Abstract
Background
Polymyalgia rheumatica (PMR) is a common systemic inflammatory disease of the elderly; however, the clinical characteristics and therapeutic response of PMR in Korea have been rarely studied.
Methods
We reviewed the medical records of 54 Korean patients diagnosed with PMR between January 2009 and February 2017 in a locomotive pain clinic of one tertiary referral hospital. We analyzed epidemiologic and clinical characteristics, therapeutic responses, and prognostic factors for remission-failure at one-year after oral prednisolone treatment.
Results
In 54 patients with PMR, 32 (59.3%) were female. The average age at diagnosis was 65.0 ± 10.5 years. Duration of symptoms before diagnosis was 8.1 ± 8.6 months. All patients had shoulder pain (54 patients, 100.0%); 49 patients (90.7%) had hip girdle pain, while 19 patients (35.2%) had peripheral joint pain. Four patients (7.4%) were accompanied by the giant cell arteritis (GCA). There was no seasonal preference for symptom development. Only 19 patients were diagnosed with PMR at initial symptom presentation. At one-year follow-up after oral prednisolone treatment, the remission rate was 35.3% (12/34). Multivariate analysis showed that history of relapse (odds ratio, 6.81; 95% confidence interval, 1.035–44.804) was a significant predictor of remission-failure.
Conclusion
The rate of remission (35.3%) after oral prednisolone treatment was similar to previous reports in western countries; and GCA is not a rare condition in Korean PMR patients. Misdiagnosis of PMR is common, and heightened consideration for PMR is needed in elderly patients who present inflammatory features of bilateral shoulder pain.
Keywords: Polymyalgia Rheumatica, Giant Cell Arteritis, Prednisolone, Treatment Outcome
Graphical Abstract
INTRODUCTION
In 1957, Barber used the term polymyalgia rheumatica (PMR) to describe sudden onset of inflammatory features characterized by shoulder and pelvic girdle pain and stiffness.1 Since that description, PMR has become a common systemic disease in the elderly, characterized by inflammatory pain and stiffness of the shoulder and/or pelvic girdles combined with laboratory evidence of inflammation.2,3,4
There is lack of standardized diagnostic criteria for PMR, but sets of classification criteria have been proposed by several groups of investigators. Recently, the 2012 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria were developed to standardize the definition of PMR and thereby address the uncertainty that commonly surrounds its diagnosis.5 Although the 2012 EULAR/ACR classification criteria were developed, chronic inflammatory and autoimmune disease, degenerative disease, infections, and malignancies can mimic PMR. Heterogeneity in clinical manifestation and the lack of diagnostic criteria of PMR have led to misdiagnosis.
There are many reports on the various symptoms of PMR. The clinical features that appear in PMR vary by country.4,6,7 Bilateral shoulder pain with morning stiffness is the reported symptom in 70%–95% of PMR patients.2 Peripheral arthritis was reported in 25% of patients, while 40% of PMR patients have constitutional manifestations including low-grade fever, depression, fatigue, and weight loss.3,8,9 In previous Korean epidemiology studies, the incidence of constitutional symptoms and peripheral arthritis was similar to those reported in Caucasian populations. However, the incidence of hip girdle pain was higher in the Korean studies than reported from western countries.6,10 In addition, western population studies reported concomitant giant cell arteritis (GCA) in 16%–21% of the study population.11 However, no GCA case were reported in the Korean epidemiology study populations.6,10
Although PMR is well controlled by a low dose of oral glucocorticoid, not all patients respond adequately, and relapse and long-term glucocorticoid dependency are common.12 The treatment response to oral prednisolone for PMR also varies. In a prospective western population cohort study of PMR, 56% of prednisolone-treated patients achieved complete response.13 However, in previous Korean studies, the remission rate was reported as about 20%.6,10 Demographic and clinical characteristics, along with outcomes, in PMR patients have been studied extensively in western countries. However, Korean PMR patient studies are still sparse.
The aim of our study was to investigate the clinical and demographic characteristics, including related factors, of therapeutic responses in Korean PMR patients.
METHODS
Patients and methods
We retrospectively reviewed the medical records of 54 Korean patients diagnosed with PMR between January 2009 and February 2017 in a locomotive pain clinic in one tertiary referral hospital. Patients were included based on the 2012 EULAR/ACR classification criteria for PMR. Baseline demographic characteristics, clinical characteristics, and past medical history were reviewed. Initial laboratory tests included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC), platelet, hemoglobin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), rheumatoid factor (RF), anti-citrullinated protein antibody (anti-CCP), antinuclear antibody (ANA), and human leukocyte antigen (HLA)-B27. Patients who had satisfied the classification criteria for rheumatoid arthritis (RA), had fulfilled criteria for inflammatory spondyloarthropathy, or had systemic infection were excluded. We also collected data on previous diagnoses made in primary medical clinics prior to referral to our locomotive pain clinic.
Analysis of response to oral prednisolone treatment was performed in 34 patients without GCA. The initial oral prednisolone regimen and tapering schedule were based on the British Society for Rheumatology (BSR) and the British Health Professional in Rheumatology (BHPR) guidelines, which recommend an initial 15 mg daily dose continued for 3 weeks with a gradual tapering schedule. We used of vitamin D and bisphosphonates routinely when initiating oral prednisolone for PMR to prevent the complications of osteoporosis.14 Twenty of 54 patients were excluded for the following reasons: 13 patients were transferred to patients' regional hospital for oral prednisolone, lost to follow-up or referred to rheumatologic specialist in case of GCA; 7 patients were excluded due to short treatment duration (1 week to 9 months). Thirty-four patients who received oral prednisolone treatment for at least 3 months were divided according to the treatment response at one year follow-up. Remission was defined as the absence of clinical symptoms and laboratory evidence of PMR (CRP < 0.3 mg/dL) at one year after oral prednisolone was administered. Relapse was defined as aggravation or reappearance of clinical symptoms with elevated level of CRP or ESR (CRP > 0.3 mg/dL; ESR > 40 mm/hr) during prednisolone tapering.
Statistical methods
Variables of continuous outcomes were presented as the mean (standard deviation) for normally distributed data, or median (interquartile range) for not normally distributed data. Normal distribution was evaluated by visual inspection of the variable distribution and statistical methods using Shapiro-Wilk test. Frequency count and percentages were provided for the categorical variables. Comparisons between groups were made using an independent t-test or a Mann-Whitney U test for continuous variables and either Fisher's exact test or χ2 test for categorical variables. Univariate logistic regression was used to evaluate the factors related with remission failure presented by unadjusted odds ratio (OR) and 95% confidence interval (CI). We conducted a multivariate logistic regression analysis to confirm independent predictive factors of remission-failure such as sex, diabetes mellitus, history of relapse, initial CRP ≥ 5 mg/dL, time elapsed to normalization of CRP > 4 weeks presented by adjusted OR and 95% CI. All statistical analysis was performed using IBM SPSS statistical software (ver. 24.0; IBM Corp., Armonk, NY, USA). P values < 0.05 were considered significant.
Ethics statement
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2016-12-152). Informed consent was waived by the board.
RESULTS
Demographics
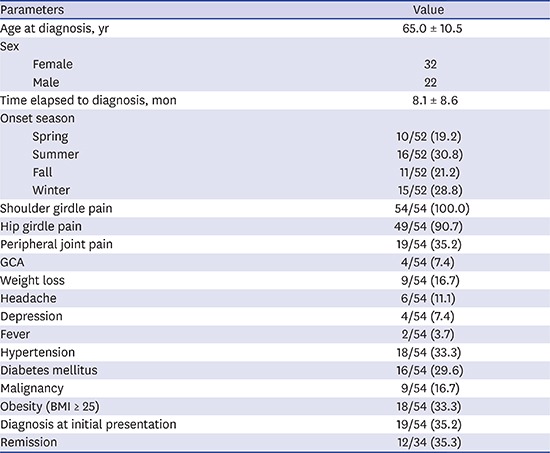
Among the 54 patients with PMR, 32 (59.3%) were female. The average age at diagnosis was 65.0 ± 10.5 (range 46–87) years. Two 46-year-old females were diagnosed with PMR and had shown inflammatory feature of bilateral shoulder and hip pain with elevated acute phase reactants, and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) showed characteristic findings of PMR without satisfying the criteria of RA or spondyloarthropathy.15 The average duration of symptoms before diagnosis was 8.1 ± 8.6 (range 1–36) months. There was no seasonal preference for symptom development: 10 patients developed symptoms in the spring, 16 patients in the summer, 11 patients in the fall, and 15 patients in the winter.
Clinical characteristics
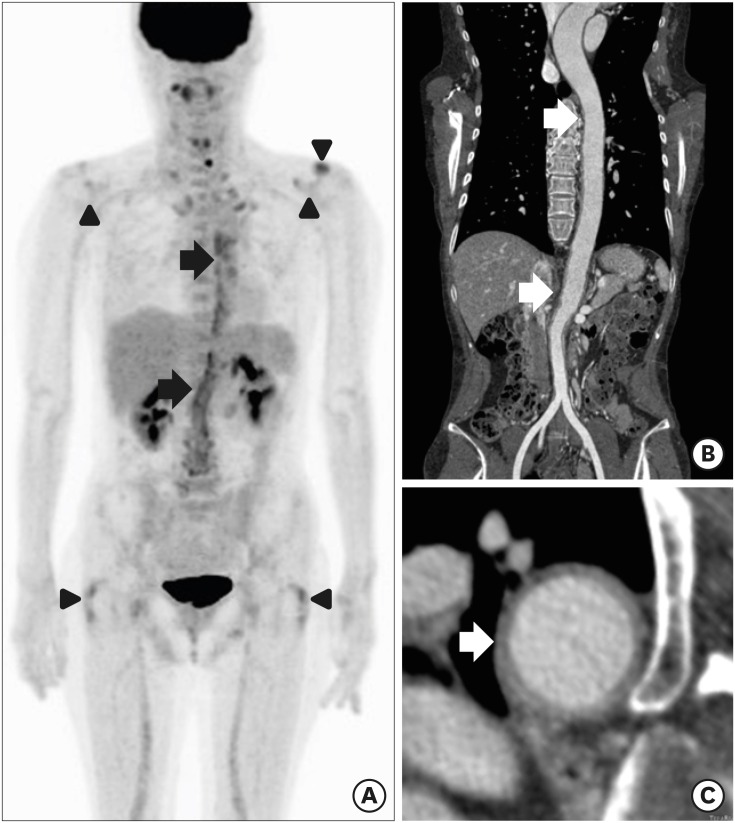
All 54 patients had shoulder girdle pain (100%), 49 patients (90.7%) had hip girdle pain, and 19 patients (35.2%) had peripheral joint pain. The timing of hip and shoulder pain varied. In 22 cases (41.5%) hip and shoulder pain presented simultaneously, 10 patients (18.9%) reported hip pain before shoulder pain, and 20 patients (39.6%) reported hip pain after shoulder pain. Forty-eight patients (96%) reported stiffness in the morning. Other manifestations were weight loss (9 patients), headache (6 patients), depression (4 patients), and fever (2 patients) (Table 1). Only one female patient of six patients with headache was classified with GCA based on the American Rheumatology Association (ARA) classification criteria for GCA.16 There were three cases that showed increased FDG uptake of characteristic finding on PET/CT without clinical symptoms of GCA. Thus, four patients were accompanied by the GCA. In one of three cases with the GCA showed increased uptake of FDG in the aorta that then spread to the right common iliac artery, suggesting active inflammation (Fig. 1).
Table 1. Baseline characteristics in 54 patients with polymyalgia rheumatica.
| Parameters | Value | |
|---|---|---|
| Age at diagnosis, yr | 65.0 ± 10.5 | |
| Sex | ||
| Female | 32 | |
| Male | 22 | |
| Time elapsed to diagnosis, mon | 8.1 ± 8.6 | |
| Onset season | ||
| Spring | 10/52 (19.2) | |
| Summer | 16/52 (30.8) | |
| Fall | 11/52 (21.2) | |
| Winter | 15/52 (28.8) | |
| Shoulder girdle pain | 54/54 (100.0) | |
| Hip girdle pain | 49/54 (90.7) | |
| Peripheral joint pain | 19/54 (35.2) | |
| Knee | 7/54 (16.7) | |
| Wrist | 6/54 (11.1) | |
| Hand | 5/54 (9.3) | |
| Elbow | 3/54 (5.6) | |
| Ankle | 3/54 (5.6) | |
| The sequence of pain | ||
| Simultaneous | 22/52 (41.5) | |
| Shoulder → Hip | 20/52 (39.6) | |
| Hip → Shoulder | 10/52 (18.9) | |
| Morning stiffness duration, min | ||
| 0 | 2/50 (4.0) | |
| < 30 | 5/50 (10.0) | |
| 30–44 | 5/50 (10.0) | |
| ≥ 45 | 38/50 (76.0) | |
| GCA | 4/54 (7.4) | |
| Weight loss | 9/54 (16.7) | |
| Headache | 6/54 (11.1) | |
| Depression | 4/54 (7.4) | |
| Fever | 2/54 (3.7) | |
| Hypertension | 18/54 (33.3) | |
| Diabetes mellitus | 16/54 (29.6) | |
| Malignancy | 9/54 (16.7) | |
| Obesity (BMI ≥ 25) | 18/54 (33.3) | |
Values are mean ± standard deviation (range) or numbers of patients with characteristic/total number of patients (%).
GCA = giant cell arteritis, BMI = body mass index.
Fig. 1. 18F FDG-PET/CT and CT angiography findings of a 56-year-old female diagnosed with GCA. Coronal FDG-PET/CT (A) image demonstrates a significant uptake in aortic wall from the level of thoracic spine to aortic bifurcation (black arrows). Uptake of the tracer of the bilateral glenohumeral joints, bilateral greater trochanter area and left acromio-clavicle joint is also seen (black arrowheads). CT angiography image (B) and (C) shows the diffuse thickening with enhancement in thoracic aorta and abdominal aorta (white arrows).
FDG-PET/CT = fluorodeoxyglucose positron emission tomography/computed tomography, CT = computed tomography, GCA = giant cell arteritis.
Laboratory findings
The laboratory findings of PMR patients are presented in the Table 2. Elevated CRP (> 0.3 mg/dL) was observed in 48 patients (88.9%), and elevated ESR (> 40 mm/hr) was reported in 42 patients (77.8%). RF was screened in 51 patients, and 9 (17.6%) were determined to be RF positive. Titers of RF positive patients were 40 ± 9.4. The presence of anti-CCP antibodies was tested in 52 patients, and 3 (5.8%) showed positive results. Among 39 patients, ANA was positive in 21 (53.9%). The ANA titer was 1:40 in 20 patients and 1:80 in one patient (Table 2). Diagnosis of systemic lupus erythematous, elderly onset RA, and late-onset seronegative spondyloarthropathy was excluded according to ACR classification criteria.
Table 2. Laboratory findings in patients with polymyalgia rheumatica.
| Parameters | No. (%) |
|---|---|
| Elevate CRP, > 0.3 mg/dL | 48/54 (88.9) |
| Elevated ESR, > 40 mm/hr | 42/54 (77.8) |
| Leukocytosis, ≥ 12,000/µL | 4/54 (7.4) |
| Thrombocytosis, ≥ 400,000/uL | 11/54 (20.4) |
| Anemia, < 10.0 g/dL | 4/54 (7.4) |
| Elevated AST, AST > 40U/L | 0/54 (0.0) |
| Elevated ALT, ALT > 40U/L | 3/54 (5.6) |
| Elevated ALP, > 120 U/L | 5/54 (9.3) |
| Positive RF | 9/51 (17.6) |
| Positive anti-CCP | 3/52 (5.8) |
| Positive ANA | 21/39 (53.9) |
| Positive HLA-B27 | 3/35 (8.6) |
Numbers of patients with characteristic/total number of patients (%).
CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, AST = aspartate transaminase, ALT = alanine transaminase, ALP = alkaline phosphatase, RF = rheumatoid factor, anti-CCP = anti-citrullinated protein antibody, ANA = antinuclear antibody, HLA = human leukocyte antigen.
Previous diagnosis
Previous diagnoses made at primary medical clinics before referral to our locomotive pain clinic are presented in Table 3. Only 19 patients initially presenting to our hospital with symptoms were diagnosed with PMR. The remaining patients had different previous diagnoses before visiting our hospital. Spondyloarthropathy (6 patients), adhesive capsulitis (6 patients), and cervical radiculopathy (4 patients) were the most frequent previous diagnoses. Three patients were misdiagnosed with an infectious disease such as infectious arthritis of the shoulder and lumbar osteomyelitis. Nine patients had no specific diagnosis prior to PMR diagnosis.
Table 3. Previous diagnoses before referral to our locomotive pain clinic.
| Previous diagnoses | Patients No. (%) | |
|---|---|---|
| Shoulder | ||
| Adhesive capsulitis | 6 (11.1) | |
| Infectious arthritis | 2 (3.7) | |
| Calcific tendinitis | 1 (1.9) | |
| Rotator cuff syndrome | 1 (1.9) | |
| Back | ||
| Spondyloarthropathy | 6 (11.1) | |
| Lumbosacral radiculopathy | 2 (3.7) | |
| Lumbar osteomyelitis | 1 (1.9) | |
| Cervical radiculopathy | 4 (7.4) | |
| Myofascial pain syndrome | 2 (3.7) | |
| Knee osteoarthritis | 1 (1.9) | |
| No specific diagnosis | 9 (16.7) | |
| Total | 35 (64.8) | |
Values are presented as number (%).
Responses to oral prednisolone
In 34 patients, 15 mg of oral prednisolone was administered initially according to the BSR and the BHPR guidelines. Analysis of response to oral prednisolone treatment was performed in 34 patients without GCA. We categorized the groups according to the therapeutic response. In our study, the remission rate was 35.3% (12/34) at one-year follow-up after administering oral prednisolone. Twenty-three of 34 patients (67.6%) had relapsed at least once during tapering of oral prednisolone dose. We evaluated the clinical and laboratory findings between patients with remission and remission-failure (Table 4). There were no differences in the clinical characteristics and laboratory findings between the groups, except levels of AST and ALT, which were significantly higher in the remission-failure group (P = 0.019 and P = 0.044, respectively).
Table 4. Comparison of characteristics according to remission or not in patients with polymyalgia rheumatica.
| Parameters | Remission (n = 12; 35.3%) | Remission-failure (n = 22; 64.7%) | P value |
|---|---|---|---|
| Age at diagnosis | 66.8 ± 14.0 | 62.8 ± 10.4 | 0.352 |
| Sex, female, No. (%) | 9/12 (75.0) | 14/22 (63.6) | 0.705 |
| Onset to diagnosis, mon | 5.2 ± 4.3 | 8.6 ± 7.8 | 0.109 |
| Peripheral joint pain, No. (%) | 3/12 (25.0) | 5/22 (22.7) | 1.000 |
| Use of NSAID, No. (%) | 9/12 (75.0) | 10/22 (45.5) | 0.097 |
| Hypertension, No. (%) | 4/12 (33.3) | 6/22 (27.3) | 10.000 |
| Diabetes mellitus, No. (%) | 2/12 (16.7) | 9/22 (40.9) | 0.252 |
| Malignancy, No. (%) | 1/12 (8.3) | 4/22 (18.2) | 0.635 |
| Obesity (BMI ≥ 25) | 4/12 (33.3) | 7/22 (31.8) | 1.000 |
| WBC, /µL | 8,065.8 ± 2,414.2 | 8,787.7 ± 1,717.3 | 0.318 |
| Hemoglobin, g/dL | 11.8 ± 1.7 | 12.3 ± 1.8 | 0.459 |
| Platelet, 1,000/ µL | 290.9 ± 73.1 | 338.6 ± 97.1 | 0.147 |
| AST, U/L | 16.5 ± 2.6 | 20.6 ± 6.8 | 0.019 |
| ALT, U/L | 13.0 (9.5–15.5) | 17.0 (13.0–25.0) | 0.044 |
| ALP, U/L | 81.0 (63.0–88.5) | 91.0 (70.0–107.0) | 0.345 |
| Initial CRP, mg/dL | 3.74 (1.73–4.43) | 2.44 (1.43–5.79) | 0.261 |
| Initial ESR, mm/hr | 73.0 ± 21.2 | 64.5 ± 30.5 | 0.395 |
| BMI, kg/m2 | 22.9 ± 3.3 | 23.3 ± 3.2 | 0.754 |
| RF, IU/mL | 9.4 (8.1–33.9) | 8.4 (7.3–14.6) | 0.427 |
| Positive RF, No. (%) | 2/12 (16.7) | 4/20 (20.0) | 1.000 |
| Positive ANA, No. (%) | 3/9 (33.3) | 11/18 (61.1) | 0.236 |
| Time elapsed to normalization of CRP, wk | 4.0 (2.5–6.0) | 5.0 (2.0–11.0) | 0.083 |
| Time elapsed to normalization of ESR, wk | 4.0 (2.5–12.5) | 5.0 (4.0–9.0) | 0.286 |
Values area mean ± standard deviation or median (interquartile range), or numbers of patients with characteristic/total number of patients (%).
NSAID = nonsteroidal anti-inflammatory drug, BMI = body mass index, WBC = white blood cell, AST = aspartate transaminase, ALT = alanine transaminase, ALP = alkaline phosphatase, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, RF = rheumatoid factor, ANA = antinuclear antibody.
We examined the independent predictive factors of remission-failure using logistic regression. Univariate analyses indicated that history of relapse (unadjusted OR, 12.67; 95% CI, 2.292–70.105) and longer than four weeks to CRP normalization (unadjusted OR, 6.67; 95% CI, 1.162–38.247) were significantly more likely to result in remission-failure (Table 5). Multivariate logistic regression analysis showed that history of relapse (adjusted OR, 6.81; 95% CI, 1.035–44.804) was a significant predictor of remission-failure (Table 6).
Table 5. Univariate analyses of risk factors of remission-failure in polymyalgia rheumatica.
| Parameters | Unadjusted OR | 95% CI | P value |
|---|---|---|---|
| Age, ≥ 65 yr | 0.971 | 0.913–1.032 | 0.342 |
| Sex, female | 0.501 | 0.121–2.801 | 0.501 |
| Diabetes mellitus | 3.462 | 0.608–19.719 | 0.162 |
| Malignancy | 2.444 | 0.241–24.778 | 0.449 |
| History of relapse | 12.667 | 2.292–70.015 | 0.004 |
| Initial CRP ≥ 5 mg/dL | 1.125 | 0.225–5.620 | 0.886 |
| Initial ESR ≥ 70 mm/hr | 0.857 | 0.207–3.552 | 0.832 |
| Time elapsed to normalization of CRP > 4 wk | 6.667 | 1.162–38.247 | 0.033 |
| Time elapsed to normalization of ESR > 4 wk | 1.925 | 0.445–8.331 | 0.381 |
OR = odds ratio, CI = confidence interval, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate.
Table 6. Multivariate analyses of risk factors of remission-failure in polymyalgia rheumatica.
| Parameters | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Sex, female | 0.895 | 0.098–8.195 | 0.922 |
| Diabetes mellitus | 2.862 | 0.224–36.522 | 0.418 |
| History of relapse | 6.808 | 1.035–44.804 | 0.046 |
| Initial CRP ≥ 5 mg/dL | 0.761 | 0.090–6.465 | 0.803 |
| Time elapsed to normalization of CRP > 4 wk | 2.977 | 0.388–33.820 | 0.294 |
R2 = 0.41, OR = odds ratio, CI = confidence interval, CRP = C-reactive protein.
DISCUSSION
In this study, we described the demographic and clinical features of 54 Korean patients with PMR. Furthermore, we analyzed the therapeutic response to oral prednisolone and predictive factors of remission-failure. The rate of remission on oral prednisolone treatment was 35.3%, and history of relapse was a predictor of remission-failure. Four of 54 patients (7.4%) were also diagnosed with GCA.
In our study, 12 of 34 patients (35.3%) were in remission after oral prednisolone treatment at the one-year follow-up. Twenty-three of 34 patients (67.6%) had relapsed at least once during treatment. Because relapse and long-term glucocorticoid dependency are common, remission is difficult to achieve in PMR treatment.14,17 The remission rate of our study was 35.3%, which is comparable to previous reports in western countries. In 94 Italian PMR subjects, Salvarani et al.18 had found that 50% of patients were in remission after oral prednisolone at a mean starting dosage of 17.5 mg/day. In addition, a multicenter prospective cohort study in 10 European countries and the United States reported a 56% complete response rate to corticosteroids at week 26. In contrast, previous Korean studies have reported remission rates of 19.5%–20.5%, which is lower than the rate in our study. In 10 multicenter study of 51 Korean subjects, the remission rate was 19.5%.10 Lee et al.6 evaluated the clinical data of 78 patients with PMR from 5 hospitals and reported a 20.5% remission rate. One explanation for these lower rates is that in previous multicenter-based Korean studies, the treatment strategies of PMR varied between centers, and the initial oral prednisolone dosage and the tapering schedule were not standardized. Another possible explanation is that the definition of remission among studies was not consistent. In our study, we standardized assessment and the initial dose and tapering schedule of oral prednisolone according to the BSR and the BHPR guidelines. This standardization may have led to better outcome in comparison to previous Korean studies. Our findings indicate that the remission rate of PMR patients on oral prednisolone is not low in the Korean population.
GCA and PMR are conditions of elderly that frequently overlap. GCA predominantly affects large- and medium-sized blood vessels with systemic vasculitis in individuals over the age of 50. The most commonly affected areas are the aorta and its major braches.19 It is associated with increased thrombotic events, both arterial and venous thromboses. Aortic involvement usually remains unnoticed until there are fatal complications. The diagnosis of GCA is based on clinical symptoms and laboratory findings consistent with systemic inflammation and confirmed with histologic analysis. Also, FDG-PET/CT has a major role in assessing the extent of disease.20 Previous western population studies have shown that GCA is present in 16%–21% of PMR patients.11 However, in epidemiology studies on PMR patients within the Korean population, no GCA cases were reported.6,10 In our present study, four patients (7.4%) were diagnosed with GCA. One patient was clinically diagnosed and three patients were diagnosed with characteristic findings of GCA, increased FDG uptake in the aorta and its major branch vessel as determined by FDG-PET/CT. In this regard, GCA is not a rare condition in Korean PMR patients. Therefore, all patients with PMR should be carefully assessed for GCA.
In this study, history of relapse was a significant independent risk factor (P < 0.05) for remission-failure of PMR (OR, 6.81; 95% CI, 1.035–44.804). In previous studies, high initial acute phase reactant level, female sex, older age, and longer duration of symptoms were reported as prognostic factors for treatment response.21 However, the factors related to treatment are still not clear. Our study showed that older age, female sex, diabetes mellitus, malignancy, and initially high levels of CRP and ESR were not risk factors of remission failure in univariate logistic analysis. History of relapse was the only significant predictor of remission-failure in multivariate analysis (OR, 6.81; 95% CI, 1.035–44.804). In our study, 23 of 34 patients (67.6%) had relapsed at least once during oral prednisolone treatment. This is consistent with a previous report; Kim et al.10 found that the relapse rate was 68.8% and the frequency of relapse was significantly higher in patients without remission (P < 0.02). These findings suggest that the presence of relapse might be an important parameter for predicting remission in PMR patients. Larger prospective studies are needed to determine predictive factors of remission in PMR patients.
The treatment for PMR is primarily based on oral glucocorticoid. The EULAR and the ACR recommend oral glucocorticoid treatment in PMR for a minimum of 12 months.12 Long-term treatment of glucocorticoid treatment is related to cardiovascular side effects and risk factors for the development of glucocorticoid-induced diabetes mellitus. Patients with an older age, higher HbA1c level and lower eGFR require close monitoring for the development of glucocorticoid-induced diabetes mellitus, regardless of the dose of glucocorticoids.22 Also, glucocorticoid induced osteoporosis is the most common cause of secondary osteoporosis. A treatment with 10 mg/day of prednisone or equivalent for more than 3 months leads to a 7-fold increase in hip fractures and a 17-fold increase in vertebral fractures.23 In a large cohort of patients from the National Database of the German Collaborative Arthritis Centers, median glucocorticoid doses ≤ 5 mg/day were reached at a 13–18 months disease duration in PMR patients. And the prevalence of osteoporosis increased within 3 years in a national rheumatology database.24 To prevent glucocorticoid induced osteoporosis the use of bone protection when initiating glucocorticoid treatment for PMR is recommended.14 We used of vitamin D and bisphosphonates routinely when initiating oral prednisolone for PMR to prevent the complications of osteoporosis and monitoring for the development of glucocorticoid-induced diabetes mellitus by blood sugar test and HbA1c level. PMR requires long-term glucocorticoid treatment longer than. If 1-year and patients are older, careful examination and monitoring of the side effects of glucocorticoid is necessary.
The diagnosis of PMR is clinically based on proximal inflammatory pain associated with increased ESR and/or CRP in the elderly. Elderly onset RA, late onset spondyloarthropathy, inflammatory myopathies, adhesive capsulitis, fibromyalgia, subacute infections, and occult malignancies can mimic PMR.25 In PMR patients, magnetic resonance imaging or ultrasonography typically shows bilateral bursitis or synovitis in the hips or shoulders.2,3 However, these radiologic findings may also occur in other inflammatory or degenerative joint diseases. In our study, only 19 patients (35.2%) were diagnosed with PMR when symptoms initially presented. Twenty-six patients (48.1%) had varying previous diagnoses before visiting our hospital. Nine patients (16.7%) had no specific diagnosis prior to diagnoses of PMR at our facility. Because of inflammatory nature of the spinal pain, 6 patients (11.1%) were misdiagnosed as spondyloarthropathy. Clinical features such as limitation of motion in the shoulder and pain aggravated by movement may lead to a misdiagnosis of adhesive capsulitis; this was the case for 6 patients (11.1%) in this study. Elevated acute phase reactants in PMR can mimic an infectious disease such as infectious arthritis or lumbar osteomyelitis. Three patients fell into this category in our study (5.6%). There are several explanations for misdiagnosis of PMR. As previously mentioned, it is difficult to exclude a variety of conditions that mimic the clinical features of PMR. New biomarkers and imaging techniques specifically related to PMR would be useful to clarify these clinical features. Lower incidence of PMR may be attributable to a lack of awareness on the part of physicians. A precise and specific approach is essential for the accurate diagnosis of PMR, and higher physician awareness may be needed.
In this present study, morning stiffness was reported in 48 patients, and it lasted 45 minutes or more in 38 patients (76%). Morning stiffness is one of the classification criteria for PMR, but the durations vary depending on the classification criterion and include > 1 hour, > 30 minutes, presence, not included as criterion.26 The 2012 EULAR/ACR criteria include > 45 minutes of morning stiffness as 2 of the clinical criteria points, and there is no consideration given to a shorter duration of morning stiffness. In this study, 20.0% of patients suffered from morning stiffness for less than 45 minutes, and 4.0% of patients did not complain of morning stiffness. Morning stiffness should be included as one classification criterion; however, 45 minutes may be too long and might decrease the sensitivity of PMR diagnosis.
Interestingly, the incidence of hip girdle pain (90.7%) in our study was higher than the incidence in previous studies.7,8,10,27,28 Mori et al.8 reported that 34% of Japanese PMR patients showed pelvic girdle pain, and a study of Korean PMR patients reported 59.6% pelvic girdle pain.10 In this study, positive provocation tests for the hip girdle, such as the Patrick test and evoked pain during squatting, were included in hip girdle pain. Provocation tests for the hip girdle could explain the higher incidence of hip girdle pain in our study. Hip girdle pain is one of the clinical features of PMR, and the 2012 EULAR/ACR classification criteria highlight the importance of hip involvement since it is 1 point on the clinical criteria. Thus, the provocation test for hip pain could be useful to facilitate diagnosis of PMR.
The incidence of PMR appears to vary in the world, with higher rates observed in studies performed in northern European populations. Variation in incidence suggests environmental factors, such as seasonal effects. Indeed, previous epidemiological studies have shown a regular cyclical pattern of PMR incidence. In a retrospective study of 201 PMR patients, the onset of PMR showed a significant winter periodicity.29 Also, Kim et al.10 reported that 45.1% of PMR patients were diagnosed in winter. A possible mechanism suggests a role for precipitating environmental factors, such as Mycoplasma pneumoniae, Chlamydia pneumoniae, and parvovirus B19. Moreover, some authors have reported seasonal variation in the immune system and inflammatory responsiveness associated with several infectious agents. However, in the present study, no seasonal preferences were observed. In several studies, authors had failed to observe seasonal variation and infectious etiology associated with PMR onset.30,31 Cimmino et al.32 had found 62% of PMR cases developed in the summer, suggesting that onset of PMR correlated with outside temperature and hours of sunshine. Peris31 had reported that onset of PMR symptoms is unrelated to the season, and Parvovirus B19 infection was unrelated to PMR onset in a 4-year prospective study. Our study findings do not support a seasonal pattern for PMR diagnosis. Since studies on seasonal patterns and PMR onset have been inconsistent, a large prospective study is needed to estimate seasonal variation.
The BSR and the BHPR recommended that atypical presentations, such as patients younger than 60 years and those with peripheral inflammatory arthritis, systemic symptoms, or very high or normal inflammatory markers, should be considered for early specialist referral, including those patients with incomplete glucocorticoid response or at high risk of therapy-related side effects.12,14 In our study, six PMR patients (11.1%) were referred to a rheumatologic specialist for further treatment. Three of six patients were also diagnosed with GCA. Remaining three patients were refractory to prednisolone therapy with uncontrolled musculoskeletal pain.
There were several limitations of this study. The first is the retrospective study design. Although we reviewed the past 8 years of data, we had some insufficient demographic data, clinical characteristics and comorbidities such as cardiovascular disease and osteoporosis. A second limitation is that 20 patients were excluded in the analysis of response to oral prednisolone treatment. And, we collected data of PMR for more than 8 years, but did not have large sample sized because our retrospective study is a single center experience. Due to small sample size, in logistic regression to analysis of predictive factors of treatment response the range of 95% CI is wide. Despite these limitations, the present study identifies more precise clinical characteristics and therapeutic response in PMR, because of the relatively large sample size in an Asian population and a standardized treatment protocol for PMR. Larger prospective studies will be needed to precisely ascertain the clinical characteristics and therapeutic response in PMR.
In conclusion, the rate of remission (35.3%) after oral prednisolone treatment was similar to previous reports from western countries, and GCA is not a rare condition in Korean PMR patients. Misdiagnosis of PMR is common; therefore, heightened awareness of PMR is needed in elderly patients with bilateral hip and/or shoulder pain. Physicians dealing with locomotive system pain should always consider PMR in elderly patients who present with the inflammatory feature of bilateral shoulder pain.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Sung DH, Do JG, Park JY. Data curation: Sung DH, Do JG, Park JY. Formal analysis: Do JG. Methodology: Sung DH, Do JG. Writing - original draft: Sung DH, Do JG, Park JY. Writing - review & editing: Sung DH, Do JG, Park JY.
References
- 1.Barber HS. Myalgic syndrome with constitutional effects; polymyalgia rheumatica. Ann Rheum Dis. 1957;16(2):230–237. doi: 10.1136/ard.16.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen CE, Buchanan RR, Hoi A. Recent advances in polymyalgia rheumatica. Intern Med J. 2015;45(11):1102–1108. doi: 10.1111/imj.12823. [DOI] [PubMed] [Google Scholar]
- 3.Pipitone N, Salvarani C. Update on polymyalgia rheumatica. Eur J Intern Med. 2013;24(7):583–589. doi: 10.1016/j.ejim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Soriano A, Landolfi R, Manna R. Polymyalgia rheumatica in 2011. Best Pract Res Clin Rheumatol. 2012;26(1):91–104. doi: 10.1016/j.berh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71(4):484–492. doi: 10.1136/annrheumdis-2011-200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Choi ST, Kim JS, Yoon BY, Kwok SK, Kim HS, et al. Clinical characteristics and prognostic factors for relapse in patients with polymyalgia rheumatica (PMR) Rheumatol Int. 2013;33(6):1475–1480. doi: 10.1007/s00296-012-2580-4. [DOI] [PubMed] [Google Scholar]
- 7.Li WL, Lo Y, Leung MH, Wong WS, Mok MY. The clinical course of polymyalgia rheumatica in Chinese. Clin Rheumatol. 2010;29(2):199–203. doi: 10.1007/s10067-009-1315-8. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Koga Y, Ito K. Clinical characteristics of polymyalgia rheumatica in Japanese patients: evidence of synovitis and extracapsular inflammatory changes by fat suppression magnetic resonance imaging. Mod Rheumatol. 2007;17(5):369–375. doi: 10.1007/s10165-007-0595-6. [DOI] [PubMed] [Google Scholar]
- 9.Salvarani C, Cantini F, Olivieri I. Distal musculoskeletal manifestations in polymyalgia rheumatica. Clin Exp Rheumatol. 2000;18(4) Suppl 20:S51–S52. [PubMed] [Google Scholar]
- 10.Kim HA, Lee J, Ha YJ, Kim SH, Lee CH, Choi HJ, et al. Induction of remission is difficult due to frequent relapse during tapering steroids in Korean patients with polymyalgia rheumatica. J Korean Med Sci. 2012;27(1):22–26. doi: 10.3346/jkms.2012.27.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Gay MA. Giant cell arteritis and polymyalgia rheumatica: two different but often overlapping conditions. Semin Arthritis Rheum. 2004;33(5):289–293. doi: 10.1016/j.semarthrit.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, et al. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheumatol. 2015;67(10):2569–2580. doi: 10.1002/art.39333. [DOI] [PubMed] [Google Scholar]
- 13.Matteson EL, Maradit-Kremers H, Cimmino MA, Schmidt WA, Schirmer M, Salvarani C, et al. Patient-reported outcomes in polymyalgia rheumatica. J Rheumatol. 2012;39(4):795–803. doi: 10.3899/jrheum.110977. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta B, Borg FA, Hassan N, Barraclough K, Bourke B, Fulcher J, et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology (Oxford) 2010;49(1):186–190. doi: 10.1093/rheumatology/kep303a. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Sung DH. Early onset polymyalgia rheumatica: two rare cases under age of 50. Skeletal Radiol. 2017;46(6):837–840. doi: 10.1007/s00256-017-2618-5. [DOI] [PubMed] [Google Scholar]
- 16.Bloch DA, Michel BA, Hunder GG, McShane DJ, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum. 1990;33(8):1068–1073. doi: 10.1002/art.1780330803. [DOI] [PubMed] [Google Scholar]
- 17.Soubrier M, Dubost JJ, Ristori JM. Polymyalgia rheumatica: diagnosis and treatment. Joint Bone Spine. 2006;73(6):599–605. doi: 10.1016/j.jbspin.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Salvarani C, Cantini F, Niccoli L, Macchioni P, Consonni D, Bajocchi G, et al. Acute-phase reactants and the risk of relapse/recurrence in polymyalgia rheumatica: a prospective followup study. Arthritis Rheum. 2005;53(1):33–38. doi: 10.1002/art.20901. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61(10):1454–1461. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- 20.Crespo RR, Menezes Falcão L. Approach to giant cell arteritis and recent evidence on its relation with cardiovascular risk. A review. Eur Geriatr Med. 2016;7(6):591–596. [Google Scholar]
- 21.Mackie SL, Hensor EM, Haugeberg G, Bhakta B, Pease CT. Can the prognosis of polymyalgia rheumatica be predicted at disease onset? Results from a 5-year prospective study. Rheumatology (Oxford) 2010;49(4):716–722. doi: 10.1093/rheumatology/kep395. [DOI] [PubMed] [Google Scholar]
- 22.Katsuyama T, Sada KE, Namba S, Watanabe H, Katsuyama E, Yamanari T, et al. Risk factors for the development of glucocorticoid-induced diabetes mellitus. Diabetes Res Clin Pract. 2015;108(2):273–279. doi: 10.1016/j.diabres.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Mazzantini M, Di Munno O. Glucocorticoid-induced osteoporosis: 2013 update. Reumatismo. 2014;66(2):144–152. doi: 10.4081/reumatismo.2014.787. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht K, Huscher D, Buttgereit F, Aringer M, Hoese G, Ochs W, et al. Long-term glucocorticoid treatment in patients with polymyalgia rheumatica, giant cell arteritis, or both diseases: results from a national rheumatology database. Rheumatol Int. 2018;38(4):569–577. doi: 10.1007/s00296-017-3874-3. [DOI] [PubMed] [Google Scholar]
- 25.González-Gay MA, Matteson EL, Castañeda S. Polymyalgia rheumatica. Lancet. 2017;390(10103):1700–1712. doi: 10.1016/S0140-6736(17)31825-1. [DOI] [PubMed] [Google Scholar]
- 26.Nesher G. Polymyalgia rheumatica--diagnosis and classification. J Autoimmun. 2014;48-49:76–78. doi: 10.1016/j.jaut.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Bird HA, Leeb BF, Montecucco CM, Misiuniene N, Nesher G, Pai S, et al. A comparison of the sensitivity of diagnostic criteria for polymyalgia rheumatica. Ann Rheum Dis. 2005;64(4):626–629. doi: 10.1136/ard.2004.025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawley A, Prior JA, Muller S, Helliwell T, Hider SL, Dasgupta B, et al. Association between characteristics of pain and stiffness and the functional status of patients with incident polymyalgia rheumatica from primary care. Clin Rheumatol. 2018;37(6):1639–1644. doi: 10.1007/s10067-017-3730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perfetto F, Moggi-Pignone A, Becucci A, Cantini F, Di Natale M, Livi R, et al. Seasonal pattern in the onset of polymyalgia rheumatica. Ann Rheum Dis. 2005;64(11):1662–1663. doi: 10.1136/ard.2005.038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narváez J, Clavaguera MT, Nolla-Solé JM, Valverde-Garcia J, Roig-Escofet D. Lack of association between infection and onset of polymyalgia rheumatica. J Rheumatol. 2000;27(4):953–957. [PubMed] [Google Scholar]
- 31.Peris P. Polymyalgia rheumatica is not seasonal in pattern and is unrelated to parvovirus b19 infection. J Rheumatol. 2003;30(12):2624–2626. [PubMed] [Google Scholar]
- 32.Cimmino MA, Caporali R, Montecucco CM, Rovida S, Baratelli E, Broggini M. A seasonal pattern in the onset of polymyalgia rheumatica. Ann Rheum Dis. 1990;49(7):521–523. doi: 10.1136/ard.49.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]