Since the unique features of the three-layered glomerular capillary wall between the blood and the urine were first visualized by electron microscopy, the identity of the definitive components that restrict the filtration of albumin, Igs, and other large plasma constituents has been controversial. Because proteinuria is the single strongest predictor of progression from CKD to renal failure,1 an understanding of what constitutes the ultrafiltration barrier and how it is maintained is critical.
It is evident that all three layers of the glomerular capillary wall—fenestrated endothelium and its associated glycocalyx, glomerular basement membrane (GBM), and podocyte foot processes with their slit diaphragms—participate in the maintenance of the glomerular filtration barrier (GFB).2 However, the physical chemistry of the GFB is not completely understood. Moreover, the mechanisms of action of the most commonly used and most effective treatments to reduce proteinuria, such as renin-angiotensin system blockade and calcineurin inhibitors, remain incompletely defined. New mechanism-based treatments for renal disease will rely on new mechanistic insights into an old problem.
Comprehensive models of the GFB should explain all of the following clinical and laboratory observations of glomerular anatomy and physiology in both health and disease.
Nearly complete retention of albumin and other macromolecules in both health and nephrotic syndrome, with severe nephrotic syndrome resulting from a quantitatively minor change in glomerular permselectivity.
Exclusion of albumin and antibodies from the subepithelial GBM if foot processes are intact but their appearance in Bowman’s space when either foot processes become effaced or blood flow is halted.
Accumulation of certain antibodies (e.g., anti-phospholipase A2 receptor) at the interface between podocytes and the GBM.
Near-perfect fidelity of glomerular permselectivity over decades without deterioration, clogging, or fouling.
Absence of nephrotic syndrome even with defects in GBM composition (with the exception of laminin).
Heavy proteinuria either accompanied or caused by derangement of foot processes.
Increase in GFR associated with decreased glomerular capillary wall macromolecular permeability in healthy rats.
Increase in existing proteinuria with increase in systolic BP.
Many models, ranging from simple to complex, have been proposed to explain the extraordinary GFB. Despite intriguing and highly suggestive ultrastructural data regarding the slit diaphragm,3 before the mid-1990s, hindered transport through the unique double-thickness GBM was the concept that attracted attention as the likely dominant factor in plasma protein retention. Recent super-resolution imaging of the GBM reveals a highly ordered superstructure with two distinct laminin-521 and two distinct collagen IV networks4 that are organized separately by podocytes and endothelial cells. Although laminin and collagen IV networks do not interact with each other directly, there are multiple bridging molecules that stabilize them, presumably forming a dense, gel-like structure that impedes the passage of macromolecules. Additional data combining laminin and collagen IV mutations support an indirect physical interaction that cross-stabilizes the two networks to maintain GBM architecture and glomerular permselectivity.5
In 1998, the identification of nephrin, a glomerular slit diaphragm protein that, when mutated, causes catastrophic nephrotic syndrome, brought the slit diaphragm back to the forefront. In the subsequent two decades, the list of podocyte proteins essential to healthy podocyte architecture and maintenance of the GFB has grown substantially due mainly to breakthroughs in the genetics of nephrotic syndrome. It is indisputable that proper podocyte architecture is necessary to maintain the GFB over the long term. This led to a simple heuristic model wherein slit diaphragms, stabilized by connections to the actin cytoskeleton, form a molecular-scale colander separating plasma from urine.
This model, however, predicts a spatial distribution of plasma proteins in the GBM at odds with observations by multiple investigators, and it is not easily reconciled with continuous, clogfree operation over the 2.5 billion heartbeats of a lifetime.6–8 Several other explanations have emerged. Here, we expand on an older proposition9 and then link podocyte biology to the physical chemistry of the GBM.
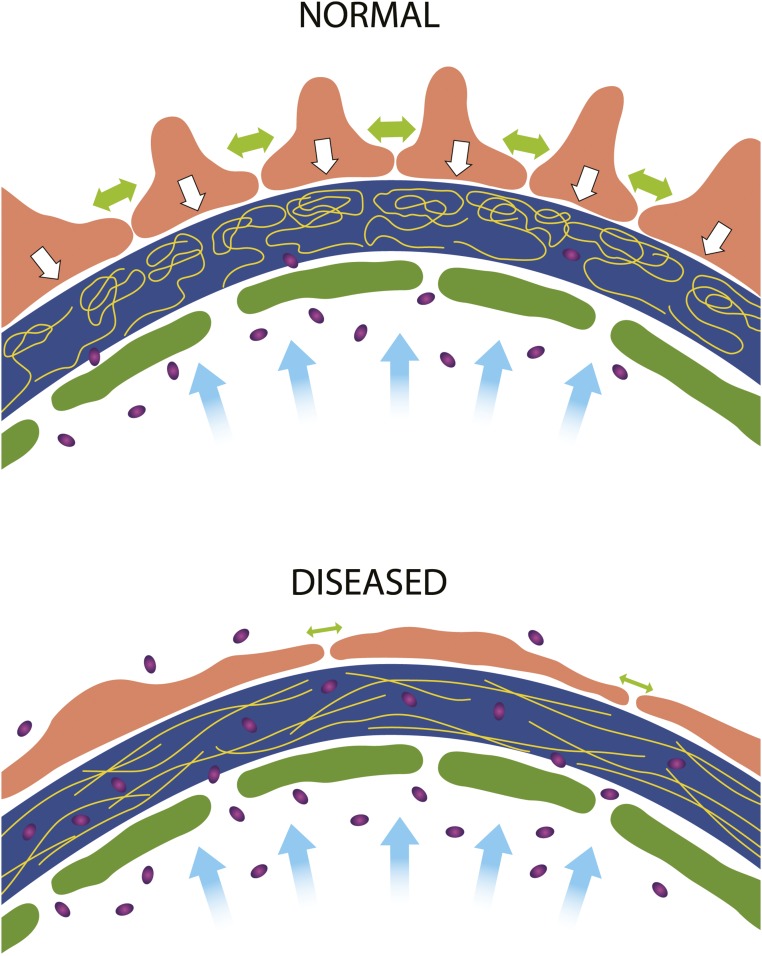
In our model (Figure 1), the fluid flow of glomerular filtration compresses the GBM against the restraining foot processes, likely in an increasing gradient from the lumen to the podocyte. This “gel compression” is well documented in other biologic systems.10 The resulting anisotropic density of the GBM explains the observed spatial distribution of macromolecules therein and the very limited—but not zero—transmission of plasma proteins to the primary urine. With disease or injury and the cytoskeletal rearrangements that typically accompany them, the decreased contractile strength of injured podocytes allows for a subtle shift from radial compression to circumferential tension within the GBM, opening the meshwork of the GBM gel, increasing average pore size, and permitting leakage of macromolecules into Bowman’s space (Figure 1).
Figure 1.
A new model of the glomerular filtration barrier links glomerular basement membrane (GBM) compression to permselectivity. Schematics of hydrodynamic forces in the glomerular capillary wall. In a healthy glomerulus (upper panel), water filtration (blue arrows) through the GBM (dark blue) compresses the GBM constituents (gold lines) against the podocyte foot processes (orange). Podocyte cell-cell attachment and contraction forces (large green double arrows) act to buttress (white arrows) the GBM against the distending force of circulation pressure. The compressed GBM cannot admit albumin (purple ovals) except possibly in the subendothelial space, where gel compression is least pronounced. In proteinuric glomerular disease with podocyte foot process effacement (lower panel), cell contraction is decreased (small green arrows), and podocytes no longer form as strong a buttress against which the GBM is compressed. With reduced gel compression, the mesh size of the GBM gel becomes large enough to allow albumin to transit through to Bowman’s space.
In this model, the compressed GBM hinders transport of macromolecules but allows water and small solutes to pass freely. The slit diaphragms and whatever aberrant cell-cell junctions form after effacement are nonrestrictive, as supported by cryoelectrontomography data.11 This model explains why mutations that affect the slit diaphragm, its linkage to the cytoskeleton, or the cytoskeleton itself cause nephrotic syndrome and importantly, takes into account mechanisms involving cellular regulation of GBM protein organization through integrin-α3β1. The model, like a recently presented one,12 also has explanatory power regarding the varied phenotypes arising from mutations in GBM components.13 Mutations affecting laminin-521 disproportionately derange the GFB compared with collagen IV and agrin.13 Laminin-521 is the primary tether for podocytes to the matrix and may uniquely mediate transmission of podocyte traction forces to the GBM while at the same time, ensuring the proper organization of other GBM components.5 By analogy, laminin is to the GBM what nephrin is to the slit diaphragm.
Disclosures
None.
Acknowledgments
The authors regret being unable to discuss and cite additional relevant literature due to space constraints and acknowledge funding from National Institutes of Health grants U01EB021214 (to W.H.F.), R01DK058366 (to J.H.M.), and R01DK078314 (to J.H.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Palmer BF: Proteinuria as a therapeutic target in patients with chronic kidney disease. Am J Nephrol 27: 287–293, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Haraldsson B, Nyström J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60: 423–433, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, et al. : Nanoscale protein architecture of the kidney glomerular basement membrane. eLife 2: e01149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk SD, Bayer RH, Malone AF, McKee KK, Yurchenco PD, Miner JH: Pathogenicity of a human laminin β2 mutation revealed in models of Alport syndrome. J Am Soc Nephrol 29: 949–960, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B: Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol Renal Physiol 284: F1226–F1234, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ryan GB, Karnovsky MJ: Distribution of endogenous albumin in the rat glomerulus: Role of hemodynamic factors in glomerular barrier function. Kidney Int 9: 36–45, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Fujigaki Y, Nagase M, Kobayasi S, Hidaka S, Shimomura M, Hishida A: Intra-GBM site of the functional filtration barrier for endogenous proteins in rats. Kidney Int 43: 567–574, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Robinson GB, Walton HA: Glomerular basement membrane as a compressible ultrafilter. Microvasc Res 38: 36–48, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Holmes MH, Mow VC: The nonlinear characteristics of soft gels and hydrated connective tissues in ultrafiltration. J Biomech 23: 1145–1156, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Grahammer F, Wigge C, Schell C, Kretz O, Patrakka J, Schneider S, et al. : A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes. JCI Insight 1: e86177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, et al. : Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A 114: 2958–2963, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh JH, Miner JH: The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]