Abstract
Background
Expression of genes regulating fatty acid metabolism is reduced in tubular epithelial cells from kidneys with tubulointerstitial fibrosis (TIF), thus decreasing the energy produced by fatty acid oxidation (FAO). Acetyl-CoA carboxylase (ACC), a target for the energy-sensing AMP-activating protein kinase (AMPK), is the major controller of the rate of FAO within cells. Metformin has a well described antifibrotic effect, and increases phosphorylation of ACC by AMPK, thereby increasing FAO.
Methods
We evaluated phosphorylation of ACC in cell and mouse nephropathy models, as well as the effects of metformin administration in mice with and without mutations that reduce ACC phosphorylation.
Results
Reduced phosphorylation of ACC on the AMPK site Ser79 occurred in both tubular epithelial cells treated with folate to mimic cellular injury and in wild-type (WT) mice after induction of the folic acid nephropathy model. When this effect was exaggerated in mice with knock-in (KI) Ser to Ala mutations of the phosphorylation sites in ACC, lipid accumulation and fibrosis increased significantly compared with WT. The effect of ACC phosphorylation on fibrosis was confirmed in the unilateral ureteric obstruction model, which showed significantly increased lipid accumulation and fibrosis in the KI mice. Metformin use was associated with significantly reduced fibrosis and lipid accumulation in WT mice. In contrast, in the KI mice, the drug was associated with worsened fibrosis.
Conclusions
These data indicate that reduced phosphorylation of ACC after renal injury contributes to the development of TIF, and that phosphorylation of ACC is required for metformin’s antifibrotic action in the kidney.
Keywords: fibrosis, renal fibrosis, metformin, ampk, fatty acid oxidation
Recent studies have demonstrated that tubulointerstitial fibrosis (TIF) in both humans and mice is associated with reduced mRNA for genes important in fatty acid metabolism.1 This has been observed in both whole kidneys and cultured human tubular epithelial cells (TECs), and is predicted to reduce fatty acid oxidation (FAO), mainly in proximal tubular cells of the kidney.1 A subsequent study from Han et al.2 showed that specific tubular deletion of LKB-1, one of the upstream regulators of AMPK, was associated with the spontaneous development of TIF. Confirmatory evidence supporting a causal role for reduced FAO in the development of TIN includes the antifibrotic effect of drugs that increase FAO, such as fenofibrate, AICAR, or metformin,1,3–7 and the use of mice overexpressing the gene proliferator-activated receptor-γ (PPAR-γ) coactivator 1-α (PGC-1α), the master regulator of mitochondrial biogenesis.8
Oxidation and synthesis of fatty acids are linked in tissues, and regulated at multiple levels.9,10 One of the major controllers of FAO is acetyl-CoA carboxylase (ACC), which converts acetyl CoA to malonyl CoA in the cytoplasm of cells. Malonyl CoA is the first step in the synthesis of fatty acids but also acts as an inhibitor of carnitine palmitoyl transferase 1 (Cpt1), found on the surface of mitochondria.11,12 When ACC is active, levels of malonyl CoA are high, entry of fatty acids into mitochondria via Cpt1 is low, and most of the malonyl CoA is diverted to fatty acid synthesis. When the activity of ACC is low, increased entry of fatty acids into mitochondria is associated with increased FAO. A number of factors control ACC activity, including abundance, intracellular citrate, and phosphorylation.13
Phosphorylation of ACC is linked to intracellular energy stores by the energy sensing kinase AMPK.11,12 AMPK is activated by a number of stimuli, but the best known is high intracellular AMP, which occurs when ATP has been extensively utilized. AMPK then phosphorylates ACC to inhibit enzyme activity, leading to reduced intracellular malonyl-CoA and increased FAO, so replenishing intracellular energy stores. There are two isoforms of ACC, the product of separate genes.14 In the kidney, the predominant isoform by western blot and real-time PCR (qRT-PCR) is ACC1, with ACC2 expressed at a much lower level or not at all.15 ACC2 is more abundant in tissues such as the heart, liver, and skeletal muscle. Whereas ACC1 is cytoplasmic, ACC2 localizes to the mitochondria due to an N-terminal amino acid extension.
Metformin alters fatty acid metabolism by activation of the energy-sensing kinase AMPK,16 which phosphorylates acetyl-CoA carboxylase 1 (ACC1) at Ser79 and ACC2 at Ser212 to inhibit activity of these enzymes.9,16,17 Activation of AMPK by metformin is indirect, mediated by an increased AMP-to-ATP ratio due to its inhibitory effect on the respiratory chain.18 Metformin is reported to reduce renal fibrosis in several experimental models.3,5 A number of the known actions of metformin have been proposed to explain its antifibrotic effect but, so far, there are no studies directly evaluating the role of the AMPK-ACC pathway in this phenomenon.
To evaluate the proposed causal role of reduced FAO in the pathogenesis of TIF, we have used ACC 1/2 knock-in (KI) mice with Ser to Ala mutations at ACC1 Ser79 and ACC2 Ser212.19 The ACC 1/2 KI mice are slightly hyperglycemic and hyperinsulinemic, and have increased hepatic fatty acid synthesis, although the phenotype is not severe.19 Using these ACC 1/2 KI mice, Fullerton et al.19 have demonstrated that phosphorylation of ACC1 and ACC2 at these two sites was required for the insulin-sensitizing effect of metformin in mice fed a high-fat diet. In these studies, we have utilized these mice to determine whether the inability of AMPK to phosphorylate ACC worsens the fibrotic response to renal injury. In addition, we have attempted to determine whether the antifibrotic action of metformin is dependent on its ability to increase phosphorylation of ACC and so increase FAO in damaged renal tissues.
Methods
Animal Studies
All experiments were approved by the Austin Health Animal Ethics Committee. Homozygous lines of wild type (WT) and ACC 1/2 whole body KI mice19 on a C57/Bl6 background were used. To derive the two lines, ACC1 KI heterozygotes were crossed with ACC2 KI heterozygotes. From the generation of double heterozygous mice, mice homozygous for ACC1 and ACC2 KI mutations and mice with homozygous WT ACC1 and ACC2 were generated by brother-sister matings. They were maintained as two separate lines from then on. To prevent genetic drift between the two groups, they were backcrossed to C57BL/6 once a year with mice ordered from the local animal supplier and the whole process again. The folic acid nephropathy (FAN) model was used to create an experimental model of renal fibrosis, as has been described.20 The FAN model was used to assess the effect of metformin on renal fibrosis. Unilateral ureteric obstruction (UUO) was also used to produce a model of TIF, as has been described.3
Western Blot Analysis
Kidney lysates were prepared and western blots performed as we have previously described.21 Quantification of western blots was by densitometry with analysis using Image J software.22 Use of GAPDH as a loading control was compared with β-tubulin and β-actin and found to be more consistent (Supplemental Figure 1A).
Histology
Kidneys were sliced in half transversely and fixed in formalin. Kidney sections were stained using Picro-Sirius Red Stain Kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Masson’s trichrome staining was performed by the Department of Anatomic Pathology, Austin Health. For Oil Red O staining, kidney halves were embedded in plastic and snap frozen in liquid nitrogen. Samples were processed by the Department of Anatomic Pathology, Austin Health.
Quantification
The area occupied by collagen in picro-sirius red– or Masson’s trichrome–stained sections and lipid droplets in Oil Red O–stained sections was measured using Image J software. The values obtained were expressed as a percentage of the whole cortical area.
Triglyceride Assay
Kidney tissue from the UUO model was processed according to the triglyceride (TG) assay protocol in the ab65336 kit from Abcam.
Cell Culture Studies
Primary cultures of renal TECs were prepared by sieving whole mouse kidneys. It is worth noting that these cultures will contain cells of a variety of tubular lineages, but only the proximal tubule uses FAO extensively as an energy source. To mimic injury, cells were treated with 10 mM folic acid in serum-free KI media for 24 hours.23 ATP was assayed using the Abcam kit ab83355 according to the manufacturer’s instructions.
qRT-PCR
Total RNA was purified from whole mouse kidney samples and reverse transcribed as previously described.21 Primer sequences are shown in Supplemental Table 1. Use of GAPDH as a loading control was compared with 18S ribosomal RNA and β-actin and found to be more consistent (Supplemental Figure 1B).
Statistical Analyses
Statistical analyses were performed using Prism version 7.0a for Mac OS X (GraphPad Software, San Diego, CA). Data are presented as mean±SD. RT-PCR data are presented as SEM. Multiple group means were compared by ANOVA followed by a post hoc test. Comparison of means from two groups was performed by an unpaired t test. P values of <0.05 were considered significant.
Results
In Vitro Toxicity of Folate in TECs
Primary cultures of murine TECs were isolated from female C57BL/6 mouse kidneys and used when 70% confluent. Phosphorylation of ACC1 on Ser79 was detected in mouse TECs, whereas ACC2 was not observed (Figure 1A). Exposure to folate was associated with a 60% reduction in phosphorylation of ACC1 on Ser79 (Figure 1B). This was associated with a reduction in mRNA encoding two of the key enzymes involved in FAO, ACox and Cpt1a (Figure 1, C and D). These data suggest that toxic injury to TECs reduces use of FAO as an energy source.
Figure 1.
Expression of ACC, pACC and FAO enzymes in TEC from WT mice. (A) Representative western blots showing reduced expression of pACC-Ser79 in WT TECs treated with 10 mM folic acid compared with WT control cells and (B) quantitated by densitometry. (C, D) Expression of mRNA transcripts for key regulatory enzymes required for FAO; (C) Cpt1a (n=6) and (D) ACox1 (n=6) were reduced in WT TECs after treatment with 10 mM folic acid. qRT-PCR was normalized to 18S and represented as fold changes. **P<0.01; ***P<0.001.
Fibrosis in the FAN Model
To determine the role of phosphorylation of ACC in the response to tissue injury, and to determine whether it was deleterious, mice with a Serine to Alanine mutation of the Ser79 site in ACC1 and the Ser212 site in ACC2 (ACC 1/2 KI mice) were used. There was no morphologic abnormality in their kidneys by light microscopy (not shown). At day 14 of the FAN model, there was a 33% reduction in phosphorylation of Ser79 on ACC1 in WT mice with no reduction in ACC1 expression (P<0.05) (Figure 2, A–C). As we have previously reported, ACC2 expression in the murine kidney is minimal,15,21 and it was not detected by western blot in this study (data not shown). Ser79 phosphorylation on ACC1 was not seen in the ACC 1/2 KI mice (Figure 2A). As has been previously reported,1 mRNA for Cpt1a and ACox was reduced in mice with FAN (Figure 2, D and E). These enzymes are critical for FAO. There was, however, no difference in their expression between ACC 1/2 KI and WT mice.
Figure 2.
Expression of ACC, pACC and FAO enzymes in kidneys from WT and ACC 1/2 KI mice with FAN. (A) Representative western blots showing absent pACC-Ser79 in ACC 1/2 KI mice compared with WT mice. WT folate mice have a significantly reduced level of pACC-Ser79 compared with WT control mice as analyzed by western blot and (B) quantitated by densitometry (n=10). (C) There was no significant difference in expression of total ACC between groups as shown by densitometry (n=8). (D, E) Expression of mRNA transcripts for key regulatory enzymes in FAO; (C) Cpt1a (n=5) and (D) ACox (n=5) were reduced in WT and ACC 1/2 KI mice after folic acid treatment. qRT-PCR was normalized to GAPDH and represented as fold changes. *P<0.05; **P<0.01; ***P<0.001.
Reflecting the reduction in FAO, there was increased staining for lipid by Oil Red O in the kidneys of WT mice and ACC 1/2 KI mice receiving folate compared with vehicle-injected mice (P<0.05 and <0.001, respectively) (Figure 3, A and B). There was also significantly increased lipid staining in the ACC 1/2 KI mice with FAN compared with WT (P<0.05).
Figure 3.
Markers of lipid accumulation and fibrosis in kidneys from WT and ACC 1/2 KI mice with FAN. (A) Images of Oil Red O–stained kidney sections from control and folic acid–treated WT and ACC 1/2 KI mice. (B) Quantification of Oil Red O–stained kidney sections analysis showing increased staining in the ACC 1/2 KI FAN group compared with the WT FAN group (n=7). (C) Representative images of picro-sirius red–stained kidney sections from control and folic acid–treated WT and ACC 1/2 KI mice. (D) Quantification of picro-sirius red–stained kidney sections showing increased staining in ACC 1/2 KI mice with FAN compared with WT mice with FAN (n=7). (E) Images of kidney sections from control and folic acid–treated WT and ACC 1/2 KI mice stained with Masson’s trichrome. (F) Quantification of Masson’s trichrome–stained kidney sections analysis showing increased staining in the ACC 1/2 KI FAN group compared with the WT FAN group (n=7). *P<0.05; **P<0.01; ***P<0.001.
The functional effect of reduced ACC phosphorylation was further investigated by studies of renal fibrosis. At day 14 of the FAN model, there was significantly increased fibrosis detected by staining for picro-sirius red in WT and ACC 1/2 KI mice treated with FAN compared with sham-injected mice (P<0.001) (Figure 3, C and D). Notably, fibrosis in the FAN model was more severe in ACC 1/2 KI than in WT mice (P<0.05) (Figure 3, C and D). These findings were confirmed by analysis of Masson’s trichrome staining, another collagen stain (Figure 3, E and F). Expression analysis of fibrosis genes found that ACC 1/2 KI mice with FAN had greater expression of α–smooth muscle actin (α-SMA) (P<0.05) and collagen 1 (P<0.01), and a trend to greater collagen 3 (Figure 4A), compared with WT mice. Increased expression of α-SMA protein in the ACC 1/2 KI FAN mice was confirmed by western blot (P<0.05) (Figure 4B). Together, these data indicate increased renal fibrosis in the FAN model in the ACC 1/2 mice when compared with WT.
Figure 4.
Expression of fibrotic markers in kidneys from WT and ACC 1/2 KI mice with FAN. (A) mRNA expression of α-SMA, fibronectin, collagen3α1, and collagen1α1 was assessed using qRT-PCR (mean+SD). mRNA expression for α-SMA (n=7) and collagen1α1 (n=7) in ACC 1/2 KI mice with FAN increased significantly compared with WT mice with FAN. Quantitative qRT-PCR data were normalized to GAPDH and expressed as the fold change compared with WT control. (B) ACC 1/2 KI mice with FAN have increased α-SMA expression compared with WT mice with FAN as analyzed by western blot and quantitated by densitometry (n=6). *P<0.05; **P<0.01.
Fibrosis in the UUO Model
To confirm that a failure to regulate fatty acid metabolism by ACC was associated with increased fibrosis, the UUO model of renal fibrosis was used. This confirmed the pattern of lipid accumulation and increased TIF after injury in the ACC 1/2 KI mice (Figure 5, Supplemental Figure 2). There was a significant worsening of lipid accumulation in the ACC 1/2 KI mice with UUO compared with the sham-operated group (P<0.001) (Figure 5, A and B). There was a nonsignificant trend to an increase in the WT mice with UUO compared with WT sham, and a significant increase in ACC 1/2 KI mice with UUO compared with WT with UUO (P<0.01). Assay of TGs was also performed and showed a significant fall in TGs in WT UUO mice compared with WT sham mice (P<0.05), with no change in ACC1/2 KI compared with ACC1/2 KI sham. The difference between WT UUO and ACC1/2 KI UUO mice was significant, confirming the result of the Oil Red O staining (P<0.05). There is, however, an unexplained discrepancy between Oil Red O staining and TG assay for the WT UUO mice. Possibly, it is due to accumulation of other neutral lipids. The data for TIF showed a significant increase in the ACC 1/2 KI mice compared with the sham-operated group (P<0.001) (Figure 5C). There was also a significant increase in the WT mice with UUO compared with sham-treated WT mice (P<0.001). Most notably, there was a significant increase in fibrosis in ACC 1/2 KI UUO mice compared with WT UUO mice (P<0.001).
Figure 5.
Markers of lipid accumulation and fibrosis, mitochondrial number and activity, and pro-inflammatory cytokines in WT and ACC 1/2 KI mice with UUO. (A) Quantification of Oil Red O–stained kidney sections showing increased staining in ACC 1/2 KI UUO compared with WT UUO mice (n=7–8). (B) Assay of TGs in kidney tissue from ACC1 KI and WT mice after UUO or sham procedure. (C) Quantification of picro-sirius red–stained kidney sections showing increased staining in ACC 1/2 KI UUO compared with WT UUO mice (n=7). (D) Assay of mRNA relevant to mitochondrial biogenesis and function by qRT-PCR in kidneys from ACC 1/2 KI and WT mice. (E) Assay of mRNA relevant to mitochondrial biogenesis and function by qRT-PCR in kidneys from ACC 1/2 KI and WT mice. *P<0.05; **P<0.01; ***P<0.001.
Expression of mitochondrial genes was assessed using primer pairs described by Romanino et al.24 (Figure 5D). Although PGC-1α mRNA was not altered, mRNA encoding cytochrome C oxidase 4 subunit (Cox4) was increased in the WT UUO mice compared with sham (P<0.05). It did not increase in the ACC1/2 KI mice, an indication of reduced transfer of terminal electrons in the mitochondria and reduced mitochondrial activity. mRNA encoding citrate synthase, a protein that is generally considered a marker of mitochondrial number, was increased in both WT UUO and ACC1/2 KI UUO compared with sham controls (P<0.01 and 0.05, respectively). Expression of mRNA encoding proinflammatory molecules was similar in both UUO groups, suggesting that inflammation is not central to the differences seen in neutral fat and fibrosis (Figures 5E).
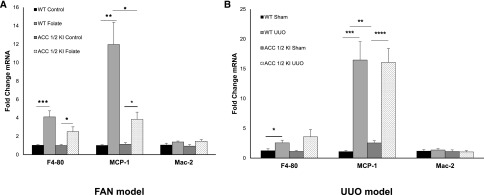
Macrophage Infiltrates in the FAN and UUO Models
Cellular infiltration in FAN was detected using qRT-PCR for the macrophage marker F4/80, as well as the M1 macrophage subset marker MCP-1 and the M2 marker Mac-2.4 As expected, there was a significant increase in macrophage numbers in mice with FAN (Figure 6A). The M1 marker MCP-1 was increased, indicating predominant infiltration with this macrophage subset. There was a nonsignificant trend to reduced numbers of macrophages in ACC 1/2 KI mice with FAN compared with WT, and this was significant for the M1 macrophage subset (P<0.05) (Figure 6A). These data suggest that the worse fibrosis in the ACC 1/2 mice was not caused by increased inflammation.
Figure 6.
Macrophage infiltration in kidneys from WT and ACC 1/2 KI mice with FAN or UUO. (A) RT-PCR analysis showing increased mRNA expression of F4–80, MCP-1, and Mac-2 (n=7) after administration of folic acid in both WT and ACC 1/2 KI mice. (B) mRNA transcripts for F4–80, MCP-1, and Mac-2 (n=7) were assessed by qRT-PCR and significantly increased in both WT UUO and ACC 1/2 KI UUO compared with sham groups. Quantitative PCR data were normalized to GAPDH and expressed as the fold change compared with WT control. *P<0.05; **P<0.01; ***P<0.001.
In the UUO model, there was also an increase in M1 macrophages in WT and ACC 1/2 KI with UUO compared with sham controls (P<0.001) (Figure 6B). However, there was no difference in total or M1 macrophage infiltration between ACC 1/2 KI mice and WT mice with UUO. Interestingly, there was a significant increase in M1 macrophages between WT and ACC 1/2 KI mice after a sham operation.
ATP Levels in TEC Treated with Folic Acid
To determine whether ATP levels were different following injury in WT and ACC 1/2 KI cells, TEC from both mouse lines were treated with folic acid, and ATP levels assayed. At both 3 and 24 hours, ATP levels were lower in cells treated with folic acid, but there was no difference between cells from WT and ACC1/2 KI mice (Figure 7).
Figure 7.
ATP levels in TEC from WT and ACC1/2 KI mice. Cells were treated with serum-free media (SFM) or 10 mM folate in serum-free media (SFM+FA). n=23–30. ***P<0.001.
Metformin Administration in the FAN Model
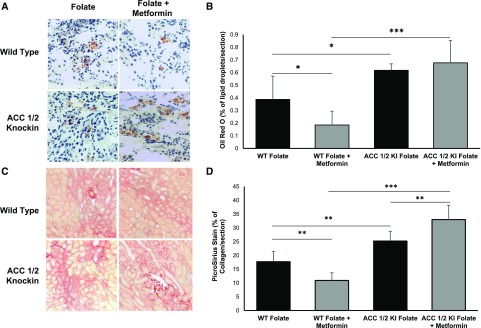
Metformin has been reported to have an antifibrotic effect in the UUO model, although the mechanism is obscure.3,5 Because the antidiabetic effects of metformin in the liver are dependent on phosphorylation of ACC,19 we attempted to determine whether the same is also true of its antifibrotic effects in the kidney. All mice in the study were given folic acid, and metformin was commenced from day 1. WT mice with FAN receiving metformin had increased Ser79 phosphorylation compared with mice not receiving metformin (Figure 8, A and B), as has previously been described in mice with the UUO model.3 There was reduced lipid accumulation (P<0.05) and fibrosis (P<0.01) in WT mice receiving metformin compared with untreated WT mice (Figure 9). The protective effects of metformin did not occur in the ACC 1/2 KI FAN mice, which had equivalent lipid accumulation and significantly worse TIF when receiving metformin compared with untreated ACC 1/2 KI mice (P<0.01) (Figure 9).
Figure 8.
Effect of metformin on pACC-S79 in kidneys from WT mice with FAN. Expression of pACC-Ser79 was significantly increased in WT folate mice after administration of metformin as (A) analyzed by western blot and (B) quantitated by densitometry (arbitrary units, n=6). *P<0.05.
Figure 9.
Effect of metformin on markers of lipid accumulation and fibrosis in WT and ACC 1/2 KI mice with FAN. Representative images of (A) Oil Red O– and (C) picro-sirius red–stained kidney sections of mice receiving folate to induce FAN or folate+metformin. (B) Quantification of Oil Red O–stained kidney sections showing increased staining in ACC 1/2 KI folate+metformin compared with WT mice receiving folate+metformin (n=8). (D) Quantification of picro-sirius red-stained kidney sections shows increased staining in kidney sections from ACC 1/2 KI mice receiving folate+metformin compared with both ACC 1/2 KI mice receiving folate and WT mice receiving folate+metformin (n=8). *P<0.05; **P<0.01; ***P<0.001.
Discussion
In this study, renal injury was associated with reduced phosphorylation of ACC1 on Ser79. Complete absence of ACC phosphorylation in transgenic mice was associated with increased fibrosis, indicating that phosphorylation of ACC by AMPK reduces renal injury and that the response of the injured kidney to reduce ACC phosphorylation is maladaptive. That proposal is supported by studies of the administration of metformin, which has been reported to reduce renal fibrosis.3,5 Metformin indirectly activates AMPK, which leads to phosphorylation of ACC. When this phosphorylation event was blocked by mutation in the ACC 1/2 KI mice, renal fibrosis in the FAN model was worse. That result appears at variance with the study of Christensen et al.,4 who were unable to find any change in the renoprotective effects of metformin in AMPK β1 null mice. In contrast, Feng et al.25 noted a partial effect in AMPK α2 null mice. Neither of these transgenic mice have a complete knockout of AMPK activity, due to the presence of alternative subunits, and this might be responsible for the difference.
These data are similar to studies in the liver, where inhibition of ACC1 and ACC2,26 and knockout of ACC1, have been consistently associated with reduced de novo lipogenesis.27 The kidney is very different metabolically in its heavy use of FAO for energy generation,28 even though it does not express ACC2 at significant levels.21 In the heart and liver, by contrast, ACC2 is considered the most important isozyme in regulation of FAO, being localized to the mitochondrial surface via an N-terminal amino acid sequence.10,12 Presumably, ACC1 fulfills a similar role in the kidney, although there is no evidence on this point.
This study strengthens the concept, previously advanced by others,1 that reduced FAO contributes to renal fibrosis after injury. There have been suggestions that the diseased or damaged kidney transitions its metabolism to aerobic glycolysis,29,30 the so-called Warburg effect first described in cancerous cells.25,31 This is a state where the cells utilize glycolysis and reduce mitochondrial oxidation of the pyruvate generated, which is then converted to lactate, despite the presence of oxygen that should permit complete mitochondrial oxidation with significantly greater generation of ATP. The specific advantages of metabolic reprogramming to malignant cells have been debated, but include maintenance of “stemness” or an ability to repopulate damaged cells, reduced metabolic stress, and a reduced susceptibility to immune surveillance.26 Whether these effects are important to damaged kidney cells is unclear, but the outcome of this study suggests that the response is disadvantageous, at least in the experimental models so far used.
In this study, we were unable to demonstrate a reduction in ATP in folate-treated TECs from ACC1 KI mice compared with WT. This could indicate that another mechanism is responsible for increased fibrosis in the ACC1 KI mice. Direct toxicity of neutral lipids, so-called lipotoxicity, has been proposed as a mechanism of injury in various models of renal disease, although the precise pathogenesis remains unclear.32,33 Certainly, there was a significant increase in both TGs and neutral lipids in the ACC1 KI mice with kidney disease compared with WT, and this could be the mechanism of injury rather than reduced generation of ATP. In their study of the ACC1/2 KI mice, Fullerton et al.19 noted that there were increased diacylglycerol and triacylglycerol levels in the liver and skeletal muscle of ACC1/2 KI mice compared with WT. They also argued that the increase in diacylglycerol could lead to activation of protein kinase C isoforms. Whether this also occurs in the kidney is unknown but provides a possible direction for future study.
There has also been a great deal of interest in the role of FAO in macrophage function, including polarization into M1 and M2 phenotypes. There has been a view, recently characterized as simplistic,34 that M1 macrophages rely on glycolysis,35 whereas M2 macrophages utilize FAO.36 In this study, where absence of ACC phosphorylation is predicted to reduce FAO, there was reduced infiltration by M1 macrophages in the FAN model in ACC 1/2 KI mice compared with WT. This is the reverse of the result predicted if M1 macrophages do not utilize FAO. In contrast, ACC 1/2 KI mice undergoing sham surgery had significantly increased numbers of M1 macrophages in the kidney compared with WT. The significance of these changes is unclear, and it would be wrong to assume they are due to events within macrophages alone. Presumably, other unmeasured changes occur in the ACC 1/2 KI mice that affect macrophage infiltration, such as differences in chemokine secretion by kidney cells. Perhaps more importantly, though, the discrepancy between macrophage infiltration and fibrosis in the FAN model suggests that increased TIF is likely to be due to impaired energy metabolism in the tubular cells of the kidney, rather than increased inflammation. This suggestion is supported by the lack of changes in mRNA encoding proinflammatory molecules such as VCAM-1, ICAM-1, IL-1β, IL-6, and TNF-α.
One curious aspect of the results is that mutation of the ACC phosphosites not only removed the antifibrotic effect of metformin, as it does for the antidiabetic effect in the liver, but the fibrosis was actually worse. This suggests that there is an effect of metformin on another pathway that is deleterious and usually balanced by the effect of increased FAO. AMPK increases glycolysis through phosphorylation of the glycolytic regulator PFK211; much of the glucose is metabolized to lactate rather than entering the oxidative pathway. It is possible that the local effect of lactate is deleterious, but there is no evidence on the point. The ACC1/2 KI mice also have a relatively mild metabolic phenotype, described by Fullerton et al.,19 characterized by mild hyperglycemia and hyperinsulinemia. Whether this phenotype influenced the results is unclear.
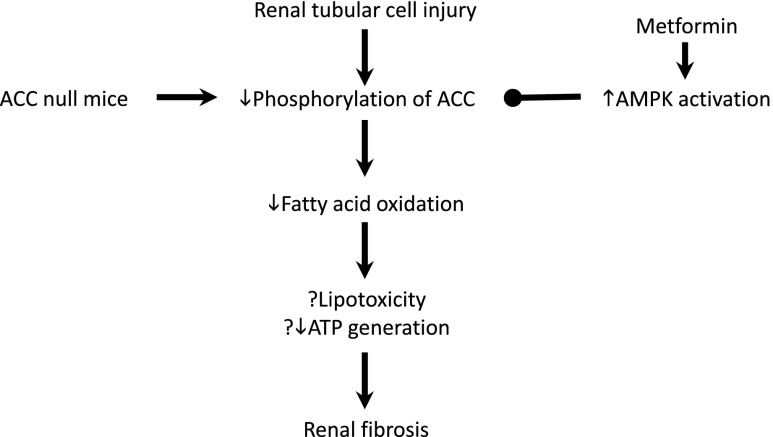
In conclusion, inhibition of the regulation of FAO by AMPK increases renal fibrosis, supporting the hypothesis that reduced FAO is deleterious after renal injury (Figure 10). Moreover, the antifibrotic effect of metformin is dependent on its ability to increase FAO through regulation of ACC by AMPK. These studies support the view that therapeutic approaches designed to increase FAO in the damaged kidney may be useful in modifying the long-term outcome of renal injury.
Figure 10.
Schematic drawing showing the role of reduced phosphorylation of ACC, leading to reduced energy generation through FAO, the major energy source for renal tubular cells. Reduced FAO is proposed to lead to death or transformation of epithelial cells to fibroblasts, either through lack of ATP or accumulation of lipids that are not utilized in energy metabolism. The effect is enhanced in mice with an inactivating mutation of the phosphorylation sites in ACC. By contrast, metformin activates AMPK, which increases ACC phosphorylation, lipid utilization, and energy generation. The antifibrotic effect is not seen, therefore, in mice that do not possess the phosphorylation sites for AMPK in ACC. ↓, decreased; ↑, increased; ?, possible.
Disclosures
D.A.P. has received honoraria from AstraZeneca and is a paid advisor (including share options) for Dimerix Ltd. P.F.M. is a paid member of an Advisory Board for Vifor Pharma.
Supplementary Material
Acknowledgments
The assistance of the Microscopy Unit, Florey Institute of Neuroscience and Mental Health is gratefully acknowledged.
M.L. was supported by a Postgraduate Scholarship from NHMRC. B.E.K. and S.G. were supported by Fellowships and a Project Grant from the National Health and Medical Research Council (NHMRC) (1068813 and 1085460) and the Victorian Government Operational Infrastructure Support Scheme. B.E.K. is an NHMRC Fellow.
D.A.P. and P.F.M. designed the study; M.L., M.K., and K.G. carried out experiments; S.G. and B.E.K. derived and provided mice; M.L., M.K., K.G., and D.A.P. analyzed the data; M.L., M.K., K.G., and D.A.P. made the figures; M.L., M.K., B.E.K., P.F.M., and D.A.P. drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010050/-/DCSupplemental.
References
- 1.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al.: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han SH, Malaga-Dieguez L, Chinga F, Kang HM, Tao J, Reidy K, et al.: Deletion of Lkb1 in renal tubular epithelial cells leads to CKD by altering metabolism. J Am Soc Nephrol 27: 439–453, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaglieri RC, Day RT, Feliers D, Abboud HE: Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Mol Cell Endocrinol 412: 116–122, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Christensen M, Jensen JB, Jakobsen S, Jessen N, Frøkiær J, Kemp BE, et al.: Renoprotective effects of metformin are independent of organic cation transporters 1 &2 and AMP-activated protein kinase in the kidney. Sci Rep 6: 35952, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Miao N, Xu J, Gan X, Xu D, Zhou L, et al.: Metformin prevents renal fibrosis in mice with unilateral ureteral obstruction and inhibits Ang II-induced ECM production in renal fibroblasts. Int J Mol Sci 17: 17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satriano J, Sharma K, Blantz RC, Deng A: Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol 305: F727–F733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Moon SY, Kim JS, Baek CH, Kim M, Min JY, et al.: Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol 308: F226–F236, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Houten SM, Auwerx J: PGC-1alpha: Turbocharging mitochondria. Cell 119: 5–7, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ruderman NB, Carling D, Prentki M, Cacicedo JM: AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123: 2764–2772, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg GR, Kemp BE: AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Angin Y, Beauloye C, Horman S, Bertrand L: Regulation of carbohydrate metabolism, lipid metabolism, and protein metabolism by AMPK. EXS 107: 23–43, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Saha AK, Ruderman NB: Malonyl-CoA and AMP-activated protein kinase: An expanding partnership. Mol Cell Biochem 253: 65–70, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM: Regulation of acetyl-CoA carboxylase. Biochem Soc Trans 34: 223–227, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Abu-Elheiga L, Jayakumar A, Baldini A, Chirala SS, Wakil SJ: Human acetyl-CoA carboxylase: Characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci U S A 92: 4011–4015, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser SA, Choy SW, Pastor-Soler NM, Li H, Davies MR, Cook N, et al.: AMPK couples plasma renin to cellular metabolism by phosphorylation of ACC1. Am J Physiol Renal Physiol 305: F679–F690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al.: Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien D, Dean D, Saha AK, Flatt JP, Ruderman NB: Malonyl-CoA content and fatty acid oxidation in rat muscle and liver in vivo. Am J Physiol Endocrinol Metab 279: E259–E265, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Rena G, Hardie DG, Pearson ER: The mechanisms of action of metformin. Diabetologia 60: 1577–1585, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al.: Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19: 1649–1654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H-C, Zuo Y, Fogo AB: Models of chronic kidney disease. Drug Discov Today Dis Models 7: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mount PF, Gleich K, Tam S, Fraser SA, Choy SW, Dwyer KM, et al.: The outcome of renal ischemia-reperfusion injury is unchanged in AMPK-β1 deficient mice. PLoS One 7: e29887, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Santos S, Bosch RJ, Ortega A, Largo R, Fernández-Agulló T, Gazapo R, et al.: Up-regulation of parathyroid hormone-related protein in folic acid-induced acute renal failure. Kidney Int 60: 982–995, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Romanino K, Mazelin L, Albert V, Conjard-Duplany A, Lin S, Bentzinger CF, et al.: Myopathy caused by mammalian target of rapamycin complex 1 (mTORC1) inactivation is not reversed by restoring mitochondrial function. Proc Natl Acad Sci U S A 108: 20808–20813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Wang S, Zhang Y, Xiao H: Metformin attenuates renal fibrosis in both AMPKα2-dependent and independent manners. Clin Exp Pharmacol Physiol 44: 648–655, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Kim CW, Addy C, Kusunoki J, Anderson NN, Deja S, Fu X, et al.: Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: A bedside to bench investigation. Cell Metab 26: 576, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, et al.: Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A 103: 8552–8557, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kone BC: Brenner & Rector’s the Kidney, Vol. 1, edited by Brenner BM, Chapter 4, 130–155, Philadelphia, Saunders Elsevier, 2008 [Google Scholar]
- 29.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, et al.: Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol 313: F561–F575, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Riwanto M, Kapoor S, Rodriguez D, Edenhofer I, Segerer S, Wüthrich RP: Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 11: e0146654, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee N, Kim D: Cancer metabolism: Fueling more than just growth. Mol Cells 39: 847–854, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, et al.: Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab 25: 713–726, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Bobulescu IA: Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19: 393–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namgaladze D, Brüne B: Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim Biophys Acta 1861: 1796–1807, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, et al.: Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 194: 6082–6089, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al.: Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4: 13–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.