Abstract
Background
The slit diaphragm is a specialized adhesion junction between opposing podocytes, establishing the final filtration barrier that prevents passage of proteins from the capillary lumen into the urinary space. Nephrin, the key structural and signaling adhesion molecule expressed in the slit diaphragm, contains an evolutionally conserved, atypical PDZ-binding motif (PBM) reported to bind to a variety of proteins in the slit diaphragm. Several mutations in NPHS1 (the gene encoding nephrin) that result in nephrin lacking an intact PBM are associated with glomerular diseases. However, the molecular basis of nephrin-PBM–mediated protein complexes is still unclear.
Methods
Using a combination of biochemic, biophysic, and cell biologic approaches, we systematically investigated the interactions between nephrin-PBM and PDZ domain–containing proteins in the slit diaphragm.
Results
We found that nephrin-PBM specifically binds to one member of the membrane-associated guanylate kinase family of scaffolding proteins, MAGI1, but not to another, MAGI2. The complex structure of MAGI1-PDZ3/nephrin-PBM reveals that the Gly at the −3 position of nephrin-PBM is the determining feature for MAGI1-PDZ3 recognition, which sharply contrasts with the typical PDZ/PBM binding mode. A single gain-of-function mutation within MAGI2 enabled nephrin-PBM binding. In addition, using our structural analysis, we developed a highly efficient inhibitory peptide capable of specifically blocking the nephrin/MAGI1 interaction.
Conclusions
MAGI1 interacts with nephrin-PBM with exquisite specificity. A newly developed, potent inhibitory peptide that blocks this interaction may be useful for future functional investigations in vivo. Our findings also provide possible explanations for the diseases caused by NPHS1 mutations.
Keywords: nephrin, nephrotic syndrome, glomerular filtration barrier, Cell Signaling, cell adhesion
Slit diaphragm is a highly specialized cell-cell junction formed by the neighboring podocytes and functions as the final filtration barrier that prevents passage of macromolecules from the capillary lumen into the urinary space.1,2 Disruption of the architecture of slit diaphragm can lead to severe nephrotic syndrome, with symptoms including massive proteinuria, hypoalbuminemia, and edema.3,4
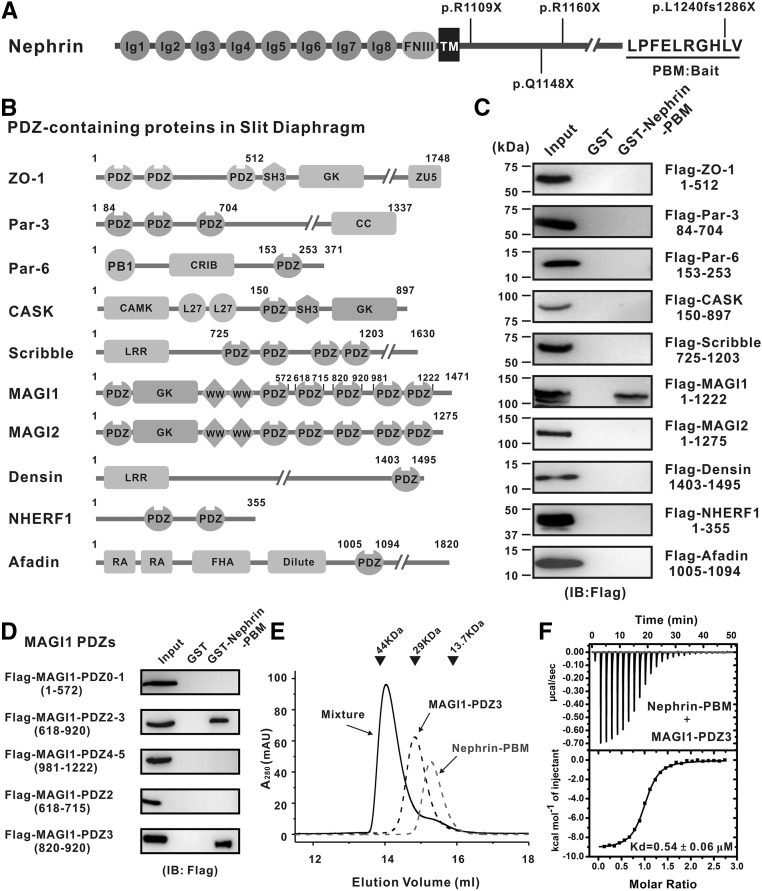
NPHS1, encoding the defining adhesion molecule in Nephrin in the slit diaphragm, was identified as the major causative gene for congenital nephrotic syndrome of the Finnish type (CNF).5 To date, over 160 mutations in NPHS1 have been discovered in the patients with CNF.6 NPHS1 knockout mice showed defective assembly of the slit diaphragm and impaired barrier function of podocytes.7 Nephrin consists of extracellular eight Ig-like domains, a fibronectin type III-like domain, and an unstructured intracellular domain (Figure 1A). Nephrins form the unique zipper-like structure via the homotypic interactions between the Ig domains from the adjacent podocytes.8 The intracellular domain of Nephrin (Nephrin-CT) contains an evolutionarily conserved atypical PDZ domain-binding motif (PBM; “−LPFELRGHLV”) at the C-terminus, not consistent with the canonical type I, type II, or type III PBMs (Figure 1A).9 Mutations of NPHS1 lacking partial intracellular domain including the C-terminal PBM lead to congenital nephrotic syndrome.6 Specifically, a truncating mutation of Nephrin (p.L1240fs1286×) that lacks the very C-terminal Valine of PBM and instead bears an additional 45 amino acids was identified in patients with steroid-resistant nephrotic syndrome (SRNS) (Figure 1A, Supplemental Figure 1).10 These genetic data implied that Nephrin, in addition to playing key roles in the assembly of the slit diaphragm, also serves as an intracellular signaling hub to orchestrate the dynamic regulation of the slit diaphragm.
Figure 1.
Nephrin-PBM specifically interacts with PDZ3 domain of MAGI1. (A) Domain organization of Nephrin. Sequence of Nephrin-PBM (-LPFELRGHLV) is shown. Nephrotic syndrome-causing mutations located at the cytoplasmic region of Nephrin are listed. (B and C) GST pull-down analyses of the interactions between the Nephrin-PBM and various PDZ-containing proteins expressed in the slit diaphragm. The domain organizations of PDZ-containing proteins are shown (B). (D) GST pull-down analyses of the interactions between various MAGI1 fragments and Nephrin-PBM. (E) Analytical gel filtration-based analysis showing that MAGI1-PDZ3 forms a 1:1 stoichiometric complex with Nephrin-PBM. (F) Quantitative measurement of the binding affinity between MAGI1-PDZ3 and Nephrin-PBM using an ITC-based assay.
There is much evidence indicating that Nephrin forms multicomponent complexes with a variety of PDZ domain–containing proteins in the slit diaphragm, including membrane-associated guanylate kinase family proteins (e.g., MAGI1/2, ZO-1, and CASK),11,12 the Par6/Par3 complex,13 scribble,14 etc. Nephrin-PBM–mediated protein complexes play essential roles in maintenance of glomerular filtration barrier integrity.11 However, little is known about whether the atypical PBM of Nephrin directly interacts with these PDZ-containing proteins. If it does, how is it specifically recognized by its target(s)? We believe that a better understanding of the molecular basis underlying the Nephrin-PBM–mediated complex formation can provide valuable insights into the physiologic roles of Nephrin in the assembly and dynamic regulation of the slit diaphragm, as well as the pathogenesis of nephrotic syndrome caused by alterations of NPHS1.
In this study, we systematically studied the interactions between Nephrin-PBM and PDZ domain–containing proteins in slit diaphragm and found that only MAGI1 bound directly to Nephrin-PBM. We further discovered that MAGI1-PDZ3 binds to Nephrin-PBM with high specificity and affinity. The precise molecular basis underlying this interaction was elucidated by solving the complex structure. Unexpectedly, the atypical PBM of Nephrin binds to MAGI1-PDZ3 with a mode distinct from the canonical PDZ/PBM interactions. In addition to the canonical binding interface at the αB/βB groove, the N-terminus of Nephrin-PBM adopts a short helix structure to engage the hydrophobic surface formed by βB/βC of PDZ3. Interestingly, the highly conserved residue Gly(−3) of Nephrin-PBM plays a critical role in the formation of the MAGI1/Nephrin complex, which is different from the typical PDZ/PBM binding modes. We further developed a potent inhibitory peptide that can specifically block the MAGI1/Nephrin interaction in vitro and in vivo. Our biochemic, biophysic, and cell biologic studies could provide valuable insights into Nephrin-mediated protein complex in slit diaphragm assembly and signaling. The potent inhibitory peptide could serve as a useful manipulating tool to dissect differential roles of MAGI isoforms in future investigations.
Methods
Protein Expression and Purification
The coding sequences of MAGI1-PDZ3 (residues 820–920) and Nephrin-PBM (residues 1247–1256) were amplified from mouse brain complementary DNA. For the isothermal titration calorimetry (ITC) assay, wild-type or various mutants of MAGI1-PDZ3 and Nephrin-PBM were cloned into a modified version of pET15b vector and pET32a vector, respectively. His6-tagged proteins were purified with Ni2+-NTA agarose affinity chromatography, followed by size-exclusion chromatography (SEC). For the glutathione-S-transferase (GST) pull-down assay, MAGI1-PDZ3 or Nephrin-PBM was fused to the C-terminus of GST using the pGEX-4T-1 vector and purified by GSH-Sepharose affinity chromatography, followed by another round of SEC. All of the constructs were expressed in Escherichia coli BL21 (DE3) host cells at 16°C for 16–18 hours.
For the reconstitution of the MAGI1-PDZ3/Nephrin-PBM complex, His6-MAGI1-PDZ3 was firstly purified as described above, and the His6-tag was then removed by incubation with human rhinovirus 3C protease overnight, followed by another round of SEC. The commercial synthetic Nephrin-PBM peptide was mixed with MAGI1-PDZ3 in a molar excess of 3:1 (approximately 15 mg/ml total complex protein in PBS buffer; pH 7.4).
All of the peptides used in this study were commercial synthesized by ChinaPeptide Co., Ltd.
GST Pull-Down Assay
GST-tagged MAGI1-PDZ3 was incubated with thioredoxin-tagged Nephrin-PBM in the assay buffer (50 mM Tris [pH 8.0], 100 mM NaCl, 1 mM DTT, and 1 mM EDTA) for 1 hour at 4°C. For inhibitory peptide competition experiments, GST-tagged Nephrin-PBM was incubated with His-tagged MAI1-PDZ3 in the presence of the PBM_H-2T peptide or the PBM_G-3A peptide in the assay buffer. After incubation, the mixture was centrifuged at 13,000× rpm for 5 minutes and the supernatant was then loaded into 20 µl GSH-Sepharose 4B slurry beads pre-equilibrated with the assay buffer, to incubate for 15 minutes at 4°C. After washing three times, the complex was boiled with 20 µl 2× SDS-PAGE loading dye and detected by Coomassie brilliant blue staining.
Flag-tagged PDZ-containing proteins and various MAGI1 fragments were overexpressed in HEK293T cells. Cells were lysed in ice-cold cell lysis buffer (50 mM Hepes [pH 7.4], 150 mM NaCl, 10% glycerol, 1 mM EGTA, 1% Triton and protease inhibitor cocktail) for 1 hour at 4°C. After centrifugation at 13,000× rpm for 10 minutes at 4°C, the supernatants were incubated with GST-Nephrin-PBM on preloaded GSH-Sepharose 4B slurry beads individually for 30 minutes. After washing three times, the bound proteins were eluted by boiling with 20 µl 2× SDS-PAGE loading dye and detected by Western blotting with anti-Flag antibody (Sigma).
ITC Assay
The ITC assay was performed on a MicroCal ITC-200 (Malvern Panalytical, UK) at 25°C. All of the protein samples were in the buffer containing 50 mM Tris (pH 8.0), 100 mM NaCl, 1 mM EDTA, and 1 mM DTT. Wild-type and various mutants of Nephrin-PBM (approximately 0.6 mM) and MAGI1-PDZ3 (approximately 0.04 mM) were placed in a syringe and the sample cell, respectively. The Nephrin-PBM fragments were injected into the MAGI1-PDZ3 proteins with 2 µl of Nephrin-PBM fragments at a time, at 2-minute intervals. The titration data were analyzed and fitted by Origin 7.0 from MicroCal, using the one-site binding model.
Crystallography
The best crystals of the MAGI1-PDZ3/Nephrin-PBM complex were obtained by the sitting-drop vapor diffusion method at 16°C. The crystals were grown in 0.5 M sodium chloride, 0.01 M magnesium chloride hexahydrate, and 0.01 M hexadecyltrimethylammonium bromide. The crystals were cryoprotected with the mother liquor supplemented with 25% glycerol and flash-frozen in liquid nitrogen. The diffraction data were collected at 100 K with a wavelength of 0.97791 Å and processed with the HKL3000 package.15 The structure of the complex was solved by the molecular replacement method using the structure of MAGI1-PDZ3 (protein data bank code: 2Q9V) using the software suits of PHASER.16 The initial model was rebuilt automatically and refined by PHENIX.17 Further model building and adjustment were completed using COOT.18 The final refinement statistics of the complex structure are listed in Supplemental Table 1. Structural diagrams were prepared by Pymol.
Coimmunoprecipitation Assay
Flag-tagged full-length MAGI1 was cotransfected with GFP-tagged wild-type or mutants of Nephrin full-length constructs into HEK293T cells. At 24 hours after transfection, the cells were lysed in ice-cold cell lysis buffer for 1 hour at 4°C, followed by centrifugation at 13,000× rpm for 10 minutes. The supernatants were each incubated with anti–Flag-conjugated agarose beads (Sigma) for 1 hour at 4°C. For inhibitory peptide competition experiments, the supernatants were incubated with the PBM_H-2T peptide or the PBM_G-3A peptide before incubation of anti–Flag-conjugated agarose beads. After washing three times, the samples were resuspended with SDS-PAGE dye and analyzed by Western blotting using anti-Flag and anti-GFP (Santa Cruz Biotechnology) antibodies.
Cellular Localization Assay
HEK293T cells were transiently transfected with 0.5 µg of each plasmid per well using a lipofectamine 2000 reagent according to the manufacturer’s instructions (Invitrogen, Thermo Fisher Scientific) in 12-well plates. Cells were cultured for 12 hours in DMEM containing 10% FBS in 5% CO2, before fixation. The cell images were acquired on a Leica TCS SP8 confocal microscope (Hyvolution) with a 63× oil immersion lens. Fluorescence intensities of each image were analyzed using ImageJ software. To quantify membrane colocalization of Nephrin and MAGI1, we used the Pearson correlation coefficient (R). Data were analyzed using a t test. Twenty cells were randomly selected as quantification for each group and values (means±SD) were calculated from three independent experiments.
Accession Code
The atomic coordinate of MAGI1-PDZ3/Nephrin-PBM complex has been deposited to the Protein Data Bank under the accession code 5ZYS.
Results
Specific Interaction between Nephrin-PBM and MAGI1-PDZ3
Various scaffold proteins have been found to link Nephrin with downstream cytoskeleton components in slit diaphragm. To uncover the detailed composition of Nephrin-PBM–assembled protein complexes, we first systematically studied the interactions between Nephrin-PBM and the PDZ-containing proteins expressed in slit diaphragm. Flag-tagged PDZ domain(s) of ten PDZ-containing proteins expressed in slit diaphragm were tested individually for their bindings to GST-tagged Nephrin-PBM in vitro (Figure 1, A and B). Surprisingly, none of these PDZ domain(s) bound to Nephrin-PBM except for MAGI1 (Figure 1C). Most notably, MAGI2, the closest homolog to MAGI1, did not bind to Nephrin-PBM, indicating that the MAGI1/Nephrin-PBM interaction is highly specific. MAGI1 is a unique member of the membrane-associated guanylate kinase family of proteins, which is enriched in the glomerular podocytes.19 MAGI1 was reported to bind to Nephrin and the interaction was required for Rap1 activation in podocyte to maintain long-term slit diaphragm structure.20,21 Membrane localization of Nephrin was diminished when MAGI1 was depleted in podocytes.20 The combination of heterozygous deletion of NPHS1 and homozygous deletion of MAGI1 resulted in spontaneous glomerulosclerosis,20 indicating a genetic complementation of the two genes (NPHS1 and MAGI1).
Previous studies have demonstrated that PDZ2–3 tandems of MAGI1 binds to Nephrin-CT.20,21 We further demonstrated that the PDZ3 domain is sufficient for MAGI1 to bind to Nephrin-PBM through a truncation-based approach (Figure 1D). MAGI1-PDZ3 and Nephrin-PBM formed a stable 1:1 stoichiometric complex in solution (Figure 1E). ITC-based assay showed that Nephrin-PBM binds to MAGI1-PDZ3 with a Kd of approximately 0.54 µM (Figure 1F), which is much stronger than those of canonical PDZ/PBM interactions (Kd approximately 10 µM).
Structural Characterization of Nephrin-PBM/MAGI1-PDZ3 Complex
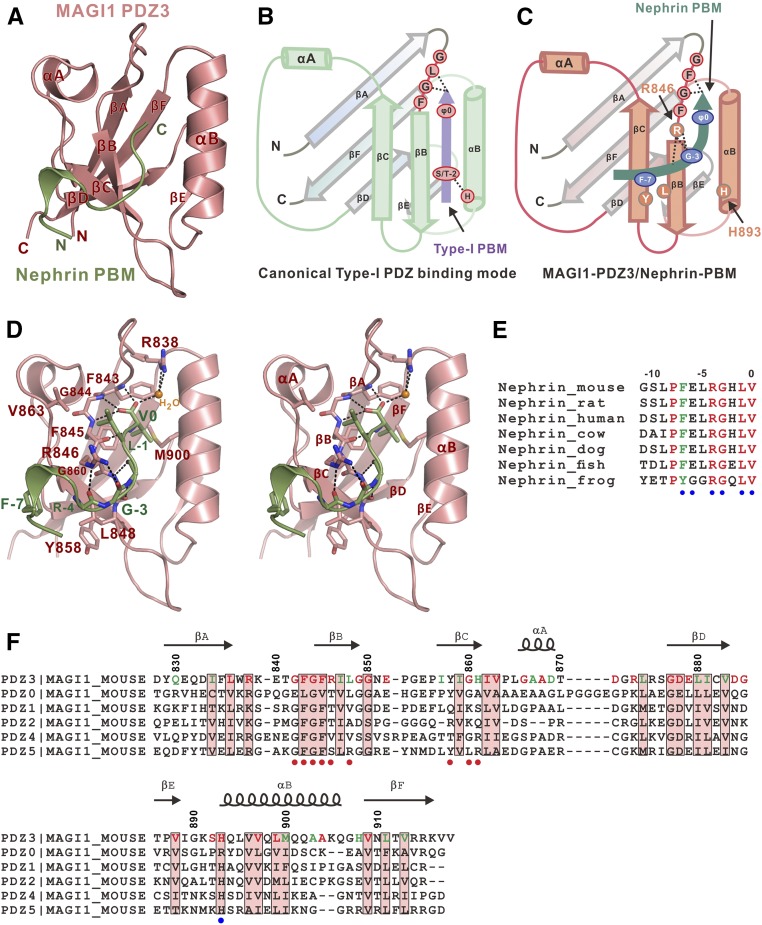
We further determined the crystal structure of the Nephrin-PBM/MAGI1-PDZ3 complex at 1.8-Å resolution (Supplemental Table 1). In the complex structure, MAGI1-PDZ3 adopts a canonical PDZ domain folding consisting of six β-strands (βA–F) and two helices (αA and αB) (Figure 2A); unlike the canonical PBM, which folds into a β-strand to insert into the concave groove formed by αB/βB of PDZ (Figure 2B), Nephrin-PBM adopts a unique conformation to contact PDZ3 with the C-terminus of PBM, binding to the αB/βB groove and N-terminus of PBM, which forms a short α-helix coupling with the βB/βC surface of PDZ3 (Figure 2, A and C).
Figure 2.
Complex structure of MAGI1-PDZ3/Nephrin-PBM reveals a unique binding mode of PDZ/PBM interaction. (A) Ribbon diagram of the MAGI1-PDZ3/Nephrin-PBM complex structure. MAGI1-PDZ3 and Nephrin-PBM are colored in pink and green, respectively. (B) A cartoon showing the canonical type-I PDZ binding mode. The carboxyl group of type-I PBM (purple) forms extensive hydrogen bonds with the “−GLGF” motif of PDZ. The residue at −2 position (Ser or Thr) of type-I PBM determines the binding specificity by forming the hydrogen bond with His from αB of PDZ. (C) A cartoon showing the unexpected MAGI1-PDZ3/Nephrin-PBM (green) binding mode. N-terminus of Nephrin-PBM contacts with the hydrophobic surface of βB/βC of PDZ3. Note that His893 from αB of PDZ3 has no contact with Nephrin-PBM. ψ represents hydrophobic residue. (D) Stereo view of detailed interface of MAGI1-PDZ3 (pink)/Nephrin-PBM (green) complex. Dotted lines represent hydrogen bonds. A water molecule is shown in brown. (E) Sequence alignment of Nephrin-PBM from different spices. In this drawing, totally conserved and conserved residues are colored in red and green, respectively. Residues involved in binding to Nephrin-PDZ3 are shown with a blue dot at the bottom. (F) Structure-based sequence alignment of different PDZ domains of MAGI1. Conserved residues between different PDZ domains are highlighted with pink boxes. The totally conserved and conserved residues in MAGI1-PDZ3 form different species are colored in red and green, respectively (related to Supplemental Figure 2). Residues involved in binding to Nephrin-PBM and Ser/Thr at −2 position of canonical PBM are shown with red and blue dots at the bottom, respectively.
Analysis of the PDZ3/PBM binding interface revealed several unique features (Figure 2D): (1) the N-terminus of PBM forms another hydrophobic core (interaction between Phe(−7)PBM and Leu848PDZ3/Tyr858PDZ3) in addition to the canonical PDZ core (interaction between Val(0)PBM and Phe843PDZ3/Phe845PDZ3/Met900PDZ3); (2) His(−2)PBM is not involved in the binding, although the residue at −2 position usually determines the binding type of a typical PBM9; and (3) Arg846PDZ3 forms hydrogen bonds with the backbones of Gly(−3)PBM and Arg(−4)PBM. Importantly, the residues involved in the binding interface are conserved in MAGI1 (Supplemental Figure 2) and Nephrin (Figure 2E) from different species, suggesting that the interaction is highly conserved during evolution.
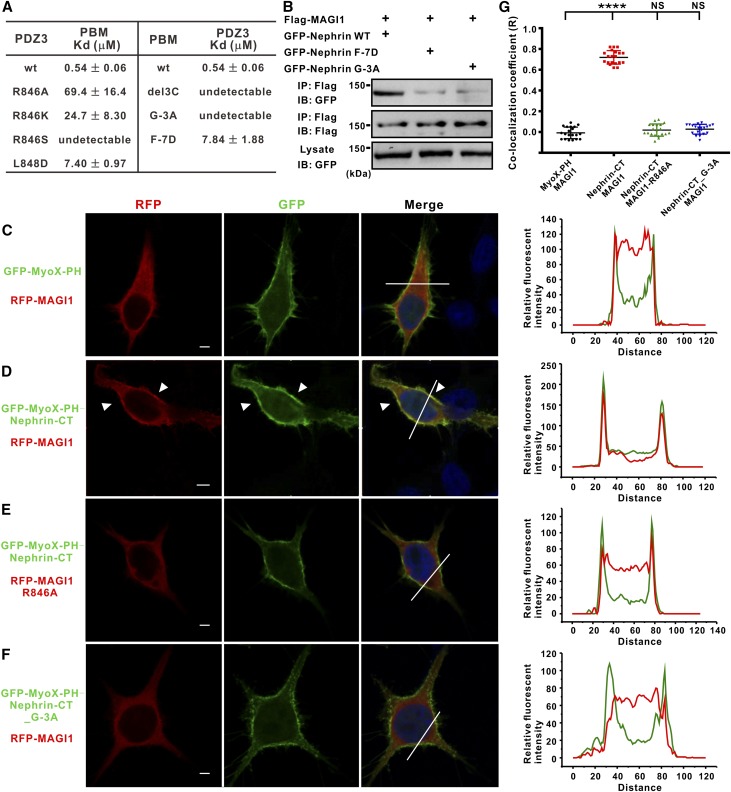
To probe the role of the hydrophobic interaction between the N-terminus of PBM and the βB/βC surface of PDZ3, mutations of residues in this interface (i.e., L848DPDZ3 and F(−7)DPBM) were introduced. These mutants led to significant weakening of both the PDZ3/PBM interaction (Figure 3A) and the full-length Nephrin/MAGI1 interaction (Figure 3B).
Figure 3.
Mutations of specific residues in the interface disrupt Nephrin/MAGI1 interaction. (A) A summary of the binding affinities between PDZ3 (or its mutants) and PBM (or its mutants) measured by ITC assays. (B) A single mutation in MAGI1 and Nephrin significantly impaired the interactions between full-length MAGI1 and Nephrin in the co-immunoprecipitation assay. (C–F) The cellular colocalization analyses of Nephrin, MAGI1, and their mutations in transfected HEK293T cells. Because the expression level of the full-length Nephrin is extremely low, a chimera protein with the Nephrin-CT fused to the C-terminal end of GFP-Myosin X-PH123 (GFP-MyoX-PH) was effectively localized at the membrane. Fluorescence intensities of the area marked by the white lines (left panels) are shown in the right panels. Green and red curves represent the fluorescence intensities of GFP and RFP, respectively. Colocalizations of Nephrin and MAGI1 are indicated by white arrows. Scale bars, 5 μm. (G) Quantification of colocalization coefficient (Pearson) (R) between wild-type and mutants of Nephrin and MAGI1. Values are means±SD from three independent experiments (n=20 for each group), analyzed with t test; ****P<0.001.
Substitution of Arg846PDZ3 with Ala, Ser, or even Lys led to obvious decreased PDZ3/PBM interaction (Figure 3A). Arg846 is totally conserved in the PDZ3 domain from different species of MAGI1 (Supplemental Figure 2), but not conserved among different PDZ domains of the MAGI1 protein (Figure 2F), which may explain why other PDZ domains of MAGI1 did not bind to Nephrin-PBM.
It is noted that, in addition to forming a hydrogen bond with Arg846PDZ3, the backbone of Gly(−3)PBM (adopting unique dihedral angles of ϕ [82.24°] and ψ [−177.40°], which are not permitted by other residues) allows the PBM peptide to adopt a sharp turn to facilitate the upstream of PBM to interact with βB/βC of PDZ3. Importantly, substitution of Gly(−3) with Ala completely disrupted the PDZ3/PBM interaction, substantiating the critical structural role of Gly(−3) in the binding process (Figure 3A). Consistently, the G(−3)A mutant also blocked the binding of the full-length Nephrin to MAGI1 (Figure 3B). Analysis of PBMs of other reported targets of MAGI1-PDZ3, such as endothelial cell-selective adhesion molecule (ESAM)22 and coxsackievirus and adenovirus receptor,23 showed that the residues at −3 position are both Gly (Supplemental Figure 3A). ESAM-PBM binds to MAGI1-PDZ3 with a Kd of approximately 2.1 µM, whereas G(−3)AESAM showed undetectable binding to MAGI1-PDZ3 (Supplemental Figure 3B). These data collectively suggest that Gly at the −3 position of PBMs may be a determining feature in their specific binding to MAGI1-PDZ3.
We next wanted to investigate the roles of the above key elements for the MAGI1/Nephrin interaction in living cells. Because of the relatively low expression level of the full-length Nephrin in the heterologous cells, we hypothesize that fusion of the Nephrin-CT to a membrane-associated protein could perfectly mimic the native Nephrin protein at plasma membrane. Pleckstrin homology (PH) domain, one of the largest domain families for membrane association, is often used as lipid marker or membrane recruitment tag. Myosin X (MyoX) contains three PH domains that form a tandem and cooperatively bind to the cellular membrane, with extremely high affinity.24,25 Therefore, we made the MyoX-PH123-Nephrin-CT (MyoX-PH-Nephrin-CT) chimera protein. As expected, MyoX-PH-Nephrin-CT effectively recruits MAGI1 to the cell membrane (Figure 3, D and G), whereas MyoX-PH alone could not (Figure 3, C and G). Fully consistent with the in vitro binding data, neither the R846A mutant of MAGI1 nor the G(−3)A mutant of Nephrin could colocalize with wild-type Nephrin or MAGI1 at plasma membrane, respectively (Figure 3, E–G).
A Potent Inhibitory Peptide Capable of Blocking Nephrin/MAGI1 Interaction
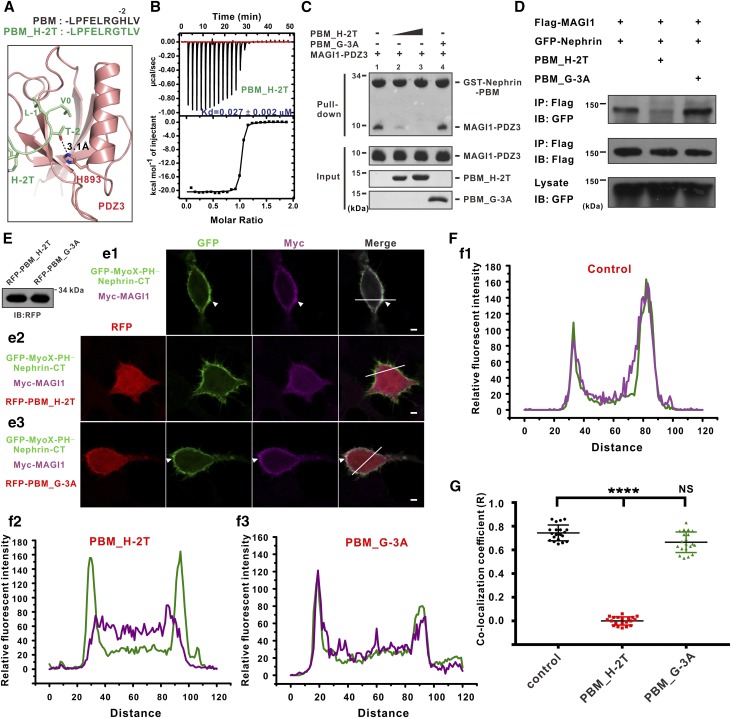
MAGI1-PDZ3 is a type I PDZ domain, with a His (H893) at αB capable of forming a hydrogen bond with a Ser or Thr at −2 position of a canonical type I PBM (Figure 2, B and C). However, the residue at the −2 position of Nephrin-PBM is a His that does not bind to His893PDZ3 (Figure 2C). Thus, we reason that substitution of His(−2)PBM with a Thr would further enhance the binding between PDZ3 and PBM. Our modeled structure also suggested that the sidechain of T(−2)PBM_H-2T would form a hydrogen bond with the sidechain of His893PDZ3 (Figure 4A). The PBM_H-2T peptide binds to MAGI1-PDZ3 with a Kd of approximately 27 nM, which is about 20-fold higher than that of wild-type PBM/PDZ3 interaction (Figures 1F and 4B).
Figure 4.
A potent inhibitory peptide is capable of disrupting Nephrin/MAGI1 interaction. (A) Combined stick and ribbon representation showing a structural homology model (on the basis of the Nephrin-PBM/MAGI1-PDZ3 structure in this study) for PBM_H-2T/MAGI1-PDZ3 complex using the SWISS MODEL tool. The sequences of Nephrin-PBM and PBM_H-2T are shown above. (B) ITC-based measurements of binding affinity between the PBM_H-2T peptide and MAGI1-PDZ3. (C) The PBM_H-2T peptide effectively blocked Nephrin-PBM/MAGI1-PDZ3 interaction in a GST pull-down assay. (D) The PBM_H-2T peptide effectively blocked full-length Nephrin/MAGI1 interaction in a coimmunoprecipitation assay. (E and F) The membrane colocalization analyses of Nephrin and MAGI1 in living cells when expressing the PBM_H-2T peptide or the PBM_G-3A mutant peptide. Fluorescence intensities of the area marked by the white lines in e1, e2, and e3 are shown in f1, f2, and f3, respectively. The expression levels of the RFP-PBM_H2T and RFP-PBM_G-3A peptides are also included. (G) Quantification of colocalization coefficient (Pearson) (R) between Nephrin and MAGI1 revealing that the PBM_H-2T peptide significantly blocks the Nephrin/MAGI1 in living cells. Values are means±SD from three independent experiments (n=20 for each group), analyzed with t test; ****P<0.001.
We next tested whether the PBM_H-2T peptide can block the Nephrin/MAGI1 interaction in vitro. As expected, GST-tagged Nephrin-PBM could robustly pull down MAGI1-PDZ3 (Figure 4C, lane 1). Addition of the PBM_H-2T peptide in the reaction mixture significantly reduced the interaction between Nephrin-PBM and MAGI1-PDZ3 (Figure 4C, lanes 2 and 3), whereas the PBM_G-3A mutant peptide had no effect on the binding (Figure 4C, lane 4). We further evaluated whether the PBM_H-2T peptide can block the full-length Nephrin/MAGI1 interaction. Similarly, the PBM_H-2T peptide significantly reduced the interaction between full-length Nephrin and MAGI1, whereas the PBM_G-3A mutant peptide had no effect on the binding in a coimmunoprecipitation assay (Figure 4D), further confirming the specificity of the PBM_H-2T peptide in blocking the Nephrin/MAGI1 interaction in vitro.
We next investigated whether the PBM_H-2T peptide can effectively modulate the membrane colocalization of Nephrin and MAGI1 in vivo. Satisfyingly, the membrane localization of MAGI1 in cells expressing the PBM_H-2T peptide was significantly reduced compared with those expressing the PBM_G-3A peptide (Figure 4, E and G), indicating that expression of the PBM_H-2T peptide, most likely through disrupting the interaction between Nephrin-atypical PBM and MAGI1, can block membrane colocalization of Nephrin and MAGI1 in living cells.
A Gain-of-Function Mutant of MAGI2 Rescues Nephrin-PBM Binding
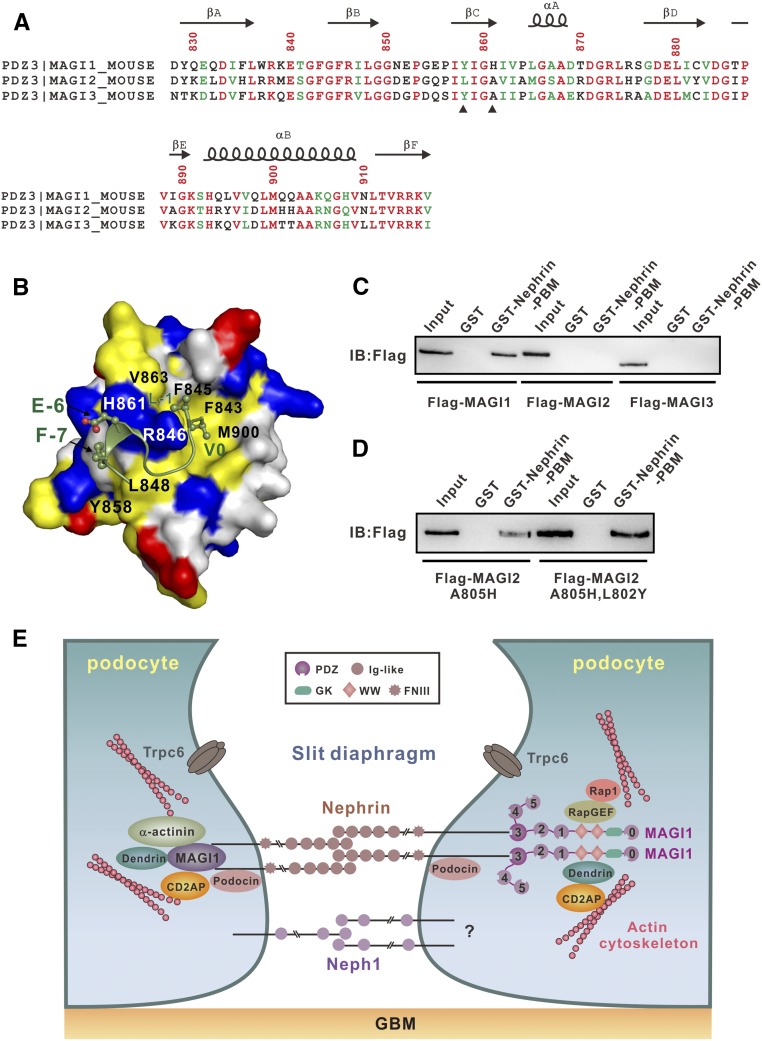
An unexpected finding in our study was that MAGI2 does not bind to Nephrin-PBM, although the amino acid sequences of PDZ3 domains from MAGI1 and MAGI2 are highly conserved (Figure 5A). We further found that MAGI3 also does not bind to Nephrin-PBM (Figure 5C). Detailed analysis of the sequence alignment, together with careful analysis of complex structure, revealed that only two residues (Tyr858MAGI1 and His861MAGI1) are different between MAGI1-PDZ3 and MAGI2-PDZ3 (Figure 5A). In the structure, His861MAGI1 forms a hydrogen bond with Glu(−6)PBM; Tyr858MAGI1, together with L848MAGI1, forms hydrophobic interactions with Phe(−7)PBM (Figure 5B). We first mutated Ala805 (the corresponding residue in MAGI1 is His861) in MAGI2 to His (A805HMAGI2). Surprisingly, the A805HMAGI2 mutant partial rescued the binding between MAGI2 and Nephrin-PBM (Figure 5D). Further substitution of Leu802 (the corresponding residue in MAGI1 is Tyr858) into Tyr (i.e., A805H/L802YMAGI2 double mutant) completely rescued MAGI2/Nephrin-PBM interaction (Figure 5D). The fact that changing only one residue of MAGI2-PDZ3 to the corresponding residue in MAGI1-PDZ3 can convert MAGI2 into a Nephrin-PBM binding protein further illustrates the exquisite specificity of the binding between MAGI1 and Nephrin.
Figure 5.
A single mutation converts MAGI2 into a Nephrin-PBM binder. (A) Structure-based sequence alignment of PDZ3 domains of MAGI1–3. The totally conserved and conserved residues are colored in red and green, respectively. The residues involved in the binding but not conserved among MAGI1–3 are highlighted by a black triangle at the bottom. (B) The combined surface and ribbon representations of the PDZ3/PBM complex. The hydrophobic residues, positively charged residues, and negatively charged residues of PDZ3 are colored in yellow, blue, and red, respectively. (C) GST pull-down assay showing that Nephrin-PBM did not bind to MAGI2 and MAGI3. (D) Substitution of Ala805 of MAGI2 with His would partially rescue the binding to Nephrin-PBM. Further replacement of Leu802 to Tyr would enhance the MAGI2/Nephrin-PBM interaction. (E) The proposed schematic model illustrating the molecular basis of Nephrin/MAGI1 interaction in slit diaphragm assembly and signaling.
Discussion
In this study, we systematically investigated the interaction between the evolutionarily conserved atypical PBM of Nephrin and various PDZ-containing proteins in the slit diaphragm and demonstrated that only MAGI1 could bind to Nephrin-PBM. We further elucidated the molecular basis of the exquisite binding specificity of MAGI1-PDZ3/Nephrin-PBM complex via combination of the biochemic, biophysic, and cell biologic approaches. In the podocytes, MAGI1 connects Nephrin with cytoskeletons via binding to various proteins such as dendrin, actinin, CD2AP, etc., which is essential for stable assembly of slit diaphragm (Figure 5E). It is worth noting that the specific Nephrin/MAGI1 interaction is not only required for slit diaphragm assembly, but also critical for Nephrin’s role as an intracellular signaling hub in slit diaphragm homeostasis. A recent study suggested that Nephrin/MAGI1 interaction is crucial for proper activity of Rap1 in the podocytes.20 Rap1 activation is tightly balanced to sustain proper podocyte function. How does Nephrin/MAGI1 complex promote the activation of Rap1? We recent discovered that MAGI1 can bind directly to RapGEF2, a guanine nucleotide exchange factor of Rap1 that is required for Rap1 activation (Z.F. Weng, J.W. Zhu, unpublished data). Thus, it is most likely that the Nephrin-MAGI1-RapGEF2-Rap1 axis plays critical role in maintaining the long-term slit diaphragm structure (Figure 5E).
Notably, although global MAGI1 knockout mice demonstrated normal glomerular histology and function into adulthood, NPHS1 but not NEPH1 heterozygosity is capable of providing podocytes with a “second hit” when combined with loss of MAGI1, leading to spontaneous FSGS.20 These data suggested a specific genetic interaction between NPHS1 and MAGI1. Such a multihit scenario is typical for glomerular disease initiation and progress, where two or more insults may be necessary for glomerular disease pathogenesis.20 For example, bigenic heterozygosity of Cd2ap and either Synaptopodin or Fyn resulted in spontaneous proteinuria and in FSGS-like glomerular damage26; combinations of heterozygous NPHS2 mutations and a heterozygous NPHS1 mutation lead to FSGS.27 Interestingly, MAGI2, the closest homolog to MAGI1, did not bind to Nephrin-PBM in our biochemic study, although the MAGI2 knockout mice presented with progressive proteinuria, diffused podocyte foot process effacement, and died of kidney failure.28 Mutations of MAGI2 have recently been identified to cause congenital nephritic syndrome in human.29 MAGI2 is tightly associated with Nephrin in the regulation of podocyte cytoskeleton and slit diaphragm dynamics.11 Loss of MAGI2 function led to a significant decrease of Nephrin and dendrin at slit diaphragm.28 Although our data indicate that Nephrin-PBM does not bind to MAGI2, it could not be ruled out that MAGI2 interacts with other regions of Nephrin than PBM, or it may associate with Nephrin via other adaptor protein(s). Further work is required to dissect the differential roles of MAGI1 and MAGI2 in the podocytes. We believe that the potent inhibitory peptide capable of blocking the Nephrin/MAGI1 interaction would offer a valuable manipulating tool in these studies.
Intriguingly, a protein-truncating mutation (p.L1240fs1286×) of Nephrin was identified in patients with steroid-resistant nephrotic syndrome. The mutant protein lacks the very C-terminal Valine of PBM and instead bears an additional 45 amino acids at the C-terminus (Supplemental Figure 1, A and B). On the basis of our structure, the mutant would diminish the Nephrin/MAGI1 interaction, thus impairing Nephrin/MAGI1-mediated function in the podocytes. Indeed, the impaired PBM of the p.L1240fs1286× mutant did not bind to MAGI1-PDZ3 (Supplemental Figure 1C). In addition, several truncation mutations (e.g., p.R1109×, p.Q1148×, and p.R1160×; Figure 1A) have been identified in patients with the congenital nephrotic syndrome.6 These mutations all lack the PBM and would definitely disrupt the Nephrin/MAGI1 interaction, leading to improper podocyte function. Therefore, our work may provide possible explanations for the glomerular disease-associated mutants in NPHS1.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the Shanghai Synchrotron Radiation Facility Beamline 18U1 for x-ray beam time, the National Center for Protein Science Shanghai (Protein Expression and Purification system, and Molecular Imaging System) for their instrument support and technical assistance, Professor Qing Lu (Shanghai Jiao Tong University) for technical support during imaging processes, and Professor Mingjie Zhang (Hong Kong University of Science and Technology) for the critical reading of the manuscript.
J.Z. and R.Z. designed the experiments. Z.W. and Z.J. performed the experiments. Y.S., L.L., F.Y., and Z.J. contributed to x-ray data collection and structure determination. Z.W., R.Z., and J.Z. analyzed the data. J.Z. wrote the manuscript. All authors approved the final version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (U1532121, 31470733, and 31770779) to J.Z., the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08030104) and the Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences to R.Z.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121275/-/DCSupplemental.
References
- 1.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Perico L, Conti S, Benigni A, Remuzzi G: Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12: 692–710, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Assady S, Wanner N, Skorecki KL, Huber TB: New insights into podocyte biology in glomerular health and disease. J Am Soc Nephrol 28: 1707–1715, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al.: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Schoeb DS, Chernin G, Heeringa SF, Matejas V, Held S, Vega-Warner V, et al.; Gesselschaft für Paediatrische Nephrologie (GPN) Study Group : Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS). Nephrol Dial Transplant 25: 2970–2976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Conti S, Perico L, Grahammer F, Huber TB: The long journey through renal filtration: New pieces in the puzzle of slit diaphragm architecture. Curr Opin Nephrol Hypertens 26: 148–153, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Wang W: Organization of signaling complexes by PDZ-domain scaffold proteins. Acc Chem Res 36: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Philippe A, Nevo F, Esquivel EL, Reklaityte D, Gribouval O, Tête MJ, et al.: Nephrin mutations can cause childhood-onset steroid-resistant nephrotic syndrome. J Am Soc Nephrol 19: 1871–1878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG: Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 102: 9814–9819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG: Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20: 1491–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartleben B, Schweizer H, Lübben P, Bartram MP, Möller CC, Herr R, et al.: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, et al.: Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One 7: e36705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otwinowski Z, Minor W: [20] Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276: 307–326, 1997 [DOI] [PubMed] [Google Scholar]
- 16.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ: Phaser crystallographic software. J Appl Cryst 40: 658–674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, et al.: PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emsley P, Cowtan K: Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Patrie KM, Drescher AJ, Goyal M, Wiggins RC, Margolis B: The membrane-associated guanylate kinase protein MAGI-1 binds megalin and is present in glomerular podocytes. J Am Soc Nephrol 12: 667–677, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Ni J, Bao S, Johnson RI, Zhu B, Li J, Vadaparampil J, et al.: MAGI-1 interacts with nephrin to maintain slit diaphragm structure through enhanced Rap1 activation in podocytes. J Biol Chem 291: 24406–24417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirabayashi S, Mori H, Kansaku A, Kurihara H, Sakai T, Shimizu F, et al.: MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest 85: 1528–1543, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S: Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res 300: 121–133, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J: A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci 117: 4401–4409, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lu Q, Yu J, Yan J, Wei Z, Zhang M: Structural basis of the myosin X PH1(N)-PH2-PH1(C) tandem as a specific and acute cellular PI(3,4,5)P(3) sensor. Mol Biol Cell 22: 4268–4278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, et al.: Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Huber TB, Kwoh C, Wu H, Asanuma K, Gödel M, Hartleben B, et al.: Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest 116: 1337–1345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löwik M, Levtchenko E, Westra D, Groenen P, Steenbergen E, Weening J, et al.: Bigenic heterozygosity and the development of steroid-resistant focal segmental glomerulosclerosis. Nephrol Dial Transplant 23: 3146–3151, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Ihara K, Asanuma K, Fukuda T, Ohwada S, Yoshida M, Nishimori K: MAGI-2 is critical for the formation and maintenance of the glomerular filtration barrier in mouse kidney. Am J Pathol 184: 2699–2708, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Bierzynska A, Soderquest K, Dean P, Colby E, Rollason R, Jones C, et al.; UK study of Nephrotic Syndrome : MAGI2 mutations cause congenital nephrotic syndrome. J Am Soc Nephrol 28: 1614–1621, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.