Abstract
Background
Current definitions of AKI do not take into account serum creatinine’s high variability in children.
Methods
We analyzed data from 156,075 hospitalized children with at least two creatinine tests within 30 days. We estimated reference change value (RCV) of creatinine on the basis of age and initial creatinine level in children without kidney disease or known AKI risk, and we used these data to develop a model for detecting pediatric AKI on the basis of RCV of creatinine. We defined pediatric AKI according to pediatric reference change value optimized for AKI in children (pROCK) as creatinine increase beyond RCV of creatinine, which was estimated as the greater of 20 μmol/L or 30% of the initial creatinine level.
Results
Of 102,817 children with at least two serum creatinine tests within 7 days, 5432 (5.3%) had AKI as defined by pROCK compared with 15,647 (15.2%) and 10,446 (10.2%) as defined by pediatric RIFLE (pRIFLE) and Kidney Disease Improving Global Outcomes (KDIGO), respectively. Children with pROCK-defined AKI had significantly increased risk of death (hazard ratio, 3.56; 95% confidence interval, 3.15 to 4.04) compared with those without AKI. About 66% of patients with pRIFLE-defined AKI and 51% of patients with KDIGO-defined AKI, mostly children with initial creatinine level of <30 μmol/L, were reclassified as non-AKI by pROCK, and mortality risk in these children was comparable with risk in those without AKI by all definitions.
Conclusions
pROCK criterion improves detection of “true” AKI in children compared with earlier definitions that may lead to pediatric AKI overdiagnosis.
Keywords: children, acute kidney injury, criterion
AKI is common in hospitalized children. It is associated with increased mortality and adverse outcomes,1–4 including development of hypertension and CKD.5–7 Definitions for AKI were originally derived for application in critically ill adults, and although some studies have adapted these definitions for children,8–11 the ideal way of defining AKI in children remains an evolving field.12,13 The current definitions of pediatric AKI include the pediatric RIFLE (pRIFLE) and the Kidney Disease Improving Global Outcomes (KDIGO).5,14–17 Both are on the basis of the observation that even a small rise in serum creatinine (SCr) is associated with worsening short- and long-term outcomes,7,18–21 and both define AKI by a given magnitude of rise in SCr within a short period of time.
Neither pRIFLE nor KDIGO criteria take into account the biologic and analytic variability of changes in SCr, which does not reflect a real change in renal function.22 Only an increase in SCr that is greater than the normal variability can (with high probability) represent a true decline in renal function. The reference change value (RCV) of SCr has been estimated at 14%–25% in adults.23–25 Incorporating RCV into the definition of AKI is more important in children, because both the level and the variability of SCr depend on age.26 The distribution of change in SCr and its relationship with age and baseline SCr levels have not been established in children, but we speculate that both affect the distribution of change in SCr in children. Therefore, we hypothesize that a definition of AKI on the basis of RCV of SCr adjusted for age and baseline SCr may improve the reliability of detecting pediatric AKI.
To test this hypothesis, we estimated the RCV of creatinine given age and initial SCr level in 100,081 hospitalized children ages 1 month old to 18 years old without kidney diseases and known risk of AKI. We derived a simple formula for RCV of SCr on the basis of the 95th percentile of the distribution and proposed a pediatric reference change value optimized for AKI in children (pROCK). We validated pROCK in 94,715 children and compared its performance with pRIFLE and KDIGO.
Methods
Study Population and Data Source
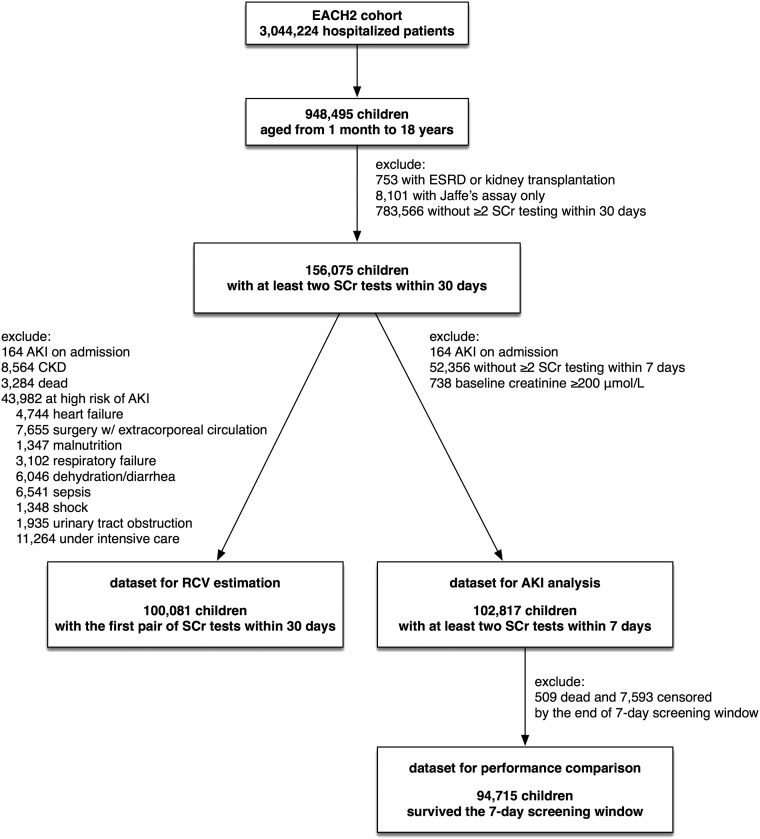
The study population was drawn from the Epidemiology of AKI in Chinese Hospitalized Patients Study. This is a multicenter, retrospective cohort study conducted in 25 regional medical centers, including nine children’s hospitals, with 3,044,224 patients admitted from January 1, 2013 to December 31, 2015. This study included 156,075 pediatric inpatients ages between 1 month old and 18 years old who had at least two SCr measurements taken ≤30 days apart. Children with ESRD or those receiving maintenance dialysis or renal transplantation were excluded. Patient-level data were extracted from the electronic hospitalization databases and laboratory databases from the participating centers. The hospitalization records consisted of patients’ age, sex, date and diagnosis code on admission and at discharge, operation procedures and dates, need for intensive care, in-hospital death, and total hospitalization cost. The laboratory data included value and time of patients’ SCr tests. Only creatinine tests that used an enzymatic assay calibrated to be isotope dilution mass spectrometry traceable were included. All of the laboratories of the participating hospitals had passed the annual External Quality Assessment by the Chinese National Center for Clinical Laboratories. The flowchart of the study population and the patients selected for subsequent analyses is presented in Figure 1. The Medical Ethics Committee of Nanfang Hospital approved the study.
Figure 1.
The flowchart of patient selection. A total of 156,075 pediatric inpatients ages between 1 month old and 18 years old who had at least two SCr measurements within 30 days were selected from EACH2 study and divided into different datasets for RCV estimation, AKI detection and definition comparison. EACH2, Epidemiology of AKI in Chinese Hospitalized Patients; RCV, reference change value; SCr, serum creatinine.
Distribution of SCr Ratio by Age and Initial SCr in Children
Children were included if they had at least one pair of SCr values measured within 30 days, but they were excluded if they had AKI on admission, kidney diseases, or known risks of AKI. AKI on admission and coexisting conditions were determined using the diagnosis codes on admission and at discharge, respectively. The first available pair of SCr measurements within 30 days was selected to derive the distribution of creatinine ratio.
Let c1 and c2 denote the values from two consecutive creatinine tests. Because SCr is log-normally distributed, the log of the ratio, log(c2/c1) = log(c2) − log(c1), is also normally distributed with a mean of μ and a variance of σ2. The distribution of log(c2/c1) was examined in strata of age and c1 by density plots and quantile-quantile plots. The maximum likelihood method was used to estimate the distribution parameters μ and σ2 as a function of age and c1 under Gaussian location-scale additive models.27 The 95th percentile of creatinine ratio (c2/c1) and absolute change (c2 − c1) were calculated at a given age and c1 as 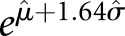 and
and 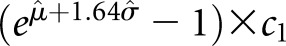 , respectively, where
, respectively, where 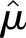 and
and 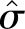 2 were the maximum likelihood estimates of the mean and the variance of log(c2/c1) given age and c1.
2 were the maximum likelihood estimates of the mean and the variance of log(c2/c1) given age and c1.
Definition of AKI on the Basis of RCV of SCr
We defined RCV of SCr as the 95th percentile of c2 − c1 given age and c1. The smoothed curves of RCV with respect to age and c1 were used to derive a simplified formula that approximated the RCV as 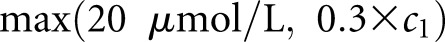 , where c1 is in units of micromoles per liter. We used this as the cutoff value for the definition of AKI. This definition, termed as pROCK, could also be expressed as both an absolute increase of SCr of ≥20 μmol/L and a relative increase of ≥30% at the same time.
, where c1 is in units of micromoles per liter. We used this as the cutoff value for the definition of AKI. This definition, termed as pROCK, could also be expressed as both an absolute increase of SCr of ≥20 μmol/L and a relative increase of ≥30% at the same time.
Comparison of the Performance of Three AKI Definitions
Detecting AKI
We selected patients with at least two SCr results within 7 days, excluding those with AKI on admission and those with an initial SCr value of ≥200 μmol/L. We used the first 7-day window that had at least two SCr tests (the AKI screening window) to define the AKI status according to pROCK, pRIFLE, and KDIGO. The baseline of SCr was defined as the mean of the SCr measurements within the 90 days before the start of the AKI screening window (including the first SCr in the screening window). The pROCK defined AKI by an increase in SCr of both ≥20 μmol/L and ≥30% within 7 days. The pRIFLE defined AKI by a ≥25% decrease in estimated creatinine clearance (equivalent to a ≥33% increase in SCr according to the SCr-based Schwartz equation) within 7 days.16 The KDIGO defined AKI by a ≥50% increase in SCr within 7 days or an absolute increase of ≥26.5 μmol/L within 2 days17 (Supplemental Table 1). SCr values below 5 μmol/L were reset to 5 μmol/L.
The incidences of AKI during the 7-day screening window were computed according to the above three definitions in strata of initial SCr level. Because there are two components in the KDIGO criteria (SCr increase ≥0.3 mg/dl within 48 hours or ≥50% within 7 days), we also compared the incidences of AKI using pROCK and each subcomponent of KDIGO.
Comparison of Performance in Predicting the Risk of Death
The risk of death was analyzed in children who survived the 7-day screening window. About 46.7% of the patients had personal identifiers that allowed their survival status after discharge to be tracked using the national mortality surveillance system from the Chinese Center for Disease Control and Prevention.28
The smoothed curves of the cumulative death hazard with respect to both the absolute change and the relative change (SCr ratio) in SCr were estimated by Cox proportional hazard models that were stratified by whether the SCr changes were within normal range (less than RCV). The performance of the three AKI definitions was compared in predicting the risk of death after the 7-day screening window using Cox proportional hazard models with adjustment for age, sex, initial creatinine value, and need for intensive care. The Harrell c index of each Cox model was computed to assess the concordance between the predicted risk and the survival time.29 The difference in the c indices between any pair of models was compared using a nonparametric test.30 The performance of the three AKI definitions was also compared in predicting the risk of death by the fixed time (15, 30, 60, and 90 days after the screening window) using logistic regression models with AKI status, age, sex, initial SCr level, and need for intensive care as predictors. The Akaike information criterion (AICs), the areas under the receiver operating characteristic curve (AUCs), and the sensitivities of the models given a specificity of 0.95 were computed and compared.31
We also examined the effects of AKI by different definitions on log-transformed length of stay and average daily cost during hospitalization and on progression to CKD in regression analyses with adjustment for confounders. Progression to CKD was defined as an increase in SCr of ≥20 μmol/L and 30% from the baseline and to a level of ≥1.5 times the age- and sex-specific reference of SCr (Supplemental Figure 1) lasting for at least 90 days.
Staging of AKI
Three criteria for staging AKI were compared by visually inspecting the separation of the survival curves among different staging strata. The KDIGO classified AKI stages 2 and 3 as SCr increases of ≥100% and ≥200%, respectively, whereas the pRIFLE classified AKI stages 2 and 3 as 50% and 75% decreases, respectively, in estimated creatinine clearance (equivalent to SCr increases of ≥100% and ≥300%, respectively). The pROCK classified AKI stages 2 and 3 as SCr increases of ≥40 μmol/L and ≥60% and ≥80 μmol/L and ≥120%, respectively.
Statistical analyses were performed using R version 3.3.1, including packages mgcv version 1.8–12, survival version 2.41–3, pROC version 1.8, and compareC version 1.3.1.32
Results
Characteristics of the Study Cohort
The study population included 156,075 hospitalized children who were between 1 month old and 18 years old and had at least one pair of SCr measurements within 30 days (Figure 1). In the population, 31%, 52%, and 17% were in infancy (1 month old to 1 year old), childhood (>1–10 years old), and adolescence (>10–18 years old), respectively. The median age was 4.8 years old, and 62% of the children were boys. The characteristics of the study cohort and the analysis sets are presented in Table 1.
Table 1.
Characteristics of analysis datasets by age strata
| Characteristics | 1 mo to 1 yr | >1–10 yr | >10-18 yr | All Ages |
|---|---|---|---|---|
| Dataset for estimation of RCV of creatinine | ||||
| N | 25,087 | 55,131 | 19,863 | 100,081 |
| Boys, n (%) | 15,785 (62.9) | 33,620 (61.0) | 12,309(62.0) | 61,714 (61.7) |
| From children’s hospital, n (%) | 20,995 (83.7) | 38,149 (69.2) | 6335 (31.9) | 65,479 (65.4) |
| Initial creatinine, μmol/La | 21(17, 27) | 30 (24, 37) | 50 (41, 62) | 30 (22, 41) |
| Log creatinine ratio, mean (SD) | 0.018 (0.49) | −0.010 (0.38) | −0.030 (0.25) | −0.007 (0.39) |
| Dataset for performance comparison of AKI definitions | ||||
| N | 30,981 | 46,817 | 16,917 | 94,715 |
| Boys, n (%) | 19,125 (61.7) | 27,644 (59.0) | 10,381 (61.4) | 57,150 (60.3) |
| From children’s hospital, n (%) | 23,664 (76.4) | 29,604 (63.2) | 4904 (29.0) | 58,172 (61.4) |
| Initial creatinine, μmol/La | 23 (18, 29) | 29 (23, 37) | 51 (41, 64) | 29 (22, 41) |
| Need for intensive care, n (%) | 6717 (21.7) | 5850 (12.5) | 1548 (9.2) | 14,115 (14.9) |
| Length of hospital stay, da | 14 (9, 22) | 14 (10, 23) | 15 (10, 23) | 14 (10, 22) |
| Follow-up length, da,b | 18 (6, 295) | 49 (6, 388) | 130 (9, 455) | 38 (6, 371) |
| Death, n (per 100,000 person-years) | 663 (4800) | 789 (2982) | 403 (3627) | 1855 (3610) |
RCV, reference change value.
Initial creatinine and follow-up length are presented as median (quartile 25, quartile 75).
Follow-up was counted from the end of the 7-day screening window.
Distribution of SCr Change in Children
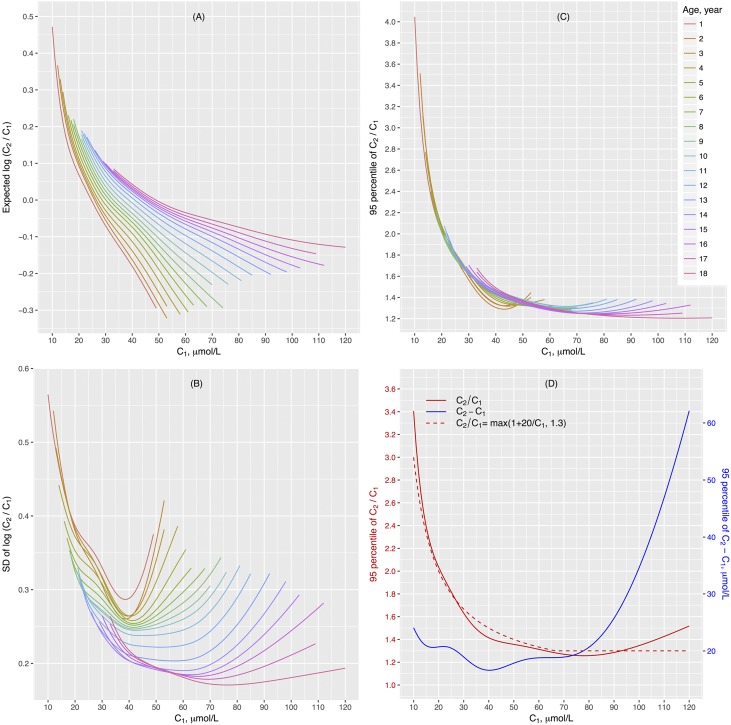
We estimated the distribution of SCr change in 100,081 hospitalized children ages 1 month old to 18 years old who had at least two SCr measurements within 30 days and did not have kidney diseases or known risk of AKI. The majority of the children included in the analysis were inpatients admitted for surgery without extracorporeal circulation, respiratory tract infections, or malignant tumor. SCr was log-normally distributed and varied greatly with age (Supplemental Figures 1–3). The distribution of the ratio between two SCr measurements (c1 and c2 in sequential order) from the same individual was also log-normally distributed, and it varied with age and c1 (Supplemental Figures 4 and 5). The smoothed curves of the mean and the SD of log(c2/c1) with respect to c1 and age are presented in Figure 2, A and B. The corresponding 95th percentile of the distribution of SCr ratio had an L-shaped relationship with c1. The curves were remarkably similar across the age spectrum (Figure 2C). The RCV curves with respect to c1, expressed as SCr ratio (red solid line in Figure 2D) and absolute change (c2 − c1) (blue solid line in Figure 2D), are shown in Figure 2D. The reference value for SCr ratio was relatively stable at about 1.3 when c1 was above 60 μmol/L. However, it rose sharply in the lower ranges of c1. Meanwhile, the corresponding reference value for the absolute change of SCr remained within a narrow window around 20 μmol/L when c1 was below 60 μmol/L. From these results, RCV of SCr, defined as the 95th percentile of the distribution of absolute SCr change given age and initial SCr, approximated the greater of an increase of 20 μmol/L and 30% of c1. We proposed a new creatinine criterion for pediatric AKI (termed pROCK), by which AKI is defined as an SCr increase of both ≥20 μmol/L and ≥30% over the initial value within 7 days.
Figure 2.
Distribution and the reference change value (RCV) of serum creatinine (SCr). (A) The mean and (B) SD of log SCr ratio (c2/c1) given age and initial SCr values estimated in 100,081 children who had at least two SCr measurements (c1 and c2 in sequential order) within 30 days and did not have kidney diseases or known risks of AKI. We assumed log(c2/c1) to be normally distributed with a mean of μ and a variance of σ2 given age and c1, and we used Gaussian location-scale additive models to estimate the distribution parameters μ and σ2 as a function of age and c1. (C) The estimated 95th percentile of SCr ratio given age and initial creatinine value. The curves were remarkably similar across the age spectrum. (D) The smooth curves of 95th percentile of SCr ratio (red solid line) and the corresponding absolute change in creatinine (also known as RCV; blue solid line) given initial creatinine value. The dotted red line is the SCr ratio given by the formula max(1+20/c1, 1.3), with the corresponding SCr change (c2−c1) being max(20 μmol/L, 0.3×c1), where c1 is in units of micromoles per liter. The formula approximates the empirical curve (red solid line) well.
Detecting the Incidence of AKI by pROCK
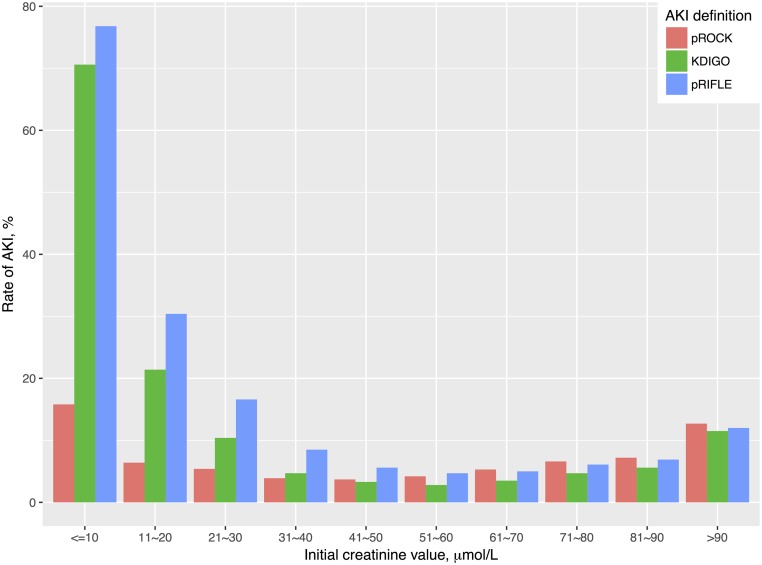
Among 102,817 children who had at least two SCr tests taken ≤7 days apart, 5432 (5.3%), 10,446 (10.2%), and 15,647 (15.2%) AKI events were detected by pROCK, KDIGO, and pRIFLE, respectively, during the 7-day screening window (Supplemental Table 2). Over 95% of children with pROCK-defined AKI also had pRIFLE- and KDIGO-defined AKI, whereas 51% of children with KDIGO-defined AKI and 66% of children with pRIFLE-defined AKI were classified as non-AKI according to pROCK. Most discrepancies in the detected AKI between pROCK and the other definitions were found in children with lower initial SCr values (Figure 3). The incidence rates of AKI defined by KDIGO or pRIFLE were remarkably high in children with initial SCr value of ≤30 μmol/L. Over 70% of children with initial SCr value of ≤10 μmol/L were classified as having AKI by KDIGO and pRIFLE. In comparison, the incidence rate of AKI defined by pROCK in this low-creatinine group was much less influenced by the initial SCr value.
Figure 3.
The incidence rates of AKI during the 7-day screening window varied with the baseline serum creatinine (SCr) level. The incidence rates of AKI defined by KDIGO or pRIFLE were remarkably higher than that defined by pROCK in children with initial SCr value of ≤30 μmol/L. KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, pediatric RIFLE; pROCK, pediatric reference change value optimized for AKI in children.
Of the two components of the KDIGO criteria (SCr increase ≥0.3 mg/dl within 48 hours or ≥50% within 7 days), the 7-day criterion produced a much higher discordance in AKI classification when comparing with pROCK (Supplemental Table 3).
Comparing the Performance of AKI Definitions for Predicting the Risk of Death
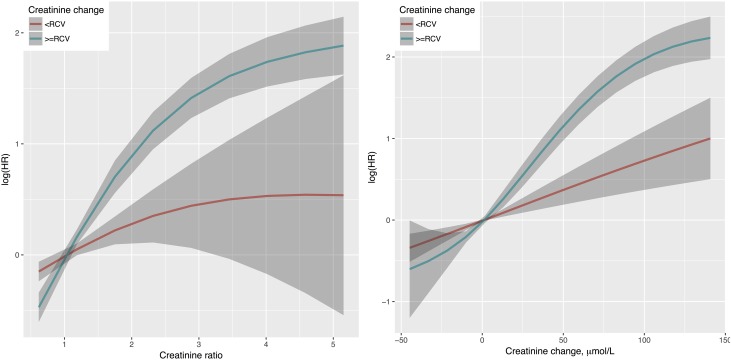
A total of 1855 deaths were observed among 94,715 children who survived the 7-day AKI screening window (Table 1). Both absolute and relative changes in SCr were associated with increased mortality risk, and the associations were significantly greater when the size of change was beyond RCV (Figure 4). At the same size of absolute or relative change in SCr, children whose SCr changed beyond RCV had a much higher mortality risk than those who remained within the range.
Figure 4.
The associations between SCr change and mortality risk were significantly greater when the size of change was beyond RCV. Stratified smooth curves of death risk with respect to the ratio (c2/c1) and the absolute change (c2 − c1) of serum creatinine (SCr) were illustrated. The effects were estimated from Cox proportional hazard models with adjustment for age, sex, initial SCr value, and need for intensive care, and they were stratified by whether the SCr change was within normal range (less than the reference change value [RCV]). HR, hazard ratio.
AKI defined by pROCK was associated with an increased risk of death (Supplemental Table 4) and a higher cumulative hazard (Supplemental Figure 6). We compared the performance of three Cox proportional hazard models for death incorporating AKI defined by one of three AKI criteria as well as age, sex, initial SCr value, and need for intensive care as predictors. The model with AKI defined by pROCK produced the highest c index (Supplemental Table 4). Notably, the superiority of pROCK was more evident in children who needed intensive care, giving rise to c indices of 0.68, 0.65, and 0.65 for pROCK, KDIGO, and pRIFLE, respectively (P<0.001 for comparison). In children with AKI defined by pRIFLE or KDIGO who did not meet the pROCK criteria, the risk of death was comparable with that of those children without AKI defined by all definitions, with hazard ratios of 1.19 (95% confidence interval, 1.00 to 1.41) and 1.13 (95% confidence interval, 0.90 to 1.42), respectively (Table 2). This phenomenon holds in both children with and without intensive care (Table 2) and for both mild and severe AKI (Supplemental Table 5).
Table 2.
Death risk stratified by status of different AKI definitions
| pROCK | KDIGO | pRIFLE | Total, N | Dead, N | Person-yr | Death Ratea | HR (95% CI) |
|---|---|---|---|---|---|---|---|
| All patients | |||||||
| − | − | 84,905 | 1453 | 46,834 | 3102 | — | |
| − | + | 4919 | 91 | 2188 | 4159 | 1.13 (0.90 to 1.42) | |
| + | − | 266 | 15 | 161 | 9341 | 2.40 (1.43 to 4.02) | |
| + | + | 4625 | 296 | 2197 | 13,471 | 3.69 (3.25 to 4.19) | |
| − | − | 80,276 | 1365 | 44,679 | 3055 | — | |
| − | + | 9548 | 179 | 4343 | 4122 | 1.19 (1.00 to 1.41) | |
| + | − | 34 | 1 | 27 | b | b | |
| + | + | 4857 | 310 | 2331 | 13,300 | 3.68 (3.24 to 4.17) | |
| Initial creatinine ≤30 μmol/L | |||||||
| − | − | 38,554 | 623 | 20,823 | 2992 | — | |
| − | + | 8371 | 148 | 3789 | 3906 | 1.05 (0.87 to 1.27) | |
| + | − | 0 | 0 | 0 | b | b | |
| + | + | 2942 | 119 | 1325 | 8980 | 2.63 (2.16 to 3.21) | |
| − | − | 38,554 | 623 | 20,823 | 2992 | — | |
| − | + | 8371 | 148 | 3789 | 3906 | 1.05 (0.87 to 1.27) | |
| + | − | 0 | 0 | 0 | b | b | |
| + | + | 2942 | 119 | 1325 | 8980 | 2.63 (2.16 to 3.21) | |
| Initial creatinine >30 μmol/L | |||||||
| − | − | 42,727 | 767 | 24,320 | 3154 | — | |
| − | + | 172 | 6 | 91 | 6628 | 2.07 (0.92 to 4.65) | |
| + | − | 266 | 15 | 161 | 9341 | 2.56 (1.53 to 4.29) | |
| + | + | 1683 | 177 | 872 | 20,297 | 5.00 (4.22 to 5.91) | |
| − | − | 41,722 | 742 | 23,856 | 3110 | — | |
| − | + | 1177 | 31 | 554 | 5595 | 1.64 (1.14 to 2.36) | |
| + | − | 34 | 1 | 27 | b | b | |
| + | + | 1915 | 191 | 1006 | 18,994 | 4.79 (4.07 to 5.64) | |
| With intensive care | |||||||
| − | − | 11,965 | 388 | 7264 | 5431 | — | |
| − | + | 1161 | 28 | 719 | 3893 | 0.76 (0.50 to 1.14) | |
| + | − | 60 | 8 | 29 | 27,852 | 3.62 (1.75 to 7.54) | |
| + | + | 929 | 123 | 515 | 23,890 | 4.09 (3.33 to 5.02) | |
| − | − | 11,055 | 357 | 6703 | 5326 | — | |
| − | + | 2071 | 59 | 1280 | 4608 | 0.97 (0.72 to 1.31) | |
| + | − | 10 | 0 | 6.4 | b | b | |
| + | + | 979 | 131 | 537 | 24,385 | 4.18 (3.41 to 5.12) | |
| Without intensive care | |||||||
| − | − | 72,940 | 1065 | 39,569 | 2691 | — | |
| − | + | 3758 | 63 | 1469 | 4289 | 1.48 (1.13 to 1.95) | |
| + | − | 206 | 7 | 132 | 5308 | 1.73 (0.82 to 3.67) | |
| + | + | 3696 | 173 | 1682 | 10,283 | 3.50 (2.97 to 4.12) | |
| − | − | 69,221 | 1008 | 37,976 | 2654 | — | |
| − | + | 7477 | 120 | 3063 | 3918 | 1.38 (1.13 to 1.69) | |
| + | − | 24 | 1 | 20.6 | b | b | |
| + | + | 3878 | 179 | 1794 | 9980 | 3.43 (2.91 to 4.03) |
pROCK, pediatric reference change value optimized for AKI in children; KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, pediatric RIFLE; HR, hazard ratio; 95% CI, 95% confidence interval; —, reference.
Per 100,000 person-years.
Did not estimate due to insufficient number of observations.
pROCK also outperformed KDIGO and pRIFLE in predicting the mortality risk at the fixed time points examined, especially in children requiring intensive care, consistently producing models with the lowest AICs, the highest AUCs, and the highest sensitivities given a specificity of 0.95 (Supplemental Table 6, Table 3).
Table 3.
Performance of death risk predictive models incorporating different AKI definitions
| Definition | ORa | 95% CI of OR | AICb | AUC | P Value Difference in AUC | Sensitivity,c % | 95% CI for Sensitivity, % | P Value Difference in Sensitivity |
|---|---|---|---|---|---|---|---|---|
| Day 15: 54,857 survivors and 765 deaths | ||||||||
| pROCK | 6.73 | 5.70 to 7.94 | — | 0.750 | — | 31.9 | 28.8 to 35.2 | — |
| KDIGO | 4.84 | 4.11 to 5.72 | 88.5 | 0.742 | 0.04 | 26.9 | 23.8 to 30.3 | <0.001 |
| pRIFLE | 4.22 | 3.60 to 4.95 | 102.1 | 0.743 | 0.15 | 25.0 | 21.7 to 28.5 | <0.001 |
| Day 30: 48,094 survivors and 985 deaths | ||||||||
| pROCK | 6.24 | 5.35 to 7.29 | — | 0.743 | — | 30.9 | 27.9 to 33.7 | — |
| KDIGO | 4.33 | 3.73 to 5.04 | 107.9 | 0.735 | 0.01 | 25.3 | 22.3 to 28.0 | <0.001 |
| pRIFLE | 3.69 | 3.20 to 4.26 | 132.2 | 0.734 | 0.02 | 24.4 | 21.6 to 27.2 | <0.001 |
| Day 60: 44,035 survivors and 1182 deaths | ||||||||
| pROCK | 5.70 | 4.92 to 6.60 | — | 0.729 | — | 28.6 | 25.8 to 31.6 | — |
| KDIGO | 3.89 | 3.38 to 4.49 | 119.4 | 0.721 | <0.01 | 23.8 | 21.3 to 26.3 | <0.001 |
| pRIFLE | 3.32 | 2.90 to 3.80 | 143.6 | 0.720 | 0.02 | 22.4 | 20.2 to 25.0 | <0.001 |
| Day 90: 41,734 survivors and 1295 deaths | ||||||||
| pROCK | 5.26 | 4.55 to 6.07 | — | 0.718 | — | 26.4 | 23.9 to 29.0 | — |
| KDIGO | 3.57 | 3.10 to 4.10 | 120.3 | 0.710 | 0.004 | 22.2 | 19.8 to 24.6 | <0.001 |
| pRIFLE | 3.04 | 2.67 to 3.46 | 144.9 | 0.710 | 0.01 | 20.5 | 18.4 to 22.8 | <0.001 |
OR, odds ratio; 95% CI, 95% confidence interval; AIC, Alkaike information criterion; AUC, area under the receiver operating characteristic curve; pROCK, pediatric reference change value optimized for AKI in children; —, reference; KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, pediatric RIFLE.
Logistic models with AKI status, age, sex, initial creatinine value, and need for intensive care as predictors.
AIC with pROCK as reference.
Sensitivity given a specificity of 0.95.
pROCK was also associated with longer length of hospital stay, higher daily cost, and increased risk of progression to CKD, even after adjustment for confounders (Supplemental Tables 7 and 8).
Staging of AKI by pROCK
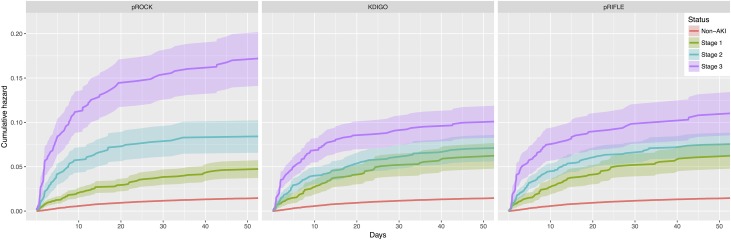
pROCK defines an SCr increase of both ≥40 μmol/L and ≥60% as stage 2 and an increase of both ≥80 μmol/L and ≥120% as stage 3 (Supplemental Table 1). Among the children with AKI defined by pROCK, 23% and 17% were classified as stages 2 and 3, respectively, using the pROCK staging criteria. In comparison, 36% and 31% were stages 2 and 3, respectively, according to the KDIGO criteria, and 48% and 19% were stages 2 and 3, respectively, according to the pRIFLE criteria. The pROCK staging criteria applied to the study population yielded the best separation among non-AKI and different stages of AKI on the survival curves (Figure 5).
Figure 5.
Kaplan–Meier curves of cumulative death hazard by AKI stage using different staging criteria pROCK yielded the best separation among non-AKI and different stages of AKI on the Kaplan–Meier curves of cumulative death hazard. KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, pediatric RIFLE; pROCK, pediatric reference change value optimized for AKI in children.
Discussion
This large multicenter study in children confirmed that the change in SCr varied greatly with age and initial creatinine value. This is the first report to establish the RCV of creatinine given initial SCr and age in children without kidney diseases and known risk of AKI. This was used to propose a simplified RCV-based criterion for pediatric AKI (pROCK). Among 100,081 children, more than one half of the children with AKI defined by the creatinine criteria of pRIFLE or KDIGO were reclassified as non-AKI by pROCK. Importantly, the risk of death in these patients approached the risk in children without AKI by all definitions, indicating that the pROCK classification had enriched the population with a higher risk for adverse outcomes. The performance of pROCK compared with KDIGO and pRIFLE in predicting the risk of death was validated in a large sample of hospitalized children. It was particularly discriminatory in children with lower initial SCr levels as well as those requiring intensive care.
Both KDIGO and pRIFLE define AKI by a fixed percentage increase in SCr. These criteria lack precision in individuals with lower creatinine levels that are characteristic of young children. For example, the median SCr in our study population in children from 1 month old to 1 year old was 21 μmol/L. At this level, increases of 10.5 and 7 μmol/L in SCr in this group would meet the AKI criteria by KDIGO and pRIFLE, respectively. However, an increase of SCr of this level can well be a result of analytic and biologic variation rather than an indication of kidney function loss. This explains the highly inflated rate of AKI diagnosed by KDIGO and pRIFLE in children with SCr level of ≤30 μmol/L. It extends the findings in adults33 that the incidence of AKI, defined by percentage increase in SCr, is markedly higher in those with SCr levels <0.6 mg/dl (approximately 53 μmol/L). AKI was not associated with death in those with baseline SCr lower than 0.4 mg/dl (approximately 35 μmol/L).33 The crux of the issue is the unreliability of relative change in SCr when the baseline SCr is low, a challenge not confined to children.34
A major strength of pROCK is that it incorporates an experimentally determined RCV of SCr. The definition of AKI by pROCK is on the basis of the simple concept that an acute increase in SCr above the upper limit of normal variability should be a consistent index of a decline in renal function. The RCV defined by the 95th percentile of the distribution in a large sample of hospitalized children without kidney diseases and known risk of AKI was approximately the greater of 20 μmol/L and 30% of the baseline SCr. Importantly, our data indicate that this refinement in the population diagnosed as having AKI by pROCK via eliminating those diagnosed by pRIFLE and KDIGO was valuable, because the mortality of those excluded was close to that of children without AKI. The pROCK had better precision in identifying children at risk of death, especially those with low SCr or requiring intensive care. In our performance comparison, models incorporating AKI defined by pROCK consistently outperformed those incorporating AKI defined by the creatinine criteria of KDIGO and pRIFLE in predicting both the survival time and the survival status up to 90 days after AKI, yielding the lowest AICs, the highest AUCs, and the highest sensitivities given a specificity of 0.95.
Within-individual variability in SCr has been studied previously in adults, with reported RCVs ranging from 14% to 25%, but it has not been studied in children.23–25 Deriving RCV in children is challenging, because it varies with age and initial SCr. Our method for estimating the RCV of SCr in children has several unique features. First, we estimated RCV of SCr as a function of initial SCr and age. Second, we incorporated the mean of change value in calculating the RCV. Most RCV calculations presume that the mean of change value is zero and ignore the effect of regression to the mean. Third, our analysis set consisted of >100,000 hospitalized children without kidney diseases and without known risk of AKI. This large sample size allowed us to estimate RCVs with decent accuracy in hospitalized children over a wide spectrum of initial SCr and age. Although the RCVs derived from such population may be slightly higher than those from “healthy” children, they could capture the potential variability in SCr change associated with the environment and interventions during hospitalization, and hence, they might be a more appropriate index for defining hospital-acquired AKI in children. Fourth, we used log-transformed SCr in the calculation to achieve a better normality, thereby reducing the effect of extreme values.
There are some limitations of this study. First, pROCK was validated by predicting the short-term (median follow-up length of 38 days) risk of death in hospitalized children due to the limited follow-up period of this cohort. Later studies should compare the performance of pROCK, pRIFLE, and KDIGO in predicting the long-term risk of death in children. Second, we compared the performance of the AKI definitions using the SCr criteria only because urine output data were not available from our database. It would be interesting to compare with the definitions using both SCr and urine output, although quantification of urine output in a large pediatric cohort is difficult. Third, the formula for RCV was derived from a Chinese population. Whether it is applicable to other ethnic populations needs further validation. Fourth, although there was some overlap between the datasets used for estimation of the RCV and the outcome analysis, the risk of overfitting the prediction models in the outcome analysis would be limited, because pROCK was derived on the basis of the RCV of SCr without consideration of any outcomes.
In conclusion, we propose pROCK on the basis of the RCV of SCr derived in a large population to identify pediatric AKI. Our data suggested that more than one half of the patients with AKI identified by KDIGO and pRIFLE had an SCr increase within the normal range of variability, and these patients were not associated with a significantly increased risk of death. pROCK may avoid overdiagnosis of AKI, particularly in children with lower baseline SCr and those requiring intensive care. It helps to obtain more accurate disease burden of pediatric AKI to reduce unnecessary procedures and treatment and save medical resources in patient care. pROCK is easy to apply and outperforms current definitions for detecting “true” AKI in children. Further validation of pROCK in other populations is warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China grant 81770683 (to X.X.), National Innovation Team Program grant 81521003 (to Y.L.), National Key Technology Support Program of China grant 2015BAI12B07 (to F.F.H.), National Natural Science Foundation of China (Key Program) grant 81430016 (to F.F.H.), Major Scientific and Technological Planning Project of Guangzhou grant 201504010027 (to F.F.H.), and Major International (Regional) Joint Research Project grant 81620108003 (to F.F.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A New Pediatric AKI Definition: Implications of Trying to Build the Perfect Mousetrap,” on pages 2259–2261.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010090/-/DCSupplemental.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL: AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al.: Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGregor TL, Jones DP, Wang L, Danciu I, Bridges BC, Fleming GM, et al.: Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: A retrospective observational study. Am J Kidney Dis 67: 384–390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpon LC, Sugo EK, Consulin JC, Tavares TL, Aragon DC, Carlotti AP: Epidemiology and outcome of acute kidney injury according to pediatric risk, injury, failure, loss, end-stage renal disease and kidney disease: Improving global outcomes criteria in critically ill children-a prospective study. Pediatr Crit Care Med 17: e229–e238, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al.: Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al.: AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10: 554–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Yi ZW, Zhang H, Dang XQ, Wu XC, Huang AW: Etiology and outcomes of acute kidney injury in Chinese children: A prospective multicentre investigation. BMC Urol 13: 41–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al.: AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland SM, Kwiatkowski DM: Acute kidney injury in children. Adv Chronic Kidney Dis 24: 380–387, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Ingelfinger JR, Kalantar-Zadeh K, Schaefer F: World Kidney Day Steering Committee : Averting the legacy of kidney disease: Focus on childhood. Nephrol Dial Transplant 31: 327–331, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Lameire N, Van Biesen W, Vanholder R: Epidemiology of acute kidney injury in children worldwide, including developing countries. Pediatr Nephrol 32: 1301–1314, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, et al.: Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med 40: 1481–1488, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL: Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3: 948–954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 17.KDIGO AKI Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 18.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 Year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SL, Devarajan P: Pediatrics: Acute kidney injury leads to pediatric patient mortality. Nat Rev Nephrol 6: 393–394, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al.: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al.: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al.: The definition of acute kidney injury and its use in practice. Kidney Int 87: 62–73, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Reinhard M, Erlandsen EJ, Randers E: Biological variation of cystatin C and creatinine. Scand J Clin Lab Invest 69: 831–836, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Pineda-Tenor D, Laserna-Mendieta EJ, Timón-Zapata J, Rodelgo-Jiménez L, Ramos-Corral R, Recio-Montealegre A, et al.: Biological variation and reference change values of common clinical chemistry and haematologic laboratory analytes in the elderly population. Clin Chem Lab Med 51: 851–862, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, et al.: Biological variation of plasma and urinary markers of acute kidney injury in patients with chronic kidney disease. Clin Chem 62: 876–883, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al.: Closing the gaps in pediatric laboratory reference intervals: A CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58: 854–868, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Wood SN: Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99: 673–686, 2004 [Google Scholar]
- 28.Liu S, Wu X, Lopez AD, Wang L, Cai Y, Page A, et al.: An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ 94: 46–57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA: Regression modelling strategies for improved prognostic prediction. Stat Med 3: 143–152, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Kang L, Chen W, Petrick NA, Gallas BD: Comparing two correlated C indices with right-censored survival outcome: A one-shot nonparametric approach. Stat Med 34: 685–703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 32.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2016 [Google Scholar]
- 33.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS: Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9: 12–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.