Abstract
Background Complement-fixing antibodies against donor HLA are considered a contraindication for kidney transplant. A modification of the IgG single-antigen bead (SAB) assay allows detection of anti-HLA antibodies that bind C3d. Because early humoral graft rejection is considered to be complement mediated, this SAB-based technique may provide a valuable tool in the pretransplant risk stratification of kidney transplant recipients.
Methods Previously, we established that pretransplant donor-specific anti-HLA antibodies (DSAs) are associated with increased risk for long-term graft failure in complement-dependent cytotoxicity crossmatch-negative transplants. In this study, we further characterized the DSA-positive serum samples using the C3d SAB assay.
Results Among 567 pretransplant DSA-positive serum samples, 97 (17%) contained at least one C3d-fixing DSA, whereas 470 (83%) had non–C3d-fixing DSA. At 10 years after transplant, patients with C3d-fixing antibodies had a death-censored, covariate-adjusted graft survival of 60%, whereas patients with non–C3d-fixing DSA had a graft survival of 64% (hazard ratio, 1.02; 95% confidence interval, 0.70 to 1.48 for C3d-fixing DSA compared with non–C3d-fixing DSA; P=0.93). Patients without DSA had a 10-year graft survival of 78%.
Conclusions The C3d-fixing ability of pretransplant DSA is not associated with increased risk for graft failure.
Keywords: chronic allograft failure, kidney transplantation, anti-HLA antibodies, complement-fixing antibodies
The presence of complement-fixing antibodies against donor HLA before transplantation is considered a contraindication for kidney transplantation. HLA antibody detection by single-antigen bead (SAB) assays is much more sensitive than detection by complement-dependent cytotoxicity (CDC) crossmatch (XM), and it allows for more detailed definition of antibody specificity. Because not all SAB-defined anti-HLA antibodies seem to be clinically relevant, further characterization of donor-specific anti-HLA antibodies (DSAs) is required for better risk stratification in individual kidney transplant recipients. Modifications of the IgG SAB assay allow for detection of complement-fixing HLA antibodies binding C1q,1 C4d,2 and C3d.3 Loupy et al.4 showed that the C1q-fixing ability of DSA is strongly associated with short-term kidney graft loss after kidney transplant but not pretransplant. The risk of graft loss according to the DSA-C1q status at day 0 and the status after transplantation revealed that patients with C1q-binding DSA after transplantation (n=77) had the highest risk of graft loss compared with patients with pretransplant C1q-binding DSA (n=22). Their study has gained considerable attention for SAB assays detecting complement-fixing HLA antibodies, because the authors show that this technique can be added to a risk model to identify patients at risk for graft failure. Studies defining the potential of C3d as a marker of complement fixation are scarce and include low numbers of patient sera.5–7 In the context of the Dutch Profiling Consortium of Antibody Repertoire and Effector functions Study, we investigated whether the C3d assay could aid in pretransplant risk stratification.
Methods
Study Population
In this Dutch multicenter study, we included 6097 kidney transplantations performed with a negative CDC-XM between January 1995 and December 2005 (Figure 1). In that era, historic cytotoxic HLA antibodies were assigned as unacceptable for allocation within the Eurotransplant region. SAB assay–defined DSAs were not available; they were not considered as risk factors in the matching procedure at that time, and therefore, they had no influence on allocation or immunosuppressive treatment. The use of sera and experimental protocols were approved by the Research Ethics Committee for Biobanks and the Medical Ethics Committee of the University Medical Center Utrecht, and experimental protocols were performed in accordance with the Foundation Federation of Dutch Medical Scientific Societies Code of Conduct. The study was conducted in accordance with the 2013 Declaration of Helsinki and the 2008 Declaration of Istanbul.
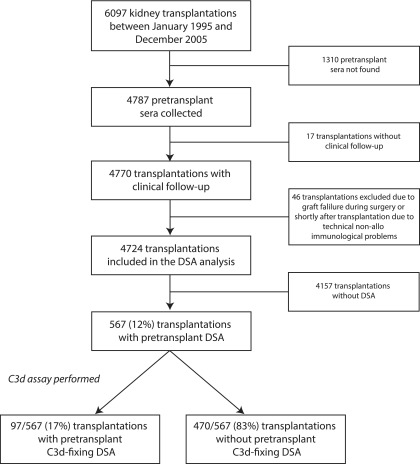
Figure 1.
Description of the study cohort for which the C3d single-antigen bead assay was performed. Among 567 pretransplant DSA-positive serum samples, 97 (17%) contained at least one C3d-fixing DSA, whereas 470 (83%) had non–C3d-fixing DSA. DSA, donor-specific anti-HLA antibody.
Clinical data were obtained from the Netherlands Organ Transplant Registry (NOTR). We retrospectively collected 4787 (78%) of the pretransplant sera; 17 transplantations were lost to follow-up by the NOTR, and 46 transplantations were excluded, because the kidney failed during surgery or shortly thereafter due to technical nonimmunologic problems. Thus, we included 4724 transplantations as described elsewhere (Figure 1).8
Detection and Characterization of Anti-HLA Antibodies
First, heat-inactivated sera long-term stored at −20°C or colder were passed through a 96-well 1.2-μm MultiScreen filter plate (Millipore, Billerica, MA) to clear debris, and we retrospectively determined the presence of anti-HLA antibodies in the pretransplant sera in one central laboratory as described previously.8,9 Bead positivity was defined according to the manufacturer’s instructions, requiring a minimum signal-to-background ratio to be reached (described in ref. 9), which leads to virtually identical results to an absolute Median Fluorescence Intensity (MFI) cutoff of 750. The presence of SAB DSA was assigned by comparing the SAB HLA-A, -B, -DR, and -DQ antibody specificities on serologic level with the split-level HLA typing of the donor. Second, only the sera with DSA were analyzed further for the presence of C3d-fixing DSA using the SAB C3d assay (Immucor, Herentals, Belgium) (described in Supplemental Material). Beads were defined as positive when two or more of the three adjusted values were above the cutoff level according to the manufacturers’ recommendations (described in Supplemental Material). Immucor donated reagents but was not involved in either the conduct of the study or the preparation of the manuscript.
Statistical Analyses
Differences in patient, donor, and transplant characteristics between the no DSA group, the non–C3d-fixing group, and the C3d-fixing DSA group were assessed by the chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables. Death-censored graft survival was assessed using the adjusted Kaplan–Meijer estimator (AKME) on the basis of inverse probability weighting.10 Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were derived using multivariable Cox regression. We adjusted in both the AKME and Cox regression for recipient age (quadratic) and donor age (quadratic), donor type (living or deceased), cold ischemia time (for donation after brain death and donation after cardiac death), time on dialysis in years (quadratic), and induction therapy with IL-2 receptor blocker. More detailed descriptions are in the work by Kamburova et al.8 Statistical analyses were performed with R (version 3.2.2) and SAS (version 9.4; SAS Institute, Cary, NC) software.
Results
In this study, 4724 transplantations were included for DSA analysis (Figure 1).8 The median follow-up time for all transplantations was 11.4 years, with a maximum of 21.2 years. In this study, we performed additional antibody characterization in the 567 transplantations with pretransplant DSA against HLA-A, -B, -DR, and -DQ antigens. In 97 of 567 (17%) transplantations with DSA, one or more of the DSA specificities were C3d fixing, and in 470 of 567 (83%) of the transplantations with DSA, there was no C3d-fixing activity. Characteristics of patient, donor, and transplantation as stratified according to the presence of C3d-fixing DSA are summarized in Table 1. The mean recipient and donor ages, type of donor, number of HLA-A/B/DR broad mismatches, use of induction therapy, and initial immunosuppression were comparable between patients with C3d-fixing DSA and those with non–C3d-fixing DSA. Patients in the C3d-fixing DSA group were more often women and had a higher percentage of CDC panel reactive antibody. Moreover, the proportion of retransplantations was higher in the C3d-fixing DSA group.
Table 1.
Patient, donor, and transplant characteristics
| Characteristics | Non–C3d-Fixing DSA, n=470 | C3d-Fixing DSA, n=97 | P Value | Total DSA Cohort, n=567 |
|---|---|---|---|---|
| Patient | ||||
| Age at transplantation, yr, mean±SD | 44.5±14.1 | 42.7±12.8 | 0.16a | 44.2±13.9 |
| Women, no. (%) | 286 (60.9) | 47 (48.5) | 0.02b | 333±58.7 |
| CDC-PRA at the time of transplantation, %, mean±SD | 22.3±29.6 | 34.6±33.8 | <0.001a | 24.4±30.7 |
| Highest CDC-PRA (%, mean±SD) | 42.7±36.5 | 48.1±35.5 | 0.16a | 43.6±36.4 |
| Dialysis, no. (%) | 0.09b | |||
| No | 38 (8.1) | 5 (5.2) | 43 (7.6) | |
| Yes: hemodialysis | 279 (59.4) | 53 (54.6) | 332 (58.6) | |
| Yes: peritoneal dialysis | 150 (31.9) | 36 (37.1) | 186 (32.8) | |
| Unknown | 3 (0.6) | 3 (3.1) | 6 (1.1) | |
| Time on dialysis, yr, mean±SD | 3.4±2.9 | 3.4±3.2 | 0.68a | 3.4±3.0 |
| Donor | ||||
| Age, yr, mean±SD | 43.8±15.8 | 44.7±16 | 0.58a | 44.0±15.8 |
| Women, no. (%) | 208 (44.3) | 50 (51.6) | 0.19b | 258±45.5 |
| Type of donor, no (%) | 0.49b | |||
| Living | 116 (24.7) | 21 (21.7) | 137±24.2 | |
| Deceased: DBD | 292 (62.1) | 59 (60.8) | 351±61.9 | |
| Deceased: DCD | 62 (13.2) | 17 (17.5) | 79±13.9 | |
| Cold ischemia time, h, mean±SD | ||||
| Deceased donors | 22.9 (6.7) | 22.4 (7.5) | 0.24a | 22.8 (6.8) |
| Living donors | 2.4 (0.9) | 2.9 (1.4) | 0.23a | 2.5 (1) |
| Transplant | ||||
| Retransplantation, no. (%) | 204 (43.4) | 66 (68.0) | <0.001b | 270±47.6 |
| HLA-A/B/DR broad mismatches, mean±SD | 2.5±1.3 | 2.3±1.4 | 0.39a | 2.4±1.3 |
| Induction therapy | ||||
| IL-2 receptor blocker, no. (%) | 85 (18.1) | 24 (24.7) | 0.13b | 109±19.2 |
| T cell–depleting antibody,c no. (%) | 36 (7.7) | 3 (3.1) | 0.11b | 39±6.9 |
| Initial immunosuppression, no. (%) | ||||
| Steroids | 457 (97.2) | 90 (92.8) | 0.03b | 547±96.5 |
| MMF/azathioprine | 362 (77.0) | 80 (82.5) | 0.24b | 442±78 |
| Cyclosporin/tacrolimus | 451 (94.3) | 91 (93.8) | 0.35b | 542±95.6 |
| Sirolimus | 24 (5.1) | 2 (2.1) | 0.19b | 26±4.6 |
| Other | 45 (9.6) | 8 (8.3) | 0.19b | 53±9.4 |
| Unknown | 0 (0) | 3 (3.1) | <0.001b | 3±0.5 |
DSA, donor-specific anti-HLA antibody; CDC, complement-dependent cytotoxicity; PRA, panel reactive antibody; DBD, donation after brain death; DCD, donation after cardiac death; MMF, mycophenolate mofetil.
Mann–Whitney U test for continuous variables.
Chi-squared test for categorical variables.
T cell–depleting antibody therapy: ALG, ATG, OKT3 mAb.
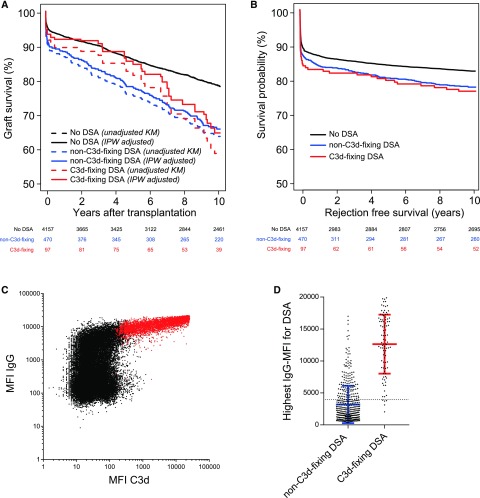
The AKME showed 10-year death-censored graft survival rates of 78% (95% CI, 74% to 81%) for the 4157 patients without DSA, 64% (95% CI, 62% to 66%) for the 470 of 567 patients with non–C3d-fixing DSA, and 60% (95% CI, 49% to 70%) for the 97 patients with C3d-fixing DSA in pretransplant serum (Figure 2A). The multivariable analysis, also adjusted for the same covariables, showed that the risk of graft failure was comparable for C3d-fixing and non–C3d-fixing DSA (HR, 1.02; 95% CI, 0.70 to 1.48 for C3d-fixing DSA compared with non–C3d-fixing DSA; P=0.93). At 5 years after transplant, the HR was 0.69 (95% CI, 0.41 to 1.17) for the C3d-fixing DSA compared with non–C3d-fixing DSA (P=0.17). Similar results were observed in separate AKME analyses for living and deceased donor transplantations (data not shown).
Figure 2.
Pretransplant C3d-fixing Donor-specific anti-HLA Antibodies are not associated with increased risk for kidney graft failure. Graft survival of kidney transplants and MFI values according to the presence of pretransplant C3d-fixing donor-specific anti-HLA antibody (DSA). (A) Adjusted Kaplan–Meier estimate (AKME) for death-censored graft survival according to the presence of pretransplant C3d-fixing DSA for the total cohort (n=4724). AKME was adjusted for the following covariates: recipient age (quadratic) and donor age (quadratic), donor type (living or deceased), cold ischemia time (for donation after brain death and donation after cardiac death), time on dialysis in years (quadratic), and induction therapy with IL-2 receptor blocker. The use of different MFI cutoffs for DSA positivity resulted in comparable effects on long-term graft survival (the work by Kamburova et al.8). In this cohort, we have a total of 97 transplants with C3d-fixing DSA: 86 transplants have one isolated C3d-fixing DSA, nine transplants have two C3d-fixing DSAs, and two transplants have three C3d-fixing DSAs. (B) Rejection-free survival according to the presence of pretransplant C3d-fixing DSA for the total cohort. (C) The MFI values for both the IgG and C3d assay are plotted for all 51,646 measured single-antigen beads. The C3d-positive beads as determined by the manufacturer’s instructions are depicted in red. (D) The MFI of the highest DSA is plotted according to patients’ C3d status. The means±SD are plotted for the non–C3d-fixing and C3d-fixing DSA groups in blue and red, respectively. The dotted line is set at an absolute MFI of 4000. IPW, inverse probability weighting; KM, Kaplan–Meier.
Unfortunately, rejection data available for this consortium study were limited to treatment for rejection episodes with the date and whether a biopsy was performed. Using these data, we observed that the rejection-free survival of patients with C3d-fixing DSA is comparable with that in the non–C3d-fixing DSA group (Figure 2B). In addition, we investigated graft function at month 3 and years 1, 3, 5, and 10 after transplant. Proteinuria and serum creatinine levels were comparable between the non–C3d-fixing and C3d-fixing DSA groups (Supplemental Table 1).
Because previous studies suggested that binding capacity of C3d is correlated with the MFI of the pan-IgG, we plotted the MFI values of both assays for all 51,646 beads (Figure 2B). According to the manufacturer’s instructions, the determination of C3d-positive beads was on the basis of different ratios using the negative control serum (described in Supplemental Material). The MFI values of the C3d assay correlated with the MFI values of the IgG assay, with the highest IgG MFI values for C3d-positive beads (depicted in red in Figure 2). For 92 of 97 (95%) C3d-positive sera, the highest IgG MFI for any of the DSA was 4000 or more (Figure 2C). However, only 4762 of 8461 (56%) of the beads with an IgG MFI of 4000 or higher were C3d positive. Consequently, the C3d-binding capacity correlated with the IgG MFI, but positivity cannot be completely predicted on the basis of the MFI values of the IgG assay, and a clear cutoff could not be defined.
DISCUSSION
In this study, we show that the C3d-fixing ability of pretransplant SAB-defined DSA in transplantations with a negative CDC-XM is not associated with the risk of long-term kidney graft loss. In another retrospective analysis of 48 pretransplant sera from kidney transplant recipients with negative CDC-XM, the presence of C1q-binding DSA was not associated with antibody-mediated rejection or graft loss in the first 4 years after transplantation.11 In a small Dutch cohort, no difference in graft survival between patients with or without C1q-fixing pretransplant DSA was observed.12 In a third study including 15 transplantations with C1q-positive DSA and 13 with C1q-negative DSA, the C1q-binding status was not able to predict acute rejection, renal function, or graft loss after 5 years.13 Taken together, an effect of pretransplant in vitro complement-fixing antibodies, as detected by SAB C1q or C3d assays, on graft survival has not been shown so far.
In contrast, several studies showed a negative effect on graft survival of de novo DSA, mostly found at the time of a rejection episode.14–17 Loupy et al.4 studied the risk of kidney graft loss according to the C1q-binding status at time of transplantation and after transplantation. A significant effect on graft survival was only shown for C1q-fixing DSA detected after transplantation. Assessment of C1q- and C3d-fixing de novo DSA in pediatric kidney transplantations revealed that C3d-fixing DSA (and not C1q-fixing DSA) was associated with decreased kidney function after transplantation.16 At the time of detection of de novo DSA, the 5-year kidney graft survival was shown to be significantly lower when DSAs were C3d fixing, especially if the C3d-fixing DSAs were detected against both HLA classes 1 and 2 antigens.17 In comparison with all published studies that used the Lifecodes C3d assay to determine C3d-fixing DSA in patients with kidney transplants, we found a lower percentage of patients who were C3d positive. This difference could be due to patient selection, time of serum sample, and/or IgG DSA positivity cutoff (Supplemental Table 2). There might be a difference in the effect of C3d-fixing DSA pretransplant versus post-transplant, because de novo DSAs seem to be more detrimental, and if these are also C3d fixing, this might increase the risk of graft failure.
Detection of C3d may be a more valid reflection of in vivo complement activation compared with detection of C1q, because C3d is positioned downstream in the complement cascade. However, there are multiple factors that regulate complement activation: antigen expression and density, antibody titer, avidity, subclass, glycosylation, and local complement concentration.18 In addition, the prevalence of C3d-fixing DSA may be higher post-transplant compared with pretransplant. Others have shown that post-transplant C3d-fixing DSAs are associated with increased risk of graft failure. Although we have analyzed pretransplant sera for the presence of C3d-fixing DSA in a much larger cohort compared with previous studies, our findings do not indicate that the C3d-binding status of DSA at the time of transplantation can contribute to risk stratification of renal transplant recipients.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by research funding from Dutch Kidney Foundation project code CP12.23 (risk assessment of kidney graft failure by HLA antibody profiling).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018020205/-/DCSupplemental.
References
- 1.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, et al. : Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant 30: 158–163, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lachmann N, Todorova K, Schulze H, Schönemann C: Systematic comparison of four cell- and Luminex-based methods for assessment of complement-activating HLA antibodies. Transplantation 95: 694–700, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Visentin J, Vigata M, Daburon S, Contin-Bordes C, Fremeaux-Bacchi V, Dromer C, et al. : Deciphering complement interference in anti-human leukocyte antigen antibody detection with flow beads assays. Transplantation 98: 625–631, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen J-P, Mooney N, et al. : Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. : Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26: 457–467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, et al. : Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitized pediatric kidney recipients. Am J Transplant 16: 2106–2116, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Eskandary F, Bond G, Kozakowski N, Regele H, Marinova L, Wahrmann M, et al. : Diagnostic contribution of donor-specific antibody characteristics to uncover late silent antibody-mediated rejection-results of a cross-sectional screening study. Transplantation 101: 631–641, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. : Differential effects of donor-specific HLA antibodies in living versus deceased donor transplant [published online ahead of print February 21, 2018]. Am J Transplant doi:10.1111/ajt.14709. [DOI] [PMC free article] [PubMed]
- 9.Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. : How can we reduce costs of solid-phase multiplex-bead assays used to determine anti-HLA antibodies? HLA 88: 110–119, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Liu C: Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 24: 3089–3110, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Thammanichanond D, Wiwattanathum P, Mongkolsuk T, Kantachuvesiri S, Worawichawong S, Vallipakorn SA, et al. : Role of pretransplant complement-fixing donor-specific antibodies identified by C1q assay in kidney transplantation. Transplant Proc 48: 756–760, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Otten HG, Verhaar MC, Borst HPE, Hené RJ, van Zuilen AD: Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant 12: 1618–1623, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Crespo M, Torio A, Mas V, Redondo D, Pérez-Sáez MJ, Mir M, et al. : Clinical relevance of pretransplant anti-HLA donor-specific antibodies: Does C1q-fixation matter? Transpl Immunol 29: 28–33, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Freitas MCS, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, et al. : The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation 95: 1113–1119, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB: Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant 16: 12–17, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Shaw O, Martin C, Michaelides G, Balasubramaniam R, Sebire NJ, et al. : Clinical risk stratification of paediatric renal transplant recipients using C1q and C3d fixing of de novo donor-specific antibodies. Pediatr Nephrol 33: 167–174, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelletier RP, Balazs I, Adams P, Rajab A, DiPaola NR, Henry ML: Clinical utility of C3d binding donor-specific anti-human leukocyte antigen antibody detection by single antigen beads after kidney transplantation-a retrospective study. Transpl Int 31: 424–435, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Lan JH, Tinckam K: Clinical utility of complement dependent assays in kidney transplantation. Transplantation 102[Suppl 1]: S14–S22, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.