriptomic and proteomic profiles of plaques and plasma from patients undergoing carotid surgery to discover circulating proteins likely originating from unstable lesions and, thus, with a causal relationship to the disease. The results were validated in independent cohorts and normal individuals and complemented by functional studies. We found enrichment of BLVRB in plaques and plasma from symptomatic patients, suggesting it may represent a clinically useful marker of carotid atherosclerosis. Furthermore, BVLRB was associated with IPH and CD68+ cells in situ and in vitro, confirming a functional link to the pathology of the vulnerable plaque.
Unique to our approach, the use of antibody bead arrays (29) here permitted analysis of small plasma volumes retrieved locally around the lesion during vessel clamping prior to arteriotomy. This method facilitated the discovery of a more specific candidate likely originating from the lesions and directly associated with the disease, as opposed to peripheral plasma, where proteins arising from the lesion would be more diluted. Following the development of a dual binder assay, we could also demonstrate enrichment of BLVRB in peripheral blood of patients with carotid atherosclerosis compared with a control group of healthy individuals, as well as in an independent population cohort stratified by CAC score as a surrogate marker of coronary artery disease. Although these cohorts were not stratified for symptoms, elevation of BLVRB in peripheral plasma of patients with atherosclerosis suggests a potential for clinical application of BLVRB as a disease biomarker, and demonstrates how a rational selection of candidates can be achieved through our alternative translational design.
Detection of IPH by magnetic resonance imaging has been suggested to predict stroke risk in patients with carotid stenosis 30, 31, 32, and IPH has been proposed as a potential source of biomarkers for plaque instability (4). Moreover, it has been observed that IPH in carotid lesions can predict adverse events in other vascular territories, which underlined the notion that it should be investigated as a target for noninvasive imaging and diagnostic approaches (33). We recently demonstrated that Hb-haptoglobin (Hp) binding as well as Hb metabolism were some of the most enriched functional categories in a global transcriptomic analysis of carotid plaques and peripheral blood mononuclear cells (11), and the importance of Hb metabolism in unstable atherosclerosis has also been supported by epidemiological findings linking a common genetic variant of Hp to cardiovascular risk 34, 35, 36. Here, the notion that IPH and Hb metabolism are central elements in end-stage unstable carotid atherosclerosis was confirmed as enrichment of many IPH-related molecules was found in plaques, whereas BLVRB was the only component of this pathway significantly reflected in plasma.
In IPH areas, released Hb is cleared by binding to Hp and catabolism of heme through the Hp-CD163-HMOX pathway, which prevents the toxic and proinflammatory effects of Hb (5). Although both active HMOX isozymes (HMOX1 and HMOX2) catalyze the same reaction, they may have different functions. HMOX2 is the constitutive form, whereas HMOX1 is the stress responsive cognate, which is normally expressed at low levels and induced in atherosclerosis by free radicals and hypoxia (37). The levels of HMOX1 were found to be the highest in patients with previous myocardial infarction, but it is unclear whether this represents a protective mechanism or a pathophysiological consequence of the disease (38). BLVR is another major enzyme of heme catabolism in macrophages, resulting in the degradation products carbon monoxide, ferrous iron, and biliverdin (28). BLVR occurs in the form of 2 isozymes (BLVRA and BLVRB), which have redundant as well as distinct functions and differ in their reduction specificity (27). BLVRB is expressed in the early fetal stages, whereas BLVRA expression increases later in gestation (39) and remains ubiquitously expressed in adults (40). In the context of atherosclerosis, this study is to our knowledge the first report of a role for BVLRB.
Although both HMOX1 and BLVRB enzymes are normally attenuated in adult healthy tissues, we demonstrated their association in CD68+ cells at sites of IPH in plaques and induction by iron in macrophages in vitro. Induction of HMOX1 was previously reported in foam cells and macrophages stimulated with oxidized low-density lipoprotein (41). We were able to replicate our findings in a public dataset from an independent cohort of carotid patients, while we also noted that enrichment of BLVRB and HMOX1 was not associated with other features of late-stage plaques such as calcification (data not shown). Clinical associations supported a prognostic potential of both BLVRB and HMOX1 in end-stage carotid atherosclerosis, because expression of these genes in plaques associated with previous and future adverse vascular events, as well as with high plaque echolucency, signifying vulnerable lesions (42). Whereas validations are necessary to confirm the value of BLVRB as a biomarker for unstable carotid atherosclerosis, our approach emphasizes BLVRB as a candidate biomarker in circulation, with a causal relationship to disease pathophysiology.
With stringent statistical criteria applied in this study, only BVLRB was found to be consistently up-regulated in all 3 datasets. One other candidate, apolipoprotein C1, was below the multiple testing threshold in plasma, but significantly enriched in tissue and thus of potential interest as a molecular signature of carotid plaques. Apolipoprotein C1 is expressed primarily by the liver, activated during macrophage differentiation and shown to be crucially involved in lipopolysaccharide-induced atherosclerosis in apolipoprotein E knockout mice (43).
Study limitations
Consensus is lacking in the field regarding the selection of appropriate control tissues to atherosclerotic plaques, and arteries of various embryonic origins have been used for this purpose as well as adjacent macroscopically intact parts of lesions. The BiKE cohort contains only advanced late-stage lesions; therefore, we could not study early and atheroprogression-related changes. Of note, IPH in plaques was localized using Perls reaction, which predominantly detects ferric compared with ferrous iron. Primarily, our findings are restricted by the current performance characteristics (such as sensitivity, selectivity, and throughput) of the profiling platforms used. To overcome the drawback that omics technologies can lead to false-positive findings, we considered candidates only if they were significant in all 3 discovery datasets. Our findings should be replicated in larger independent cohorts that will permit adjustment for traditional cardiovascular risk factors and evaluation within subgroups of interest such as defined by age, sex, symptoms, and comorbidities. This will require multicenter study design and development of sensitive yet cost-effective assays that would allow for such analyses.
Conclusions
In summary, using a novel translational pipeline combining proteomics and transcriptomics for the discovery of disease-related signature molecules in carotid atherosclerosis, we identified enrichment of BLVRB in plaque tissue and plasma, which was related to IPH and processes previously associated with plaque instability. From a clinical perspective, our approach is important because it also generates candidates for investigations of peripheral plasma from patients with coronary artery disease, where local sampling is less feasible; BLVRB may be evaluated for early diagnosis of cardiovascular disease prior to increase in acute markers of tissue damage (e.g., troponins); and BLVRB levels may be informative about an atherothrombotic origin of ischemic stroke in general. Further studies are warranted to evaluate our approach and explore the predictive potential of BLVRB, especially for the detection of vulnerable individuals and lesions.
Perspectives.
COMPENTENCY IN MEDICAL KNOWLEDGE: Using a novel translational pipeline combining proteomics and transcriptomics of plaques and plasma for the discovery of signature molecules of unstable carotid atherosclerosis, we identified enrichment of BLVRB, which was related to intraplaque hemorrhage and processes previously associated with plaque instability. The approach we used generates biomarker candidates with a causal relationship to the disease and opens up further investigations to determine the value of BLVRB in prediction of ischemic stroke but also of atherothrombotic events in other vascular territories, such as coronary artery disease and myocardial infarction.
TRANSLATIONAL OUTLOOK: The diagnostic potential of BLVRB as a surrogate marker for intraplaque hemorrhage and carotid plaque instability will be further investigated in patients with carotid atherosclerosis where plasma levels of BLVRB will be correlated to the presence of intraplaque hemorrhage by carotid cardiac magnetic resonance imaging. In addition, population-based longitudinal studies will be performed to address the value of BLVRB in risk prediction of myocardial infarction and ischemic stroke.
Acknowledgments
The authors would like to acknowledge the Karolinska Hospital and Södersjukhuset Vascular Surgery Teams for their dedicated help in collection of human samples.
Footnotes
This work was conducted with support from the Swedish Heart and Lung Foundation, the Swedish Research Council (K2009-65X-2233-01-3, K2013-65X-06816-30-4, and 349-2007-8703), Uppdrag Besegra Stroke (P581/2011-123), the Strategic Cardiovascular Programs of Karolinska Institutet and Stockholm County Council, the Stockholm County Council (ALF2011-0260 and ALF-2011-0279, HMT-2013-0741, and HMT-2015-0924), the Foundation for Strategic Research, and the European Commission (INTRICARE, CarTarDis, AtheroRemo, VIA, and AtheroFlux projects). Financial support was also provided by the Knut and Alice Wallenberg Foundation, Science for Life Laboratory, and the KTH Center for Applied Proteomics funded by the Erling-Persson Family Foundation. Data related to this study is available from Gene Expression Omnibus (GEO) datasets (accession number GSE21545). Dr. Matic has received fellowships from the Swedish Society for Medical Research and the Heart and Lung Foundation. Dr. Hansson has received lecture honoraria and consulting fees for biotech investments during 2016-2017 from Carnegie, SAB worth 10, 000 USD per year. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Matic and Iglesias are joint first authors. Drs. Odeberg and Hedin contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Jacob Odeberg, Email: jacob.odeberg@scilifelab.se.
Ulf Hedin, Email: Ulf.Hedin@ki.se.
Appendix
References
- 1.Rothwell P.M. Endarterectomy for symptomatic and asymptomatic carotid stenosis. Neurol Clin. 2008;26:1079–1097. doi: 10.1016/j.ncl.2008.09.013. x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A., Marshall R.S. Moving beyond luminal stenosis: imaging strategies for stroke prevention in asymptomatic carotid stenosis. Cerebrovasc Dis. 2015;39:253–261. doi: 10.1159/000381108. [DOI] [PubMed] [Google Scholar]
- 3.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 4.Michel J.B., Martin-Ventura J.L., Nicoletti A., Ho-Tin-Noe B. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis. 2014;234:311–319. doi: 10.1016/j.atherosclerosis.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Levy A.P., Moreno P.R. Intraplaque hemorrhage. Curr Mol Med. 2006;6:479–488. doi: 10.2174/156652406778018626. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati E., Magnoni M., Camici P.G. Need for new non-invasive imaging strategies to identify high-risk asymptomatic patients with carotid stenosis. Int J Cardiology. 2013;168:4342–4343. doi: 10.1016/j.ijcard.2013.05.079. [DOI] [PubMed] [Google Scholar]
- 7.Hoefer I.E., Steffens S., Ala-Korpela M., for the ESC Working Group Atherosclerosis and Vascular Biology Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J. 2015;36:2635–2642. doi: 10.1093/eurheartj/ehv236. [DOI] [PubMed] [Google Scholar]
- 8.Addona T.A., Shi X., Keshishian H. A pipeline that integrates the discovery and verification of plasma protein biomarkers reveals candidate markers for cardiovascular disease. Nat Biotechnol. 2011;29:635–643. doi: 10.1038/nbt.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta-Martin A.E., Panchaud A., Chwastyniak M. Quantitative mass spectrometry analysis using PAcIFIC for the identification of plasma diagnostic biomarkers for abdominal aortic aneurysm. PLoS One. 2011;6:e28698. doi: 10.1371/journal.pone.0028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivanco F., Padial L.R., Darde V.M. Proteomic biomarkers of atherosclerosis. Biomark Insights. 2008;3:101–113. doi: 10.4137/bmi.s488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perisic L., Aldi S., Sun Y. Gene expression signatures, pathways and networks in carotid atherosclerosis. J Intern Med. 2016;279:293–308. doi: 10.1111/joim.12448. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell P.M., Pendlebury S.T., Wardlaw J., Warlow C.P. Critical appraisal of the design and reporting of studies of imaging and measurement of carotid stenosis. Stroke. 2000;31:1444–1450. doi: 10.1161/01.str.31.6.1444. [DOI] [PubMed] [Google Scholar]
- 13.Halliday A., Mansfield A., Marro J., for the MRC Asymptomatic Carotid Surgery Trial (ASCT) Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 14.Perisic L., Hedin E., Razuvaev A. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:2432–2443. doi: 10.1161/ATVBAHA.113.301743. [DOI] [PubMed] [Google Scholar]
- 15.Folkersen L., Persson J., Ekstrand J. Prediction of ischemic events on the basis of transcriptomic and genomic profiling in patients undergoing carotid endarterectomy. Mol Med. 2012;18:669–675. doi: 10.2119/molmed.2011.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GALA Trial Collaborative Group. Lewis S.C., Warlow C.P. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet. 2008;372:2132–2142. doi: 10.1016/S0140-6736(08)61699-2. [DOI] [PubMed] [Google Scholar]
- 17.Razuvaev A., Ekstrand J., Folkersen L. Correlations between clinical variables and gene-expression profiles in carotid plaque instability. Eur J Vasc Endovasc Surg. 2011;42:722–730. doi: 10.1016/j.ejvs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom G., Berglund G., Blomberg A. The Swedish CArdioPulmonary BioImage Study: objectives and design. J Intern Med. 2015;278:645–659. doi: 10.1111/joim.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.Branca R.M., Orre L.M., Johansson H.J. HiRIEF LC-MS enables deep proteome coverage and unbiased proteogenomics. Nat Methods. 2014;11:59–62. doi: 10.1038/nmeth.2732. [DOI] [PubMed] [Google Scholar]
- 21.Anderson J.D., Johansson H.J., Graham C.S. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Taranto M.D., Morgante A., Bracale U.M. Altered expression of inflammation-related genes in human carotid atherosclerotic plaques. Atherosclerosis. 2012;220:93–101. doi: 10.1016/j.atherosclerosis.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Papaspyridonos M., Smith A., Burnand K.G. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:1837–1844. doi: 10.1161/01.ATV.0000229695.68416.76. [DOI] [PubMed] [Google Scholar]
- 24.Rocchiccioli S., Pelosi G., Rosini S. Secreted proteins from carotid endarterectomy: an untargeted approach to disclose molecular clues of plaque progression. J Transl Med. 2013;11:260. doi: 10.1186/1479-5876-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisniewski J.R., Zougman A., Mann M. Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res. 2009;8:5674–5678. doi: 10.1021/pr900748n. [DOI] [PubMed] [Google Scholar]
- 26.Uhlen M., Fagerberg L., Hallstrom B.M. Proteomics: tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 27.Pereira P.J., Macedo-Ribeiro S., Parraga A. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nat Struct Biol. 2001;8:215–220. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen J.H., Etzerodt A., Svendsen P., Moestrup S.K. The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid Med Cell Longev. 2013;2013:523652. doi: 10.1155/2013/523652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwenk J.M., Gry M., Rimini R., Uhlen M., Nilsson P. Antibody suspension bead arrays within serum proteomics. J Proteome Res. 2008;7:3168–3179. doi: 10.1021/pr700890b. [DOI] [PubMed] [Google Scholar]
- 30.Treiman G.S., McNally J.S., Kim S.E., Parker D.L. Correlation of carotid intraplaque hemorrhage and stroke using 1.5 T and 3 T MRI. Magn Reson Insights. 2015;8 Suppl 1:1–8. doi: 10.4137/MRI.S23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prati F., Di Vito L. Imaging of intraplaque haemorrhage. J Cardiovasc Med. 2012;13:640–644. doi: 10.2459/JCM.0b013e328357a665. [DOI] [PubMed] [Google Scholar]
- 32.Naylor A.R., Sillesen H., Schroeder T.V. Clinical and imaging features associated with an increased risk of early and late stroke in patients with symptomatic carotid disease. Eur J Vasc Endovasc Surg. 2015;49:513–523. doi: 10.1016/j.ejvs.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Hellings W.E., Peeters W., Moll F.L. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–1950. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 34.Ijas P., Saksi J., Soinne L. Haptoglobin 2 allele associates with unstable carotid plaque and major cardiovascular events. Atherosclerosis. 2013;230:228–234. doi: 10.1016/j.atherosclerosis.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Degraba T.J., Hoehn G.T., Nyquist P.A. Biomarker discovery in serum from patients with carotid atherosclerosis. Cerebrovasc Dis Extra. 2011;1:115–129. doi: 10.1159/000334477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingrove J.A., Daniels S.E., Sehnert A.J. Correlation of peripheral-blood gene expression with the extent of coronary artery stenosis. Circ Cardiovasc Genet. 2008;1:31–38. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- 37.Siow R.C., Sato H., Mann G.E. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–394. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 38.Idriss N.K., Blann A.D., Lip G.Y. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal S.G., Stucker T., Rasmussen R.D. Changes in bilirubins in human prenatal development. Biochem J. 1980;186:693–700. doi: 10.1042/bj1860693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komuro A., Tobe T., Nakano Y., Yamaguchi T., Tomita M. Cloning and characterization of the cDNA encoding human biliverdin-IX alpha reductase. Biochim Biophys Acta. 1996;1309 doi: 10.1016/s0167-4781(96)00099-1. 89–9. [DOI] [PubMed] [Google Scholar]
- 41.Nakayama M., Takahashi K., Komaru T. Increased expression of heme oxygenase-1 and bilirubin accumulation in foam cells of rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:1373–1377. doi: 10.1161/hq0801.093592. [DOI] [PubMed] [Google Scholar]
- 42.Doonan R.J., Gorgui J., Veinot J.P. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J Vasc Surg. 2016;64:671–677.e8. doi: 10.1016/j.jvs.2016.03.423. [DOI] [PubMed] [Google Scholar]
- 43.Westerterp M., Berbee J.F., Pires N.M. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.