Abstract
Background
Pseudoxanthoma elasticum (PXE) is a genetic disease caused by mutations in the ABCC6 gene that result in low pyrophosphate levels and subsequent progressive soft tissue calcifications. PXE mainly affects the skin, retina, and arteries. However, many patients with PXE experience kidney stones. We determined the prevalence of this pathology in patients with PXE and examined the possible underlying mechanisms in murine models.
Methods
We conducted a retrospective study in a large cohort of patients with PXE and analyzed urine samples and kidneys from Abcc6−/− mice at various ages. We used Yasue staining, scanning electron microscopy, electron microscopy coupled to electron energy loss spectroscopy, and Fourier transform infrared microspectroscopy to characterize kidney calcifications.
Results
Among 113 patients with PXE, 45 (40%) had a past medical history of kidney stones. Five of six computed tomography scans performed showed evidence of massive papillary calcifications (Randall plaques). Abcc6−/− mice spontaneously developed kidney interstitial apatite calcifications with aging. These calcifications appeared specifically at the tip of the papilla and formed Randall plaques similar to those observed in human kidneys. Compared with controls, Abcc6−/− mice had low urinary excretion of pyrophosphate.
Conclusions
The frequency of kidney stones and probably, Randall plaque is extremely high in patients with PXE, and Abcc6−/− mice provide a new and useful model in which to study Randall plaque formation. Our findings also suggest that pyrophosphate administration should be evaluated for the prevention of Randall plaque and kidney stones.
Keywords: calcium, genetic renal disease, mineral metabolism
Pseudoxanthoma elasticum (PXE; Online Mendelian Inheritance in Man 264800, prevalence 1/25,000–1/50,000) is an autosomal recessive disease resulting from mutations in ABCC6 gene that encodes an ATP binding cassette transporter mainly expressed in the liver and to a lesser extent, the kidney.1,2 This disease is characterized by progressive calcification with destruction of the elastic fibers in the skin, retinal Bruch membrane, and the medial layers of large- and medium-sized peripheral arteries.
Recent studies have suggested that the hepatic ABCC6 transporter facilitates the release of extracellular ATP, which is quickly converted to inorganic pyrophosphate (PPi) by ectonucleotide pyrophosphatase phosphodiesterase-1 in the hepatic vasculature. This process is the main source of circulating PPi, a physiologic anticalcifying molecule that counteracts the effect of inorganic phosphate on hydroxyapatite crystal growth.3 Patients with PXE as well as Abcc6−/− mice have a reduced plasma PPi level, explaining, at least in part, their mineralization disorder.4–6
Renal involvement in PXE has been poorly documented in the literature, and only sporadic cases of kidney stones in patients with PXE have been reported.7,8 The presence of typical nephrocalcinosis has been reported in one patient with PXE.9 Recently, data from a PXE cohort suggested that nephrolithiasis was an unrecognized but a prevalent feature of PXE, affecting at least 10% of patients with PXE.10 Nevertheless, the prevalence and features of nephrolithiasis have never been specifically assessed.
To better characterize the renal calcifications affecting patients with PXE, we conducted a retrospective study in one of the largest cohorts of patients with PXE. We focused on the prevalence of kidney tissue calcifications and kidney stones. In addition, urine samples and kidney tissues from Abcc6−/− mice were analyzed to better understand the pathophysiologic mechanisms underlying the development of kidney calcifications in a mouse modeling the human pathology.
Methods
Study Population
A national survey was conducted in 164 patients with PXE from the PXE Consultation Center of the University Hospital of Angers, France from December 2016 to June 2017.
The diagnosis of PXE was on the basis of a combination of established criteria for indisputable PXE, including clinically suggestive skin lesions, angioid streaks, and histologically proven fragmented and calcified elastic fibers on skin biopsy.11 Moreover, ABCC6 genotyping was systematically performed, and all exons of the ABCC6 gene have been sequenced to confirm the diagnosis.12 Sequence changes were identified in about 85% of alleles. This mutation rate is superimposable to the rate reported in most large cohorts.
The patients with PXE were contacted by mail to complete a self-questionnaire. All patients with a typical PXE were eligible for inclusion. All patients gave informed written consent, and the study was approved by the local ethics committee (CPP Ouest II, Angers, France) and registered at ClinicalTrials.gov (identifier: NCT01446393).
Six patients with PXE who previously experienced renal colic had a computed tomography (CT) scan performed to evaluate the presence of remnant stones and Randall plaque in various radiology units, and five of them also had urine and blood sample analyses to assess kidney stone risk factors as recommended by the French Urological Association. Patients sent CT scans and biologic data to Angers reference center managers.
Data Collection
The presence of kidney stones and/or nephrocalcinosis, past medical history of kidney stones, renal colic, and surgical procedures were recorded. Familial past medical history and stone analysis were also collected when available. Age, height, and weight were collected. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation by using the last available plasma creatinine.13
Animal Studies
Mice with a genetically invalidated Abcc6 gene are designated as Abcc6tm1Aabb. They were generated on a 129/Ola background and backcrossed into a C57Bl/6J background more than ten times in Professor A.A. Bergen’s laboratory.14 These mice, which were maintained at Angers University Mouse Facility (France) and the John A. Burns School of Medicine, University of Hawaii, are herein designated Abcc6−/−.
The development of renal calcifications was assessed in 15 Abcc6+/+ and 17 Abcc6−/− female mice housed and bred in similar conditions (five mice per cage) at the Angers University Mouse Facility. All animal procedures were performed in accordance with the European Union and National Institutes of Health guidelines for the care and use of laboratory animals and in accordance with the local ethics committee (CEEA.2012.21). Urine PPi, calcium, and phosphate excretion were assessed in nine Abcc6+/+ and eight Abcc6−/− female mice (12 months old). Urine was collected by using metabolic cages with free access to water for 24 hours.
In addition, a cDNA encoding the normal human ABCC6 and a PXE-causing mutant ABCC6 cDNA (R1314W) were cloned into delivery plasmids used for transgenic animal production, and transgenic mice expressing the normal human ABCC6 protein and the PXE-causing R1314W mutant in the liver were generated at the University of Hawaii as previously decribed.15
Study of the Human Papillae
Papillae from healthy parts of human kidneys removed for cancer have been analyzed as described in a previous study.16 This study showed that many patients are affected by incipient Randall plaque, even in the absence of kidney stones. Patients gave written consent. Papillae were anonymously collected, and no data relative to patients have been recorded in accordance with French legislation and the Helsinki Declaration for Patient Safety.
Morphoconstitutional Analysis of Renal Stones
Morphologic examination and classification of renal stone surface and section were combined with Fourier transform infrared spectroscopy (FTIR; Bruker).
Histology and Yasue Staining of Renal Tissue
Human and mice kidney tissues were fixed in 4% formalin and embedded in paraffin. Four-micrometer tissue sections were performed and stained by Yasue procedure to reveal tissue calcifications. Eosin staining was also performed in addition to Yasue staining to identify red blood cells and vasa recta. The mice papilla calcification ratio was assessed by isolating the papilla (1.3 mm from the tip) and performing morphometry by Analysis Software (proportion of calcified tissue to whole papilla).
Field Emission Scanning Electron Microscopy
Tissue sections (4 μm) were investigated with a Zeiss SUPRA55VP field emission scanning electron microscope. Measurements were performed at a low voltage (1.4 keV).
Transmission Electron Microscopy
Small pieces of papilla tip were fixed in 2.5% glutaraldehyde in 0.1 mmol/L cacodylate buffer (pH 7.4) at 4°C. Fragments were then postfixed in 1% osmium tetroxide, dehydrated using graded alcohol series, and embedded in epoxy resin. Semithin sections (0.5 μm) were stained using toluidine blue. Ultrastructure sections (80 nm) were contrast enhanced using uranyl acetate and lead citrate, and they were examined using a JEOL 1010 electron microscope (JEOL, Ltd., Tokyo, Japan) with a MegaView III camera (Olympus Soft Imaging Systems GmbH, Munster, Germany).
Electron Energy Loss Spectroscopy Experiments
Electron energy loss spectroscopy experiments were performed in a VGHB01 scanning transmission electron microscope equipped with a cold field emission gun operated at 100 keV. Electron energy loss spectroscopy data were collected on a low-noise/low-temperature charge-coupled device camera (Princeton Instruments) optically coupled to a scintillator in the image plane of a Gatan 666 magnetic sector. The sample was cooled down to liquid nitrogen temperature using a homemade cryostage.
FTIR Microspectroscopy
Microcalcifications were characterized using Fourier transform infrared microspectroscopy. Tissue sections (4 μm) were deposited on low-emission microscope slides (MirrIR; Keveley Technologies, Tienta Sciences, Indianapolis, IN). FTIR hyperspectral images were recorded with a Spectrum spotlight 400 FT-IR Imaging System (Perkin Elmer Life Sciences, Courtaboeuf, France), with a spatial resolution of 6.25 μm and a spectral resolution of 8 cm−1. Each spectral image covering a substantial part of the tissue consisted of about 30,000 spectra.
Mice Urine Assays: PPi Assay
Freshly collected urine samples were centrifuged (10 minutes at 20,000×g at 4°C), and supernatant was diluted (1/10 in distilled water) and frozen until use. To quantify PPi, we used ATP sulfurylase to convert PPi into ATP in the presence of excess of adenosine 5′ phosphosulfate (A5508; Sigma-Aldrich). Generated ATP was subsequently quantified using the ATP Determination Kit (A22066; Molecular Probes) according to the manufacturer’s instructions.
Urinary creatinine and phosphate were assessed on an ISYS analyzer from ImmunoDiagnostic Systems. Calcium urinary levels were measured with the Perkin Elmer 3300 Atomic Absorption Spectrometer.
Statistical Analyses
Clinical data are presented as mean (SD), median (25th–75th percentiles), and percentages, and they were compared with t tests and chi-squared tests. Experimental data are presented as mean (SEM) and were compared with nonparametric (Mann–Whitney) tests. A P value <0.05 was considered significant for all statistics.
Results
High Prevalence of Kidney Stones in Patients with PXE
Among the 170 patients participating in the Angers PXE cohort, 164 received the questionnaire, and 113 answered the survey (men: 33; women: 80). Among these 113 patients, 45 (39.8%) had a past medical history of renal colic and/or kidney stones. Forty-one patients had kidney stones, and four patients had renal colic without evidence of kidney stone by imaging. Twenty-eight patients (24.7%) reported at least one case of kidney stone disease in their close family. Kidney stone former and nonkidney stones former characteristics, including estimated renal function (eGFR by the CKD-EPI formula), are summarized in Table 1. There was no significant difference between the two groups.
Table 1.
Characteristics and eGFR (the Chronic Kidney Disease Epidemiology Collaboration formula) in patients with pseudoxanthoma elasticum affected or not affected by kidney stones
| General Characteristics of Patients with PXE | All Patients with PXE Who Filled Out the Questionnaire, n=113 | Patients with Medical History of Kidney Stone, n=45 | Patients without Medical History of Kidney Stone, n=68 |
|---|---|---|---|
| Age, yr | 50.1±16.5 | 50.3±12.5 | 49.9±18.8 |
| Sex, men/women | 33/80 (29% men) | 13/32 (29% men) | 20/48 (29% men) |
| Weight, kg | 67.6±17.5 | 69.9±21.0 | 66.1±14.8 |
| Height, cm | 163.6±9.1 | 164.8±8.8 | 162.9±9.4 |
| Plasma creatinine, μmol/L | 66.1±12.2 | 68.4±12.1 | 64.5±12.2 |
| CKD-EPIcr, ml/min per 1.73m2 | 98.6±17.8 | 96±14.4 | 100.2±19.7 |
Data are mean±SD. PXE, pseudoxanthoma elasticum; CKD-EPIcr, eGFR by the Chronic Kidney Disease Epidemiology Collaboration formula.
Study of the Renal Calcifications and Urine Biochemistry in Patients with PXE
Six patients with PXE from the Angers cohort who previously experienced renal colic had a CT scan to assess disease evolution. Urine biochemistry was analyzed in five of these six kidney stone formers. None of these patients had remnant kidney stones, but five patients had papillary calcifications (Randall plaque) as evidenced by CT scan (Figure 1, A–D). One patient had both papillary calcifications and cortical and medullary calcifications (incipient nephrocalcinosis) (Figure 1E). Urine biochemistry showed mild hypercalciuria in some patients (median, 237 [122–277] mg/d; normal value <250 mg/d), normal urine phosphate excretion (median, 1020 [626–1067] mg/d), normal urine oxalate excretion (median, 0.36 [0.20–0.41] mmol/d), and intermediate urine pH (median, 6.2 [6.0–6.9]).
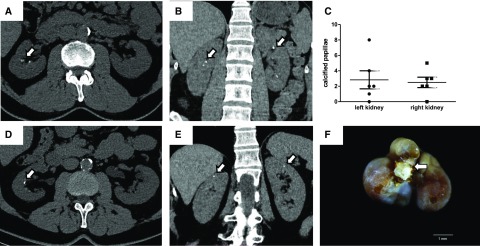
Figure 1.
Patients with pseudoxanthoma elasticum (PXE) are affected by massive Randall plaque. (A–D) Computed tomography (CT) scans showed papilla calcifications in patients with PXE who have been previously affected by kidney stones (arrows; representative CT scans from three individuals). Among six patients with PXE who underwent CT scans, five had several papillary calcifications in both right and left kidneys (C shows the number of calcified papilla in each individual). (E) One patient with PXE was affected by moderate and atypical nephrocalcinosis (cortical calcifications; arrows). (F) Monohydrate calcium oxalate stone (brown crystalline phase) from a patient with PXE with evidence of papilla umbilication and Randall plaque fragments (white crystalline phase; arrow).
Stone Analyses
According to the survey, four stones have been analyzed in various laboratories, and all were made of calcium oxalate, although no detail on stone morphology was obtained. Two other stones that patients with PXE passed spontaneously were collected at the Tenon Hospital Laboratory, and a morphoconstitutional analysis of the stone was performed, revealing the presence of massive Randall plaque remnant within stone umbilication (Figure 1F).
Study of the Renal Papilla Calcifications in Abcc6−/− Mice
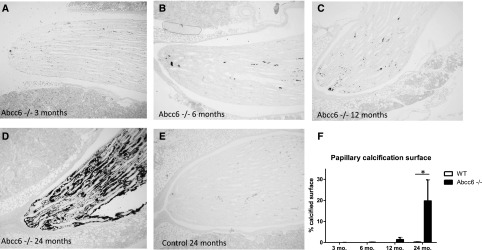
In view of the dramatically high proportion of kidney stone formers among patients with PXE and the presence of Randall plaque at the origin of these stones, we analyzed whether Abcc6−/− mice also developed similar kidney calcifications. Analyses were performed in kidneys from 17 Abcc6−/− and 15 wild-type mice at various ages: 3±0.5, 6±1, 12±2, and 24±2 months old (Figure 2). Yasue staining did not reveal the presence of significant calcifications at any time in wild-type animals (Figure 2E). However, renal calcifications appeared and worsened with aging in Abcc6−/− mice papillae (Figure 2, A–D and F). At 6 months, these calcifications were sparse and located at the most distal part of the papilla in the interstitium. At 12 months, interstitial round-shaped calcifications were developed around tubular structures at the tip of the renal papilla; at 24 months, calcifications were important, and some tubules were obstructed (n=4 Abcc6−/− mice kidneys and n=5 wild-type mice kidneys at 24 months) (Figure 2, A–D).
Figure 2.
Yasue staining evidences the development of murine Randall plaque with age. (A–E) Representative papilla from Abcc6−/− mice at various ages exhibit increasing Yasue staining with aging, which was absent in control mice Magnification, ×100. (F) The percentage of papillary tissue calcification was assessed by morphometry in 15 wild-type (WT) and 17 Abcc6−/− mice. At 24 months, calcified surface was significantly increased in Abcc6−/− mice (n=4 Abcc6−/− mice kidneys and n=5 WT mice kidneys). *P=0.01.
Abcc6−/− Mice Papilla Calcifications Are Similar to Human Randall Plaque
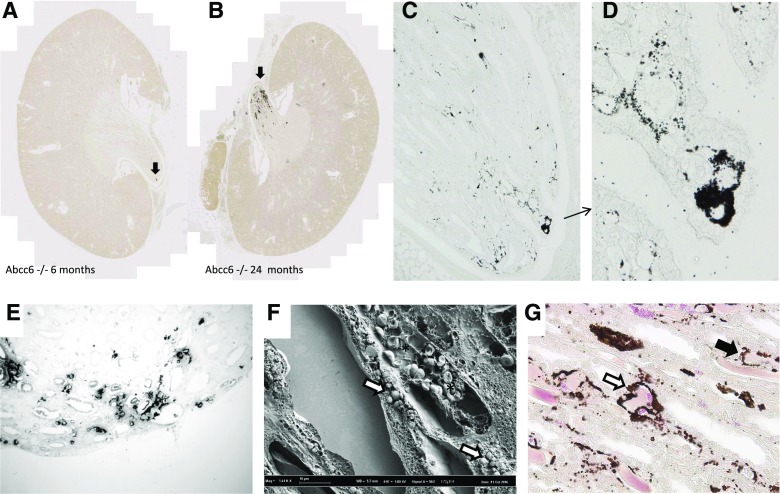
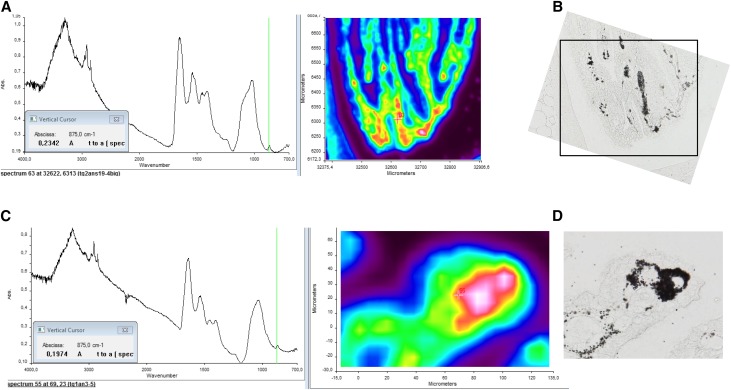
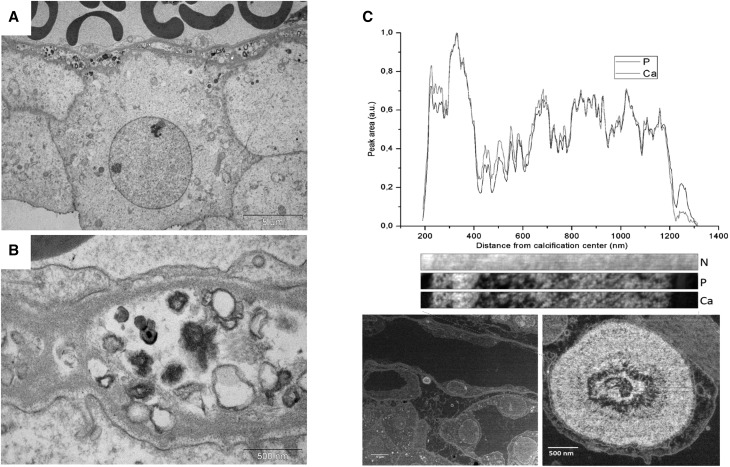
Aging Abcc6−/− mice developed renal calcifications that were located at the tip of the renal papilla (Figure 3, A and B), resembling human Randall plaque. Calcification that developed in the kidney interstitial tissue of the Abcc6−/− mice was often round shaped and surrounding loops of Henle and vasa recta, similar to calcification observed in human Randall plaques (Figure, 3 C–E). These calcifications were made of spherulites as revealed by scanning electron microscopy, predominating in the interstitial tissue and surrounding vascular and tubular lumens (Figure 3F). Eosin/Yasue staining confirmed that round-shaped calcifications developed around both vasa recta (containing red blood cells) and tubular structures with thin epithelium (loops of Henle) (Figure 3G). Fourier transform infrared microspectroscopy with an imaging system was used to characterize the chemical phases. The analysis of the absorption spectrum revealed some features specific to the presence of different absorption bands of the apatite [Ca5(PO4)3(OH)], especially the ν3 P-O stretching vibration mode measured at 1035–1045 cm−1 (Figure 4, A and B). Carbonate ions were detected together with apatite by their ν3 C-O stretching vibration mode around 1420 cm−1 and the ν2 C-O bending mode at 875 cm−1. The presence of amorphous calcium phosphate (CaP), revealed by the partial disappearance of the shoulder of the ν3 P-O absorption band of apatite, was also evidenced (Figure 4, C and D). Finally, calcification was analyzed at the nanometer scale by using transmission electron microscopy coupled to electron energy loss spectroscopy. Interestingly, deposits were located beneath the loops of Henle and vasa recta basement membranes. Their structure was frequently concentric and laminated, with mineral layers containing calcium and phosphate but also, organic layers. These features were similar to human Randall plaque (Figure 5).
Figure 3.
Calcification is electively located at the tip of the papilla, mainly in the interstitial tissue around capillaries (vasa recta) and loops of Henle. Calcification in Abcc6−/− mice was specifically located at the tip of the papilla, as shown by digital slide scanning (arrows, Figure 3A, B). Yasue staining performed on papilla slices revealed round-shaped and circular structures surrounding tubules (Magnification ×200 and zoom, Figure 3C, D). The structures are similar observed in human papillae from patients affected by Randall’s plaque (Magnification ×200, Figure 3E). Scanning electron microscopy revealed spherulites in the interstitial tissue, around vasa recta containing red blood cells (*) and loops of Henle (Figure 3F). Eosin plus Yasue staining confirmed that calcifications affect both vasa recta containing red blood cells (white arrow, Figure 3G) and loops of Henle (black arrow, Figure 3G).
Figure 4.
Calcification is made of apatite and amorphous calcium phosphate. Fourier transform infrared microspectroscopy analysis and imaging of plaques. Fourier transform infrared microspectroscopy spectra revealed (A and B) the presence of apatite and also, (C and D) the presence of mixed apatite with amorphous calcium phosphate. B and D are Yasue-stained sections corresponding to the areas analyzed by Fourier transform infrared spectroscopy.
Figure 5.
Nanostructure of calcifications is similar to human Randall plaque. Transmission electron microscopy and electron energy loss spectroscopy analysis of murine Randall plaques. (A and B) Transmission electron microscopy revealed the presence of many small dense deposits often close to endothelial cells and vasa recta. (C) Some concentric and laminated microcalcifications classically described in Randall plaque have also been observed. Electron energy loss spectroscopy analysis revealed the presence of calcium phosphate laminations in these particles alternating with nitrogen-rich organic layers. Ca, calcium cartography; N, nitrogen cartography; P, phosphorus cartography.
PPi Urinary Excretion in Abcc6−/− Mice
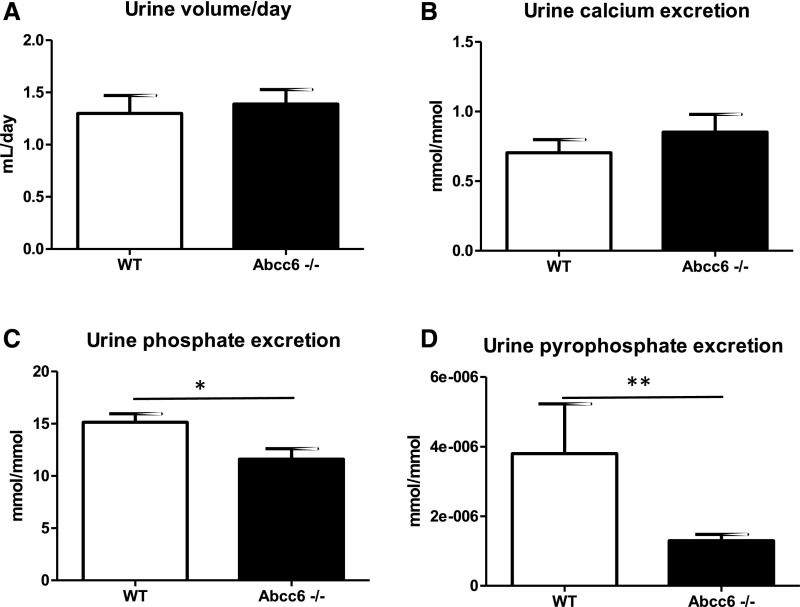
Urine volume and calcium excretion in Abcc6−/− mice did not differ from wild-type mice (Figure 6, A and B). Conversely, urine phosphate excretion was slightly but significantly lower in Abcc6−/− mice (P=0.03) (Figure 6C). Urine PPi excretion was dramatically reduced in Abcc6−/− mice (P<0.01) (Figure 6D).
Figure 6.
Abcc6−/− mice have a low urine pyrophosphate excretion. (A–D) urine volume, phosphate, calcium (indexed to urine creatinine), and pyrophosphate urine excretion have been assessed in Abcc6−/− and wild-type (WT) mice (n=8 and n=9 animals, respectively). (D) Pyrophosphate excretion was significantly lower in Abcc6−/− mice. **P<0.01. (C) Urine phosphate excretion was also significantly decreased but to a lower extent. *P=0.03.
Restoration of Systemic PPi Synthesis in Abcc6−/− Mice Protects against Randall Plaque Formation
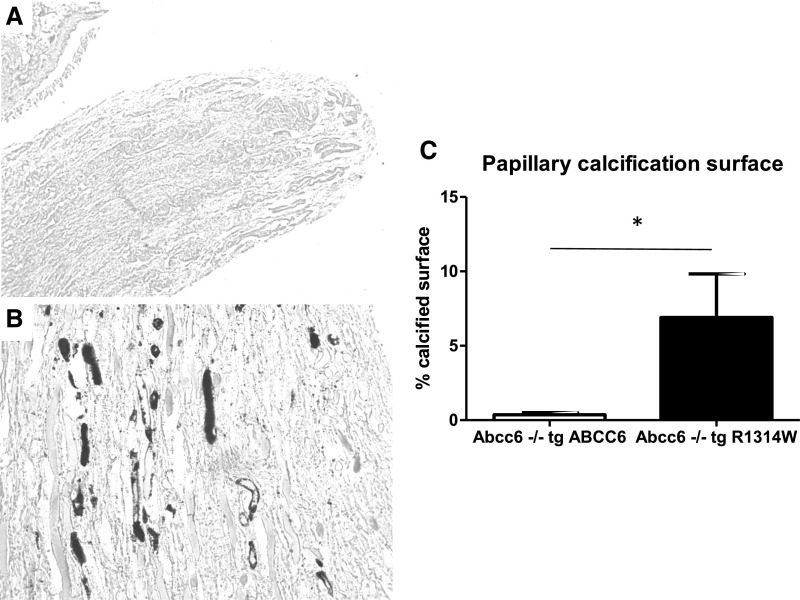
Analyses were performed in kidneys from several 18-month-old transgenic Abcc6−/− animals: three Abcc6−/− mice expressing a PXE-causing ABCC6 mutant protein (R1314W) in the liver and five Abcc6−/− mice expressing the normal functional human ABCC6 protein in the liver. Yasue staining revealed that interstitial round-shaped calcifications developed around tubular structures at the tip of the renal papilla in Abcc6−/− mice expressing the inactive R1314W mutant. By contrast, mice expressing the normal ABCC6 protein in the liver showed no calcification (Figure 7) (P=0.02).
Figure 7.
Transgenic (tg) ABCC6 expression protects Abcc6−/− mice against kidney calcification. (A) Expression of the functional human ABCC6 protein in Abcc6−/− mice protected against papillary calcification (Yasue staining). Magnification, ×100. (B) Expression of the pseudoxanthoma elasticum–causing ABCC6 mutant protein (R1314W) did not prevent the development of calcifications in the papilla, which was similar to that observed in Abcc6−/− mice (Yasue staining). Magnification, ×400. (C) The percentage of papillary tissue calcification was assessed by morphometry in five Abcc6−/− tg ABCC6 mice and three Abcc6−/− tg R1314W mice. At 18 months of age, calcified surface was significantly decreased in Abcc6−/− tg ABCC6 mice. *P=0.02.
Discussion
To our knowledge, this is the first study investigating renal stone formation in PXE and suggesting an inhibiting role of PPi in Randall plaque formation.
There is evidence that renal stone incidence increased during the past decades in industrialized countries, with a current lifetime prevalence in Western countries nearing 10%.17,18 Kidney stone prevalence is dramatically increased in patients with PXE, affecting approximately 40% of them. Of note, kidney stone prevalence in the patients’ close family members was much lower. The proportion of kidney stone formers in patients with PXE is even more remarkable considering that, in the reported literature of PXE, approximately two thirds of the patients are women. Indeed, nephrolithiasis generally affects men more frequently than women.19 Renal function was normal in almost all patients with PXE, independent of the presence of kidney stones (Table 1). Remarkably, a recent study identified kidney stones as a feature of PXE, but the prevalence was probably underestimated because of the study design.10
Randall plaque is a common mechanism initiating kidney stone formation, but the genetic and environmental determinants of Randall plaque formation are still unknown.20–22 The observation of massive Randall plaque by CT scan in five of six patients with PXE is a remarkable feature. Only the presence of massive papillary calcifications can be determined by imaging. CT scans have been performed only in patients with past medical history of kidney stones, but patients with PXE without kidney stones are also probably affected by asymptomatic papillary calcifications. The presence of massive Randall plaque fragments in the kidney stones from patients with PXE suggests that stone formation in these individuals was actually initiated by these plaques. Randall plaques were first described by Alexander Randall in 1937, who proposed an original theory relative to “the origin and growth of renal calculi.”20 He was the first to identify interstitial CaP deposits at the tip of renal papillae with growth that resulted in urothelium rupture. Calcium oxalate stones expelled by patients affected by plaques have, in many cases, a depression due to papilla imprinting and CaP residues, evidence that stones originate from Randall plaque.22,23
Since the discovery of the implication of ABCC6 mutations in PXE, a key element in the etiology of this disease has been the recent implication of hepatic and to a lesser extent, renal ABCC6-mediated ATP release as a major source of circulating PPi, a potent calcification inhibitor.3,24 In the absence of functional ABCC6, patients with PXE have low circulating PPi levels leading to ectopic calcifications in the elastic fiber-rich tissues.
Because PPi deficiency increases CaP precipitation, ABCC6 deficiency could increase CaP crystallization at the tip of renal papillae and thereby, promote Randall plaque formation in patients with PXE.
Urine PPi is not measured in clinical practice, and we were not able to determine PPi urine concentration in PXE stone formers in the absence of a specific protocol. Several studies have described lower PPi concentration in the urine of kidney stone formers.25–27 Decrease of other calcification inhibitors has also been reported in the urine of kidney stone formers. However, this is the first time to our knowledge that a monogenic defect affecting a calcification inhibitor is associated with an increased risk of Randall plaque and kidney stone formation in humans.
To test the hypothesis that ABCC6 deficiency and low PPi concentrations would result in Randall plaque formation, we used the Abcc6−/− mouse model that recapitulates many of the pathophysiologic and phenotypical characteristics of the human PXE disease. Interestingly, renal calcifications have been previously described in the cortex of young Abcc6−/− animals, although the medulla and the tip of the renal papilla were not assessed.28 Kidney calcifications can be “accelerated” by a specific diet (high phosphorus and vitamin D and low magnesium in the diet) in Abcc6−/− young mice; however, these calcifications were predominantly located in renal tubules.29 It is very likely that the systemic PPi deficiency in these mice in combination with the acceleration diet resulted in massive tubular precipitation of CaP. These tubular calcifications clearly differ from Randall plaques observed with aging in Abcc6−/− mice, which were mainly found in interstitial tissues and began at the tip of the renal papilla. Remarkably, the renal calcification phenotype that we described in aging Abcc6−/− mice meets the four essential criteria of Randall plaque: (1) interstitial deposits around tubular and vessels lumen, (2) appearing and spreading at the tip of the renal papilla (i.e., different from medullary nephrocalcinosis), (3) made of carbonated apatite and amorphous CaP as previously described in human papillae, and (4) forming nanometer-scale spherulites with both mineral and organic layers.16
Interestingly, the development of Randall plaque in human papillae has been attributed to CaP supersaturation in the interstitial tissue at the tip of the papilla. Asplin et al.30 have hypothesized that CaP supersaturation in the thin limb of the Henle loop could promote CaP particle precipitation. The main determinants of CaP supersaturation in vivo are actually high pH, high calcium and phosphate concentrations, and possibly, low concentration of calcification inhibitor.31 To date, only high urine calcium concentration (hypercalciuria) has been related to the development of plaques at the surface of renal papillae.32 Because calcium and phosphate concentrations are predicted to be extremely high at the tip of the papilla, inhibitors of crystallization are probably physiologically essential to prevent calcifications in this specific area of the kidney. Our previous attempt to develop Randall plaques in murine models by inducing hypercalciuria through high vitamin D and calcium intakes has failed. As observed in other hypercalciuric rat models, animals developed CaP stones but no Randall plaque, despite high CaP supersaturation in urine.33,34 Of note, patients with PXE affected by Randall plaque had only mild increase in calcium and normal oxalate and phosphate urine concentration, suggesting that hypercalciuria was probably not the main determinant of CaP supersaturation and plaque formation in these patients. On the basis of our results, it seems that calcification inhibitors are probably essential to prevent tissue mineralization in kidney. It is remarkable that the invalidation of a single gene (ABCC6) promotes Randall plaque formation, suggesting that PPi is a significant inhibitor of Randall plaque and kidney stone formation in vivo.
PXE is a rare disease, but Randall plaque and related stones are extremely frequent in the general population. Further studies will be necessary to assess whether patients affected by Randall plaques have low PPi concentration in urine. In our experience, these studies will require special experimental procedures because of low PPi concentration in urine and preanalytic pitfalls in our experience (E. Letavernier, G. Leftheriotis, and M. Daudon, unpublished data). Of note, PPi level in Abcc6−/− mice urine was dramatically reduced, but we also observed a small but significant decrease in urine phosphate excretion. Further study of phosphate and PPi homeostasis in patients with PXE and Abcc6−/− mice is warranted. ABCC6 is expressed in renal proximal tubular cells at a relatively low level.35 It may be hypothesized that renal ABCC6 could play a role in renal calcifications. However, transgenic Abcc6−/− mice expressing a functional human ABCC6 protein were protected against kidney calcifications at 18 months of age, suggesting that raising plasma PPi in these mice, even modestly, exerts a systemic protection.15 Interestingly, urolithiasis is independently associated with vascular calcifications and increased cardiovascular risk.36 Whether plasma PPi deficiency could increase both kidney stone and vascular calcifications in kidney stone formers deserves further studies.
Our work presented some limitations. There was a potential bias from an over-representation of kidney stone–related episodes in the patient surveys. However, 46 patients reported kidney stones: even if considering the (unlikely) hypothesis that none of the 51 patients who did not reply to the questionnaire were affected by stones, at least 28% (46 of 164) of patients with PXE were affected by stones. This proportion is still extremely high compared with in the general population. Furthermore, even as we observed plaque imprints on kidney stones, we have no evidence that all stones originated from a Randall plaque. Finally, this type of survey is not adequate to collect extensive clinical data (history of stones, number of episodes, familial medical history, etc.).
In conclusion, we report a dramatic prevalence of kidney stones in patients affected by PXE, a disease notably characterized by low circulating PPi levels. When available, kidney stones and CT scans revealed the presence of massive Randall plaques, the first step of kidney stone formation. Patients with PXE should be aware that they are at high risk to form kidney stones, and preventive measures should now be considered (increased diuresis, lowering calcium urinary excretion, etc.). Moreover, Abcc6−/− mice, which are also characterized by low circulating and urine PPi levels, develop Randall plaque spontaneously with age at the tip of the renal papilla. These observations from a rare monogenic disease suggest that PPi is a major protective factor against the development of Randall plaque and urolithiasis, a condition affecting 10% of the population. It has been recently shown that PPi administration increases blood PPi levels and protects against tissue calcifications in Abcc6−/− mice.15 Further studies would be useful to assess whether PPi administration may also protect against the development of Randall plaque in murine models and could, therefore, be a potential new treatment against kidney stones.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Prof. Claude Marsault for his kind relecture of patient computed tomography scans. We thank the patients with PXE, the PXE France support group, and Mrs. Hélène Humeau for her help (data collection).
Financial support for E.L. came from Agence Nationale pour la Recherche grant ANR-13-JSV1-0010-01, Société de Néphrologie (the Genzyme grant), Académie Nationale de Médecine (the Nestlé–Waters award), and Convergence-Université Pierre et Marie Curie grants CVG1205 and CORDDIM-2013-COD130042. Financial support for D.B. came from Agence Nationale pour la Recherche grant ANR-12-BS08-0022. Financial support for O.L.S. came from National Institutes of Health grants HL108249 and P20GM113134.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J: Pseudoxanthoma elasticum: Clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol 18: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al.: Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 25: 223–227, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Váradi A, et al.: ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 34: 1985–1989, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanakker OM, Leroy BP, Coucke P, Bercovitch LG, Uitto J, Viljoen D, et al.: Novel clinico-molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum Mutat 29: 205, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, et al.: Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med 9: 393, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Kingman J, Sundberg JP, Uitto J, Li Q: Plasma PPi deficiency is the major, but not the exclusive, cause of ectopic mineralization in an Abcc6-/- mouse model of PXE. J Invest Dermatol 137: 2336–2343, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabre B, Bayle P, Bazex J, Durand D, Lamant L, Chassaing N: Pseudoxanthoma elasticum and nephrolithiasis. J Eur Acad Dermatol Venereol 19: 212–215, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mallette LE, Mechanick JI: Heritable syndrome of pseudoxanthoma elasticum with abnormal phosphorus and vitamin D metabolism. Am J Med 83: 1157–1162, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Seeger H, Mohebbi N: Pseudoxanthoma elasticum and nephrocalcinosis. Kidney Int 89: 1407, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Legrand A, Cornez L, Samkari W, Mazzella JM, Venisse A, Boccio V, et al.: Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with pseudoxanthoma elasticum. Genet Med 19: 909–917, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Lebwohl M, Neldner K, Pope FM, De Paepe A, Christiano AM, Boyd CD, et al.: Classification of pseudoxanthoma elasticum: Report of a consensus conference. J Am Acad Dermatol 30: 103–107, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Leftheriotis G, Kauffenstein G, Hamel JF, Abraham P, Le Saux O, Willoteaux S, et al.: The contribution of arterial calcification to peripheral arterial disease in pseudoxanthoma elasticum. PLoS One 9: e96003, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al.: Disruption of Abcc6 in the mouse: Novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet 14: 1763–1773, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al.: Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am J Pathol 187: 1258–1272, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verrier C, Bazin D, Huguet L, Stéphan O, Gloter A, Verpont MC, et al.: Topography, composition and structure of incipient randall plaque at the nanoscale level. J Urol 196: 1566–1574, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y: Epidemiology of stone disease across the world. World J Urol 35: 1301–1320, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Daudon M: [Epidemiology of nephrolithiasis in France]. Ann Urol (Paris) 39: 209–231, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Randall A: The origin and growth of renal calculi. Ann Surg 105: 1009–1027, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, et al.: Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daudon M, Bazin D, Letavernier E: Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 43[Suppl 1]: 5–11, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Letavernier E, Vandermeersch S, Traxer O, Tligui M, Baud L, Ronco P, et al.: Demographics and characterization of 10,282 Randall plaque-related kidney stones: A new epidemic? Medicine (Baltimore) 94: e566, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen RS, Küçükosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al.: ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A 110: 20206–20211, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleisch H: Inhibitors and promoters of stone formation. Kidney Int 13: 361–371, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Roberts NB, Dutton J, Helliwell T, Rothwell PJ, Kavanagh JP: Pyrophosphate in synovial fluid and urine and its relationship to urinary risk factors for stone disease. Ann Clin Biochem 29: 529–534, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Muñoz JA, López-Mesas M, Valiente M: Minimum handling method for the analysis of phosphorous inhibitors of urolithiasis (pyrophosphate and phytic acid) in urine by SPE-ICP techniques. Anal Chim Acta 658: 204–208, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al.: Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 25: 8299–8310, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Chou DW, Price TP, Sundberg JP, Uitto J: Genetic modulation of nephrocalcinosis in mouse models of ectopic mineralization: The Abcc6(tm1Jfk) and Enpp1(asj) mutant mice. Lab Invest 94: 623–632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asplin JR, Mandel NS, Coe FL: Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol 270: F604–F613, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Bird VY, Khan SR: How do stones form? Is unification of theories on stone formation possible? Arch Esp Urol 70: 12–27, 2017 [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, et al.: Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 64: 2150–2154, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Letavernier E, Verrier C, Goussard F, Perez J, Huguet L, Haymann JP, et al.: Calcium and vitamin D have a synergistic role in a rat model of kidney stone disease. Kidney Int 90: 809–817, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Bushinsky DA, Parker WR, Asplin JR: Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 57: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD: The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem 51: 887–902, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Shavit L, Girfoglio D, Vijay V, Goldsmith D, Ferraro PM, Moochhala SH, et al.: Vascular calcification and bone mineral density in recurrent kidney stone formers. Clin J Am Soc Nephrol 10: 278–285, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.