Fabry disease (FD) is an X-linked lysosomal storage disorder (LSD) caused by mutations of the α-galactosidase A gene. The lysosomal enzyme α-galactosidase A (GLA) mediates the hydrolysis of the terminal α-galactosyl moiety from globotriaosylceramide (Gb3). An enzymatic defect leads to the lysosomal accumulation of mainly Gb3 in different cells and tissues, causing a multisystemic disease. The first formulation of recombinant enzyme replacement therapy (ERT) was approved in Europe in 2001. Although ERT has been demonstrated to improve patient outcomes and disease course, especially when initiated early in the course of the disease, the intravenous infusion of the recombinant proteins can trigger a humoral immune response, resulting in infusion-associated reactions. Moreover, recent studies provide evidence that neutralizing antidrug antibodies (ADAs) seem to attenuate therapy efficacy in affected male patients, mediating disease progression despite ERT. The deleterious effect of ERT-related ADAs is well known in other LSDs. This review focuses on the efficacy of ERT, especially on the humoral response to continuous infusion of recombinant proteins in patients with FD, and considers the evidence about the effect of ERT-related neutralizing ADAs during ERT infusions and their effect on disease progression. It also proposes a model to explain how ADAs might mitigate therapy efficiency in FD. Finally, it discusses how clinical knowledge derived from immune tolerance induction protocols used in other LSDs might translate to FD, providing an approach to prevent ADA formation in ERT-naïve patients and decrease or supersaturate existing antibody titers in affected patients, leading to an improved disease course.
Fabry disease (FD) is an X-linked lysosomal storage disorder (LSD) on the basis of mutations within the α-galactosidase A (GLA; Xq21.3-q22) gene. The worldwide incidence of FD has been estimated at 1 in 40,000 to 1 in 117,000 live male births.1 However, FD is probably underestimated because symptoms are often nonspecific. More than 900 GLA mutations have been identified, according to the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/), resulting in a wide spectrum of clinical symptoms and manifestations.2 The reduction or loss of enzymatic GLA activity results in a progressive lysosomal accumulation, mainly of globotriaosylceramide (Gb3).3 The deacetylated and, therefore, soluble form of Gb3, lyso-Gb3, seems to be a reliable biomarker for both disease progression and therapy efficacy, and can be detected in blood plasma as well as urine.4–10 The systemic cellular accumulation of Gb3 leads to progressive renal failure, cardiomyopathy, and recurrent cerebrovascular events, significantly limiting life expectancy in affected patients.3 The most common cause of death is cardiovascular disease.11–13
Although FD is an X-linked condition, females can also be severely affected,14 likely due to a skewed X-inactivation.15 However, onset of first symptoms in females is generally 5–10 years later than in males.16 In male patients, a definitive diagnosis of FD involves demonstrating a GLA deficiency of <5% of wild-type activity in leukocytes and the presence of a GLA mutation.17,18 In females, because of the high residual GLA activity, the diagnostic gold standard requires genetic analyses; in patients with an uncertain diagnosis, biopsy of an affected organ that demonstrates pathognomonic FD-typical deposits (zebra bodies) can confirm FD.17,18
Current treatment options for FD include two different recombinant enzyme replacement therapies (ERTs) (agalsidase-α and agalsidase-β), as well as chaperone therapy (migalastat). The latter, used in patients with certain amenable mutations, facilitates cellular clearance of Gb3 and an overall improvement of disease burden.19–21 The annual costs of FD-specific treatment in Europe are about €€250,000 per year. However, ERT can lead to infusion-associated reactions (IARs) in females and males, as well as the formation of neutralizing antidrug antibodies (ADAs) in about 40% of all ERT-treated males, leading to an attenuation of therapy efficacy.19,20,22–25
This review discusses the use of agalsidase-α and agalsidase-β ERT in FD, with a special focus on the humoral response to the infused recombinant proteins that leads to the formation of neutralizing ADAs in affected patients. It also presents immune tolerance induction protocols for other LSDs that might be translated to mitigating the development of ADAs in patients with FD who receive ERT. In addition, it discusses alternative therapeutic strategies, including chaperone therapy and substrate reduction therapy, and suggests areas for future research.
FD-Specific Treatment
ERT
Depletion of accumulated Gb3 from affected cells and organs by ERT should lead to an improvement or at least a stabilization of effects in the kidney and other organs. In Europe, ERT has been in use since 2001, when agalsidase-α was approved. Two different formulations are currently available in Europe, agalsidase-α (Shire), which is produced from a modified human fibrosarcoma cell line, and agalsidase-β (Sanofi), which is produced in Chinese hamster ovary cells. The recommended dosage for agalsidase-α is 0.2 mg/kg intravenously every 2 weeks, and the advised dosage for agalsidase-β is 1.0 mg/kg intravenously every 2 weeks. In addition, two new plant-based products are currently in development: pegunigalsidase α, (Protalix Biotherapeutics),26 which is produced in BY2 tobacco cells, and moss-aGal (Greenovation),27 which is produced in moss.
Both agalsidase-α and agalsidase-β have been demonstrated to benefit patients in the short term and, in observational studies, patients treated for up to 10 years showed stabilization of eGFR or a slowing of the progression of eGFR decline.19,20,28–36 The natural course of disease is associated with a rapid decline of renal function (eGFR decline up 12 ml/min per 1.83 m2 per year) and left ventricular hypertrophy.37 Tables 1 to 5 provide a general overview of the effects of both ERTs on symptoms and manifestations in patients with FD.
Table 1.
Effect of ERT in patients with FD
| Organ | Background/Setting/Measures | Effect on Renal Function | Reference |
|---|---|---|---|
| Kidney | |||
| Agalsidase-α | Randomized, placebo-controlled study, 26 males treated for 6 mo, eGFR improved by +2.1 ml/min per 1.73 m2 (P=0.02) | Stabilization of eGFR | 20 |
| Retrospective FOS analysis, 201 patients treated for 24 mo, eGFR stabilized in 13 patients with CKD stages 2 and 3 during treatment (P>0.05) | Stabilization of eGFR | 28 | |
| Retrospective FOS analysis, 268 (135 males) patients treated for 5 yr, compared with untreated patients eGFRs in males with <60 and ≥60 ml/min per 1.73 m2 changed by −2.86 (95% CI, −3.90 to −1.83) and −1.68 (95% CI, −2.05 to −1.31) ml/min per 1.73 m2 per year, respectively, and in females with <60 ml and ≥60 ml/min per 1.73 m2 by 0.36 (95% CI, −0.47 to 1.19) and −0.43 (95% CI, −0.83 to −0.02) ml/min per 1.73 m2 per year, respectively | Stabilization of eGFR | 31 | |
| Observational single-center study, 45 (21 males) patients treated for 10 yr, eGFR analyzed as ml/min per 1.73 m2 per year reported to be stable | Stabilization of eGFR | 32 | |
| Observational single-center study, 12 male patients switched from biweekly to weekly infusions and observed for 10 yr, biweekly infusion resulted in yearly eGFR loss of −7.92±2.88 ml/min per 1.73 m2, weekly infusion resulted in an amelioration of −3.84±4.08 ml/min per 1.73 m2 per year (both P=0.01) | Stabilization of eGFR | 34 | |
| Retrospective FOS analysis, 188 patients treated for 12 mo, eGFR changes in patients with an eGFR 30 to 60 ml/min per 1.73 m2 as well as 60 to 90 ml/min per 1.73 m2 were NS | Stabilization of eGFR | 42 | |
| Single-center prospective open-label study, 24 male patients treated for up to 4.5 yr, eGFR changed by approximately −3 ml/min per 1.73 m2 per year (P=0.04) | Slowing the progression of eGFR decline | 43 | |
| Retrospective FOS analysis, 165 (115 males) patients treated for 3 yr, in males eGFR changed by −2.66±5.07 ml/min per 1.73 m2 per year (P<0.01) and in females by −1.20±3.28 ml/min per 1.73 m2 per year (P<0.01) | Slowing the progression of eGFR decline | 44 | |
| Prospective, randomized, placebo-controlled trial, 85 male patients treated for 2.5 yr, eGFR changed by −2.9±8.7 ml/min per 1.73 m2 per year (P=0.002) (patients with hyperfiltration were excluded) | Slowing the progression of eGFR decline | 45 | |
| Retrospective FOS analysis, 208 (134 males) patients treated for 7.4 yr, in males eGFR changed by −2.2 ( 95% CI, −2.8 to −1.7) ml/min per 1.73 m2 per year (P=0.01) and in females by −0.7 (95% CI, −1.4 to 0.0) ml/min per 1.73 m2 per year (P=0.05) | Slowing the progression of eGFR decline | 46 | |
| Open-label, multicenter study, 11 (ten males) pediatric patients treated for 6.5 yr, eGFR changed by +0.22 (95% CI, −2.84 to 3.28) ml/min per 1.73 m2 per year | Stabilization of eGFR | 47 | |
| Agalsidase-β | Retrospective Fabry Registry analysis, 52 (50 males) patients treated for 10 yr, in patients with LRI eGFR changed by −1.89 ml/min per 1.73 m2 per year (P=0.001) and in patients with HRI eGFR changed by −6.82 ml/min per 1.73 m2 per year (P<0.001) | Slowing the progression of eGFR decline | 33 |
| Observational multicenter study, 24 (21 males) patients treated at least for 24 mo, eGFR changed by 0.13 (95% CI, −3.75 to 5.00) ml/min per 1.73 m2 per year (P>0.05) | Stabilization of eGFR | 35 | |
| Multicenter phase 3 trial, 55 patients, treated for up to 3 yr, serum creatinine levels reported to be stable over time | Stabilization of eGFR | 48 | |
| Prospective single-center open-label study, 25 (19 males) patients treated for up to 2 yr, in eight patients with a baseline mGFR<90 ml/min per 1.73 m2, mGFR declined by −11 ml/min per 1.73 m2 (P=0.04), in 24 patients with a baseline mGFR≥90 ml/min per 1.73 m2 mGFR remained stable (P>0.05) | Slowing the progression of mGFR decline | 49 | |
| Open-label phase 3 extension study, 52 (50 males) patients treated for 4.5 yr, eGFR changed by −0.4 ml/min per 1.73 m2 per year (P=0.68) | Stabilization of eGFR | 50 | |
| Retrospective Fabry Registry analysis, 213 (151 males) patients treated for 2 yr, the highest risk factor for renal disease progression was an averaged urinary protein-to-creatinine ratio ≥1 g/g (OR, 112; 95% CI, 4 to 3109; P=0.01) and a longer time from symptom onset to treatment (OR, 19; 95% CI, 2 to 184; P=0.01) | Stabilization/preservation of renal function due to early treatment and adjustment of risk factors | 51 | |
| Observational multicenter study, 38 (26 males) patients treated for 12 mo, eGFR changed by +2 ml/min per 1.73 m2 per year (P=0.49) | Stabilization of eGFR | 52 | |
| Prospective observational study, 24 (15 males) patients treated for 2 yr, eGFR changed in patients with controlled proteinuria by −3.6 (95% CI, −4.8 to −1.1) ml/min per 1.73 m2 per year (P=0.01) and in patients with uncontrolled proteinuria by −7 (95% CI, −5.6 to −2.0) ml/min per 1.73 m2 per year (P=0.02) | Stabilization of eGFR due to controlled proteinuria | 79 | |
FOS, Fabry outcome survey; 95% CI, 95% confidence interval; LRI, low renal impairment; HRI, high renal impairment; mGFR, measured glomerular filtration rate; OR, odds ratio.
Table 5.
Effect of ERT in patients with FD
| Organ | Background/Setting/Measures | Effect on GI Symptoms/Frequencies | Reference |
|---|---|---|---|
| Gastrointestinal system | |||
| Agalsidase-α | Single-center open-label study, 11 (nine males) patients treated for 6 mo, questionnaire for the presence of abdominal pain and the frequency of diarrhea revealed a significant reduction (both P<0.02) | Reduction of abdominal pain and frequency of diarrhea | 72 |
| Retrospective FOS analysis, 58 (33 males) patients treated for 24 mo, frequency of abdominal pain decreased significantly (P<0.05) | Reduction of abdominal pain | 73 | |
| Agalsidase- β | Single-center clinical trial, four male patients treated for 6–7 mo to 3 yr, patients reported decreased frequencies of abdominal pain and diarrhea and experienced weight gains of 3–8 kg | Reduction of abdominal pain and frequency of diarrhea and weight gain | 74 |
| Retrospective Fabry Registry analysis, 168 female patients treated for up to 5.7 yr, presence of abdominal pain changed by −14% (P<0.01) and frequency of diarrhea by −12% (P<0.01) | Reduction of abdominal pain and frequency of diarrhea | 75 | |
Table 3.
Effect of ERT in patients with FD
| Organ | Background/Setting/Measures | Effect on CNS Function/Symptoms | Reference |
|---|---|---|---|
| Central nervous system | |||
| Agalsidase-α | Placebo-controlled study, 26 male patients treated for 6 mo, resting global cerebral blood flow changed by −3.48±4.57 (treated, n=14) versus +1.25±5.11 (placebo, n=12) ml/min per 100 g of tissue (P=0.03) | Improvement of cerebral blood flow | 63 |
| Open-label study, 63 male patients treated for 18 mo, Doppler blood flow parameters (peak flow velocity, mean flow velocity, end-diastolic velocity, flow acceleration) in certain vessels improved (all P<0.01) | Improvement of cerebral blood flow | 67 | |
| Agalsidase-β | Post hoc analysis of phase 4 study, 31 patients treated for 12 mo, in patients≤50 yr of age, normalized WML diameter increased significantly in placebo group (n=13, P=0.03) and remained stable in the treated group (n=18, P>0.5) | Stabilization of WMLs | 68 |
CNS, central nervous system; WML, white matter lesion.
Table 4.
Effect of ERT in patients with FD
| Organ | Background/Setting/Measures | Effect on Pain | Reference |
|---|---|---|---|
| Peripheral nervous system | |||
| Agalsidase-α | Randomized, placebo-controlled study, 26 males treated for 6 mo, BPI (worst) changed by −1.9 (P=0.02) | Reduction of BPI (worst) | 20 |
| Retrospective FOS analysis, 25 patients treated for up to 2 yr, BPI (average) reported to decrease | Reduction of BPI (average) | 42 | |
| Retrospective FOS analysis, 53 patients treated for up to 5 yr, BPI (average) changed by −1.2±2.7 (n=53, P=0.002), BPI (worst) changed by −1.3±3.5 (n=49, P=0.01) | Reduction of BPI (average and worst) | 56 | |
| Single-center prospective open-label study, 36 females treated for 4 yr, BPI (worst) changed after 12 mo from 4.6±2.9 to 3.3±2.9 (P=0.001) and remained stable | Reduction of BPI (worst) | 57 | |
| Retrospective FOS analysis, 20 (16 males) patients treated for up to 2 yr, BPI (average, now, worst) decreased after the second year (all P<0.05) | Reduction of BPI (average, now, worst) | 69 | |
| Retrospective FOS analysis, 62 patients treated for 3 yr, BPI (average, least, worst) decreased (P<0.05) | Reduction of BPI (average, least, worst) | 70 | |
| Agalsidase-β | Single-center prospective open-label study, 22 male patients treated for up to 23 mo, total symptom score for neuropathic pain changed from 1.76±1.97 to 0.83±1.53 (P=0.04), quantitative sensory testing revealed improvements for VDT (P<0.05) and HP (P<0.01) | Reduction of pain, improvement of nerve function | 71 |
BPI, brief pain inventory; FOS, Fabry outcome survey; VDT, thresholds of vibration; HP, heat-pain.
Table 6.
Overview of successful therapy strategies for prevention or elimination of ADAs in patients with LSDs
| Disease | Target | Drug Components | Reference |
|---|---|---|---|
| Immune tolerance induction in ERT-naïve patients | |||
| FD | Immunosuppression due to transplantations | Methylprednisolone with azathioprine, calcineurin inhibitors, mycophenolate-mofetil/mycophenolate acid | 115 |
| PD | B cell depletion | Rituximab, MTX, IVIG | 118 |
| PD | Immunosuppression, B cell depletion, mTOR inhibition | Methylprednisolone, rituximab, rapamycin, IVIG | 112 |
| PD | B cell depletion, immunomodulation | Rituximab, MTX, IVIG | 117 |
| PD | B cell depletion, immunomodulation | Rituximab, MTX, IVIG | 114 |
| Acute reduction of neutralizing ADAs | |||
| FD | Short-term ADA reduction by immunosuppression due to transplantations | Prednisolone, tacrolimus, mycophenolate-mofetil/mycophenolate acid | 115 |
| GD | Acute ADA reduction, immunosuppression | Plasma exchange (one plasma volume), cyclophosphamide, IVIG | 116 |
| PD | B cell depletion | Rituximab, MTX, IVIG | 111 |
| PD | Immunosuppression proteasome inhibition, B cell/plasma cell depletion | Cyclophosphamide, rituximab, bortezomib, MTX, IVIG | 113 |
| PD | Acute ADA reduction, immunosuppression | Cyclophosphamide, IVIG, plasma exchange | 120 |
| PD | B cell depletion | Rituximab, MTX, IVIG | 119 |
PD, Pompe disease; IVIG, intravenous immunoglobulin; mTOR, mechanistic target of rapamycin; GD, Gaucher disease.
In principal, most studies suggest that initiating early treatment is superior to late initiation, resulting in a more pronounced long-term improvement. In more advanced stages of the disease, however, patients benefit less from ERT. Early ERT has been demonstrated to lead to Gb3 depletion in renal cells in children and adults in a dosage- and frequency-dependent manner.38–41 Furthermore, ERT has been reported to stabilize renal function in terms of eGFR, whereas ERT’s effect on proteinuria and albuminuria is inconsistent.26,29–37,42–52 If ERT is started before myocardial fibrosis has developed, a long-term improvement of myocardial morphology, function, and exercise capacity can be achieved.53–62 It also leads to a reduction of cerebrovascular and thromboembolic events63–65 and significant improvement in pain-related quality of life.67,69–71 Moreover, ERT is associated with a reduction of gastrointestinal symptoms such as abdominal pain and diarrhea72–75 and a reduction of lyso-Gb3 levels, a marker of disease load.9 Beneficial effects of ERT can be measured by different disease severity scores and tools, such as the Mainz Severity Score Index,76 the Disease Severity Scoring System,77 and the FAbry STabilization indEX.78 In addition to ERT, provision of concomitant nephroprotective and cardioprotective medication (on the basis of angiotensin-converting enzyme and angiotensin II receptor blockers) is highly warranted to preserve renal79 and cardiac function.53 However, recent reviews reported only a small benefit of the use of ERT.66,80 One mechanism suspected to play a role in curbing ERT efficacy is the formation of ADAs against infused enzymes, which is a major focus of this review.
Oral Therapy
Another treatment option, oral chaperone therapy with migalastat (Amicus Therapeutics), was approved in Europe in May of 2016 for long-term treatment of FD in adults and adolescents 16 years or older with an amenable mutation. The recommended dose is one capsule (123 mg) every other day, and food should not be consumed for at least 2 hours before and 2 hours after taking it. The drug is not currently recommended for use in patients with an eGFR<30 ml/min per 1.73 m2. Migalastat (1-deoxygalactonojirimycin), an oral small molecule chaperone that stabilizes endogenous GLA enzyme and supports proper folding in the endoplasmic reticulum, leads to increased endogenous enzymatic GLA activity in the lysosomes of patients with an amenable mutation.21 These are missense mutations that result in reduced enzymatic activity, caused by misfolding of the enzyme, which the chaperone can correct. There are at least 359 amenable and 706 nonamenable mutations (on the basis of an in vitro test in human embryonic kidney cells), according to a list published by Amicus, which markets migalastat (http://www.galafoldamenabilitytable.com, update 16th May 2018).81 For patients with amenable mutations, migalastat offers promise as a first-in-class oral monotherapy alternative treatment for patients with FD.82
In addition, recent reports describe the first attempts toward oral substrate reduction therapy, which aims to reduce production of Gb3 by inhibiting glucosylceramide synthase.83 One study demonstrated that lucerastat markedly reduced plasma GSLs such as lyso-Gb3 within 12 weeks of treatment.84 Research is also under way for a gene therapy approach to achieve tissue-specific overexpression of GLA, resulting in a clinical trial (https://clinicaltrials.gov/ct2/show/NCT02800070?term=gene+therapy&cond=fabry&rank=3).85
Immune Response to ERT
Because ERT is on the basis of the intravenous infusion of a mostly foreign recombinant protein, a humoral response is common,19,20,86 at least in males with classic FD, who lack endogenous enzyme. Such individuals are also classified as “crossreactive immunologic material” (CRIM) negative. CRIM-negative status means that there is not even a mutated or truncated form of endogenous GLA that can be recognized by the immune system as an innate molecule. In these patients, infused enzyme might be recognized as foreign, triggering an immune response. Several studies analyzing patients with LSDs demonstrate that CRIM-negative patients have a high risk for developing immune responses after ERT initiation.22,25,87,88 Because of this, it is important when treating patients with FD to distinguish an immune response caused by continuous exposure to ERT (leading to the formation of neutralizing ADAs) from IARs.
IARs frequently occur in ERT-naïve patients with FD after ERT initiation, mainly in males with nonsense or null mutations (CRIM negative).19,20,89,90 Symptoms of IARs are mostly limited to fever and chills, necessitating premedication with antihistamines and steroids, as well as prolongation of infusion times (i.e., decreased infusion rates).90 However, severe life-threatening IARs also have been reported.91,92 According to the manufacturer’s instructions, 24% of patients treated with agalsidase-α present with IARs.93 In contrast, 67% of patients treated with agalsidase-β experience this adverse effect.94
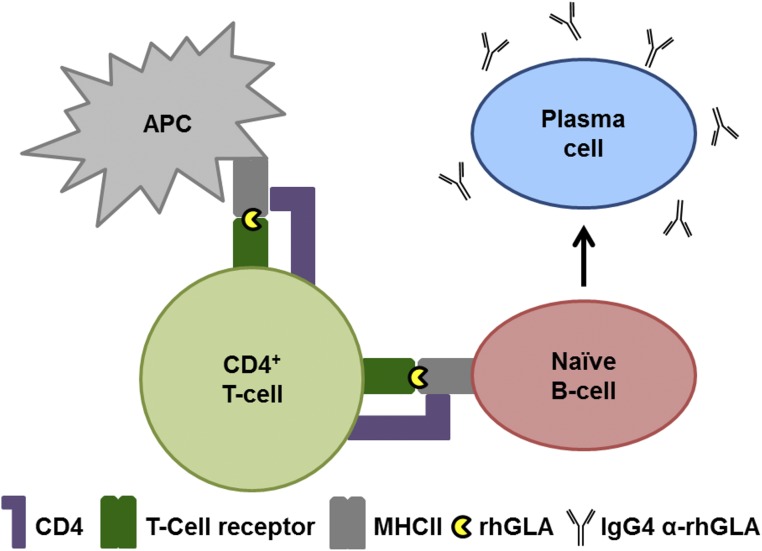
The nature of IARs is yet not completely understood but they are probably the result of anaphylactoid reactions (i.e., chemical compound–mediated) and not often anaphylactic (i.e., IgE-mediated type 1 hypersensitivity), because direct identification of IgE antibodies has been made in only a few male patients to date. In one report, IgEs were identified in one patient with severe IARs, including pain95; other reports described one patient with generalized urticaria during infusions96 and another with anaphylaxis.30 In addition, an agalsidase-β open-label safety study reported seven patients with IgEs or a positive skin test.97 Furthermore, Wilcox et al. reported three patients with IgEs identified by direct IgE measurement or a skin test.48 However, the risk of IARs seems to be higher in patients with an anti-agalsidase IgG antibody–positive status.25,90 The humoral response includes crosstalk between antigen-presenting cells, CD4+ T-helper cells, and B cells (Figure 1).98
Figure 1.
Humoral response results in IgG formation. Schematic overview of a humoral immune response to recombinant GLA resulting in IgG antibody production. In a classic T cell–dependent immune response, the recombinant human (rh) GLA is processed by antigen-presenting cells (APC) and presented to CD4+ T cells, which become activated. Activated T cells, in turn, activate naïve B cells, which maturate into IgG4-secreting plasma cells.
In patients with IgEs, the following general mechanisms might apply: After antigen presentation, an early anaphylactic response includes the formation of IgE antibodies, triggering mast cell activation and histamine release, which could explain the observed severe IARs. With ongoing antigen presentation this process is commonly followed by IgG4 isotype antibody production against similar epitopes.99 Because these antibodies compete with IgEs but do not lead to complement activation, immunization will be achieved and IARs may be attenuated or stop over time.99–101 Currently, the literature lacks comprehensive studies analyzing the presence of IgE and IgG antibodies in patients with FD.
IgG antibodies seem to develop within 3–6 months of starting ERT,22,48,90,102 but frequency and severity of IARs in affected patients may be attenuated. Smid et al.90 reported that most IARs were observed during the first 13 infusions. Although awareness of ERT-related IARs emerged in the first ERT clinical trial, to date, no standardized assays or protocols have been developed to determine IgG levels.103 Most trial studies and reports analyzing an early IgG response were performed by the ERT-manufacturing companies using ELISAs and their own antibodies.104 However, Linthorst et al.22 demonstrated that IgG antibodies (measured by ELISA) mediate an easily measurable neutralizing activity that can be used to classify neutralizing antibody–positive and –negative patients. This neutralizing activity was confirmed by several other studies,24,25,90 and the isotype of drug-neutralizing antibodies in patients with infusion-related ADAs recently was identified as IgG4, mainly.102
Recent studies have found that, once neutralizing ADAs occur, their formation seems to be irreversible, and that the majority of affected patients stay neutralizing ADA–positive over 10 years.24,25,36 About 73% of patients treated with agalsidase-β and 24% of patients treated with agalsidase-α reportedly develop ADAs.103,105 This difference might be explained by the different dosages of both drugs and the different cell lines producing them, as well as the CRIM status of the treated patients, which has not been taken into account thus far. Recent data from Arends et al.36 confirmed an increased risk for formation of neutralizing ADAs in patients treated with agalsidase-β. Although the individual CRIM status of the treated patients was not determined, their study excluded patients with nonclassic FD. However, Rombach et al.24 and later Smid et al.90 reported no significant differences in ADA formation when using the same dosage (0.2 mg/kg every 2 weeks) for both drugs at ERT initiation. Of note, the CRIM status of patients in these studies was also not determined in detail. To analyze this topic adequately, head-to-head studies with comparable dosages and a predefined CRIM status of participating patients are needed. At this point, it seems that the dosage is the most important trigger for immune response.
Although the presence of neutralizing ADAs was first described very early after ERT became available,22 the effect of ADAs on FD-specific biomarkers has been a topic of controversy. High ADA titers (measured by ELISA) have been associated with increased Gb3 deposition in endothelial cells,106 but with unclear effects on plasma Gb3 levels.23,30,43 In contrast, by using serum-mediated inhibition assays, Rombach et al.24 demonstrated that plasma (lyso-) Gb3 levels were significantly higher over time in patients with neutralizing ADAs, and that urinary Gb3 decreased only in patients without ADAs. Our group also confirmed that ADAs are associated with a poorer clearance of plasma lyso-Gb3.25 Although some previous reports have suggested that ADAs appeared to have no effects on such parameters as eGFR slopes and cardiac mass,24,43,106 there is evidence that patients with ADAs exhibit harmful clinical outcomes, including increased left ventricular mass and progressive loss of renal function.25 These differences in study outcomes might be due to an ERT dosage–dependent effect on ADA titers. Rombach et al.24 observed that in patients with ADAs who had been switched from an agalsidase-β dose of 0.2 to 1.0 mg/kg every 2 weeks, plasma lyso-Gb3 and Gb3 significantly declined after 1 year; this might be the result of a supersaturation of antibodies due to increased ERT dosages during infusion.102
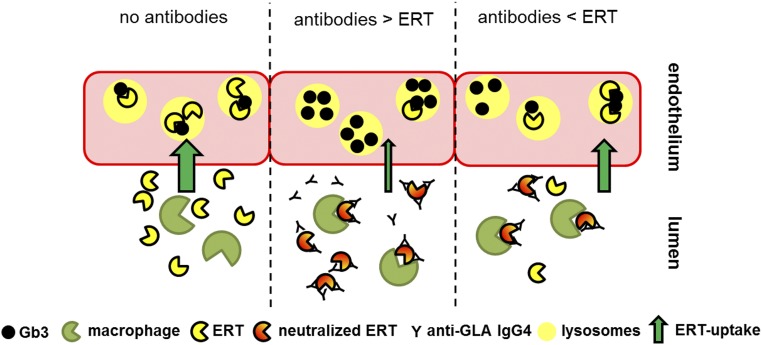
A model of ERT and neutralizing ADAs during infusion (see Figure 2), supported by findings in the literature, can potentially explain varying therapy efficiency during infusions. According to this model, during infusions in patients without ADAs, ERT enters the cell and lysosomes via the mannose-6-phosphate (M6P) receptor and results in clearance of Gb3. If neutralizing ADAs are present, ERT is directly inactivated (neutralized) by ADAs in the plasma.22,102 In addition, binding of ADAs to ERT also leads to an activation of macrophages that internalize ERT-ADA complexes,22 leading to a decrease in the cellular uptake of free ERT. If the ERT dose surmounts the antibody titer,102 more ERT can enter the lysosomes of target cells, resulting in an appropriate Gb3 clearance with subsequent therapeutic benefits. This hypothesis is supported by recent outcomes by Arends et al.36 demonstrating an improved biochemical response—decreasing lyso-Gb3—in ADA-positive patients treated with higher doses of agalsidase, such as agalsidase-β (1.0 mg/kg) compared with agalsidase-α (0.2 mg/kg). Whether the binding of ADAs to ERT further affects the cellular uptake (i.e., transport) via the M6P receptor, or independent of M6P by megalin and sortilin,107 still remains unclear and should be investigated in future studies.
Figure 2.
ADA titers affect cellular lysosomal Gb3 clearance. Current literature-based model of ERT and neutralizing ADAs during infusion. If no antibodies are present, ERT enters cells (here, endothelial cells) via the M6P receptor, leading to Gb3 clearance from lysosomes (left). If antibodies are present, they neutralize ERT activity by binding the enzyme. In addition, IgG-tagged ERT molecules will be internalized and digested by macrophages. If more antibodies than ERT are present, this results in a decreased cellular Gb3 clearance (middle). If the ERT dose exceeds antibody titers, more ERT can enter the lysosomes of target cells, resulting in increased Gb3 clearance (right).
Possible Current Therapy Strategies
Several reports for other LSDs such as Gaucher and Pompe disease have focused on the effect of neutralizing ADAs on clinical outcomes and how to adapt ERT to address ADAs. This focus is probably due to the fact that the clinical effect of ADAs in patients affected by these LSDs (especially in infantile Pompe disease) is much more obvious and severe than in FD. In principal, therapeutic strategies can be divided into the prevention of ADA formation in ERT-naïve patients and the acute reduction of existing ADAs.
Garman et al.108 demonstrated in 2004 that administration of low-dose methotrexate (MTX) during ERT initiation can prevent ADA formation and, therefore, induce immune tolerance in a murine FD model treated with agalsidase-β. A similar outcome was achieved in mice by the continuous administration of belimumab, a humoral mAb that inhibits B cell–activating factor, also known as B-lymphocyte stimulator.109 A comparable effect of low-dose MTX was also observed in treatment-naïve Pompe mice, demonstrating that transient low-dose MTX activates the formation of B-regulatory cells, which might mediate an antigen-specific tolerance to alglucosidase-α.110 To the best of our knowledge, protocols to prevent ERT-naïve patients with FD from forming neutralizing ADAs have not been reported. Reports mostly focus on how to avoid IARs after ERT initiation to minimize risks during home infusion therapy.90 Protocols for a successful reinstitution of agalsidase-α and agalsidase-β in patients with severe IARs have been published by Aydin et al.92 and Bodensteiner et al.97
Prevention of ADA Formation
Using specific B cell depletion and immunomodulation in CRIM-negative infants with Pompe disease before ERT initiation provides an appropriate immune tolerance induction to block the immune response to recombinant α-glucosidase and prevent the formation of ADAs.111–114 A recent retrospective proof-of-concept study showed that high-risk patients with FD who received immunosuppressive therapy after organ transplantation before initiating ERT demonstrated no seroconversion, even if the maintenance therapy was decreased, suggesting that an intense (and unspecific) immunosuppression as used for transplantations might also prevent ADA formation.115 To prevent ADA formation in ERT-naïve patients with FD, prospective studies are now warranted to help to establish infusion protocols with immunosuppressive therapy.
Therapeutic Options in Patients with ADAs
The conflicting outcomes in studies reporting the effect of ADAs on clinical symptoms and manifestations in patients receiving ERT demonstrate the need to determine individual antibody titers, especially because ADA titers can be supersaturated by appropriate enzyme doses at a certain point of infusion.102 The easiest method to overcome ADA titers and to increase ERT efficacy thus might be to increase infused dosages, but the maximum approved doses of agalsidase-α and agalsidase-β per kg body weight are limited, and using higher dosages of these expensive drugs would be very cost intensive. Furthermore, whether increasing dosages would also result in even higher ADA titers is unknown. Therefore, patients with severe disease progression who are running out of therapeutic options despite weight-adapted ERT might also benefit from immune-modulating therapies. Transplant-related immunotherapy in patients with FD can significantly decrease ADA titers,115 and specific immunosuppression to prevent or decrease ADAs in patients with other LSDs has been described in detail. The most successful approaches have included inhibition of the folic acid metabolism (blocking de novo DNA synthesis), by alkylation of DNA (blocking DNA replication) or by antibody-mediated specific B cell depletion. For instance, treatment periods with 2 mg/kg body wt cyclophosphamide in combination with 0.4 mg/kg body wt intravenous Ig116 or with rituximab (375 mg/m2), MTX (0.5 mg/kg weekly), and intravenous Ig (500 mg/kg every 4 weeks)111,117 resulted in a decrease of antibody titers; in the latter case, inhibitory antibodies were absent for at least 3 years.118 Moreover, a combination treatment with bortezomib (1.3 mg/m2), rituximab (375 mg/m2), and MTX (15 mg/m2) has been reported to effectively reduce antibody titers in infantile Pompe disease.113 Alternative successful approaches have also included use of inhibitors of de novo DNA synthesis or replication such as mycophenolate mofetil and cyclophosphamide.115,120 Table 2 provides an overview of therapy strategies that have been used for successful prevention or elimination of ADAs in patients with LSDs.
Table 2.
Effect of ERT in patients with FD
| Organ | Background/Setting/Measures | Effect on Cardiac Function | Reference |
|---|---|---|---|
| Heart | |||
| Agalsidase-α | Retrospective FOS analysis, 164 (71 males) patients treated for 5 yr, LVMI slopes were compared with untreated patients as 0.33 (95% CI, 0.13 to 0.53) g/m2.7 per year in males and 0.48 (95% CI, 0.30 to 0.66) g/m2.7 per year in females | Reduction of LVMI | 31 |
| Observational single-center study, 45 (21 males) patients treated for 10 yr, MWT decreased by −1.89 (95% CI, −2.58 to −1.19) mm (P<0.001) in males and by −0.48 (95% CI, −1.05 to 0.09) mm (P=0.10) in females | Reduction of MWT | 32 | |
| Retrospective FOS analysis, 52 patients treated up to 2 yr, in patients with MWT>11 mm at baseline MWT decreased (P<0.05) and in patients with LVM>50 g/m2.7 LVM also decreased (P<0.05) | Reduction of MWT and LVM | 42 | |
| Open-label multicenter study, 11 (ten males) pediatric patients treated for 6.5 yr, LVMI changed by −0.48 (95% CI, −1.33 to 0.38) g/m2.7 per year | Stabilization of LVMI | 47 | |
| Phase IIIB study, 15 female patients treated for 12 mo, LVMI changed after 27 wk by −23.0±5.78 g/m2 (P=0.003) and after 41 wk by −25.2±8.12 g/m2 (P=0.02) | Reduction of LVMI | 54 | |
| Randomized, double-blind, placebo-controlled study, 15 male patients treated for 6 mo, LVM changed by −11.5 g (treated) versus +21.8 g (placebo) (P=0.04) | Reduction of LVM | 55 | |
| Retrospective FOS analysis, 57 patients treated up to 5 yr, after 5 yr LVMI changed in patients with LVH at baseline (n=32) by −7.3±15.3 g/m2.7 (P=0.01) and without LVH (n=25) by +1.8±10.1 g/m2.7 (P>0.05) | Reduction of LVMI | 56 | |
| Single-center open-label study of 37 females treated for 4 yr, independently of LVMI at baseline, LVMI decreased over time (P<0.01) | Reduction of LVMI | 57 | |
| Agalsidase-β | Retrospective Fabry Registry analysis, 52 (50 males) patients treated for 10 yr, IVST changed in patients with LRI by +0.04 mm/yr (P=0.05) and in patients with HRI by +0.14 mm/yr (P=0.07) | Stabilization of IVST | 33 |
| Single-center open-label study, 16 (14 males) patients treated for 12 mo, end-diastolic thickness of the left ventricular posterior wall, myocardial mass, peak systolic strain rate, and end-systolic strain of the posterior wall changed by −2 mm, −21 g, +0.9 s−1, +11% (all P<0.05) | Reduction, stabilization of cardiac structures and improvement of cardiac function | 58 | |
| Single-center open-label study, nine (seven males) patients treated for 12 mo, IVST, RWT, and LVMI were significantly reduced (all at least P<0.05) after 12 mo | Reduction of IVST, RWT, and LVMI | 59 | |
| Single-center open-label study, 11 (eight males) patients treated for 45 mo, LVM changed by −35 g and left ventricular wall thickness changed by −2 mm (both P<0.001) | Reduction of LVM and LVWT | 60 | |
| Single-center open-label study, 32 (27 males) patients stratified by fibrosis treated for 3 yr, LVM and septum thickness changed in patients without fibrosis by −36 g and −1.5 mm (both P<0.02) | Reduction of LVM and septum thickness due to early treatment before fibrosis | 61 | |
| Single-center open-label study, 23 (13 males) patients treated for 14 mo, LVM, phosphocreatine, and ATP changed by −30 g (P=0.01), +2.7 mmol/kg (P=0.003), and +1.0 mmol/kg (P<0.05) | Reduction of LVM and amelioration of energetic depression | 62 | |
95% CI, 95% confidence interval; FOS, Fabry outcome survey; LRI, low renal impairment; HRI, high renal impairment; LVMI, left ventricular mass index; LVM, left ventricular mass; MWT, mean wall thickness; LVH, left ventricular hypertrophy; IVST, interventricular septum thickness; RWT, relative wall thickness; LVWT, left ventricular wall thickness.
Future Research and Treatment
Current studies suggest that treatment with 1.0 mg/kg agalsidase-β is associated with an increased risk for ADA formation.36 To explore whether this higher frequency is due to the dose or the origin (cell line) of these ERTs, prospective studies with equal doses (0.2 mg/kg) of both agalsidase-α and agalsidase-β, and including patients with defined CRIM status, should be performed to analyze the immunogenicity of the two formulations.
Because the ADA titer might affect patient outcomes,102 determining a supersaturated or nonsupersaturated status of ADAs after infusion might be one of the most important steps in future clinical practice with ERT. The establishment of recently described advanced inhibition assays to determine ADA titers before and after infusions will be useful to discriminate patients at risk,102 and preliminary results warrant validation in future studies. Furthermore, it would be helpful in clinical routine to be able to determine neutralizing ADA titers by titration from a single blood sample. This approach would allow calculation of the individualized amount of ERT required to supersaturate ADAs. The establishment of advanced inhibition assay–based titration procedures would also facilitate analysis of ADA saturation efficiencies of present and future ERTs.
In addition, given the very short reported elimination t1/2 of current ERTs in plasma (89–108 minutes for agalsidase-α and 80–120 minutes for agalsidase-β),93,94,121 future prospective studies should investigate whether a longer bioavailability would decrease the risk for ADA formation in ERT-naïve patients. For the currently approved ERTs, these approaches might be achieved by a reduced infusion interval (i.e., weekly infusion, with a half-dose), as reported by Schiffmann et al.,29,34 or by concomitant use of chaperone and ERT (until now off-label use), which is reported to increase the t1/2 of infused enzyme.121 In addition, pharmacologic analyses of novel plant-expressed ERTs such as moss-aGal and pegunigalsidase α will be of interest. The increased t1/2 of 80 hours of pegunigalsidase α (the result of PEG-mediated crosslinking of the GLA homodimer) is especially promising.122 Another potentially promising approach is the identification of GLA enzymes, as recently described by Kytidou et al.123 who reported a GLA homolog from Nicotiana benthamiana that did not crossreact with ADAs from patients treated with currently approved ERT. It will be of interest whether these approaches or the infusion of a nonapproved double-dose (infused every 4 weeks) might also lead to a reduction of existing ADA titers. Because use of immune absorption to reduce ADA titers would be an invasive, costly, and time-consuming intervention, it is unlikely to be adopted in clinical routine. However, another interesting option might be the development of a specific peptide to supersaturate ADAs before ERT infusion.
In addition to causing direct neutralization of infused enzyme, ADAs might also form ADA-ERT immune complexes (“planted antigens,” type 3 reaction), mediating complement activation and resulting in membranous nephritis (MN), as reported for other ERT-treated rare diseases.124–126 Because MN is a major cause of a nephrotic syndrome in adults, future studies using renal biopsies to detect MN developing after ERT initiation should focus not only on Gb3 accumulation but also on detection of ADA-ERT complexes and complement activation within glomeruli of patients with ADAs. Immunosuppressive therapy might benefit affected patients in two ways: by preventing neutralization of infused enzyme and by preventing alloimmune MN.125 Epitope mapping for neutralizing ADAs, comparable to that demonstrated for phospholipase-A2-receptor autoantibodies,127 will be of interest to identify potential highly antigenic epitopes within the recombinant ERTs.
Conclusions
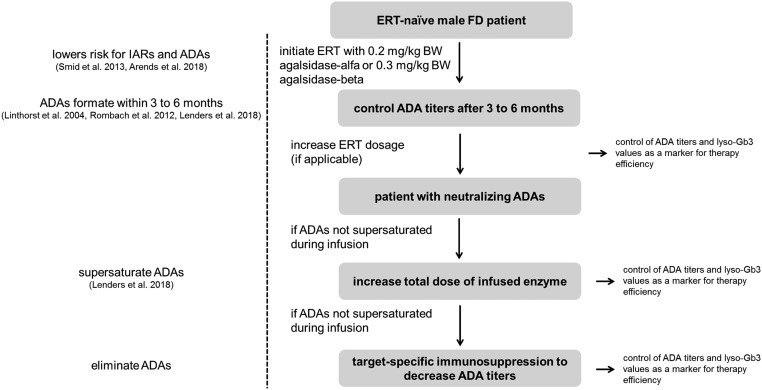
CRIM-negative males—those with classic FD, who lack GLA enzyme—are at particularly high risk for ADA formation when treated with ERT. This finding highlights the importance of refining current ERT strategies, because these patients are not treatable with current chaperone therapy. Moreover, although substrate reduction therapy might be a therapeutic option,84 it has not yet been approved for use. While awaiting the development of second-generation ERTs optimized for a decreased humoral response or increased enzymatic stability, as well as the creation of specific protocols to induce immune tolerance, the following approach might help clinicians optimize the treatment efficiency for male patients with FD (Figure 3). On the basis of findings reported in the literature,90 to minimize risk for IARs and ADAs, ERT should be initiated in male patients with an approved dose of 0.2 or 0.3 mg/kg body wt for agalsidase-α or agalsidase-β, respectively. After ERT initiation, the patient should be evaluated for the presence of neutralizing ADA titers. After 13 infusions without IARs, agalsidase-β dose could be increased to 1.0 mg/kg body wt.90 If the dose is successively increased, ADAs are typically controlled for another 3–6 months. In patients who present with ADAs, titers before and after infusions should be measured to determine whether ADAs are supersaturated by ERT.102 If ADAs are not supersaturated by ERT and the patient is free from IARs, a dose increase (within the approved range) might be an option to supersaturate ADAs.102 Chaperone therapy is an alternative option for patients with a mutation that is amenable to that approach. If a patient with ADAs has already reached the maximum limit of weight-adapted ERT or has experienced severe IARs that exclude treatment with higher infused dosages, target-specific off-label immunosuppression to eliminate ADAs, as described in recently published study findings, might be the remaining option to optimize current therapy (Figure 3, Table 2).
Figure 3.
Potential workflow for optimizing therapy management in patients with FD with neutralizing ADAs under ERT. Of note, agalsidase-β is approved only in doses of 0.3 or 1.0 mg/kg; the approved dose for agalsidase-α is 0.2 mg/kg.
We conclude that in male patients with FD being treated with ERT, the presence of neutralizing ADAs impairs the therapy efficacy and its ability to stabilize or slow disease progression. There is no specific supersaturating dose for ERT. Measurement of individual ADA titers to determine whether infused ERT doses are sufficient to supersaturate antibodies should be performed routinely in clinical practice. Researchers should implement such measurements in cohort studies that analyze the effect of FD-specific treatment on disease progression.
Disclosures
M.L. and E.B. received speaker fees and research grants from Shire, Sanofi-Genzyme, and Amicus Therapeutics. None of the funding companies had a role in writing or submission of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Meikle PJ, Hopwood JJ, Clague AE, Carey WF: Prevalence of lysosomal storage disorders. JAMA 281: 249–254, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Schiffmann R: Fabry disease. Handb Clin Neurol 132: 231–248, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Zarate YA, Hopkin RJ: Fabry’s disease. Lancet 372: 1427–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Auray-Blais C, Ntwari A, Clarke JT, Warnock DG, Oliveira JP, Young SP, et al. : How well does urinary lyso-Gb3 function as a biomarker in Fabry disease? Clin Chim Acta 411: 1906–1914, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Niemann M, Rolfs A, Störk S, Bijnens B, Breunig F, Beer M, et al. : Gene mutations versus clinically relevant phenotypes: Lyso-Gb3 defines Fabry disease. Circ Cardiovasc Genet 7: 8–16, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Auray-Blais C, Blais CM, Ramaswami U, Boutin M, Germain DP, Dyack S, et al. : Urinary biomarker investigation in children with Fabry disease using tandem mass spectrometry. Clin Chim Acta 438: 195–204, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Boutin M, Menkovic I, Martineau T, Vaillancourt-Lavigueur V, Toupin A, Auray-Blais C: Separation and analysis of lactosylceramide, galabiosylceramide, and globotriaosylceramide by LC-MS/MS in urine of Fabry disease patients. Anal Chem 89: 13382–13390, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Nowak A, Mechtler TP, Desnick RJ, Kasper DC: Plasma LysoGb3: A useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol Genet Metab 120: 57–61, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Auray-Blais C, Lavoie P, Boutin M, Ntwari A, Hsu TR, Huang CK, et al. : Biomarkers associated with clinical manifestations in Fabry disease patients with a late-onset cardiac variant mutation. Clin Chim Acta 466: 185–193, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Arends M, Wijburg FA, Wanner C, Vaz FM, van Kuilenburg ABP, Hughes DA, et al. : Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol Genet Metab 121: 157–161, 2017 [DOI] [PubMed] [Google Scholar]
- 11.MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 38: 750–760, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 38: 769–775, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P: Life expectancy and cause of death in males and females with Fabry disease: Findings from the Fabry Registry. Genet Med 11: 790–796, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lenders M, Hennermann JB, Kurschat C, Rolfs A, Canaan-Kühl S, Sommer C, et al. : Multicenter Female Fabry Study (MFFS) - clinical survey on current treatment of females with Fabry disease. Orphanet J Rare Dis 11: 88, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echevarria L, Benistan K, Toussaint A, Dubourg O, Hagege AA, Eladari D, et al. : X-chromosome inactivation in female patients with Fabry disease. Clin Genet 89: 44–54, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Beck M: Demographics of FOS – the Fabry outcome survey. In: Fabry Disease: Perspectives from 5 Years of FOS, Chapter 16, edited by Mehta A, Beck M, Sunder-Plassmann G, Oxford, Oxford PharmaGenesis, 2006 [Google Scholar]
- 17.Smid BE, van der Tol L, Cecchi F, Elliott PM, Hughes DA, Linthorst GE, et al. : Uncertain diagnosis of Fabry disease: Consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int J Cardiol 177: 400–408, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, et al. : Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J Rare Dis 10: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. : International Collaborative Fabry Disease Study Group: Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, et al. : Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, et al. : Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 375: 545–555, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM: Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int 66: 1589–1595, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, et al. : Treatment of Fabry disease with different dosing regimens of agalsidase: Effects on antibody formation and GL-3. Mol Genet Metab 94: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Rombach SM, Aerts JM, Poorthuis BJ, Groener JE, Donker-Koopman W, Hendriks E, et al. : Long-term effect of antibodies against infused alpha-galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One 7: e47805, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E: Serum-mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol 27: 256–264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kizhner T, Azulay Y, Hainrichson M, Tekoah Y, Arvatz G, Shulman A, et al. : Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metab 114: 259–267, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Shen JS, Busch A, Day TS, Meng XL, Yu CI, Dabrowska-Schlepp P, et al. : Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metab Dis 39: 293–303, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarting A, Dehout F, Feriozzi S, Beck M, Mehta A, Sunder-Plassmann G; European FOS Investigators: Enzyme replacement therapy and renal function in 201 patients with Fabry disease. Clin Nephrol 66: 77–84, 2006 [PubMed] [Google Scholar]
- 29.Schiffmann R, Askari H, Timmons M, Robinson C, Benko W, Brady RO, et al. : Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol 18: 1576–1583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, et al. : Fabry Disease Clinical Trial Study Group: Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med 146: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Beck M, Hughes D, Kampmann C, Larroque S, Mehta A, Pintos-Morell G, et al. : Fabry Outcome Survey Study Group: Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis. Mol Genet Metab Rep 3: 21–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampmann C, Perrin A, Beck M: Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: Cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis 10: 125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, et al. : Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52: 353–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffmann R, Swift C, Wang X, Blankenship D, Ries M: A prospective 10-year study of individualized, intensified enzyme replacement therapy in advanced Fabry disease. J Inherit Metab Dis 38: 1129–1136, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Lenders M, Canaan-Kühl S, Krämer J, Duning T, Reiermann S, Sommer C, et al. : Patients with Fabry disease after enzyme replacement therapy dose reduction and switch -2-year follow-up. J Am Soc Nephrol 27: 952–962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arends M, Biegstraaten M, Wanner C, Sirrs S, Mehta A, Elliott PM, et al. : Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study. J Med Genet 55: 351–358, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, et al. : Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Tøndel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, et al. : Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 24: 137–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najafian B, Tøndel C, Svarstad E, Sokolovkiy A, Smith K, Mauer M: One year of enzyme replacement therapy reduces globotriaosylceramide inclusions in podocytes in male adult patients with Fabry disease. PLoS One 11: e0152812, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skrunes R, Svarstad E, Kampevold Larsen K, Leh S, Tøndel C: Reaccumulation of globotriaosylceramide in podocytes after agalsidase dose reduction in young Fabry patients. Nephrol Dial Transplant 32: 807–813, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Skrunes R, Tøndel C, Leh S, Larsen KK, Houge G, Davidsen ES, et al. : Long-term dose-dependent agalsidase effects on kidney histology in Fabry disease. Clin J Am Soc Nephrol 12: 1470–1479, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck M, Ricci R, Widmer U, Dehout F, de Lorenzo AG, Kampmann C, et al. : Fabry disease: Overall effects of agalsidase alfa treatment. Eur J Clin Invest 34: 838–844, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO: Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant 21: 345–354, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Feriozzi S, Schwarting A, Sunder-Plassmann G, West M, Cybulla M; International Fabry Outcome Survey Investigators: Agalsidase alfa slows the decline in renal function in patients with Fabry disease. Am J Nephrol 29: 353–361, 2009 [DOI] [PubMed] [Google Scholar]
- 45.West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, et al. : Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol 20: 1132–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feriozzi S, Torras J, Cybulla M, Nicholls K, Sunder-Plassmann G, West M; FOS Investigators: The effectiveness of long-term agalsidase alfa therapy in the treatment of Fabry nephropathy. Clin J Am Soc Nephrol 7: 60–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiffmann R, Pastores GM, Lien YH, Castaneda V, Chang P, Martin R, et al. : Agalsidase alfa in pediatric patients with Fabry disease: A 6.5-year open-label follow-up study. Orphanet J Rare Dis 9: 169, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, et al. : International Fabry Disease Study Group: Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75: 65–74, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breunig F, Weidemann F, Strotmann J, Knoll A, Wanner C: Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int 69: 1216–1221, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, et al. : Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18: 1547–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Warnock DG, Ortiz A, Mauer M, Linthorst GE, Oliveira JP, Serra AL, et al. : Fabry Registry: Renal outcomes of agalsidase beta treatment for Fabry disease: Role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant 27: 1042–1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weidemann F, Krämer J, Duning T, Lenders M, Canaan-Kühl S, Krebs A, et al. : Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J Am Soc Nephrol 25: 837–849, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seydelmann N, Wanner C, Störk S, Ertl G, Weidemann F: Fabry disease and the heart. Best Pract Res Clin Endocrinol Metab 29: 195–204, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Baehner F, Kampmann C, Whybra C, Miebach E, Wiethoff CM, Beck M: Enzyme replacement therapy in heterozygous females with Fabry disease: Results of a phase IIIB study. J Inherit Metab Dis 26: 617–627, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, et al. : Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: A randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart 94: 153–158, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, et al. : Fabry Outcome Survey investigators: Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: An analysis of registry data. Lancet 374: 1986–1996, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Whybra C, Miebach E, Mengel E, Gal A, Baron K, Beck M, et al. : A 4-year study of the efficacy and tolerability of enzyme replacement therapy with agalsidase alfa in 36 women with Fabry disease. Genet Med 11: 441–449, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, et al. : Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: A prospective strain rate imaging study. Circulation 108: 1299–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Spinelli L, Pisani A, Sabbatini M, Petretta M, Andreucci MV, Procaccini D, et al. : Enzyme replacement therapy with agalsidase beta improves cardiac involvement in Fabry’s disease. Clin Genet 66: 158–165, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Imbriaco M, Pisani A, Spinelli L, Cuocolo A, Messalli G, Capuano E, et al. : Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: A prospective long-term cardiac magnetic resonance imaging study. Heart 95: 1103–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk S, et al. : Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation 119: 524–529, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Machann W, Breunig F, Weidemann F, Sandstede J, Hahn D, Köstler H, et al. : Cardiac energy metabolism is disturbed in Fabry disease and improves with enzyme replacement therapy using recombinant human galactosidase A. Eur J Heart Fail 13: 278–283, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, et al. : Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: Reversal by enzyme replacement therapy. Circulation 104: 1506–1512, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Moore DF, Altarescu G, Herscovitch P, Schiffmann R: Enzyme replacement reverses abnormal cerebrovascular responses in Fabry disease. BMC Neurol 2: 4, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenders M, Karabul N, Duning T, Schmitz B, Schelleckes M, Mesters R, et al. : Thromboembolic events in Fabry disease and the impact of factor V Leiden. Neurology 84: 1009–1016, 2015 [DOI] [PubMed] [Google Scholar]
- 66.El Dib R, Gomaa H, Ortiz A, Politei J, Kapoor A, Barreto F: Enzyme replacement therapy for Anderson-Fabry disease: A complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies. PLoS One 12: e0173358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore DF, Altarescu G, Ling GS, Jeffries N, Frei KP, Weibel T, et al. : Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke 33: 525–531, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Fellgiebel A, Gartenschläger M, Wildberger K, Scheurich A, Desnick RJ, Sims K: Enzyme replacement therapy stabilized white matter lesion progression in Fabry disease. Cerebrovasc Dis 38: 448–456, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Hoffmann B, Garcia de Lorenzo A, Mehta A, Beck M, Widmer U, Ricci R; FOS European Investigators: Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: Data from FOS (Fabry Outcome Survey). J Med Genet 42: 247–252, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann B, Beck M, Sunder-Plassmann G, Borsini W, Ricci R, Mehta A; FOS European Investigators: Nature and prevalence of pain in Fabry disease and its response to enzyme replacement therapy--a retrospective analysis from the Fabry Outcome Survey. Clin J Pain 23: 535–542, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Hilz MJ, Brys M, Marthol H, Stemper B, Dütsch M: Enzyme replacement therapy improves function of C-, Adelta-, and Abeta-nerve fibers in Fabry neuropathy. Neurology 62: 1066–1072, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Dehout F, Roland D, Treille de Granseigne S, Guillaume B, Van Maldergem L: Relief of gastrointestinal symptoms under enzyme replacement therapy [corrected] in patients with Fabry disease. J Inherit Metab Dis 27: 499–505, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann B, Schwarz M, Mehta A, Keshav S; Fabry Outcome Survey European Investigators: Gastrointestinal symptoms in 342 patients with Fabry disease: Prevalence and response to enzyme replacement therapy. Clin Gastroenterol Hepatol 5: 1447–1453, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Banikazemi M, Ullman T, Desnick RJ: Gastrointestinal manifestations of Fabry disease: Clinical response to enzyme replacement therapy. Mol Genet Metab 85: 255–259, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Wilcox WR, Feldt-Rasmussen U, Martins AM, Ortiz A, Lemay RM, Jovanovic A, et al. : Improvement of Fabry disease-related gastrointestinal symptoms in a significant proportion of female patients treated with agalsidase beta: Data from the Fabry Registry. JIMD Rep 38: 45–51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, et al. : The Mainz Severity Score Index: A new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 65: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Giannini EH, Mehta AB, Hilz MJ, Beck M, Bichet DG, Brady RO, et al. : A validated disease severity scoring system for Fabry disease. Mol Genet Metab 99: 283–290, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Mignani R, Pieruzzi F, Berri F, Burlina A, Chinea B, Gallieni M, et al. : FAbry STabilization indEX (FASTEX): An innovative tool for the assessment of clinical stabilization in Fabry disease. Clin Kidney J 9: 739–747, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warnock DG, Thomas CP, Vujkovac B, Campbell RC, Charrow J, Laney DA, et al. : Antiproteinuric therapy and Fabry nephropathy: Factors associated with preserved kidney function during agalsidase-beta therapy. J Med Genet 52: 860–866, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE: Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: A systematic review and meta-analysis: Effectiveness of ERT in different disease stages. J Inherit Metab Dis 37: 341–352, 2014 [DOI] [PubMed] [Google Scholar]
- 81.Amicus Therapeutics, 2018. Available at: http://www.galafoldamenabilitytable.com/hcp. Accessed July X, 2018.
- 82.Hughes DA, Nicholls K, Shankar SP, Sunder-Plassmann G, Koeller D, Nedd K, et al. : Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet 54: 288–296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashe KM, Budman E, Bangari DS, Siegel CS, Nietupski JB, Wang B, et al. : Efficacy of enzyme and substrate reduction therapy with a novel antagonist of glucosylceramide synthase for Fabry disease. Mol Med 21: 389–399, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guérard N, Oder D, Nordbeck P, Zwingelstein C, Morand O, Welford RWD, et al. : Lucerastat, an iminosugar for substrate reduction therapy: Tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin Pharmacol Ther 103: 703–711, 2018 [DOI] [PubMed] [Google Scholar]
- 85.Huang J, Khan A, Au BC, Barber DL, López-Vásquez L, Prokopishyn NL, et al. : Lentivector iterations and pre-clinical scale-up/toxicity testing: Targeting mobilized CD34+ cells for correction of Fabry disease. Mol Ther Methods Clin Dev 5: 241–258, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS; Kogenate Previously Untreated Patient Study Group: Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. N Engl J Med 328: 453–459, 1993 [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Lozier J, Johnson G, Kirshner S, Verthelyi D, Pariser A, et al. : Neutralizing antibodies to therapeutic enzymes: Considerations for testing, prevention and treatment. Nat Biotechnol 26: 901–908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Vries JM, van der Beek NAME, Kroos MA, Ozkan L, van Doorn PA, Richards SM, et al. : High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 101: 338–345, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Wilcox WR, Linthorst GE, Germain DP, Feldt-Rasmussen U, Waldek S, Richards SM, et al. : Anti-α-galactosidase A antibody response to agalsidase beta treatment: Data from the Fabry Registry. Mol Genet Metab 105: 443–449, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Smid BE, Hoogendijk SL, Wijburg FA, Hollak CE, Linthorst GE: A revised home treatment algorithm for Fabry disease: Influence of antibody formation. Mol Genet Metab 108: 132–137, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Nicholls K, Bleasel K, Becker G: Severe infusion reactions to fabry enzyme replacement therapy: Rechallenge after tracheostomy. JIMD Rep 5: 109–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aydin O, Kasapkara CS, Celik GE: Successful desensitization with agalsidase alfa in 2 brothers with Fabry disease. J Investig Allergol Clin Immunol 23: 367–368, 2013 [PubMed] [Google Scholar]
- 93. European Medicines Agency, 2001. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000369/WC500053612.pdf. Accessed August 3, 2018.
- 94. European Medicines Agency, 2001. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000370/WC500020547.pdf. Accessed August 3, 2018.
- 95.Tanaka A, Takeda T, Hoshina T, Fukai K, Yamano T: Enzyme replacement therapy in a patient with Fabry disease and the development of IgE antibodies against agalsidase beta but not agalsidase alpha. J Inherit Metab Dis 33[Suppl 3]: S249–S252, 2010 [DOI] [PubMed] [Google Scholar]
- 96.Wraith JE, Tylki-Szymanska A, Guffon N, Lien YH, Tsimaratos M, Vellodi A, et al. : Safety and efficacy of enzyme replacement therapy with agalsidase beta: An international, open-label study in pediatric patients with Fabry disease. J Pediatr 152: 563–570, 570.e1, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Bodensteiner D, Scott CR, Sims KB, Shepherd GM, Cintron RD, Germain DP: Successful reinstitution of agalsidase beta therapy in Fabry disease patients with previous IgE-antibody or skin-test reactivity to the recombinant enzyme. Genet Med 10: 353–358, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Doerfler PA, Nayak S, Corti M, Morel L, Herzog RW, Byrne BJ: Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol Ther Methods Clin Dev 3: 15053, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wachholz PA, Durham SR: Induction of ‘blocking’ IgG antibodies during immunotherapy. Clin Exp Allergy 33: 1171–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Wachholz PA, Soni NK, Till SJ, Durham SR: Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol 112: 915–922, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Strait RT, Morris SC, Finkelman FD: IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest 116: 833–841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lenders M, Schmitz B, Brand SM, Foell D, Brand E: Characterization of drug-neutralizing antibodies in patients with Fabry disease during infusion. J Allergy Clin Immunol 141: 2289–2292.e7, 2018 [DOI] [PubMed] [Google Scholar]
- 103.Lidove O, West ML, Pintos-Morell G, Reisin R, Nicholls K, Figuera LE, et al. : Effects of enzyme replacement therapy in Fabry disease--a comprehensive review of the medical literature. Genet Med 12: 668–679, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Mauhin W, Lidove O, Masat E, Mingozzi F, Mariampillai K, Ziza JM, et al. : Innate and adaptive immune response in Fabry disease. JIMD Rep 22: 1–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keating GM: Agalsidase alfa: A review of its use in the management of Fabry disease. BioDrugs 26: 335–354, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Bénichou B, Goyal S, Sung C, Norfleet AM, O’Brien F: A retrospective analysis of the potential impact of IgG antibodies to agalsidase beta on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab 96: 4–12, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Prabakaran T, Nielsen R, Larsen JV, Sørensen SS, Feldt-Rasmussen U, Saleem MA, et al. : Receptor-mediated endocytosis of α-galactosidase A in human podocytes in Fabry disease. PLoS One 6: e25065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garman RD, Munroe K, Richards SM: Methotrexate reduces antibody responses to recombinant human alpha-galactosidase A therapy in a mouse model of Fabry disease. Clin Exp Immunol 137: 496–502, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato Y, Ida H, Ohashi T: Anti-BlyS antibody reduces the immune reaction against enzyme and enhances the efficacy of enzyme replacement therapy in Fabry disease model mice. Clin Immunol 178: 56–63, 2017 [DOI] [PubMed] [Google Scholar]
- 110.Joly MS, Martin RP, Mitra-Kaushik S, Phillips L, D’Angona A, Richards SM, et al. : Transient low-dose methotrexate generates B regulatory cells that mediate antigen-specific tolerance to alglucosidase alfa. J Immunol 193: 3947–3958, 2014 [DOI] [PubMed] [Google Scholar]
- 111.Markic J, Polic B, Kuzmanic-Samija R, Marusic E, Stricevic L, Metlicic V, et al. : Immune modulation therapy in a CRIM-positive and IgG antibody-positive infant with Pompe disease treated with alglucosidase alpha: A case report. JIMD Rep 2: 11–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elder ME, Nayak S, Collins SW, Lawson LA, Kelley JS, Herzog RW, et al. : B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 163: 847–54.e1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banugaria SG, Prater SN, McGann JK, Feldman JD, Tannenbaum JA, Bailey C, et al. : Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: Lessons learned from Pompe disease. Genet Med 15: 123–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kazi ZB, Desai AK, Berrier KL, Troxler RB, Wang RY, Abdul-Rahman OA, et al. : Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI Insight 2: e94328, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lenders M, Oder D, Nowak A, Canaan-Kühl S, Arash-Kaps L, Drechsler C, et al. : Impact of immunosuppressive therapy on therapy-neutralizing antibodies in transplanted patients with Fabry disease. J Intern Med 282: 241–253, 2017 [DOI] [PubMed] [Google Scholar]
- 116.Brady RO, Murray GJ, Oliver KL, Leitman SF, Sneller MC, Fleisher TA, et al. : Management of neutralizing antibody to Ceredase in a patient with type 3 Gaucher disease. Pediatrics 100: E11, 1997 [DOI] [PubMed] [Google Scholar]
- 117.Banugaria SG, Prater SN, Patel TT, Dearmey SM, Milleson C, Sheets KB, et al. : Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: A step towards improving the efficacy of ERT. PLoS One 8: e67052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markic J, Polic B, Stricevic L, Metlicic V, Kuzmanic-Samija R, Kovacevic T, et al. : Effects of immune modulation therapy in the first Croatian infant diagnosed with Pompe disease: A 3-year follow-up study. Wien Klin Wochenschr 126: 133–137, 2014 [DOI] [PubMed] [Google Scholar]
- 119.Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS: Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med 360: 194–195, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Banugaria SG, Patel TT, Mackey J, Das S, Amalfitano A, Rosenberg AS, et al. : Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: Need for agents to target antibody-secreting plasma cells. Mol Genet Metab 105: 677–680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Warnock DG, Bichet DG, Holida M, Goker-Alpan O, Nicholls K, Thomas M, et al. : Oral migalastat HCl leads to greater systemic exposure and tissue levels of active α-galactosidase A in Fabry patients when co-administered with infused agalsidase. PLoS One 10: e0134341, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruderfer I, Shulman A, Kizhner T, Azulay Y, Nataf Y, Tekoah Y, et al. : Development and analytical characterization of pegunigalsidase alfa, a chemically cross-linked plant recombinant human α-galactosidase-A for treatment of Fabry disease. Bioconjug Chem 29: 1630–1639, 2018 [DOI] [PubMed] [Google Scholar]
- 123.Kytidou K, Beekwilder J, Artola M, van Meel E, Wilbers RHP, Moolenaar GF, et al. : Nicotiana benthamiana α-galactosidase A1.1 can functionally complement human α-galactosidase A deficiency associated with Fabry disease J Biol Chem 293:10042–10058, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hunley TE, Corzo D, Dudek M, Kishnani P, Amalfitano A, Chen YT, et al. : Nephrotic syndrome complicating alpha-glucosidase replacement therapy for Pompe disease. Pediatrics 114: e532–e535, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Debiec H, Valayannopoulos V, Boyer O, Nöel LH, Callard P, Sarda H, et al. : Allo-immune membranous nephropathy and recombinant aryl sulfatase replacement therapy: A need for tolerance induction therapy. J Am Soc Nephrol 25: 675–680, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 127.Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, et al. : Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26: 302–313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]