Summary
In radiation therapy for cancer, the therapeutic ratio represents an optimal balance between tumor control and normal tissue complications. As improvements in the therapeutic arsenal against cancer extend longevity, the importance of late effects of radiation increases, particularly those caused by vascular endothelial injury. Radiation both initiates and accelerates atherosclerosis, leading to vascular events like stroke, coronary artery disease, and peripheral artery disease. Increased levels of proinflammatory cytokines in the blood of long-term survivors of the atomic bomb suggest that radiation evokes a systemic inflammatory state responsible for chronic vascular side effects. In this review, the authors offer an overview of potential mechanisms implicated in radiation-induced vascular injury.
Key Words: angiogenesis, apoptosis, cytokines, senescence
Abbreviations and Acronyms: ATM, ataxia telangiectasia mutated; CD, cluster of differentiation; EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NEMO, nuclear factor kappa B essential modulator; NF-κB, nuclear factor-kappa beta; ROS, reactive oxygen species; SEK1, stress-activated protein kinase 1; TNF, tumor necrosis factor; XIAP, X-linked inhibitor of apoptosis
Central Illustration
Radiation is used to treat 50% to 60% of patients with cancer (1). Technologic improvements and the advent of multimodality therapy have extended survival times for many cancer patients, underscoring the importance of minimizing long-term treatment-induced toxicity. Radiation’s effects on cardiovascular outcomes have been documented in long-term survivors after thoracic radiation therapy (RT) for mediastinal Hodgkin lymphoma and breast malignancies (2). Head and neck RT has also been implicated in stroke (from irradiation of the carotid) and in metabolic syndrome (from irradiation of the pituitary). Epidemiologic studies of atomic bomb survivors showed that their relative risk of coronary events increased by 14% per Gy of radiation and that for stroke by 9% per Gy, in addition to increasing the incidence of cirrhosis and chronic kidney disease, both implicated in cerebrovascular and cardiovascular disease (3). Cancer clinical trials often do not prospectively evaluate cardiovascular mortality and morbidity, resulting in under-reporting of this treatment-related toxicity. Nonetheless there is accumulating clinical evidence of vascular consequences from exposure to ionizing radiation, and a comprehensive understanding of the underlying molecular and pathophysiological mechanisms is necessary, as we begin to catalog, define, and find remedies for this problem. This review provides an overview of the current knowledge about radiation-induced vascular endothelial injury.
A unifying theme in radiation injury to many organ sites (e.g., liver, heart, kidney, gastrointestinal tract, skin, brain, and lungs) is the organs’ high concentration of highly radiosensitive capillaries lined with endothelial cells (ECs). Outcomes of vascular injury at specific subsites are not fully characterized, and cause-and-effect relationships have not been fully established. The clinical manifestation of vascular injury to a specific subsite is driven by its function, extent of injury, and time lapsed since that injury. It is within this context that there is a need for greater understanding of the pathophysiology of radiation effects on the micro- and macrovasculature, particularly to guide strategies to limit long-term deleterious effects by using drugs targeted to specific molecular pathways. The abundant expression of nitric oxide, thrombomodulin, prostacyclin, endothelin, platelet-activating factor, and cell adhesion molecules by ECs suggest possible signaling cascades that may play a role in mediating anti-inflammatory, antifibrogenic, anticoagulant, antiatherogenic, and vasoactive effects (4). In addition, radiation-induced damage to the vascular endothelium could shift the balance of pro- and anti-inflammatory cytokines, release a burst of reactive oxygen species (ROS); dysregulate glycolysis, lipid metabolic pathways or angiogenesis; disrupt telomere function; and/or perturb immunity homeostasis. Like other known off-target effects of radiation (e.g., bystander effect and paracrine and clastogenic effects), these acute effects may trigger long-term vascular dysfunction if unchecked or inadequately compensated for.
In this review, drawn largely from in vitro and in vivo experiments (which often involve radiation fraction sizes not routinely used in clinical practice), we summarize current knowledge of the incidence, severity, molecular mechanisms, and consequences of radiation-induced damage to the vascular endothelium. Appreciation of the mechanistic underpinnings of such consequences of radiation could open doors to recognizing these effects, monitoring for them, and evaluating strategies to mitigate or protect against them.
Radiation Effects on Endothelial Cells
Among all blood vessels that typically have 3 layers, the tunica intima, media, and adventitia, capillaries are the most radiosensitive because they have just a single layer of endothelium, the tunica intima, which is exquisitely susceptible to ionizing radiation (5). Notably, this heightened sensitivity is not a consequence of the relative abundance of capillaries compared with that of larger caliber vessels. In comparing vessels of different calibers, capillary rarefaction with disruption, extravasation, micropetechiae formation, and inflammatory changes were more pronounced with microcascular irradiation than macrovascular irradiation.
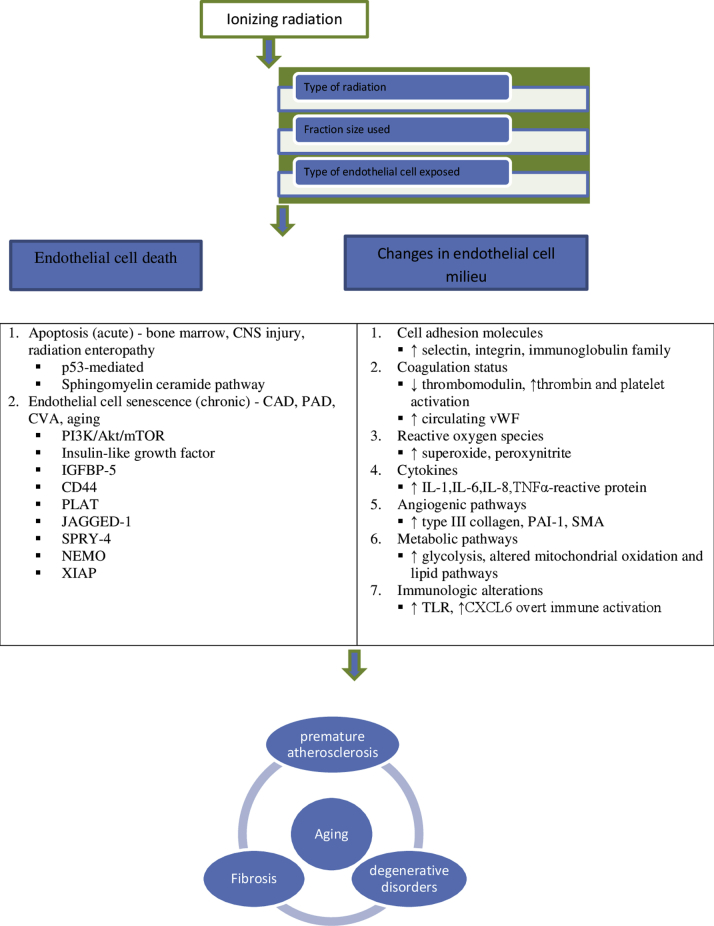
ECs respond to radiation in several ways depending on the radiation dose and the organ in which they reside. Acute effects appear in days to weeks, whereas chronic effects can take months to years to manifest. Acute radiation effects, especially in the gastrointestinal tract, hematopoietic system, and lungs, are triggered by EC apoptosis. Chronic effects reflect EC senescence in coronary arteries, cerebral circulation, and lung, liver, and peripheral arteries. In response to conventionally fractionated RT, approximately 90% of ECs experience mitotic cell death without apoptosis, whereas a larger fractional dose of radiation can induce apoptotic cell death. In addition to radiation dose, fraction size used and intrinsic nature of the vasculature that is distinct to each organ, another factor that defines the response of vessels to ionizing radiation, is the degree of differentiation of ECs. It is increasingly recognized that endothelial progenitor cells (EPCs) are seen not only in embryonic tissues but in bone marrow, and other tissues have a reservoir of EPCs that actively contribute to vascular remodeling; maladaptive functioning of EPCs contributes to vascular dysfunction (6). Notably, if higher doses (≥10 Gy) are used, EPCs undergo p53 stabilization, p21-mediated cell cycle arrest, and Bax-mediated apoptosis, whereas well-differentiated ECs undergo cellular senescence similar to aging and premature atherosclerosis (7). The mode of EC death or functional impairment could then stimulate a chronic inflammatory state or acute capillary rarefaction and, consequently, leaky and disordered vascular networks that alter normal vascular homeostasis. We highlight these physiological responses of ECs to radiation first and then explore the molecular mechanisms and signaling pathways involved.
Endothelial senescence
EC senescence has been implicated in long-term vascular dysfunctional states like premature atherosclerosis, chronic lung disease, and aging (8). Senescence results from telomere shortening (replicative senescence), increased oncogene expression, or DNA damage. Senescence induced by DNA damage from irradiation is distinct from replicative or oncogene-induced senescence in that it does not involve epigenetic aging (9). Senescence in ECs leads to a senescence-associated secretory phenotype in which cytokines, proteins, and other factors secreted by ECs cause dysfunction of adjacent cells or lead to a chronic inflammatory state.
One group of investigators quantified the responses of human cord blood and those of adult ECs to various doses and fractions of 160 kVp x-rays. Both types of ECs had negligible levels of apoptosis at 3 Gy, but as the dose increased to 10 Gy, the cord ECs exhibited high levels of annexin V staining (apoptosis), and the adult ECs showed increased β-galactosidase staining (senescence) (7). Therefore, the degree of maturation of ECs and radiation fraction size define the mode of EC death after RT. In a similar study of human umbilical vein endothelial cells (HUVECs), irradiation from 2 to 4 Gy increased the expression of β-galactosidase and several senescence-associated genes (e.g., insulin-like growth factor binding protein 5 [IGFBP-5], CD44, plasminogen activator, JAG1, and Sprout homolog 4) (10). That group also showed that IGFBP-induced senescence was mediated by p53 independent of p16 in ECs, but in other cells, p53 was required to initiate senescence, and p16 was required to maintain it (11). Others investigators have found that replicative senescence mediated by p53 with enhanced p16 expression is irreversible, but such senescence is reversible if p16 is not up-regulated (12). Another group found that chronic low-dose irradiation to HUVECs led to inactivation of the Akt/PI3K/mTOR pathway. Partial premature senescence was noted after exposure to 1.4 mGy/h, and full premature senescence was observed after 2.4 mGy/h. Total Akt showed a biphasic response, with a spike at weeks 1 and 10, whereas phosphorylated Akt, PI3K, and mTOR were reduced at week 10. Downstream of the Akt/PI3K/mTOR pathway, Rho cytoskeletal proteins were also downregulated, suggesting 1 possible mechanism by which senescence led to slower growth and perhaps increased vascular permeability (13). This finding is consistent with reports that mTOR regulates actin polymerization and interaction with cell adhesion molecules like integrin, thus affecting vascular smooth muscle contractility 13, 14.
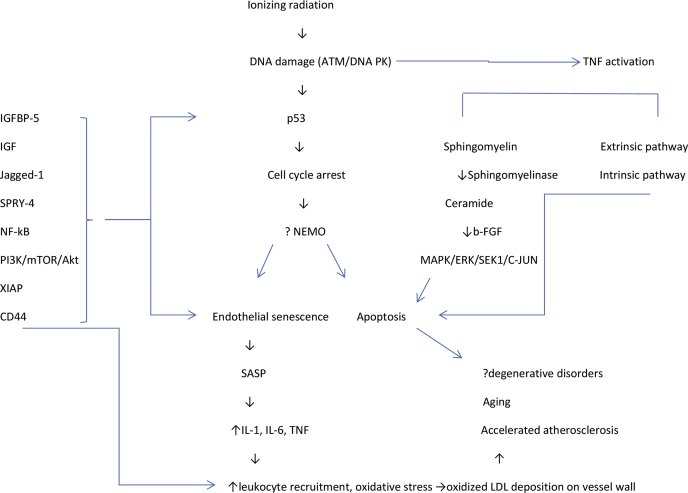
A well-described marker of age-related atherosclerotic progression is surface expression of the cell adhesion protein CD44 on senescent ECs, which evokes greater adhesion of monocytes to ECs, a key factor in atherosclerosis (15). One study showed that irradiating human coronary ECs led to increased CD44 expression and adhesion of monocytes to ECs, similar to cells undergoing replicative senescence. The initiating event for the adhesion was found to be demethylation of the CD44 promoter and, consequently, epigenetic activation of CD44 (16). Another group found that irradiating pulmonary vascular ECs with 2 to 50 Gy led to apoptotic rather than necrotic death and also accelerated senescence. Although classic features of endoplasmic reticulum stress were observed, they were not essential for the development of senescence, suggesting that radiation-induced endoplasmic reticulum stress can induce apoptosis but not contribute to senescence (17). Another gene product often upregulated in response to radiation is nuclear factor-kappa beta (NF-κB). In one study, treating HUVECs with a small-molecule inhibitor of the NF-κB essential modulator (NEMO) blocked radiation-induced activation of NF-κB and transcription of interleukin (IL)-6 (which modulates senescence) and p53-induced death domain (which modulates apoptosis). These findings suggest that DNA strand breaks induced by radiation activate NEMO/NF-κB signaling to drive a senescence-associated phenotype in ECs (18). Induction of another downstream gene product of NF-κB activation, X-linked inhibitor of apoptosis-associated factor (XAF)-1, by radiation also increased senescence in human pulmonary microvascular EC; that study further confirmed that XAF1 is transcriptionally regulated by bromodomain 7 and mediates the induction of senescence by p53, independent of p16 (19). Increased accumulation of senescent cells may overwhelm the capacity for endogenous clearance, leading to a constellation of inflammatory cytokines, chemokines, and extracellular proteases (collectively termed “senescence-associated secretory phenotype”) implicated in aging-related vascular abnormalities (20). Collectively, these findings reinforce the notion that senescence is a major contributor to radiation-induced endothelial dysfunction and that pathways of EC senescence are varied and diverse and extend beyond the ECs themselves.
Apoptotic cell death
Acute radiation side effects reflect a combination of epithelial and vascular damage, with the affected organ determining which one predominates. In addition to senescence, ionizing radiation can also induce apoptotic cell death; indeed, apoptotic cell death of vascular ECs has been implicated in acute radiation syndrome, but its role in long-term sequelae is largely unknown. Also unclear are which molecular mechanisms dictate when ECs undergo senescence, apoptosis, or both.
p53-mediated apoptosis
Radiation-induced apoptotic cell death can be mediated by p53 or by the sphingomyelin ceramide pathway. Early stages of p53-mediated apoptosis are reversible, but whether activation of the sphingomyelin ceramide pathway is reversible is unclear. p53-Mediated apoptosis of ECs is mediated predominantly by the intrinsic pathway. Radiation-induced DNA damage leads to activation of the ataxia telangiectasia-mutated (ATM) gene and DNA-dependent protein kinase, which then phosphorylates p53 and triggers cell cycle arrest. Depending on the context of EC irradiation, cells either repair the DNA damage or initiate apoptotic cell death by the cytochrome c-mediated mitochondrial pathway (intrinsic), the tumor necrosis factor (TNF) death receptor pathway (extrinsic), or the activation of the sphingomyelin ceramide pathway (21).
Sphingomyelin ceramide pathway
Apoptosis in ECs after high-dose single-fraction irradiation is modulated primarily through the sphingomyelin ceramide pathway (22). Sphingomyelin is a phospholipid present in the outer leaflet of plasma membranes; sphingomyelinase is an enzyme present outside the cell or within lysosomes that exists in acidic, neutral, and alkaline isoforms. Irradiation activates TNF, which leads to hydrolysis of sphingomyelin, thereby generating ceramide. Extracellular acidic sphingomyelinase is responsible for radiation-induced, ceramide-mediated EC apoptosis (23). Once ceramide is generated, it activates MAPK, ERK, stress-activated protein kinase/ERK kinase 1 (SEKl), and c-Jun, which modify cell membrane dynamics and prompt a cascade of events culminating in EC apoptosis (24). Radiation-induced ceramide generation can occur independently of DNA damage, as has been shown in bovine ECs devoid of nuclei. Ceramide acts as a second messenger by activating effector systems like Ras (25), RAC (26), phospholipase A2 (27), protein kinase c (28), and ceramide-activated protein phosphatase. Activation of protein kinase c by ceramide is known to cause apoptosis (29). One group found that human lymphoblasts deficient in sphingomyelinase were deficient in radiation-induced apoptosis and that this phenomenon could be reversed with sphingomyelinase supplementation. Studies of pulmonary ECs showed that ceramide-mediated apoptosis may also be responsible for acute radiation pneumonitis but could be counteracted by basic fibroblast growth factor (30), similar to another study in which this growth factor counteracted radiation-induced apoptosis in microvessel ECs from bovine adrenal glands (31). Secretory sphingomyelinase has also been implicated in the formation of atherosclerotic plaques; specifically, the interaction of secretory sphingomyelinase with subendothelial aggregates of low-density lipoprotein leads to the formation of macrophage-engulfed foam cells, which then leads to the development of atheroma 32, 33. Whether the radiation-induced apoptosis of ECs can mediate this process is unclear. However, one might envision large numbers of apoptotic cells overwhelming the renewal capability of the normal cell cycle machinery and accumulation of apoptotic cells mimicking the pathophysiology of chronic aging-related disorders. Figure 1 illustrates the dynamic interplay between radiation-induced apoptosis and senescence and its contribution to radiation-induced vascular disease
Figure 1.
Proposed Interplay Between Apoptosis and Endothelial Senescence and Its Implications for Pathogenesis
ATM = ataxia telangiectasia mutated; b-FGF = basic fibroblast growth factor; ERK = extracellular signal-regulated kinase; IGF = insulin-like growth factor; LDL = low-density lipoprotein; MAPK = mitogen-activated protein kinase; SASP = senescence-associated secretory phenotype; SEK1 = stress-activated protein kinase 1; TNF = tumor necrosis factor.
Radiation Effects on Cell Adhesion Molecules and Vascular Homeostasis
Cell adhesion molecules
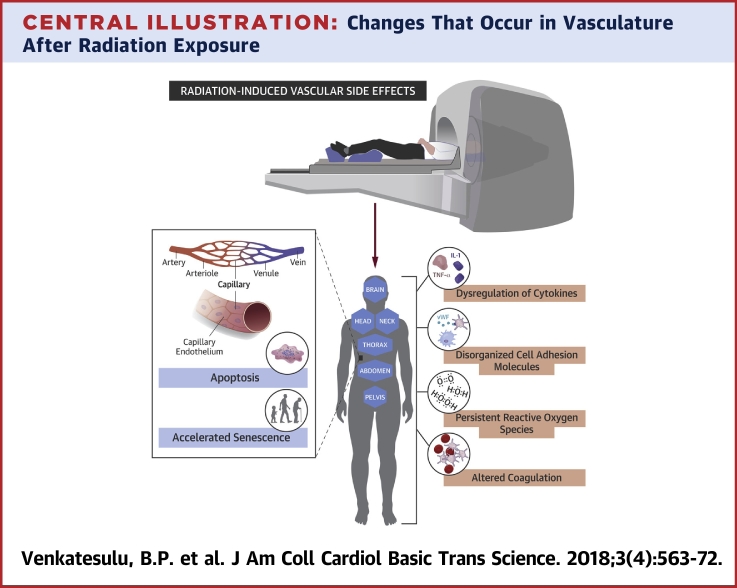
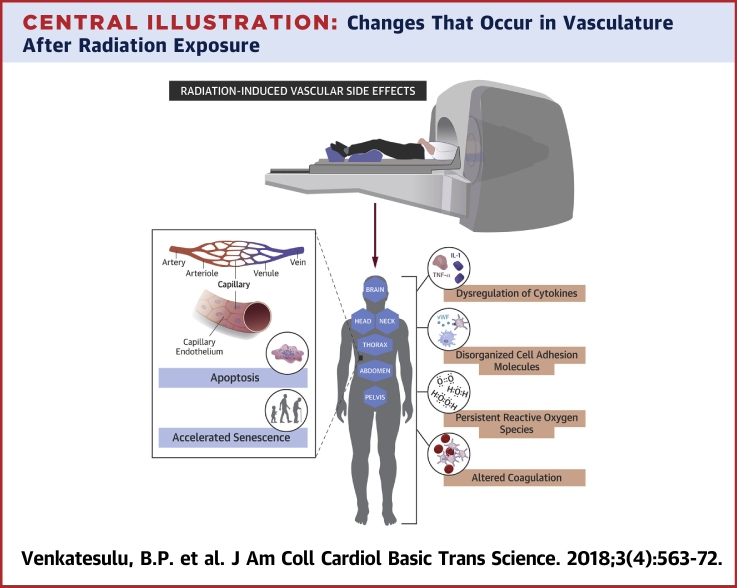
In the vascular compartment, an optimal balance is maintained between pro- and anticoagulatory and pro- and anti-inflammatory factors. Any insult, including radiation-induced EC dysfunction and activation, affects the vascular milieu. Radiation increases the expression of selectins, integrins, and immunoglobulin superfamily members like intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and platelet endothelial cell adhesion molecule-1 by the vascular endothelium (34). For example, exposing dermal microvascular cells to 10 Gy led to increased expression of E-selectin and VCAM-1 (35). Radiation also increases the adhesiveness of leukocytes and macrophages through increased expression of CD44. Radiation-induced expression of E-selectin in human EC lines is dose- and time-dependent, can occur after doses as low as 0.5 Gy, and can occur independent of cytokine activation (36). Exposure of buccal mucosal samples to 60 Gy prompted an increase in expression of ICAM-1 and E-selectin but not VCAM over baseline levels (37). All of these adhesion molecules are known mediators of acute and chronic inflammatory reactions and promote macrophage recruitment to ECs.
Radiation-induced coagulopathy
Thrombomodulin, a membrane glycoprotein expressed on the surface of intact endothelium, is a cofactor of thrombin, converting it from a procoagulant to an anticoagulant form. Oxidative damage and inflammatory mediators like TNF-α and IL-1 activated by radiation can downregulate thrombomodulin, which increases free thrombin and activates platelets to create a prothrombotic state. This process may mediate acute radiation-induced enteropathy (38) and radiation-induced liver disease that manifests as hepatic sinusoidal occlusion secondary to endothelial injury (39). Similarly, plasminogen activator inhibitor (PAI)-1 is a fibrinolytic inhibitor activated by radiation, knocking out PAI-1 reduced radiation-induced EC death to 7% to 11% versus 35% to 37% in intact cells (40). The von Willebrand factor is an acute-phase reactant constitutively expressed in normal ECs that is increased by radiation, leading to a prothrombotic state. The increased expression of cell adhesion molecules with macrophage attachment to the endothelium characteristic of a prothrombotic state can contribute to a chronic inflammatory state. Dysregulation of coagulation homeostasis can also result in hemorrhage, as noted in accidental low-dose whole-body irradiation exposures (39) and total-body irradiation in preparation for stem cell transplantation.
Radiation-Induced Effects on ROS and Cytokines
Reactive oxygen species
The ROS-induced endothelial injury resulting from radiation seems similar to reperfusion injury in myocardial ischemia in terms of increased ROS, platelet, and leukocyte activation; altered calcium homeostasis; and disordered metabolism. Intracellular levels of oxidative stress from rapid hydrolysis of water, resulting in the production of hydroxide, superoxide, and hydroxyl radicals, is modulated by xanthine oxidase and nicotinamide diphosphate, which are implicated in endothelial vascular injury 41, 42. In one study, generation of free radicals in the mesenteric circulation was noted to be secondary to leukocyte activation and adherence as determined by leukocyte blockade with anti-CD18 antibodies (43). Other studies showed that vasomotor responses mediated by nitric oxide and prostacyclin were impaired after irradiation of human cervical arteries; this impaired response was thought to contribute to vessel stenosis and impaired laminar blood flow (44). Radiation can impair vasomotor responses, both endothelium-dependent (acetylcholine and substance P) and endothelium-independent (calcitonin gene-related peptide). Finally, the reaction of nitric oxide with ROS leads to the production of peroxynitrite, which can cause nitrosylation of tyrosine residues of proteins and impair protein function (45). Thus, radiation-induced increases in ROS affect both the vessel vasomotor response and the structure of the endothelium. The cardiac myocytes have abundant plasma membrane phospholipids that are prone to lipid peroxidation, making them vulnerable to oxidative stress 46, 47. Experimental studies in cardiac myocytes have demonstrated the role of free radicals in precipitating impaired contractile function, increased muscle tension at rest, and altered energy production heralding a disordered state (48). Similarly, radiation-induced NF-κB activation can induce an inflammatory state with increased expression of adhesion molecules and cytokines, akin to induction of ICAM, TNF-α, and NF-κB following oxidative stress 49, 50. Reports have shown persistent expression of NF-κB in the vessels of the neck even 10 years post-radiation, suggesting a possible link between NF-κB and chronic fibrosis (51). In addition, the free radicals produced by macrophages tend to stimulate TGF-β and accelerate the profibrotic milieu in the vasculature. This may lead to vessel stenosis, leading to increased risk of ischemic events in the long term. A rational corollary to the recognition of the detrimental effects of radiation-induced oxidative stress is to consider blocking production or persistence of free radicals. This is, however, not entirely straightforward because the relative contribution of multiple species of radicals is not sufficiently well characterized; the intervention with a selective pharmacologic inhibitor may need to occur with each fraction of radiation and relatively contemporaneously with the radiation given the short half-life of these radicals; and the potential for these drugs to equally protect the tumor from radiation. Nonetheless, the use of superoxide dismutase mimetics with potent catalytic activity rivaling or surpassing that of the native enzyme has shown promise in multiple clinical scenarios (52) including radiation-induced oral mucositis (53). Alternative strategies could include efforts to abrogate downstream effectors of radiation-induced reactive oxygen species such as NF-κB, again, a strategy that has been explored in radiation-induced normal tissue toxicity reduction through inhibition of the inflammatory response (54) and, paradoxically, in tumor sensitization to radiation therapy through abrogation of the prosurvival signaling pathway induction 55, 56.
Cytokines
Cytokines are important signaling factors produced by ECs in response to irradiation that can mediate autocrine, paracrine, or endocrine bystander effects. Senescent and apoptotic cells also secrete cytokines that can contribute to long-term vascular dysfunction. Radiation-evoked cytokine profiles reflect radiation type, dose and dose rate, irradiated volume, and irradiated organ. One group of investigators showed that rats exposed to 10 Gy of whole-body irradiation had increased levels of IL-1, IL-6, and TNF-α mRNA at 3 and 6 h after irradiation (57). Irradiation of HUVECs in another study led to elevated levels of IL-6 and IL-8 (58). In addition to inducing these cytokine changes, a study of mice receiving a single dose of 20-Gy cardiac irradiation noted reduced contractile reserve and ejection fraction on echocardiography and left ventricular catheterization, whereas IL-1 receptor type 1 knock-out mice and IL-1 receptor antagonist (anakinra)-treated mice had preserved contractile reserve (59). Doses as low as 1 cGy can cause transient up-regulation of several cytokine genes that could mediate the acute inflammatory response to irradiation of the vasculature (60). Late radiation-induced cytokine changes in Hiroshima atomic bomb survivors included increases in C-reactive protein and IL-6, possibly accounting for the noncancer-related diseases in this population (61). Blood samples from survivors of the Chernobyl disaster showed chromosomal aberrations (clastogenic) even decades after exposure. Radiation-induced changes in cytokine genes could also contribute to long-term systemic effects (62). Cytokines recruit macrophages to the endothelium and contribute to oxidative stress, which is responsible for oxidation and deposition of low-density lipoproteins, leading to atherosclerotic plaques. Plaques are destabilized by inhibiting apoptosis in the extracellular matrix and by promoting it in smooth muscle, another possible mechanism for the accelerated atherosclerosis noted in cancer survivors exposed to radiation. Countering the cytokine changes induced by radiation as a strategy to mitigate radiation-induced endothelial injury will require judicious selecting of appropriate cytokine agonists/antagonists, careful synchronizing of the timing of cytokine blockade with radiation therapy, and monitoring of the myriad downstream pathways and physiological changes induced by these cytokines. In an era of increasing use of immunotherapy with radiation therapy, it is likely that such approaches combining radiation with cytokine therapy 63, 64 will be explored in the clinic, affording an opportunity to monitor endothelial injury and its consequences.
Radiation Effects on Angiogenic Pathways
Angiogenesis is a physiological process by which new vessels form from pre-existing mesodermal progenitors. The radiosensitivity of ECs depends on the organ site, with dermal microvascular ECs being the most radiosensitive and liver sinusoidal ECs the most radioresistant. Late effects of radiation such as radiation cystitis, radiation proctitis, and skin telangiectasia are mediated through EC injury (65). In dermal ECs, irradiation with 4 Gy inhibited capillary tube formation, but the same dose promoted capillary tube formation in liver ECs, which may contribute to late liver fibrosis. High-dose single-fraction radiation to the rat cerebral vasculature resulted in the formation of arterial aneurysms, adhesion of leukocytes to vessels, formation of thrombi, and reduced blood flow (66). Irradiation of bovine and porcine aortic ECs led to increased expression of basic fibroblast and platelet-derived growth factors, both well-known promoters of angiogenesis (67). As little as 1 Gy of low linear energy transfer radiation increased the expression of angiogenesis microRNAs and capillary formation in HUVECs in a dose-dependent manner (68). In another study, irradiation of dermal microvascular ECs promoted the expression of connective tissue growth factor, collagen type III, PAI-1, and α-smooth muscle actin in smooth muscle cells, leading to a profibrogenic phenotype (69). Such cross-talk could perturb the fine balance between pro- and anticoagulant pathways and disrupt laminar blood flow within vessels. Another unexpected finding was the differences between effects evoked by proton and those by photon irradiation: low linear energy transfer photons cause tumor angiogenesis, but low linear energy transfer protons are antiangiogenic through the down-regulation of cytokines and signaling molecules such as vascular endothelial growth factor, IL-6, IL-8, and hypoxia-inducible factor-1α 70, 71. Collectively, these observations implicate radiation-induced effects on ECs in both tumors and normal tissues in the modulation of angiogenesis and neovascularization.
Radiation Effects on Metabolic Pathways and Immunologic Bystander Effects
Radiation-induced oxidative stress affects the likelihood and severity of EC injury through its effects on glycolysis, oxidative phosphorylation, and the Kreb cycle. Excessive radiation-induced ROS can disrupt the electron transport chain and cause denaturation of proteins and changes in the electrochemical gradient of the mitochondrial membrane that lead to energy depletion, which can contribute to premature aging and degenerative abnormalities that resemble mitochondrial oxidative disorders (72). Radiation further increases the expression of monocarboxylate transporter, leading to extrusion of lactate to the exterior of the cell and activation of adenosine monophosphate–activated protein kinase, leading to cellular senescence. Metabolic perturbations such as these have been implicated in radiation-induced bystander effects (73).
In addition to directly and indirectly killing ECs, radiation also evokes an immune-mediated phenomenon involving recruitment of immune cells to a particular site. Radiation-induced EC death induces cytokines like TNF, which activate macrophages; chemokines like CXCL6, which recruit immune cells; and signals that activate Toll-like receptors on dendritic cells, all of which contribute to a tumoricidal effect (74). Although exactly how such immune activation causes vascular injury is inadequately understood. It seems reasonable to assume that, like autoimmune disorders, vasculitis, immune perturbations by radiation can contribute to long-term vascular dysfunction. Figure 2 and the Central Illustration collectively portray the mechanisms that contribute to radiation-induced vascular injury.
Figure 2.
Possible Mechanisms Implicated in Radiation-Induced Vascular Injury
CAD = coronary artery disease; CNS = central nervous system; CVA = cerebrovascular accident; CXCL = chemokine ligand; IGFBP-5 = insulin-like growth factor binding protein; NEMO = nuclear factor kappa B essential modulator; PAD = peripheral artery disease; PAI = plasminogen activator inhibitor; SMA = smooth muscle actin; SPRY = sprout homolog; TLR = Toll-like receptor; vWF = von Willebrand factor; XIAP = X-linked inhibitor of apoptosis.
Central Illustration.
Changes That Occur in Vasculature After Radiation Exposure
IL = interleukin; TNF = tumor necrosis factor; vWF = von Willebrand factor.
Conclusions
Endothelial injury is increasingly recognized as a consequence of cancer treatment. Radiation directly affects the vasculature by causing EC apoptosis and senescence and by changing aspects of normal vascular homeostasis. The altered milieu at the endothelial surface may contribute to a systemic chronic inflammatory state that, superimposed on the cascade of normal aging processes, leads to acceleration of age-related disorders such as atherosclerosis and chronic fibrosis. A greater understanding of the mechanisms underlying these processes could provide insights for devising strategies to prevent or overcome radiation-induced endothelial damage.
Acknowledgment
The authors thank Christine Wogan, MS, ELS, Division of Radiation Oncology, MD Anderson Cancer Center, for editorial contributions.
Footnotes
Supported in part by National Institutes of Health grants HL-130193, HL-123346, and HL-118462 to Dr. Abe; and grant P30 CA16672 to the University of Texas MD Anderson Cancer Center. Dr. Krishnan holds the John E. and Dorothy J. Harris Endowed Professorship. All authors have reported that they have no relationships with industry relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 2.Darby S.C., Ewertz M., Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368:252–257. doi: 10.1056/NEJMc1304601. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu Y., Kodama K., Nishi N., Kasagi F., Suyama A., Soda M. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura S., Yamaguchi M., Shimizu N., Abiko Y. Mechanical stress enhances expression and production of plasminogen activator in aging human periodontal ligament cells. Mech Ageing Dev. 2000;112:217–231. doi: 10.1016/s0047-6374(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrievich G.S., Fischer-Dzoga K., Griem M.L. Radiosensitivity of vascular tissue. I. Differential radiosensitivity of capillaries: a quantitative in vivo study. Radiat Res. 1984;99:511–535. [PubMed] [Google Scholar]
- 6.Doyle B., Metharom P., Caplice N.M. Endothelial progenitor cells. Endothelium. 2006;13:403–410. doi: 10.1080/10623320601061656. [DOI] [PubMed] [Google Scholar]
- 7.Mendonca M.S., Chin-Sinex H., Dhaemers R., Mead L.E., Yoder M.C., Ingram D.A. Differential mechanisms of x-ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. Radiat Res. 2011;176:208–216. doi: 10.1667/rr2427.1. [DOI] [PubMed] [Google Scholar]
- 8.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;2010:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe D., Horvath S., Raj K. Epigenetic clock analyses of cellular senescence and ageing. Oncotarget. 2016;7:8524–8531. doi: 10.18632/oncotarget.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.S., Kim J.E., Choi K.J., Bae S., Kim D.H. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int J Radiat Biol. 2014;90:71–80. doi: 10.3109/09553002.2014.859763. [DOI] [PubMed] [Google Scholar]
- 11.Kim K.S., Seu Y.B., Baek S.-H., Kim M.J., Kim K.J., Kim J.H. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beauséjour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yentrapalli R., Azimzadeh O., Sriharshan A., Malinowsky K., Merl J., Wojcik A. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One. 2013;8:e70024. doi: 10.1371/journal.pone.0070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M.A., Hall A. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 15.Mun G.I., Boo Y.C. Identification of CD44 as a senescence-induced cell adhesion gene responsible for the enhanced monocyte recruitment to senescent endothelial cells. Am J Physiol Heart Circ Physiol. 2010;298:H2102–H2111. doi: 10.1152/ajpheart.00835.2009. [DOI] [PubMed] [Google Scholar]
- 16.Lowe D., Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 2014;13:900–910. doi: 10.1111/acel.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panganiban R.A.M., Mungunsukh O., Day R.M. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int J Radiat Biol. 2013;89:656–667. doi: 10.3109/09553002.2012.711502. [DOI] [PubMed] [Google Scholar]
- 18.Dong X., Tong F., Qian C., Zhang R., Dong J., Wu G. NEMO modulates radiation-induced endothelial senescence of human umbilical veins through NF-κB signal pathway. Radiat Res. 2015;183:82–93. doi: 10.1667/RR13682.1. [DOI] [PubMed] [Google Scholar]
- 19.Heo J.-I., Kim W., Choi K.J., Bae S., Jeong J.-H., Kim K.S. XIAP-associating factor 1, a transcriptional target of BRD7, contributes to endothelial cell senescence. Oncotarget. 2016;7:5118–5130. doi: 10.18632/oncotarget.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seideman J.H., Stancevic B., Rotolo J.A., McDevitt M.R., Howell R.W., Kolesnick R.N. Alpha particles induce apoptosis through the sphingomyelin pathway. Radiat Res. 2011;176:434–446. doi: 10.1667/rr2472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathishkumar S., Boyanovsky B., Karakashian A.A., Rozenova K., Giltiay N.V., Kudrimoti M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4:979–986. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez I., Hughes R.T., Mayer B.J., Yee K., Woodgett J.R., Avruch J. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;1994(372):794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Yao B., Delikat S., Bayoumy S., Lin X.H., Basu S. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 26.Gulbins E., Coggeshall K.M., Brenner B., Schlottmann K., Linderkamp O., Lang F. Fas-induced apoptosis is mediated by activation of a Ras and Rac protein-regulated signaling pathway. J Biol Chem. 1996;271:26389–26394. doi: 10.1074/jbc.271.42.26389. [DOI] [PubMed] [Google Scholar]
- 27.Huwiler A., Brunner J., Hummel R., Vervoordeldonk M., Stabel S., van den Bosch H. Ceramide-binding and activation defines protein kinase c-Raf as a ceramide-activated protein kinase. Proc Natl Acad Sci U S A. 1996;93:6959–6963. doi: 10.1073/pnas.93.14.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashiwagi K., Shirai Y., Kuriyama M., Sakai N., Saito N. Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J Biol Chem. 2002;277:18037–18045. doi: 10.1074/jbc.M111761200. [DOI] [PubMed] [Google Scholar]
- 29.Fuks Z., Persaud R.S., Alfieri A., McLoughlin M., Ehleiter D., Schwartz J.L. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 30.Langley R.E., Bump E.A., Quartuccio S.G., Medeiros D., Braunhut S.J. Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer. 1997;75:666–672. doi: 10.1038/bjc.1997.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santana P., Peña L.A., Haimovitz-Friedman A., Martin S., Green D., McLoughlin M. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 32.Xu X.X., Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J Biol Chem. 1991;266:24849–24858. [PubMed] [Google Scholar]
- 33.Baluna R.G., Eng T.Y., Thomas C.R. Adhesion molecules in radiotherapy. Radiat Res. 2006;166:819–831. doi: 10.1667/RR0380.1. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakarpandian B., Goetz D.J., Swerlick R.A., Chen X., Kiani M.F. Expression and functional significance of adhesion molecules on cultured endothelial cells in response to ionizing radiation. Microcirculation. 2001;8:355–364. doi: 10.1038/sj/mn/7800105. [DOI] [PubMed] [Google Scholar]
- 35.Hallahan D., Clark E.T., Kuchibhotla J., Gewertz B.L., Collins T. E-selectin gene induction by ionizing radiation is independent of cytokine induction. Biochem Biophys Res Commun. 1995;217:784–795. doi: 10.1006/bbrc.1995.2841. [DOI] [PubMed] [Google Scholar]
- 36.Handschel J., Prott F.J., Sunderkötter C., Metze D., Meyer U., Joos U. Irradiation induces increase of adhesion molecules and accumulation of beta2-integrin-expressing cells in humans. Int J Radiat Oncol Biol Phys. 1999;45:475–481. doi: 10.1016/s0360-3016(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 37.Pathak R., Wang J., Garg S., Aykin-Burns N., Petersen K.-U., Hauer-Jensen M. Recombinant thrombomodulin (solulin) ameliorates early intestinal radiation toxicity in a preclinical rat model. Radiat Res. 2016;186:112–120. doi: 10.1667/RR14408.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sempoux C., Horsmans Y., Geubel A., Fraikin J., Van Beers B.E., Gigot J.F. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128–134. doi: 10.1002/hep.510260117. [DOI] [PubMed] [Google Scholar]
- 39.Krigsfeld G.S., Kennedy A.R. Is disseminated intravascular coagulation the major cause of mortality from radiation at relatively low whole body doses? Radiat Res. 2013;180:231–234. doi: 10.1667/RR3321.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abderrahmani R., François A., Buard V., Tarlet G., Blirando K., Hneino M. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PLoS One. 2012;7:e35740. doi: 10.1371/journal.pone.0035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallance P., Hingorani A. Endothelial nitric oxide in humans in health and disease. Int J Exp Pathol. 1999;80:291–303. doi: 10.1046/j.1365-2613.1999.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turer A.T., Hill J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panés J., Granger D.N. Neutrophils generate oxygen free radicals in rat mesenteric microcirculation after abdominal irradiation. Gastroenterology. 1996;111:981–989. doi: 10.1016/s0016-5085(96)70065-3. [DOI] [PubMed] [Google Scholar]
- 44.Sugihara T., Hattori Y., Yamamoto Y., Qi F., Ichikawa R., Sato A. Preferential impairment of nitric oxide-mediated endothelium-dependent relaxation in human cervical arteries after irradiation. Circulation. 1999;100:635–641. doi: 10.1161/01.cir.100.6.635. [DOI] [PubMed] [Google Scholar]
- 45.Beckman J.S., Crow J.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 46.Przybyszewski W.M., Widel M., Rzeszowska-Wolny J. (Cardiotoxic consequences of ionizing radiation and anthracyclines) Postepy higieny i medycyny doswiadczalnej (Online) 2006;60:397–405. [PubMed] [Google Scholar]
- 47.Antunes F., Han D., Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to HO(2) detoxification in in vivo conditions. Free Radic Biol Med. 2002;33:1260–1267. doi: 10.1016/s0891-5849(02)01016-x. [DOI] [PubMed] [Google Scholar]
- 48.Singal P.K., Khaper N., Palace V., Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res. 1998;40:426–432. doi: 10.1016/s0008-6363(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 49.Molla M., Gironella M., Salas A., Closa D., Biete A., Gimeno M. Protective effect of superoxide dismutase in radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2005;61:1159–1166. doi: 10.1016/j.ijrobp.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Akpolat M., Gulle K., Topcu-Tarladacalisir Y., Safi Oz Z., Bakkal B.H., Arasli M. Protection by L-carnitine against radiation-induced ileal mucosal injury in the rat: pattern of oxidative stress, apoptosis and cytokines. Int J Radiat Biol. 2013;89:732–740. doi: 10.3109/09553002.2013.787176. [DOI] [PubMed] [Google Scholar]
- 51.Halle M., Gabrielsen A., Paulsson-Berne G., Gahm C., Agardh H.E., Farnebo F. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol. 2010;55:1227–1236. doi: 10.1016/j.jacc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 52.Salvemini D., Riley D.P., Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 53.Anderson C.M., Sonis S.T., Lee C.M., Adkins D., Allen B.G., Sun W. Phase 1b/2a Trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2018;100:427–435. doi: 10.1016/j.ijrobp.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pordanjani S.M., Hosseinimehr S.J. The role of NF-κB inhibitors in cell response to radiation. Curr Med Chem. 2016;23:3951–3963. doi: 10.2174/0929867323666160824162718. [DOI] [PubMed] [Google Scholar]
- 55.Deorukhkar A., Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol. 2010;80:1904–1914. doi: 10.1016/j.bcp.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto Y., Gaynor R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linard C., Marquette C., Mathieu J., Pennequin A., Clarençon D., Mathé D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–434. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 58.Meeren A.V., Bertho J.M., Vandamme M., Gaugler M.H. Ionizing radiation enhances IL-6 and IL-8 production by human endothelial cells. Mediators Inflamm. 1997;6:185–193. doi: 10.1080/09629359791677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mezzaroma E., Mikkelsen R.B., Toldo S., Mauro A.G., Sharma K., Marchetti C. Role of interleukin-1 in radiation-induced cardiomyopathy. Mol Med. 2015;21:210–218. doi: 10.2119/molmed.2014.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujimori A., Okayasu R., Ishihara H., Yoshida S., Eguchi-Kasai K., Nojima K. Extremely low dose ionizing radiation up-regulates CXC chemokines in normal human fibroblasts. Cancer Res. 2005;65:10159–10163. doi: 10.1158/0008-5472.CAN-05-2015. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T., Kusunoki Y., Hakoda M., Morishita Y., Kubo Y., Maki M. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. Int J Radiat Biol. 2003;79:129–136. [PubMed] [Google Scholar]
- 62.Emerit I., Quastel M., Goldsmith J., Merkin L., Levy A., Cernjavski L. Clastogenic factors in the plasma of children exposed at Chernobyl. Mutat Res. 1997;373:47–54. doi: 10.1016/s0027-5107(96)00187-x. [DOI] [PubMed] [Google Scholar]
- 63.Pilones K.A., Formenti S., Demaria S. Peritumoral IL-15 potentiates radiation-induced anti-tumor immunity. Int J Radiat Oncol Biol Phys. 2016;96:S129. [Google Scholar]
- 64.Golden E.B., Chhabra A., Chachoua A., Adams S., Donach M., Fenton-Kerimian M. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 65.Park M.-T., Oh E.-T., Song M.-J., Lee H., Park H.J. Radio-sensitivities and angiogenic signaling pathways of irradiated normal endothelial cells derived from diverse human organs. J Radiat Res. 2012;53:570–580. doi: 10.1093/jrr/rrs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acker J.C., Marks L.B., Spencer D.P., Yang W., Avery M.A., Dodge R.K. Serial in vivo observations of cerebral vasculature after treatment with a large single fraction of radiation. Radiat Res. 1998;149:350–359. [PubMed] [Google Scholar]
- 67.Haimovitz-Friedman A., Witte L., Chaudhuri A., McLoughlin M., Fuks Z. Induction of growth factor genes in endothelial cells by ionizing radiation. Radiat Oncol Investig. 1995;3:1–8. [Google Scholar]
- 68.Vincenti S., Brillante N., Lanza V., Bozzoni I., Presutti C., Chiani F. HUVEC respond to radiation by inducing the expression of pro-angiogenic microRNAs. Radiat Res. 2011;175:535–546. doi: 10.1667/RR2200.1. [DOI] [PubMed] [Google Scholar]
- 69.Milliat F., François A., Isoir M., Deutsch E., Tamarat R., Tarlet G. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol. 2006;169:1484–1495. doi: 10.2353/ajpath.2006.060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Girdhani S., Lamont C., Hahnfeldt P., Abdollahi A., Hlatky L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat Res. 2012;178:33–45. doi: 10.1667/rr2724.1. [DOI] [PubMed] [Google Scholar]
- 71.Girdhani S., Sachs R., Hlatky L. Biological effects of proton radiation: what we know and don't know. Radiat Res. 2013;179:257–272. doi: 10.1667/RR2839.1. [DOI] [PubMed] [Google Scholar]
- 72.Azzam E.I., Jay-Gerin J.-P., Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao E.C., Hsu Y.T., Chuah Q.Y., Lee Y.J., Hu J.Y., Huang T.C. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 2014;5:e1255. doi: 10.1038/cddis.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumura S., Wang B., Kawashima N., Braunstein S., Badura M., Cameron T.O. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]