Summary
The U.S. Food and Drug Administration recently marked 10 years since first updating the labeling for warfarin (often referred to as the “poster child” of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks. Herein, we opine on the experience updating the warfarin labeling, highlighting more generally the enabling factors and challenges encountered when considering incorporation of pharmacogenomic information into the prescribing recommendations for already approved drugs. We also provide a historical perspective of implemented changes in regulatory policies related to personalized medicine.
Key Words: drug labeling, pharmacogenomics, precision medicine, regulatory perspective, warfarin
Corresponding Author

Precision medicine, as a scientific discipline, has had an interesting evolution leading up to and since the completion of the Human Genome Project in 2003. Pharmacogenomics, for example, has followed the classic hope–hype cycle that is typical of many emerging technologies: some important technological advancement leads to a significant peak of stakeholder expectations (e.g., on the part of researchers, patients, clinicians, policy makers), followed by a recalibration of these expectations informed by both successes and failures in implementation. From our standpoint, the U.S. Food and Drug Administration (FDA) was an early endorser of the promise of precision medicine since its inception (1). At the same time, we have not been immune from the challenges in bringing to bear the full potential of genomic sciences to address public health issues. The challenges have perhaps been most evident when updating prescription drug labeling to include pharmacogenomic information that emerges after a drug has been approved.
Notwithstanding, these challenges are not insurmountable. We recently marked 10 years since the FDA first updated the labeling for warfarin (often referred to as the “poster child” of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks. Herein, we opine on the experience updating the warfarin labeling, highlighting more generally the enabling factors and challenges encountered when considering incorporation of pharmacogenomic information into the prescribing recommendations for already approved drugs.
The FDA has an obligation to warn prescribers upon recognizing “a clinically significant hazard as soon as there is reasonable evidence of a causal association with a drug” (Title 21 Code of Federal Regulations 201.57) (2). Evidence supporting labeling revision can be accumulated in the pre- or post-approval setting, and revisions might be triggered by scientists at the FDA, the pharmaceutical developer, or external researchers. The strength of evidence and potential impact of a gene–drug response association is evaluated, and if supported by evidence, drug labeling is revised.
The warfarin labeling has been updated twice to include pharmacogenomic information. Before the first labeling revision in 2007, mounting evidence in the published literature prompted FDA scientists to both engage with the public on the relevance of warfarin pharmacogenomics and deliberate on the appropriate course of action. In November 2005, a meeting of the FDA Clinical Pharmacology Subcommittee of the Advisory Committee for Pharmaceutical Science was held; the committee of external experts concluded sufficient mechanistic and clinical evidence existed to include pharmacogenomic information on CYP2C9 and VKORC1 in the warfarin labeling. Based on the committee’s recommendation and a large volume of evidence from peer-reviewed literature, the FDA requested that the company (Bristol-Myers Squibb) update warfarin’s labeling to include information about the bleeding risk and warfarin dosing associated with CYP2C9 and VKORC1 variants.
In September 2006, the applicant submitted a labeling revision that included information on the association between genetic variants in CYP2C9 and VKORC1 with dosing requirements and bleeding risk. The labeling revision was primarily supported by a meta-analysis published in 2005 (3). Submitted materials were evaluated by an interdisciplinary review team of clinical pharmacologists, clinicians, biostatisticians, and in vitro diagnostic experts. Although agreement was ultimately reached on the part of the review team, challenges were identified that complicated the deliberations. Two major issues were identified. First, FDA-approved or cleared in vitro diagnostics that were explicitly developed with an intended warfarin pharmacogenetics use at the time of this labeling update were not available. Absence of a clinical diagnostic with an explicit warfarin pharmacogenetics intended use raised concern that if labeling recommendations essentially mandated testing, this mandate could create challenges for practitioners who did not have ready access to pharmacogenomic testing. Second, there was some concern that labeling recommendations were derived from the literature, which was largely composed of retrospective studies, and that paucity of evidence from prospective randomized trials limited the extent of the recommendations that could be provided in labeling.
Based on the aforementioned considerations, the revised labeling included a new Clinical Pharmacology section (i.e., Pharmacogenomics), which described results of the meta-analysis with an emphasis on increased risk of bleeding in patients carrying at least 1 copy of the CYP2C9*2 or CYP2C9*3 alleles. In addition, the Pharmacogenomics section described a single nucleotide polymorphism in the VKORC1 gene (the -1639G>A allele) and its association with lower dose requirement for warfarin based on data available from 201 individuals of European ancestry. The Precautions section provided general statements regarding an increased need for international normalized ratio (INR) monitoring in patients with genetic variants. Furthermore, the Dosage and Administration section had general information about use of lower initial doses in patients with genetic variants (CYP2C9 and/or VKORC1). At the time, inclusion of pharmacogenomic information in labeling was a way of communicating important information associated with genetic variants and warfarin safety without being prescriptive in terms of the clinical course of action to take based on a given patient’s genotype.
Incremental accumulation of pharmacogenomic evidence on the relationship between CYP2C9 and VKORC1 gene variants, warfarin dosing requirements, stability of anticoagulant response, and clinical outcomes stimulated a second pharmacogenomic labeling revision in 2010. Published literature was extensively evaluated, including data from the International Warfarin Pharmacogenetics Consortium (4). Continued discussions with external subject matter experts in conjunction with FDA research collaborations further informed the assessment of the evidence (5). The data and various approaches to address this pharmacogenetic interaction were extensively vetted; again, mixed opinions were articulated about the lack of prospective randomized clinical trials, issues with availability of approved/cleared tests, merits of testing beyond INR monitoring, and actual feasibility of genetic testing.
We deliberated over 2 key issues that have become germane to the evaluation of most pharmacogenetic interactions identified in the post-marketing setting: 1) how prescriptive to be in terms of pharmacogenetic testing; and 2) the most useful way to convey the various dose requirements among patients with different compound genotypes. Making pharmacogenetic testing a condition of prescribing warfarin (i.e., as a companion diagnostic) was not seen as a viable option given the widespread need for warfarin and the potential barriers to testing in the clinic, including the turnaround time for genotype results. As such, the decision was made to be explicit about likely maintenance dose requirements in patients whose genotypes were known. The revised labeling also reflected that prescribers should consider these variable dosing requirements in the selection of an initial warfarin dose and bear in mind that patients with certain genotypes (e.g., CYP2C9 variant carriers) may exhibit prolonged time to reach therapeutic INR.
As a part of the updated labeling, a dosing table was included that provided a range of expected therapeutic warfarin doses in known CYP2C9 and/or VKORC1 variant carriers. We chose to convey these recommendations as a function of genotype as opposed to genotype-derived phenotype (e.g., intermediate/poor/normal metabolizer). At the time, phenotypic descriptions of this sort were not standardized, and the effects of CYP2C9 variants are variable; therefore, we felt such descriptions may be a source of confusion. In addition, clinical laboratories may not uniformly use these designations when reporting results back to clinicians. In terms of including dose ranges, this approach was chosen over presenting a single dose or average dose with some measure of variability for each genotype. Eventually, it was concluded that presenting a circumscribed range of doses would offer more precision to clinicians regarding patient-specific dosing. The proposed dosing ranges could be further altered based on a patient’s clinical characteristics and health care practitioner judgment. Inclusion of a straightforward dosing table offered more actionable information for dose selection than the general statements included in the 2007 warfarin labeling.
The warfarin dosing table was intensely scrutinized and compared with empiric dosing, clinical dosing algorithms, and genotype-based dosing algorithms. Although the dosing table offered improvement over empiric dosing or clinical algorithms and affords quick and easy access to dosing range estimates 6, 7, a simplified dosing table could not capture the complexity of personalized warfarin dosing offered by more complex algorithms based on regression models. Formal pharmacogenomic algorithms were deemed more accurate to quantify the effects of multiple genetic factors in combination with clinical variables compared with the average effect provided by the dosing table. Because prescription drug labeling is static compared with clinical decision support tools, real-time incorporation of updated information regarding the interplay between various genetic and nongenetic factors could not be easily incorporated. The labeling revision certainly stimulated discussion among the pharmacogenetics community as to the appropriate comparators when assessing performance of clinical decision tools, as well as the role of labeling in advancing clinical implementation of pharmacogenetic strategies.
The experience in updating the warfarin label was very educational from our standpoint. Several enabling factors and obstacles were identified. First, the process of pharmacogenomic labeling revision is heavily reliant on the quantity, quality, and nature of available public data. Supporting evidence is often acquired from published literature. When pharmacogenetic studies are observational and heterogeneous in design, it becomes more challenging to provide specific actionable information in labeling that accounts for the range of studies evaluated. At the same time, prospective randomized trials, although important, may suffer from generalizability issues in the pharmacogenetics context and can leave unanswerable questions in terms of clinical management of a broad patient population. In some cases, such as for abacavir, unequivocal evidence of the benefit of testing is well established in controlled (and possibly randomized) clinical trials, enabling much stronger testing and prescribing recommendations to be included in labeling. Second, updating labeling requires alignment among a multidisciplinary team at the FDA, which often comprises statisticians, clinical pharmacologists, physicians, epidemiologists, and others. Team members may have differing perspectives on issues ranging from the strength of the data to the actionability of the information, akin to the deliberations of the larger scientific and clinical communities in microcosm. This situation necessitated the development of such a framework to guide discussions both internally and among various stakeholders 8, 9. Third, logistical limitations of implementation of pharmacogenetics figured prominently in our deliberations. Concern over lack of clinician access to a rapid turnaround, analytically validated assay gave us reasons to be cautious about being overly prescriptive in labeling. For example, in the case of clopidogrel, it may not be feasible to obtain a CYP2C19 genotype result in the setting of acute coronary syndromes where the drug needs to be administered rapidly. However, these issues have become much less concerning given the growing experience with rapid testing and pre-emptive genotyping. Nonetheless, they were major sources of discussion at the time. Finally, cross-labeling policy issues around implications of updating FDA-approved drug labels on FDA-cleared in vitro diagnostics raised unique challenges.
Although not without their challenges, the early experiences with warfarin and other labeling updates were very important in socializing pharmacogenetic principles throughout the FDA’s Center for Drug Evaluation and Research and Center for Devices and Radiological Health. They have been instrumental in advancing the use of precision medicine by updating the labeling of already-marketed drugs (e.g., carbamazepine, abacavir, clopidogrel) (Table 1). Furthermore, proactive approaches to evaluate pharmacogenomic influence on drug efficacy and safety have been implemented. The FDA engages early in the drug development process and encourages drug developers to collect data and deoxyribonucleic acid samples in situations when potential genetic liabilities might be present (10).
Table 1.
Selected Examples of Nononcology Drugs With Biomarker Changes Implemented Based on the Post-Marketing Evidence
| Drug (Year Approved/Initial PGx Revision)† | Therapeutic Area | Biomarkers | Outcome | Source of Evidence | Current Actions‡ |
|---|---|---|---|---|---|
| Abacavir (1998/2008) | Infectious diseases | HLA-B*57:01 | Hypersensitivity reactions | Randomized controlled trial | Recommends HLA-B∗57:01 testing before initiating treatment and avoiding use in HLA-B*57:01 carriers |
| Carbamazepine (1968/2007), (1968/2013) | Neurology | HLA-B*15:02, HLA-A*31:01 | Severe cutaneous adverse reactions (e.g., SJS, TEN) | Case-control studies, meta-analysis | Recommends HLA-B*15:02 testing before initiating treatment in patients of Asian ancestry and warns prescribers about increased risk of developing hypersensitivity reactions in the presence of HLA-A*31:01 |
| Citalopram (1998/2011) | Psychiatry | CYP2C19 | QT prolongation | PD studies | Recommends a maximum dose to be used in individuals who are CYP2C19 PMs based on QT prolongation effect |
| Clopidogrel (1997/2010) | Cardiology | CYP2C19 | Diminished antiplatelet response | PK and PD studies, retrospective case-control and cohort studies | Warns prescribers of the risk for diminished response in CYP2C19 PMs in CYP2C19 PMs and provides consideration for use of alternate treatments in “Boxed Warning” section |
| Codeine (1950/2009) | Anesthesiology | CYP2D6 | Respiratory depression, death | PK studies, case-series | Contraindicated in children <12 yrs of age and in children <18 yrs of age after tonsillectomy and/or adenoidectomy based on risk of respiratory depression and death in CYP2D6 UMs |
| Pimozide (1984/2011) | Psychiatry | CYP2D6 | QT prolongation, sudden cardiac death | PK studies | Recommends testing at a certain dose threshold that is not to be exceeded in CYP2D6 PMs and longer dose titration interval in CYP2D6 PMs |
| Rosuvastatin (2003/2012) | Endocrinology | SLCO1B1 | PK information | PK studies | Provides PK information in “Clinical Pharmacology” section; no alternative treatment strategies recommended based on SLCO1B1 genotype |
| Tramadol (1995/1999) | Anesthesiology | CYP2D6 | Respiratory depression, death | PK studies, case-series | Contraindicated in children <12 yrs of age and in children <18 yrs of age after tonsillectomy and/or adenoidectomy based on risk of respiratory depression and death in CYP2D6 UMs |
| Valproic acid (1978/2015) | Neurology | POLG | Fatal hepatic failure | Case-series | Contraindicated in children with mitochondrial disorders resulting from POLG mutations based on risk of fatal hepatic failure |
CYP = cytochrome P450; G6PD = glucose-6-phosphate dehydrogenase deficiency; PD = pharmacodynamic; PGx = pharmacogenetic; PK = pharmacokinetic; PMs = poor metabolizers; POLG = deoxyribonucleic acid polymerase γ; SJS = Stevens-Johnson Syndrome; TEN = Toxic Epidermal Necrolysis; UMs = ultra-rapid metabolizers.
Drug labeling was further modified over years.
Information included in the “Current Actions” column was based on the information available on the Drugs@FDA website. For additional examples of drugs with biomarker information included in the labeling go to https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm.
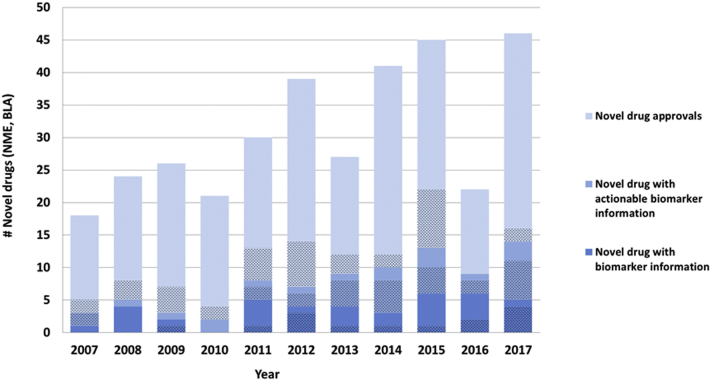
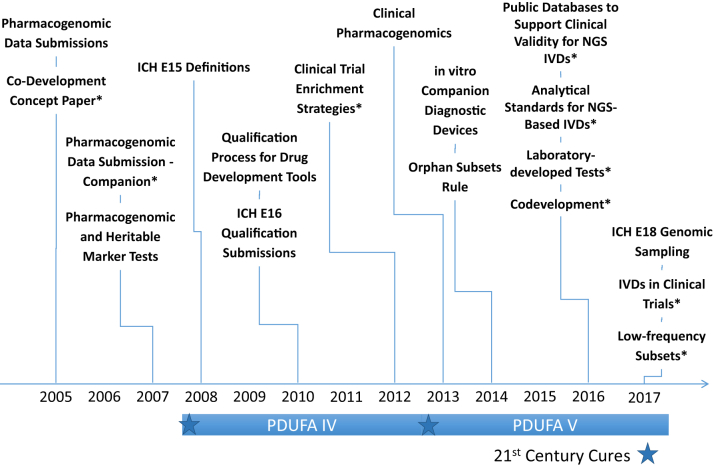
Identification of genetic biomarkers has also allowed for prospective implementation of enrichment strategies in efficacy trials. Effective collaboration between drug developers and centers within the FDA using such strategies have allowed for contemporaneous drug and device approval (e.g., enasidenib, venetoclax, vemurafenib, dabrafenib, trametinib). Indeed, over the past 10 years, many new drugs have contained genomic and other biomarker information in labeling at the time of initial approval (Figure 1). Although oncology and other genetic disorders account for much of the “precision” drug development, genome science is clearly affecting how drugs are developed and approved. Finally, there has been an exponential growth in regulatory policy related to precision medicine (Figure 2).
Figure 1.
Novel Drugs Approved (NME/BLA) Between 2007 and 2017 With Genomic and Other Selected Biomarker Information in Labeling
Shaded areas depict oncology drugs. Actionable biomarker information refers to a specific prescribing recommendation that is included in one of the following label sections: 1) Boxed Warning, 2) Indications and Usage, 3) Dosage and Administration, 4) Contraindications, or 5) Warnings and Precautions. Biomarkers may be any genomic biomarker or other selected protein biomarker that are used for patient selection. BLA = Biologic License Application; NME = New Molecular Entity.
Figure 2.
Timeline of Regulatory Guidances and Policies Related to Precision Medicine
∗Draft. ICH = The International Council for Harmonization; IVD = In vitro diagnostic; NGS = Next-generation sequencing; PDUFA = Prescription Drug User Fee Act.
It is conceivable that genome sequencing will be a routine aspect of medical care in the coming decades, and questions of utility about testing will subside. In addition, more medication options can potentially become available, changing the treatment landscape and possible approaches to manage pharmacogenetic interactions; the introduction of anti–factor Xa inhibitors for the management of atrial fibrillation and deep vein thrombosis created an alternative to warfarin, and new adenosine diphosphate receptor antagonists created an alternative to clopidogrel. Because of these eventualities, FDA often treats genomics as another factor to be considered in clinical decision-making, much like organ function, co-prescribed interacting drugs, and other factors that can alter the probability of benefit or risk. FDA strives to ensure that concrete prescribing recommendations are provided where possible, as appropriate and feasible based on the respective data and clinical context.
Acknowledgment
Padmaja Mummaneni provided additional help in constructing Figure 1.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. The paper reflects the views of the authors and should not be construed to represent the views or policies of the U.S. Food and Drug Administration.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Lesko L.J., Zineh I. DNA, drugs and chariots: on a decade of pharmacogenomics at the US FDA. Pharmacogenomics. 2010;11:507–512. doi: 10.2217/pgs.10.16. [DOI] [PubMed] [Google Scholar]
- 2.Code of Federal Regulations Title 21. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57. Accessed March 20, 2018.
- 3.Sanderson S., Emery J., Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7:97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- 4.International Warfarin Pharmacogenetics Consortium. Klein T.E., Altman R.B., Eriksson N. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlstein T.S., Goldhaber S.Z., Nelson K. The Creating an Optimal Warfarin Nomogram (CROWN) Study. Thromb Haemost. 2012;107:59–68. doi: 10.1160/TH11-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J., Kayser S.R. Accuracy of the pharmacogenetic dosing table in the warfarin label in predicting initial therapeutic warfarin doses in a large, racially diverse cohort. Pharmacotherapy. 2011;31:863–870. doi: 10.1592/phco.31.9.863. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman B.S., Gage B.F., Johnson J.A., Brensinger C.M., Kimmel S.E. Genetic warfarin dosing: tables versus algorithms. J Am Coll Cardiol. 2011;57:612–618. doi: 10.1016/j.jacc.2010.08.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zineh I., Pacanowski M.A. Pharmacogenomics in the assessment of therapeutic risks versus benefits: inside the United States Food and Drug Administration. Pharmacotherapy. 2011;31:729–735. doi: 10.1592/phco.31.8.729. [DOI] [PubMed] [Google Scholar]
- 9.Zineh I., Mummaneni P., Lyndly J. Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics. 2011;12:1741–1749. doi: 10.2217/pgs.11.131. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Guidance for Industry Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling. January 2013. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM337169.pdf. Accessed March 20, 2018.