Abstract
Cancer is the second biggest cause of death worldwide. Despite a number of studies being conducted, the effective mechanism for treating cancer has not yet been fully understood. The tumor-microenvironment such as hypoxia, low nutrients could disturb function of endoplasmic reticulum (ER) to maintain cellular homeostasis, ultimately leading to the accumulation of unfolded proteins in ER, so-called ER stress. The ER stress has a close relation with cancer. ER stress initiates unfolded protein response (UPR) to re-establish ER homeostasis as an adaptive pathway in cancer. However, persistent ER stress triggers the apoptotic pathway. Therefore, blocking the adaptive pathway of ER stress or facilitating the apoptotic pathway could be an anti-cancer strategy. Recently, natural products and their derivatives have been reported to have anti-cancer effects via ER stress. Here, we address mechanisms of ER stress-mediated apoptosis and highlight strategies for cancer therapy by utilizing ER stress. Furthermore, we summarize anti-cancer activity of the natural products via ER stress in six major types of cancers globally (lung, breast, colorectal, gastric, prostate and liver cancer). This review deepens the understanding of ER stress mechanisms in major cancers as well as the suppressive impact of natural products against cancers via ER stress.
Keywords: natural products, bioactive compounds, cancer, endoplasmic-reticulum stress, unfolded protein response, apoptosis
1. Introduction
Cancer is a group of diseases that undergo unregulated cell growth and proliferation without stopping [1]. Although overall survival term is increased slightly due to early detection, cancer-related mortality is the second biggest cause of death worldwide [2]. The International Agency for Research on Cancer (IARC) reported that 14.1 million new cancer cases and 8.2 million deaths took place worldwide in 2012, and 21.7 million cancer incidences and 13 million deaths were predicted in 2030. Among several types of cancers, lung, breast, colorectal, gastric, prostate, and liver cancer were selected as the major cancers in human being. These cancers represent 55% of the global cancer incidence burden in 2012 [3].
The mechanisms of cancer occurrence and progression are not fully understood yet. The proliferation of cancer originated from its ability to avoid programmed cell death, so-called apoptosis [4]. That is why induction of apoptosis in cancer has been identified as a target for treatment of cancer [5,6]. Up to now, induction of apoptosis is conducted by two main apoptotic pathways: intrinsic and extrinsic pathway [7]. Intrinsic pathway is mitochondria-mediated apoptosis which is mediated by cytochrome C release and activation of caspase-9, stimulating effector caspases, caspase-3. Extrinsic pathway is death receptor (DR)-mediated apoptosis which activates the FAS-associated death domain (FADD) and forms the death-inducing signaling complex (DISC), which processes downstream caspases including caspase-8,-7,-6, and -3 [8,9]. However, studies have identified that the accumulation of unfolded proteins in endoplasmic reticulum (ER) and its cellular stress response is involved in apoptosis, indicating influence of ER on cell fate as a third subcellular compartment [10].
Overview of ER Stress; A Double-Edged Sword-Cell Survival or Death?
ER is an organelle in the eukaryotic cell that is responsible for protein synthesis and calcium (Ca2+) signaling [11]. It also provides a suitable environment for lipid, steroid, and cholesterol biosynthesis. Moreover, the main roles of ER include maintenance of homeostasis in intracellular Ca2+ storage and the folding of protein destined to be secreted on the plasma membrane [12]. Proteins are translocated into ER lumen and undergo post translational modification for fidelity of synthesis, folding and correcting function. After passing ER, proteins are properly folded by a network of chaperones [13].
However, extra-cellular environmental challenges such as reactive oxygen species (ROS), hypoxia, and nutrients deprivation could induce disturbance in cellular redox regulation of ER, leading to an imbalance in homeostasis. Thus, the physiological function of ER and the ER protein-folding environment are impaired, ultimately resulting in the accumulation of unfolded protein in the lumen of the ER-namely, ER stress [10]. Prior studies have demonstrated that both ER stress and the activation of unfolded protein response (UPR) are associated with pathologic processes, including neurodegenerative diseases, cardiovascular disease, and cancer [14]. Because of the rapid expansion of malignant neoplasm, cancer cells are exposed to low nutrients, poor vascularization, and hypoxia so that ER stress-related proteins including glucose-regulated protein 78 (GRP78)/binding protein (BiP), glucose-regulated protein 94 (GRP 94), ER associated degradation (ERAD), protein disulphide isomerase (PDI), activating transcription factor 6 (ATF6), inositol-requiring protein 1 (IRE1), α-x-box binding protein 1 (XBP1), protein kinase RNA-like ER kinase (PERK) and eukaryotic initiation factor 2 (eIf2α) are overexpressed in many types of cancer cells [15,16,17]. In response to ER stress on early phase, the cell initiates UPR to modulate proper protein folding and degradation of unfolded protein as an adaptive pathway for survival. Under ER stress, GRP78/BiP, which originally binds to the luminal domain of three ER transducer sensors: IRE1, PERK, and ATF6, dissociates from these three ER transducer sensors. The three ER-localized transmembrane signal-transducers of UPR; ATF6, IRE1 and PERK detect accumulation of unfolded proteins and initiate to restore and maintain the ER homeostasis [18]. Moreover, ERAD is increased to attenuate unfolded protein accumulation, enhancing protein folding capacity in ER and ER chaperones (GRP 94, GRP78/BiP, calnexin), which are elevated to stabilize protein folding. However, if ER stress is prolonged to the extent that UPR is unable to cope with unfolded proteins, UPR is promoted to be turned into apoptotic machineries by transducing apoptotic downstream pathways through ATF6, PERK, and IRE1 signaling pathways [19].
2. Anti-Cancer Strategy Based on Adaptive Pathway of ER Stress in Cancer Growth
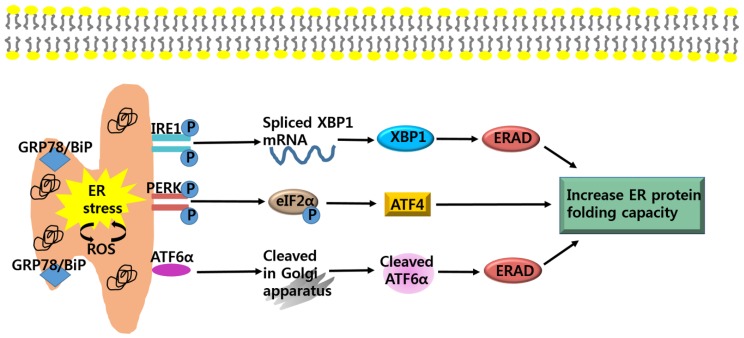
Cancer cells evolve UPR to mitigate the ER stress condition as a survival strategy for progression [20]. Also, UPR in cancer is reported to have significant roles in having resistance to chemotherapy or radiation [17,21]. Therefore, one therapeutic rationale regarding suppression of cancer is to facilitate the accumulation of unfolded proteins by inhibiting components of UPR involved in survival response, ultimately leading to apoptosis [22] (Figure 1).
Figure 1.
Adaptive pathways of endoplasmic reticulum (ER) stress. ER stress is induced by an accumulation of unfolded proteins in the ER lumen. During ER stress, glucose-regulated protein 78 (GRP78)/ binding protein (BiP) dissociates from its interaction with the three ER stress sensors, inositol-requiring protein 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which become activated. IRE1 mediates splicing of α-x-box binding protein 1 (XBP1), which is responsible for the upregulation of ER associated degradation (ERAD). PERK phosphorylates eIF2α and induce activating transcription factor 4 (ATF4), which is involved in restoring ER homeostasis. ATF6α cleaved by specific Golgi resident proteases, increase expression of UPR genes and ERAD. Eukaryotic initiation factor 2 (eIf2); protein kinase RNA-like ER kinase (PERK).
2.1. Anti-Cancer Strategy; Targeting ATF6α-GRP78/BiP Signaling
A first modulator of ER stress signaling is ATF6α-GRP78/BiP. During ER stress conditions, dissociation of GRP78/BiP from ATF6 allows this protein to translocate to Golgi apparatus where it is cleaved by specific Golgi resident proteases [23]. This process forms ATF6α, which produces active transcription factors that are involved in up-regulating ER chaperones and ERAD [24]. In addition, ATF6α contributes to transcription factor XBP1, which targets the ERAD [25] (Figure 1). Further, the expression of GRP78/BiP is elevated particularly in liver, colon, prostate, breast, lung, and gastric cancer [26,27,28]. The elevated expression of GRP78/BiP is reported to correlate with cancer proliferation, chemotherapy resistance, and poor patient survival rate by modulating accumulation of unfolded protein folding [29,30,31]. In addition, GRP78/BiP induces angiogenesis by activating the potent pro-angiogenic factor, vascular endothelial growth factor A (VEGF-A) [32]. Recently, studies have confirmed that knockdown of GRP78/BiP by siRNA can inhibit carcinogenesis and sensitize cancer cells to chemotherapeutic agents as well as ER stress [33,34,35]. The GRP78/BiP inhibitor exerted cytotoxicity against leukemia [36] and glioma cancer cells [34]. These observations suggest that during ER stress, inhibition of the ATF6-GRP78/BiP signaling pathway could be a strategy for cancer therapy [37]. For example, the knockout of GRP78 resulted in decreased proliferation rates of glioma cells and suppressed PTEN null prostate tumorigenesis and AKT oncogenic pathway [34,38].
2.2. Anti-Cancer Strategy; Targeting IRE1α-XBP1 Signaling
A second modulator of ER stress signaling is IRE1α-XBP1. Two isoforms of IRE1, IRE1α and IRE1β, have been identified. IRE1α is expressed in all cell types, but the expression of IRE1β is specifically restricted to the intestinal epithelium [39,40]. In cells undergoing ER stress, activation of IRE1α stimulates an endoribonuclease that splices XBP1 mRNA to produce mature XBP1. XBP1 encodes an active leucine zipper (bZiP) transcriptions factor that generates transcription of genes involved in ERAD components as well as genes involved in redox homeostasis and oxidative stress response (Figure 1) [41,42]. The involvement of the IRE1α-XBP1 signaling in cancer progression has been the subject of many studies. Upregulated XBP1 expression is observed in various human cancers including breast cancer and hepatocellular carcinoma [43]. XBP1-deficient cancer cells showed hypersensitivity to ER stress or hypoxia condition [41]. Moreover, expression of the dominant-negative form of IRE1α or inhibition of XBP1 by siRNA induces reduction in angiogenesis during tumorigenesis and in cancer growth in xenografts model [15,44,45]. Additionally, inhibition of IRE1α-XBP1 enhances apoptosis by down-regulating several genes involved in UPR and generates reactive oxygen species (ROS) in XBP1-deficient cells [15]. These results provide evidences that deactivation of the IRE1α-XBP1 [46] could be a target of anticancer therapy. For instance, IRE1α inhibitor rendered resistant human glioblastoma cells susceptible to oxidative stress [47]. Inhibition of IRE1α decreased endonuclease activity, increasing cytotoxic activity against human multiple myeloma in vitro and in vivo [48].
2.3. Anti-Cancer Strategy; Targeting PERK-eIF2α-ATF4 Signaling
A third modulator of ER stress signaling is the PERK-elf2α signaling pathway. Under ER stress conditions, activation of PERK phosphorylates eIF2α at Ser51 [49]. Phosphorylated eIF2α shuts down protein influx into the ER lumen and attenuates mRNA translation, reducing protein synthesis [50,51]. The activation of PERK-eIF2α increases the translation of a number of mRNAs encoding activating transcription factor 4 (ATF4) [52]. ATF4 promotes cell-survival by controlling amino acid biosynthesis and transport function, as well as antioxidant stress responses [53]. PERK-eIF2α-ATF4 signaling pathway is responsible for cancer growth and resistance against curative treatment. The UPR signaling pathway through the PERK-eIF2α-ATF4 signaling pathway also increases tolerance in cancer cells to hypoxic stress [17]. In addition, the PERK-eIF2α-ATF4 signaling pathway in cancer cells mediates the upregulation of VEGF-A transcription [17]. Recently, it has been reported that PERK-deficient cells are hypersensitive to ER stress [54], and inhibition of PERK suppressed cancer cells which are resistant to radiation in vivo [55]. These results suggest that suppression of PERK-eIF2α-ATF4 signaling pathways could be a target of cancer therapy. For example, transformed mouse embryonic fibroblasts from the PERK-deficient animals and HT29 colorectal carcinoma cells with dominant-negative PERK had lower survival rates under hypoxic conditions than wild type control cells [56]. And these cells displayed smaller cancer-formation and increased the level of apoptotic activity in hypoxic areas, compared to wildtype cells [17]. In addition, PERK-deficient cancer cells exhibited declined ability to induce angiogenesis in response to hypoxic stress [57].
3. Anti-Cancer Strategy Based on Apoptotic Pathway of ER Stress in Cancer Growth
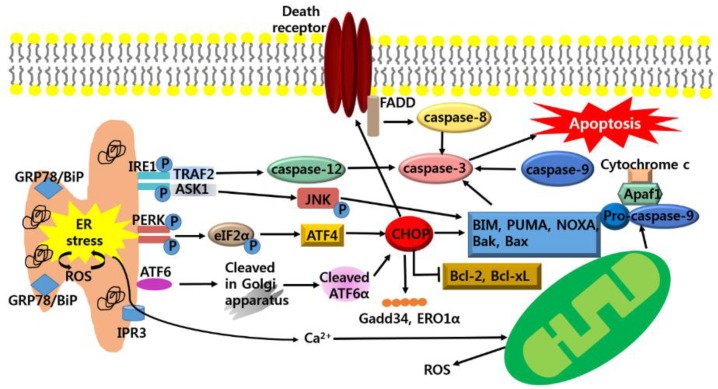
If ER stress persists or is aggravated, cancer cells fail to re-establish ER homeostasis via UPR, ER stress switches from pro-survival to pro-apoptotic condition [58]. Therefore, facilitation of ER stress to initiate the apoptosis pathway could be a therapeutic strategy for anti-cancer activity [14,59] (Figure 2).
Figure 2.
Apoptotic pathways of ER stress. If the adaptive UPR pathway fails to restore homeostasis, chronic ER stress induces apoptosis pathways. ER stress activates IRE1 and activated IRE recruits TNF receptor-associated factor2 (TRAF2) and apoptosis signal-regulating kinase 1 (ASK1). TRAF2 stimulates the activation of caspase-12 and ASK1 elicits the phosphorylation of c-Jun-N terminal kinase (JNK) to activate the pro-apoptotic protein; Bcl-2-like protein 11 (BIM), Bak, Bax and inhibits the anti-apoptotic protein; B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax). Activation of PERK and ATF6 leads to up-regulation of CHOP that regulates Gadd34, ERO1α and activates the pro-apoptotic protein; BIM, Bak, Bax, p53 upregulated modulator of apoptosis (PUMA), NOXA as well as DR which increase caspase-8. Also, ER stress generates ROS and this facilitates the accumulation of unfolded protein in the ER. ER stress-induced Ca2+ release into cytosol leads to depolarization of the inner mitochondrial membrane, resulting in Mitochondrial ROS. Apoptosis protease-activating factor 1 (Apaf1); FAS-associated death domain (FADD).
3.1. Anti-Cancer Strategy; Targeting CHOP-Mediated Apoptosis
During the prolonged ER stress condition, PERK is activated and phosphorylates elF2α [60]. Phosphorylated elF2α subsequently activates ATF4 which targets the expression of apoptotic effector, CHOP (C/EBP-homologous protein; known as GADD153; GENE name Ddit3) [61]. Furthermore, ATF6 is also activated and transcriptionally induces CHOP [62]. CHOP-mediated cell death entails the induction of a variety of genes that may potentiate apoptosis, including GADD34 and ERO1α [63]. GADD34, a transcriptional target of CHOP, encodes a subunit of protein phosphatase that promotes dephosphorylation of eIF2α that leads resumption of protein synthesis, in turn increasing the load of proteins [64]. Accordingly, ER stress activates the UPR to initiate downstream of apoptotic pathways. In addition, CHOP is thought to elevate the expression of Ero1α, which catalyzes the re-oxidation of PDI, leading to hyper-oxidizing conditions in the ER [59]. The CHOP-induced expression of ERO1α activates the ER Ca2+ release channel, inositol 1,4,5-triphosphate (IP3) receptor (IP3R) [65]. Then, cytoplasmic Ca2+ released from the ER triggers the activation of calcium/calmodulin-dependent protein kinase II (CaMKII), ultimately resulting in the induction of apoptosis pathways [66]. Furthermore, CHOP-mediated apoptosis is involved in extrinsic pathway through up-regulating DR5 along with capase-8 activation, which cleaves Bid (tBid) and translocates it to mitochondria [67,68]. In addition, CHOP is shown to decrease the expression of anti-apoptotic Bcl-2 and Bcl-xL proteins, while increasing the expression of pro-apoptotic proteins including Bak, Bax, BIM (Bcl-2-like protein 11), PUMA (p53 upregulated modulator of apoptosis) and NOXA (PMAIP1) (Figure 2) [69,70]. These studies imply that up-regulation of CHOP expression could be an attractive target for anti-cancer therapy. For example, CHOP-deficiency elevates cancer development in a KrasG12V-induced mouse model of lung carcinoma [71]. And hepatocyte-specific CHOP deletion causes cancer growth in a mouse model of hepatocellular carcinoma [72].
3.2. Anti-Cancer Strategy; Targeting IRE1-Mediated Apoptosis
Under chronic ER stress, IRE1α is induced to activate the mitogen-activated protein (MAP) kinase (MARK) [73]. The IRE1α binds with the adaptor protein, TNF receptor-associated factor2 (TRAF2). IRE1α-TRAF2 complex triggers the activation of caspase-12, which is homologous to human caspase-4 [74]. Caspase-12 translocates from the ER to the cytosol, where it initiates cleavage of procaspase-9, in turn, activating the effector caspase, caspase-3 [75,76]. IRE1α/TRAF2 activates ASK1 (apoptosis signal-regulating kinase 1), which subsequently promotes activation and phosphorylation of c-Jun-N terminal kinase (JNK) [77]. The Phosphorylation of JNK activates BIM, Bak, Bax and inhibits the Bcl-2 and Bcl-xL (Figure 2) [78,79]. These data implicate that the triggering IRE1-mediated apoptosis could be a strategy for anti-cancer therapy. For instance, ER stress-mediated apoptosis was reduced in IRE1-deficient mouse embryonic fibroblasts [80]. And deactivation of ER stress-mediated apoptosis in JNK inhibitor treated wild-type-mouse embryonic fibroblasts was confirmed [81].
3.3. Anti-Cancer Strategy; Targeting Generation of ROS-Mediated Apoptosis
When ER stress persists, it hyper-oxidizes the ER lumen, resulting in H2O2 leakage into the cytoplasm and inducing ROS [82,83]. Furthermore, under oxidative environment, leakage of Ca2+ from ER lumen is stimulated, increasing calpains and Ca2+ burden in mitochondria [84,85]. The Ca2+ uptake in mitochondria depolarizes the inner mitochondrial membrane, leading to mitochondrial ROS generation [86]. Then, BH3-only proteins, Bak and Bax, oligomerize and insert themselves into the outer mitochondrial membrane to increase permeabilization, resulting in the release of cytochrome c [87]. When cytochrome c is released with the formation of APAF-1 (apoptosis protease-activating factor 1) and procaspase-9 [88]; caspase-3 [89], apoptosis-inducing factor (AIF) and endonuclease G (EndoG) are activated [90]. In general, cancer cells intrinsically have more ROS compared to normal cells. Therefore, elevated generation of ROS render cancer cells more susceptible to ER stress, which contributes to ER stress-mediated apoptosis [91]. Thus, the generation of ROS could be a target of anti-cancer therapy. In vitro, ROS-dependent ER stress activation and mitochondrial-related apoptotic pathway were identified in MDA-MB-231 and MCF-7 cell lines with treatment of berberine [92]. Pardaxin-triggered ROS-dependent ER stress and its apoptotic activation via ER stress were inhibited by NAC treatment [93].
4. Anti-Cancer Effects of Natural Products via ER Stress
The standardized treatments of cancer consist of surgery, chemotherapy, radiation therapy and immunotherapy. These are utilized in accordance with the characters and stages of the cancers. Although the main purpose of anti-cancer treatments is to kill the cancer cells without damaging normal cells, cancer treatments possess limited efficacy and exert their actions on both malignant and normal cells, resulting in adverse effects on patients, including anemia, loss of appetite, delirium and peripheral neuropathy. Thus, the development of effective treatment which has anti-cancer activity with less adverse effects is still needed [94,95]. From the history of drug discovery, natural product-derived compounds could be a promising treatment due to their characteristic to induce apoptosis more commonly in cancers compared to normal cells [96,97]. In fact, several conventional chemotherapeutic agents including Taxol, epothilones, vinca alkaloids originate from the resource of natural products [98]. Currently, as ER stress response is proven to have both an adaptive pathway and apoptotic pathway, modulation of ER stress could be an anti-cancer strategy. Therefore, researchers have focused on the interplay between natural products and anti-cancer effect via ER stress in malignant cells in vitro and in vivo [99,100,101]. Hence, in this review article, anti-cancerous mechanisms of natural products on the most common cancer types via modulation of ER-stress are discussed; lung, breast, colorectal, gastric, prostate, liver cancer, which represent 55% of the global incidence burden in 2012 [2,3].
4.1. Search Methodology
Researches regarding the ER stress-mediated apoptotic effect of natural product on cancers were collected from PUBMED/MEDLINE and Google Scholar. Upon searching for appropriate studies, we include ‘ER stress, apoptosis, UPR, natural product, cancers’ for keywords. We collected studies which fit into the criteria: (1) researches based on in vitro or in vivo or clinical trials to prove ER-stress mediated apoptosis of natural product (2); researches that demonstrated reliable statistical analysis data (p values that were less than 0.05); and (3) researches that were not upset by subsequent studies. The family names of natural products and herbs mentioned in this review are based on a reliable source. Natural product-derived compounds in this review were double-checked from the NCBI PubChem website for precision.
4.2. Natural Products Targeting ER Stress-Mediated Apoptosis in Lung Cancer
Lung cancer is one of the leading causes of cancer-related death in the world and around 1.8 million new cases were estimated in 2012 [3]. Chemotherapy and radiation are the main treatments for lung cancer, which often become resistant to these treatments [102]. As a result, the majority of lung cancer patients suffer from severe side effects and ultimately succumb to death [103]. Therefore, there remains a need for developing a new therapeutic agent to improve the clinical outcomes. As UPR has a critical role in lung cancer viability and lung cancer death [104], researches have been implemented to prove ER stress-mediated anti-cancer activity of natural products against lung cancer cells (Table 1).
Table 1.
Bioactive compounds from natural products that induce ER-stress-mediated apoptosis in lung cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Paris polyphylla | Polyphyllin D | NCI-H460 | 1.8 µM, 8 h | CHOP ↑, GRP78/BiP ↑, PDI ↑ | [105] |
| cleavage of caspase-3,-4,-9,-12 ↑, Bax ↑, Bcl-2 ↓ | |||||
| Saussurea lappa and Aucklandia lappa | Dehydrocostuslactone | NCI-H460 | 15 μg/mL, 1 h | release of intracellular Ca2+ levels ↑, Δψm ↓, p-PERK ↑, GRP78/BiP ↑, IRE1 ↑, CHOP ↑, XBP-1 ↑ | [106] |
| Cleavage of caspase-4 ↑, JNK ↑, MAPK ↑ | |||||
| ROS ↑ | |||||
| A549 | 15 μg/mL, 1 h | release of intracellular Ca2+ ↑, Δψm ↓,p-PERK ↑, GRP78/BiP ↑, IRE1 ↑, CHOP ↑, XBP1 ↑ | |||
| JNK ↑, MAPK ↑, cleavage of caspase-4 ↑ | |||||
| ROS ↑ | |||||
| BrefeldinA | A549 | 1 µM, 36 h | p-PERK ↑, IRE1α ↑, ATF4 ↑, ATF6 ↑, CHOP ↑, GRP78/BiP ↑ | [107] | |
| cleavage of PARP ↑, cleavage of caspase-2 ↑, Bid ↑ | |||||
| Oryza officinalis | ω-Hydroxyundec-9-enoic acid | H1299 | 500 µM, 24 h | p-eIF2α ↑, CHOP ↑ | [108] |
| cleavage of caspase-6,-9 ↑, cleavage of PARP ↑ | |||||
| ROS ↑ | |||||
| Curcuma longa | Curcumin | NCI-H460 | 25 µM, 24 h | release of intracellular Ca2+ ↑, Δψm ↓, CHOP ↑, GRP78/BiP ↑ | [109] |
| Bcl-2 ↓, Bcl-xL ↓, cytochrome c ↑, cleavage of caspase-3,-8,-9 ↑, BAX ↑, BAD ↑ | |||||
| ROS ↑ | |||||
| Mylabris phalerata Pallas | Cantharidin | H460 | 10 µM, 24 h | release of intracellular Ca2+ ↑, Δψm ↓, GRP78/BiP ↑, IRE1α ↑, IRE1β ↑, ATF6α ↑, XBP1 ↑, calpain ↑, | [110] |
| Bcl-xL ↓, cleavage of caspase-3,-8,-4 ↑ cytochrome c ↑ BAX ↑, AIF ↑, Endo G ↑ | |||||
| ROS ↑ | |||||
| Curcumae Rhizoma | Furanodiene | A549, 95-D | 80 µM, 24 h | CHOP ↑, BIP ↑ | [111] |
| Tanacetum parthenium L. | Parthenolide | A549, Calu-1, H1299, H1792 | 20 µM, 24 h | ATF4 ↑, p-eIF2a ↑, eIF2α ↑ | [112] |
| cleavage of caspase-3,-8,-9 ↑, cleavage of PARP ↑ | |||||
| Anacardium occidentale | Anacardic acid | A549 | 3.0 μg/mL, 18 h | release of intracellular Ca2+ ↑, Δψm ↓, GRP78/BiP ↑, CHOP ↑, IRE1α ↑ ATF6 ↑, p-PERK ↓, p-eIF2α ↓ | [113] |
| cleavage caspase-12 ↑ |
Glucose-regulated protein 78 (GRP78); binding protein (BiP); protein disulphide isomerase (PDI); protein kinase RNA-like ER kinase (PERK); reactive oxygen species (ROS); inositol-requiring protein 1 (IRE1); ↑ - increasing concentration; ↓ - decreasing concentration; activating transcription factor 4 (ATF4); activating transcription factor 6 (ATF6); B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax); X-ox binding protein 1 (XBP-1); c-Jun-N terminal kinase (JNK); mitochondrial membrane potential (Δψm); C/EBP-homologous protein (CHOP); α-x-box binding protein 1 (XBP1); apoptosis-inducing factor (AIF); and eukaryotic initiation factor 2 (eIf2).
Enhanced expression of GRP78/BiP, CHOP, and caspase-3 in NCI-H460 cells were observed by treatment of Polyphyllin D (PD; a potent cytotoxic saponin isolated from Paris polyphylla), which suggests that Polyphyllin D-induced ER stress-mediated apoptosis [105]. Dehydrocostuslactone (DHE; sesquiterpene lactone extracted from the Saussurea lappa and Aucklandia lappa) activated PERK-CHOP and IRE1-JNK signaling pathways in both NCI-H460 and A549 cells through ER stress caused by induction of Ca2+ and ROS in the cytoplasm [106]. CHOP expression was upregulated in A549 cells, inducing apoptosis by treatment of brefeldin A (BFA; a macrocyclic lactone antibiotic synthesized from palmitate by a variety of fungi). However, NAC treatment considerably suppressed apoptosis induced by BFA treatment, indicating that BFA induced apoptosis is dependent on ROS [107]. ω-Hydroxyundec-9-enoic acid (ω-HUA; a hydroxyl unsaturated fatty acid derivative isolated from the leaves of Oryza officinalis) suppressed the viability of H1299 cells through ROS-dependent ER stress, however, the reduced expression of CHOP was detected after NAC treatment [108]. Curcumin (a phenolic compound isolated from the Curcuma longa) elevated the level of CHOP, GRP78/BiP expression in NCI-H460 cells, which is associated with release of intracellular Ca2+ [109]. Cantharidin (CTD; a natural terpenoid extracted from Mylabris phalerata Pallas) induced cytotoxic effects on NCI-H460 cells through ER stress along with the release of intracellular Ca2+, leading to mitochondrial dysfunction. Upregulation of cytochrome c, Bax, Endo G, AIF and cleaved caspase-3,-8 were observed in CTD treated NCI-H460 cells [110]. Also, CHOP expression in A549 and 95-D cells was elevated by furanodiene (FUR; a natural terpenoid isolated from Curcumae rhizome) [111]. Parthenolide (PTL; a sesquiterpene lactone derived from the feverfew) treatment upregulated ER stress by activating eIF2α-ATF4-CHOP signaling in A549, Calu-1, H1299, H1792, leading apoptosis. However, knockout of ATF4 by ATF4 siRNA reduced apoptosis, indicating that the expression of CHOP mediates PTL induced-apoptosis [112]. Anacardic acid (AA; a constituent of Anacardium occidentale) increased expression of CHOP, cleavage of caspase-12, and disturbed Ca2+ homeostasis in A549 cells, confirming AA as an ER stress inducer [113].
4.3. Natural Products Targeting ER Stress-Mediated Apoptosis in Breast Cancer
Female breast cancer is the most common malignancy in women and the most frequently diagnosed cancer globally. 1.67 million cases were estimated in 2012, ranking as the second most common cancer world-wide [3]. Until now, most of the therapeutic drugs for breast cancer target the early stage of cancer cells; still there is no available therapeutic options for advanced breast cancer [103]. Thus, the development of more effective anti-cancer agents for breast cancer is imperative. Since the UPR is involved in execution of pro-survival or pro-death decision [114], many studies have been performed to test ER stress-mediated anti-cancer activity of natural products using breast cancer cell lines [115] (Table 2).
Table 2.
Bioactive compounds from natural products that induce ER stress-mediated apoptosis in breast cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Eupenicillium brefeldianum | Brefeldin A | MDA-MB-231 | 1 μg/mL, 24 h | IRE1α ↑, PERK ↑, CHOP ↑, calnexin ↓ | [116] |
| BIM ↑, cleavage of PARP ↑, Bcl-2 ↓, | |||||
| Salvia miltiorrhiza Bunge | Cryptotanshinone | MCF7 | 10 µM, 24 h | p-eIF2α ↑, GRP94 ↑, GRP78 ↑, CHOP ↑ | [117] |
| cleavage of PARP ↑, cleavage of caspase3 ↓ | |||||
| ROS ↑ | |||||
| Androsace umbellata | Saxifragifolin | MDA-MB-231 | 5 µM, 24 h | IRE1α ↑, calnexin ↑, calpain ↑, XBP1 ↑, CHOP ↑, GRP78/BiP ↑, | [118] |
| cleavage of PARP ↑, cleavage of caspase-3,-9 ↑, Bax ↑, cytochrome C ↑, p-JNK ↑ | |||||
| ROS ↑ | |||||
| MCF7 | 5 µM, 24 h | IRE1α ↑, Calnexin ↑, calpain ↑, XBP-1 ↑, GRP78/BiP ↑, CHOP ↑ | |||
| cytochrome c ↑ | |||||
| Serratia marcescens. | Prodigiosin | MCF7 MDA-MB-231T-47D | 100 µM, 24 h | GRP78 ↑, CHOP ↑, p-IRE1 ↑, IRE1 ↑, p-eIF2a ↑, eIF2a ↑, ATF6 ↑ | [119] |
| cleavage of PARP ↑, p-JNK ↑, JNK ↑, BCL-2 ↓ | |||||
| Fucus vesiculosus | Fucoidan | MDA-MB-231 | 100 μg/mL, 72 h | CHOP ↑, ATF4 ↑, p-eIF2α ↑, GRP78/BiP ↓, p-IRE1 ↓, XBP1 ↓ | [120] |
| Bax ↑, CaMK II ↑, cleavage of caspase-3,-12 ↑, cleavage of PARP ↑ | |||||
| γ-tocotrienol | MDA-MB-231 | 40 µM, 16 h | CHOP ↑, ATF4 ↑, GRP78/BiP ↑, XBP1 ↑ | [101] | |
| cleavage of PARP ↑, cleavage of caspase-3,-8,-9 ↑, DR5 ↑, JNK ↑, p-JNK, C-Jun ↑, p38 MAPK ↑ | |||||
| MCF-7 | 40 µM, 16 h | CHOP ↑, GRP78/BiP ↑, XBP1 ↑ | |||
| cleavage of PARP ↑, cleavage of caspase-8,-9 ↑, JNK ↑, p-JNK ↑, C-Jun ↑, DR5 ↑, p38 MAPK ↑ | |||||
| Brazilian Red propolis | Ethanol extract of Brazilian Red propolis | MCF-7 | 20 μg/mL, 24 h | CHOP ↑ | [121] |
| cleavage of caspase-3 ↑, Bax ↑, BcL-xL ↓, BcL-2 ↓ | |||||
| Ampelopsis grossedentata | Ampelopsin | MCF-7 MDA-MB-231 | 60 µM, 24 h | GRP78/BiP ↑, p-PERK ↑, p-eIF2α ↑, ATF6 ↑, CHOP ↑ | [122] |
| ROS ↑ |
Bcl-2-like protein 11 (BIM); death receptor (DR5).
BFA, isolated from the fungus Eupenicillium brefeldianum, upregulated PERK, CHOP, BIM and downregulated Bcl-2 in MDA-MB-231 cells. These results suggest that mitochondrial dysfunction is involved in BFA-induced ER stress-mediated apoptosis [116]. Activation of elF2α, GRP94, GRP78/BiP, and CHOP in MCF7 cells was demonstrated by cryptotanshinone (a constituent of Salvia miltiorrhiza Bunge) treatment. Moreover, cryptotanshinone-induced ER stress sensitized MCF7 cells to anti-cancer agents including TNFα, cisplatin, etoposide and 5-FU [117]. Saxifragifolin D (SD; an oleanane type pentacyclic triterpenoid isolated from Androsace umbellate) increased expression of CHOP, JNK and cytochrome c in both MCF-7 and MDA-MB-231 cells through induction of Ca2+ and ROS in the cytoplasm, resulting in induction of ER stress [118]. Prodigiosin (a bacterial tripyrrole red-colored pigment produced by Serratia marcescens) was proven to have potent cytotoxicity against MCF7, MDA-MB-231 and T-47D cells through ER stress in terms of CHOP expression. Treatment of prodigiosin up-regulated CHOP, cleavage of PARP and decreased Bcl-2. However, decreased cleavage of PARP and increased Bcl-2 were noted after CHOP siRNA treatment, indicating CHOP-dependent apoptosis [119]. Fucoidan (a polysaccharide extracted from brown seaweed such as Cladosiphon okamuranus and Fucus evanescens) facilitated ER stress by activating eIF2α, CHOP along with suppressing IRE1, XBP1 in MDA-MB-231 cells. Additionally, Fucoidan triggered mitochondrial dysfunction, resulting in Bax and cleavage of caspase-3 [120]. Tocotrienols (a subgroup of vitamine E) promoted increased of CHOP, JNK, cleavage of caspase-3,-8 in MDA-MB-231 and MCF-7 cells. These results indicated that ER stress by Tocotrienols treatment is associated with mitochondria and DR5-mediated apoptosis [101]. Ethanol extract of Brazilian red propolis was reported to activate apoptosis against MCF-7 cells via facilitating ER stress and mitochondria-mediated apoptosis, resulting in CHOP expression, cleavage of caspase-3, Bax, and downregulation of Bcl-xL, Bcl-2 [121]. Expression of CHOP, PERK and ROS was increased in MDA-MB-231 and MCF-7 cells by treatment of ampelopsin (AMP; a main bioactive constituent of Ampelopsis grossedentata). However, NAC treatment triggered inhibition of CHOP expression, indicating that AMP-induced ER stress-mediated apoptosis is dependent on ROS [122].
4.4. Natural Products Targeting ER Stress-Mediated Apoptosis in Colorectal Cancer
Colorectal cancer is one of the leading causes of malignant neoplasm-related death in the world [3]. Around 1.3 million new cases were estimated, ranking as the third most common cancer in the world. Although early stage colon cancer is treatable, it is known to be resistant to chemotherapeutic drugs, which is linked with poor patient prognosis [123]. As UPR is involved in regulating colorectal cancer cell fates [124], many researches have been performed to test ER stress-mediated anti-cancer potential effects of natural products using colorectal cancer cells (Table 3).
Table 3.
Bioactive compounds from natural products that induce ER stress-mediated apoptosis in colorectal cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Curcuma longa | Curcumin | HT-29 | 40 μM, 24 h | release of intracellular Ca2+, Δψm ↓, CHOP ↑ | [125] |
| cleavage of caspase-3,-7,-8,-9 ↑, Fas ↑, FasL ↑ FADD ↑ Bid ↑, tBid ↑, p-JNK ↑, Bax ↑, cytochrome c ↑, Bcl-2 ↓ | |||||
| Olea europaea | 2-3,4 dihydroxyphenylethanol | HT-29 | 400 μM, 16 h, | release of intracellular Ca2+ ↑, Δψm ↓, IRE1 ↑, XBP1 ↑, GRP78/BiP ↑, PERK ↑, eIF2α ↑, CHOP ↑, | [126] |
| Bax ↑, Bak ↑, Bad ↑, cytochrome c ↑, cleavage of caspase 3 ↑, TRAF2 ↑, ASK ↑, JNK ↑, AP-1 ↑, p-JNK ↑ C-jun ↑, PI3K/Akt ↓, Bcl-2 ↓ | |||||
| Eupenicillium brefeldianum | Brefeldin A (BFA) | Colo 205 | 15 ng/mL, 24 h | GRP78 ↑, XBP1 ↑, CHOP ↑ | [127] |
| Resveratrol | HT29 | 50 μM, 24 h | GRP78/BiP ↑, CHOP ↑, XBP1 ↑, eIF2α ↑ | [128] | |
| cleavage of caspase-4 ↑, cleavage of PARP ↑ | |||||
| Zingiber zerumbet Smith | Zerumbone | HCT116-p53null | 20 μM, 24 h | ATF4 ↑, CHOP ↑, GRP78/BiP ↑, p-PERK ↑, PERK ↑ eIF2α ↑, p-eIF2α ↑ | [129] |
| DR5 ↑ | |||||
| ROS ↑ | |||||
| SW480 | 20 μM, 24 h | ATF4 ↑, CHOP ↑, GRP78/BiP ↑, p-PERK ↑, PERK ↑ eIF2α ↑, p-eIF2α ↑ | |||
| DR5 ↑ | |||||
| ROS ↑ | |||||
| Garcinia xanthochymus | Guttiferone H | HCT116 | 10 μg/mL, 24 h | ATF4 ↑, XBP1 ↑, CHOP ↑ | [130] |
| Cleavage of caspases-3,-7 ↑ | |||||
| Cladosiphon okamuranus and Fucus evanescens | Fucoidan | HCT116 | 100 μg/mL, 72 h | GRP78↑, p-CaMKII ↑, eIF2a ↑, p-eIF2α ↑, CHOP ↑, IRE1 ↓, XBP1 ↓ | [120] |
| Cleavage of PARP ↑ | |||||
| Piper nigrum Linn and Piper longum Linn | Piperine | HT-29 | 100 μg/mL, 72 h | IRE1α ↑, CHOP ↑, GPR78/BiP ↑ | [131] |
| cleavage of PARP ↑, cytochrome c ↑, JNK ↑, MAPK ↑, PI3K/Akt ↓ | |||||
| ROS ↑ | |||||
| Alpinia pricei Hayata | Flavokawain B | HCT116 | 50 μM, 8 h | CHOP ↑, ATF4 ↑ | [132] |
| Bcl-2 ↓, MAPK ↑, PARP ↑, cytochrome c ↑, BIM ↑, Bak ↑ | |||||
| ROS ↑ |
TNF receptor-associated factor2 (TRAF2); apoptosis signal-regulating kinase 1 (ASK1); calcium/calmodulin-dependent protein kinase II (CaMKII); Fas ligand (FasL).
Curcumin enhanced expression of CHOP, JNK, cytochrome c, and FADD in HT-29 cells. These results indicate that the release of intracellular Ca2+, mitochondrial dysfunction and DR5 are associated with curcumin-induced ER stress-mediated apoptosis [125]. 2-(3,4 dihydroxyphenylethanol) ethanol (DPE; phenol antioxidant derived from olive oil) induced apoptosis on HT-29 cells via ER stress, activating PERK-eIF2α-CHOP and IRE1-JNK signaling pathway. Moreover, mitochondria-mediated apoptotic factors including Bax, Bad, cytochrome c, and cleavage of caspase 3 were observed [126]. Suppression of Colo 205 cells viability was detected via BFA-induced ER stress in terms of CHOP expression [127]. Activation of eIF2α, CHOP cleavage of caspase-4 was upregulated in HT29 cells by treatment of resveratrol (natural polyphenolic compound) [128]. Expression of PERK-ATF4-CHOP signaling was activated by treatment of zerumbone (ZER; sesquiterpene purified isolated from the Zingiber zerumbet Smith). Also, ZER up-regulated DR5 expression in both HCT116-p53null cells and SW480 cells, eventually activating caspase-8 [129]. Guttiferone H (derived from Garcinia xanthochymus) elevated expression of CHOP along with cleavage of caspases-3,-7 in HCT116 cells [130]. Inhibition of HCT116 cells viability following fucoidan (derived from Cladosiphon okamuranus and Fucus evanescens) treatment was observed, which is induced by upregulation of elF2α-CHOP expression along with inhibition of IRE1-XBP1 [120]. Piperine (from Piper nigrum Linn and Piper longum Linn) generated ROS, CHOP, JNK, cytochrome c in HT-29 cells. These findings indicate that ER stress-mediated apoptosis by piperine is linked with mitochondrial dysfunction [131]. Potent anti-cancer activity of flavokawain B (derived from Alpinia pricei Hayata) against HCT116 cells was proven through ER stress in terms of CHOP expression. However, NAC treatment restored the inhibitory effects of Flavokawain B, suggesting that ROS generation is required for ER stress-mediated apoptosis [132].
4.5. Natural Product Targeting ER Stress-Mediated Apoptosis in Gastric Cancer
Gastric cancer emerged as one of the leading neoplasms in the world. Almost 1 million new cases of stomach cancer were estimated to have occurred in 2012, making it the fourth most common cancer world-wide [3]. Although progresses in surgery and chemotherapy for gastric cancer have been achieved, there is a high rate of cancer recurrences [133]. Therefore, novel effective therapeutic agents need to be developed. As UPR have a regulatory mechanism in either pro-survival or pro-apoptotic signals in gastric cancer [134], current studies have been performed to demonstrate ER-mediated anti-cancer activity of natural products (Table 4).
Table 4.
Bioactive compounds from natural products that induce ER stress-mediated apoptosis in gastric cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Curcuma longa | Curcumin | AGS | 20 µM, 24 h | Release of intracellular Ca2+ ↑, Δψm ↓, CHOP ↑ | [125] |
| cleavage of caspase-3,-7,-8,-9 ↑, cytochrome c ↑ | |||||
| Ulmus davidiana var. japonica | Ultrafine | SNU-1 | 200 μg/mL, 24 h | GRP78/BiP ↑, p-eIF2α ↑ | [135] |
| cleavage caspase-3,-6,-9 ↑, cleavage of PARP ↑ | |||||
| SNU-484 | 300 μg/mL, 24 h | p-eIF2α ↑, GRP78/BiP ↑ | |||
| cleavage of caspase-3,-6,-9 ↑, cleavage of PARP ↑, Bcl-2 ↓, Bcl-xL ↓ | |||||
| Magnolia officinalis | Honokiol | MKN45 | 40 µM, 8 h | GRP94 ↓, CHOP ↑, calpain ↑ | [136] |
| cleavage of caspase-7,-12 ↑, cleavage of PARP ↑ | |||||
| SCM-1 | 40 µM, 24 h | GRP94 ↓, CHOP ↑, calpain ↑ | |||
| cleavage of PARP ↑, | |||||
| vitamin E | Vitamin E succinate | SGC-7901 | 20 μg/mL, 24 h | release of Intracellular Ca2+ ↑, Δψm ↓ GRP78/BiP ↑, GRP94 ↓, PERK ↑, ATF4 ↑, ATF6 ↑, XBP1 ↑, CHOP ↑ |
[137] |
| cleavage of caspase-4,-7,-12 ↑, p-JNK ↑, JNK ↑, cleavage of PARP ↑ | |||||
| vitamin E | A-tocopheryl succinate | SGC-7901 | 20 μg/mL, 24 h | GRP78/BiP ↑, CHOP ↑ | [138] |
| cleavage of caspase-4 ↑ | |||||
| ROS ↑ | |||||
| Fructus viticis | Casticin | BGC-823 | 1 μmol/mL, 24 h | CHOP ↑, p-eIF2α ↑, eIF2α ↑, GRP78/BiP ↑ | [139] |
| DR5 ↑, Bax ↑, Bid ↑, cleavage of caspase-3,-8,-9 ↑ | |||||
| ROS ↑ | |||||
| Curcuma longa | 1-(4-hydroxy-3-methoxyphenyl)-5-(2-nitrophenyl)penta-1,4-dien-3-one (WZ35) | SGC-7901 | 10 μM, 12 h | ATF6 ↑, ATF4 ↑, XBP1 ↑, CHOP ↑ | [140] |
| P-JNK ↑, Bax ↑, cleavage of caspase-3 ↑ Bcl-2 ↓ | |||||
| ROS ↑ | |||||
| SGC-7901xenograft in athymic BALB/Ca-nu/nu female mice | WZ35 (orally, 50 mg/kg) for 28 days | CHOP ↑ | |||
| cleavage of caspase-3 ↑ |
Curcumin enhanced CHOP, JNK, cytochrome c, FADD, DR5 and caspase-8 in AGS cells. These results indicate that the release of intracellular Ca2+, mitochondrial dysfunction, extrinsic apoptotic pathway are involved in curcumin-induced ER stress [125]. Ultrafine (AM2; ethanolic extract of pulverized particles from Ulmus davidiana var. Japonica) upregulated GRP78/BiP, p-eIF2α, cleavage caspase-3,-6,-9 in both SNU-1 and SNU-484 cells [135]. Inhibition of MKN45 and SCM-1 cells viability through CHOP expression along with reduction of GRP94 was detected in Honokiol (isolated from the Magnolia officinalis) treatment [136]. Vitamin E succinate (RRR-a-tocopheryl succinate, VES) increased the level of CHOP, JNK, and caspase-12 and downregulated the expression of GRP94 in SGC-7901 cells [137]. Activation of CHOP, JNK and cleavage of caspase-4 were upregulated in SGC-7901 cells after treatment with a-Tocopheryl succinate (a-TOS; a derivative of natural vitamin E). NAC treatment decreased cell death by a-TOS, indicating that ER stress-mediated apoptosis by a-TOS is dependent on ROS generation [138]. Casticin (a major component obtained from Fructus viticis) induced elevation of CHOP, eIF2α, GRP78/BiP in BGC-823 cells as ER stress inducer. Additionally, Casticin upregulated DR5, leading to caspase-8 cleavage [139]. Expression of eIF2α-ATF4-CHOP signaling and JNK were enhanced in SGC-7901 cells by treatment of WZ35 (mono-carbonyl analogs of curcumin (MACs) via deletion of β-diketone moiety). Moreover, mitochondria-mediated apoptotic factor including Bax; cleavage of caspase-3 was observed. However, NAC treatment inhibited the effect of WZ35 by downregulating eIF2α-ATF4-CHOP signaling, Bax and cleavage of caspase-3. These results suggest that ROS generation may interplay with its ER stress-mediated apoptosis [140].
4.6. Natural Products Targeting ER Stress-Mediated Apoptosis in Prostate Cancer
Prostate cancer is the second most common cancer in men, and an estimated 1.1 million cases were diagnosed worldwide in 2012, making it the fourth leading cancer [3]. Due to early detection and new treatment options, quality of life of prostate cancer patients has been improved. However, most of the patients with prostate cancer suffer from aggressive and refractory cancer, with very poor prognosis [141]. Since UPR has been demonstrated to have an influence on either pro-survival or pro-death processes in prostate cancer [142]; currently, many studies have been reported to discover natural product with ER stress-mediated anti-cancer activity to develop novel therapeutic agents (Table 5).
Table 5.
Bioactive compounds from natural products that induce ER stress-mediated apoptosis in prostate cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Ardisia virens Kurz | Ardisianone | PC-3 | 10 μg/mL, 24 h | GRP78/BiP ↑ | [143] |
| cleavage ofcaspases-3,-7,-8,-9 ↑, cleavage of PARP ↑, Bcl-2 ↑, Bcl-xL ↑, Bak ↑, Bax ↑, Bid ↑, PI3K/Akt ↓, cytochrome c ↑, AIF ↑ | |||||
| ROS ↑ | |||||
| Camellia sinensis | Polyphenon E | PNT1a | 35 μg/mL, 12 h | ATF4 ↑, PERK ↑, p-eIF2α ↑, eIF2α ↑, GRP78/BiP ↑, CHOP ↑, XBP1 ↑ | [144] |
| cleavage of caspase-3,-7,-9 ↑, Bak ↑, Puma ↑, cleavage of PARP ↑ | |||||
| ROS ↑ | |||||
| PC3 | 145 μg/mL, 12 h | ATF4 ↑, PERK ↑, p-eIF2α ↑, eIF2α ↑, CHOP ↑, XBP1 ↑, | |||
| cleavage of caspase-3,-7,-9 ↑, Puma ↑, Bak ↑, Bax ↑, PARP ↑ AIF ↑ | |||||
| ROS ↑ | |||||
| Garcinia mangostana | Mangosteen Fruit Extract | LNCaP | 15 μg/mL, 24 h | PERK ↑, IRE1 ↑, CHOP ↑, GRP78/BiP ↑, Ero1 ↑, ER chaperone ↑, PDI ↑, XBP1 ↑, calnexin ↑ | [145] |
| Cleavage of caspase-3,-4 ↑, Bax ↑ | |||||
| 22Rv1 cells | 15 μg/mL, 24 h | PERK ↑, IRE1 ↑, CHOP ↑, GRP78/BiP ↑, Ero1 ↑, ER chaperone ↑, PDI ↑, XBP1 ↑, calnexin ↑ | |||
| Cleavage of caspase-3,-4 ↑, Bax ↑ | |||||
| Asterella angusta | Marchantin M | PC-3 | 10 μm, 48 h | GRP78/BiP ↑, CHOP ↑, XBP-1 ↑, p-eIF2α ↑,eIF2α ↑,ATF4 ↑, ATF6 ↑, ERAD ↓ | [146] |
| Cleavage of caspase-3,-4 ↑ | |||||
| DU145 | 10 μM, 48 h | GRP78/BiP ↑, CHOP ↑, XBP1 ↑, p-eIF2α ↑, eIF2α ↑, ATF4 ↑, ATF6 ↑, ERAD ↓ | |||
| Cleavage of caspase-3,-4 ↑ | |||||
| LNCaP | 10 μM, 48 h | GRP78/BiP, CHOP ↑, XBP1 ↑, p-eIF2α ↑, eIF2α ↑, ATF4 ↑, ATF6 ↑, ERAD ↓ | |||
| Cleavage of caspase-3,-4 ↑ | |||||
| Monascus pilosus | Monascuspiloin | PC-3 | 25 μM, 48 h | IRE1α ↑, p-eIF2α ↑, eIF2α ↑ | [147] |
| Quercetin | PC-3 | 150 μM, 48 h | Release of intracellular Ca2+ ↑, Δψm ↓, GRP78/BiP ↑, ATF4 ↑, IRE1α ↑ ATF6 ↑ | [148] | |
| Bid ↓, Bcl-2 ↓, cleavage of caspase-12 ↓Bax ↑, PARP ↑, cytochrome c ↑, AIF ↑, Endo G ↑, cleavage of caspase-3,-8,-9 ↑ | |||||
| Zingiber zerumbet Smith | Zerumbone | PC-3 | 30 μM, 24 h | Release of intracellular Ca2+ ↑, Δψm ↓, calpain ↑, GRP78, CHOP ↑ | [149] |
| cleavage of caspase-3,-7,-9 ↑, cleavage of PARP ↑, Bid ↑, Bcl-2 ↓ |
ER associated degradation (ERAD).
Ardisianone (from Ardisia virens Kurz) elevated the level of GRP78/BiP, cytochrome c, AIF, generation of ROS and cleavage of caspases-3,-9 in PC-3 cells, indicating that ER stress and mitochondria-mediated apoptosis were related to ardisianone treatment [143]. Induction of apoptosis in PNT1a and PC3 cells through CHOP expression, triggering BH3-only proteins, AIF and cleavage of caspase-3,-9 was demonstrated by the treatment of Polyphenol E (derived from Camellia sinensis) [144]. Mangosteen Fruit Extract (MFE; derived from Garcinia mangostana) enhanced the level of PERK, CHOP, IRE1 and caspase-4 in both LNCaP and 22Rv1 cells through ER stress, leading to Bax and cleavage of caspase-3 [145]. Upregulation of eIF2α-ATF4-CHOP signaling and caspase-4 in PC-3, DU145, and LNCaP cells through ER stress was observed after treatment of Marchantin M (Mar; derived from Astrella angusta) [146]. Monascuspiloin (derived from Monascus pilosus) treatment facilitated ER stress by activating eIF2α-ATF4-CHOP signaling pathway and caspase-3,-4 in PC-3 cells, which renders PC-3 cells sensitive to ionizing radiation [147]. Quercetin, a ubiquitous polyphenol found in several plants, increased the level of GRP78/BiP, ATF4, IRE1 and caspase-12 in PC-3 cells through ER-stress, which is associated with the induction of Ca2+ and ROS in the cytoplasm. Activation of Bax, cytochrome c, AIF, Endo G, and caspase-3,-9 were also detected [148]. Elevated levels of GRP78/BiP and CHOP in PC-3 cells through ER stress were induced by ZER treatment, resulting in activating cleavage of caspase-3,-7,-9, PARP and Bid along with inhibition of Bcl-2 [149].
4.7. Natural Product ER Stress-Mediated Apoptosis in Liver Cancer
Liver cancer emerged as one of the leading malignancy-related deaths globally. About 7.8 million new cases occurred in 2012, making it the sixth leading malignant neoplasm in the world [3]. Surgery is the main curative treatment for liver cancer, but a majority of patients with liver cancer are diagnosed at advanced stages. Thus chemotherapy has remained the ultimate curative approach for treatment of liver cancer [150]. However, patients tend to suffer from adverse side effect since chemotherapy agents kill both cancer cells and normal cells [151]. ER stress-mediated anti-proliferative effects of natural product have been elucidated through many studies using liver cancer cells [152] (Table 6).
Table 6.
Bioactive compounds from natural products that induce ER stress-mediated apoptosis in liver cancer.
| Family Name | Compound | Cell Line | Duration/Dosage | Mechanism | Reference |
|---|---|---|---|---|---|
| Glycyrrhiza inflate | Licochalcone A | HepG2 | 10 μM, 24 h | release of intracellular Ca2+ ↑, Δψm ↓, ATF6 ↑, eIF2α ↑, IRE1α ↑, CHOP ↑, GRP94 ↑, XBP1 ↑, GRP78/BiP ↑ | [153] |
| Cleavage of caspases-3,-4,-9 ↑, cleavage of PARP ↑ | |||||
| ROS ↑ | |||||
| Commiphora mukul | Guggulsterone | Hep3B | 30 μM, 12 h | Release of intracellular Ca2+ ↑, Δψm ↓, IRE1 ↑, JNK ↑, GRP78/BiP, PERK ↑, eIF2α ↑, ATF4 ↑, CHOP ↑ | [154] |
| DR5 ↑, cleavage of caspase-3 ↑, cleavage of PARP ↑ | |||||
| ROS ↑ | |||||
| Verrucarin A | Hep3B | 1 μM, 12 h | GRP78/BiP ↑, p-PERK ↑, p-eIF2α ↑, CHOP ↑ | [155] | |
| DR5 ↑, cleavage of caspase-3,-8 ↑, cleavage of PARP ↑ | |||||
| ROS ↑ | |||||
| HepG2 | 1 μM, 12 h | Chop ↑ | |||
| DR 5 ↑ | |||||
| 7-dimethoxyflavone | Hep3B | 5 μmol, 24 h | CHOP ↑, GPR78/BiP ↑, ATF4 ↑ | [156] | |
| DR5 ↑, cleavage of caspase-3,-8,-9 ↑ | |||||
| ROS ↑ | |||||
| Nelumbo nucifera Gaertn | Neferine | Hep3B | 20 μmol, 24 h | GRP78/BiP ↑, calnexin ↑, PDI ↑, calpain ↑ | [157] |
| cleavage of caspase-3,-6,-7,-8,-12, cleavage of PARP ↑, Puma ↑, BIM ↑, Bid ↑ | |||||
| Pycnostelma paniculatum K Schum | Paeonol | HepG2 | 31.25 mg/mL, 24 h | GRP78 ↑, CHOP ↑ | [158] |
| cleavage of caspase-3 ↑, PI3K/AKT ↓ | |||||
| Salvia miltiorrhiza Bunge | Cryptotanshinone | HepG2 | 10 μM, 24 h | eIF2α ↑, GRP94 ↑, GRP78/BiP ↑, cisplatin ↑, CHOP ↑ | [117] |
| cleavage of PARP ↑, JNK ↑, MAPK ↑ | |||||
| ROS ↑ | |||||
| Zingiber officinale Rosc | 6-Shogaol | SMMC-7721 | 20 μM, 6 h | GRP94 ↑, GRP78/BiP ↑, CHOP ↑, p-PERK ↑, PERK ↑, eIF2α ↑, p-eIF2α ↑ | [159] |
| cleavage of PARP ↑, cleavage of caspase-3 ↑ | |||||
| SMMC-7721 xenograft in Male SCID mice | 6-shogaol (orally, 10 mg/kg), 28 days | p-PERK ↓, eIF2α ↓, p-eIF2α ↓ | |||
| cleavage of caspase-3 ↑ | |||||
| Genistein | Hep3B | 100 μM, 48 h | release of intracellular Ca2+ ↑, Δψm ↓, calpain ↑, CHOP ↑, GRP78/BiP ↑ | [160] | |
| cleavage of caspase-2,-3,-7,-12 ↑, cleavage of PARP ↑, Apaf-1 ↑ cytochrome c ↑, Bad ↑ | |||||
| ROS ↑ |
Licochalcone A (derived from Glycyrrhiza inflate) elevated the expression of GRP78/BiP, ATF6, IRE1α, caspase-4, and CHOP in HepG2 cells, leading to cleavage of caspases-3,-9. However, ER stress-mediated apoptosis was inhibited by NAC treatment, indicating ROS involvement in Licochalcone A-induced ER stress [153]. Guggulsterone (originated from Commiphora mukul) increased the levels of GRP78/BiP, PERK, CHOP, JNK, DR5 in Hep3B cells through ER stress. Knockout of CHOP by CHOP siRNA reduced DR5 expression and NAC reverted a suppressive effect of guggulsterone, indicating CHOP-dependent DR5 and ROS-dependent ER-stress by guggulsterone [154]. Enhancement of CHOP-induced DR5 in Hep3B and HepG2 was noted after treatment of verrucarin A (derived from Fungal metabolite) [155]. 7-dimethoxyflavone (DMF; extracted from Myrtacea L. scoparium Forst and Piper methysticum Forst, etc) upregulated the level of ATF4, CHOP, DR5, cleavage of caspase-3,-8,-9 in Hep3B cells. This result suggests that both intrinsic and extrinsic apoptosis pathways are associated with DMF-induced ER stress [156]. GRP78/BiP, PDI, cleavage of caspase-3 and BH-3 family including BIM Bid were upregulated in Hep3B cells by treatment of neferine (isolated from Nelumbo nucifera Gaertn), indicating that ER stress and mitochondria-mediated apoptosis were induced by neferine [157]. Paenol (Pae; derived from Pycnostelma paniculatum K. Schum) elevated the level of GRP78/BiP, CHOP and cleavage of caspase-3 in HepG2 cells. This attenuated ER stress-induced resistance of HepG2 to the therapeutic agent doxorubicin [158]. Expression of elF2α, CHOP, JNK, MAPK in HepG2 cells were elevated through ER stress following cryptotanshinone treatment. However, NAC treatment down-regulated the apoptotic effect of cryptotanshinone. This result indicates that ER stress-mediated apoptosis via Cryptotanshinone is dependent on ROS generation [117]. 6-Shogaol (extracted from Zingiber officinale Rosc) inhibited SMMC-7721 cells growth via ER stress in terms of PERK-eIF2α-CHOP expression. In addition, oral administration of 6-Shogao suppressed the pro-survival pathway of p-PERK, eIF2a and p-eIF2α while it increased the cleavage of caspase-3, leading to reduction in SMMC-7721 tumor xenografts model [159]. With the induction of elevated CHOP, caspase-12 expression in Hep3B cells were noted after treatment of genistein (a flavonoid derived from soy products). In addition, activation of Apaf-1, cytochrome c, and cleavage of caspase-3,-9, PARP were observed, indicating mitochondria-mediated apoptosis [160].
5. Discussion
Cancer remains one of the leading causes of death in the world today. Although significant medical and technological developments have been achieved, still conventional cancer-targeted therapies have severe side effects and complications such as serious toxicities and development of resistance [161,162]. Therefore, one of the major goals for cancer treatment is to find novel therapeutic approaches which make it possible to selectively kill cancer cells without harming normal cells and reduce cancer cellular resistance to chemotherapeutic agents [145,147,158,163].
Upon quick expansion of the cancer tumor-microenvironment such as hypoxia, lack of glucose may have an impact on protein folding in ER of cancer cells, resulting in the accumulation of unfolded proteins, termed ER stress [22]. To overcome the hostile environment, UPR is initiated in cancer cells to attenuate ER stress for cancer survival. In fact, proteins related to UPR such as GRP78/BiP, XBP1, IRE1 were reported to be promoted in ischemic regions of cancer [17]. Also, activation of GPR78/BiP, XBP1 and ATF6 were observed in hepatocellular carcinoma cell line compared to non-cancerous liver tissues [164]. In addition, ER stress response may generate drug resistance as a survival response via activation of UPR [165]. For example, induction of the UPR in multiple myeloma cells enhanced resistance to etoposide [166]. These indicate that ER stress response may act as a key driver in tumorigenesis and the development of resistance to chemotherapy [43]. However, if the ER stress persists, apoptosis is induced in cancer cells [167]. These related findings provide clues that targeting the ER stress response, either inhibiting adaptive function or stimulating apoptotic function, may be an effective strategy for more selective cancer therapy and overcoming drug resistance [147].
As a way of cancer treatment, natural product-derived compounds are being introduced to the medical research and conducted to test the efficacy against cancers [168,169]. Especially, researches focusing on the cross link between the natural product or its derived compound and apoptosis in cancer via ER stress, are gaining interest [170,171]. Natural products or their bioactive compounds not only trigger apoptosis but also lower the resistance to chemotherapies via modulating ER stress. For example, brefeldin A derived from Eupenicillium brefeldianum was proven as an ER stress-inducing agent to overcome one of the standard drugs for leukemia fludarabine [172]. Also, Pae, which is derived from Pycnostelma paniculatum K. Schum, lowers the resistance of doxorubicin [158]. Furthermore, natural compounds were demonstrated to induce a synergistic effect with current standard therapies through ER stress regulation. For instance, Epigallocatechin 3-gallate, a polyphenolic green tea component, increased the apoptotic activity of temozolomide in glioblastoma cell death through inhibition of GPR78/BiP [173]. And falcarindiol, a natural polyyne in dietary plants, was identified to induce a synergistic effect with 5-Fluorouracil and bortezomib on suppressing breast cancer via upregulation of CHOP [174].
This present review summarizes the mechanisms of ER stress and its role in cancer development as well as apoptosis. It also delineates two approaches targeting the UPR as an anticancer strategy [19]: To inhibit ER stress–related proteins, to render cancer growing under stressful conditions to no longer be able to deal with the stress [11], and to induce chronic stress on cancer cells thereby tipping the balance towards cell death. Furthermore, the up-to-date researches were highlighted which were done with natural products in relation to the ER stress-mediated apoptosis in six types of leading cancers world-wide: lung, breast, colorectal, gastric, prostate cancer, and liver cancer, referring to the World Cancer Report 2014 by International Agency for Research on Cancer [2,3]. The related studies in this topic will not only significantly clarify our understanding of ER stress in cancer biology but also advance knowledge of interplay between natural product and ER stress-mediated apoptosis against cancer. Although cumulative evidences demonstrate that targeting ER stress as an anti-cancer strategy seems very promising. Further studies for the exact roles of ER stress in cancer and how they act on cell fate need to be investigated. Increasing knowledge in this area will be essential for pharmaceutical design toward controlling cancer through modulating UPR signaling [20,33]. Moreover, further research into ER stress could be utilized for other medical applications including the prognosis and diagnosis of multiple cancer types and could also be a solution to its involvement in chemo-resistance [26,35].
However, to successfully convert a potent natural product-derived compound to a clinically viable drug, several following questions needs to be addressed: (i) Do natural products exert selective cytotoxicity on cancer cells not on normal cells? (ii) Is it effective to target the ER stress as an anti-cancer strategy against other types of cancers? (iii) Do natural products or their derived compounds possess any special effects compared to those often seen with conventional drugs for cancer treatment? (iv) How might natural products cooperate with therapeutic agents that cause cancer cellular stress? (v) Can natural products or their derivatives induce a synergistic effect with conventional cancer treatment or unexpected side effects? Furthermore, since natural products or their molecular approaches targeting ER stress in cancer treatment are only in the early stage of research, most of the studies have focused on in vitro. Hence, additional investigation into pharmacokinetics with animal models and clinical studies are necessary. In addition, proper dosage of natural product needs to be considered to prevent the potential toxicity produced by natural products when developing natural product-derived compounds into clinically viable drugs [175]. For instance, ZER, which induces ER stress-mediated apoptosis in prostate cancer, was demonstrated to cause nephrocellular and hepatocellular damage by single intraperitoneal injection of 500 mg/kg ZER in sprague dawley (SD) rats [176]. Polyphenon E, which suppresses prostate cancer growth through ER stress, was identified to induce the mutant frequency in thymidine kinase locus and damage the myocardial fiber of Big Blue transgenic mouse when administered at a dosage of 2000 mg/kg for 28 days [177].
Eventually, more researches in this area will make clear understanding about the underlying mechanism of cross link between natural products and ER mediated-apoptosis. Also, enhanced knowledge in this area can be impetus for future developments of novel medicine not only in cancer treatment but also other diseases known to be associated with ER stress such as cardiovascular, inflammatory, and neurological diseases [178,179]. Importantly, additional evaluation of the therapeutic effects of natural products on cancer would provide us with an effective strategy to potentiate the health benefits of natural products.
Author Contributions
C.K. collected the data and C.K. and B.K. wrote and revised the manuscript.
Funding
This Research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03933656).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Hsu P.P., Sabatini D.M. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Stewart B.W., Wild C.P. World Cancer Report 2014. World Health Organization; Lyon, France: 2016. [Google Scholar]
- 3.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Reed J.C. Dysregulation of apoptosis in cancer. J. Clin. Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 5.Fisher D.E. Apoptosis in cancer therapy: Crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 6.Brown J.M., Attardi L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 7.Ghobrial I.M., Witzig T.E., Adjei A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S., Debatin K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 9.Debatin K.-M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004;53:153–159. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 11.Schröder M., Kaufman R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002;110:1389–1398. doi: 10.1172/JCI0216886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert D.N., Molinari M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 14.Yadav R.K., Chae S.-W., Kim H.-R., Chae H.J. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 2014;19:75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Ramirez L., Cao H., Nelson D., Hammond E., Lee A.-H., Yoshida H., Mori K., Glimcher L.H., Denko N.C., Giaccia A.J. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 16.Jamora C., Dennert G., Lee A.S. Inhibition of tumor progression by suppression of stress protein GRP78/BIP induction in fibrosarcoma B/C10ME. Proc. Natl. Acad. Sci. USA. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi M., Naczki C., Koritzinsky M., Fels D., Blais J., Hu N., Harding H., Novoa I., Varia M., Raleigh J. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M., Kaufman R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 19.Urra H., Dufey E., Lisbona F., Rojas-Rivera D., Hetz C. When ER stress reaches a dead end. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013;1833:3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Wang G., Yang Z.-Q., Zhang K. Endoplasmic reticulum stress response in cancer: Molecular mechanism and therapeutic potential. Am. J. Transl. Res. 2010;2:65–74. [PMC free article] [PubMed] [Google Scholar]
- 21.Sui X., Chen R., Wang Z., Huang Z., Kong N., Zhang M., Han W., Lou F., Yang J., Zhang Q. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F. Targeting endoplasmic reticulum signaling pathways in cancer. Acta Oncol. 2012;51:822–830. doi: 10.3109/0284186X.2012.689113. [DOI] [PubMed] [Google Scholar]
- 23.Schindler A.J., Schekman R. In vitro reconstitution of er-stress induced atf6 transport in copii vesicles. Proc. Natl. Acad. Sci. USA. 2009;106:17775–17780. doi: 10.1073/pnas.0910342106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 2000;20:6755–6767. doi: 10.1128/MCB.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J., Rutkowski D.T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G.D.-Y., Kaufman R.J. ATF6Α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 27.Scriven P., Coulson S., Haines R., Balasubramanian S., Cross S., Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br. J. Cancer. 2009;101:1692–1698. doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uramoto H., Sugio K., Oyama T., Nakata S., Ono K., Yoshimastu T., Morita M., Yasumoto K. Expression of endoplasmic reticulum molecular chaperone GRP78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Jiang Y., Jia Z., Li Q., Gong W., Wang L., Wei D., Yao J., Fang S., Xie K. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin. Exp. Metast. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 30.Daneshmand S., Quek M.L., Lin E., Lee C., Cote R.J., Hawes D., Cai J., Groshen S., Lieskovsky G., Skinner D.G. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum. Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Lee E., Nichols P., Spicer D., Groshen S., Mimi C.Y., Lee A.S. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 32.Abcouwer S.F., Marjon P.L., Loper R.K., Vander Jagt D.L. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Investig. Ophthalmol. Vis. Sci. 2002;43:2791–2798. [PubMed] [Google Scholar]
- 33.Backer J.M., Krivoshein A.V., Hamby C.V., Pizzonia J., Gilbert K.S., Ray Y.S., Brand H., Paton A.W., Paton J.C., Backer M.V. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia. 2009;11:1165–1173. doi: 10.1593/neo.09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyrko P., Schönthal A.H., Hofman F.M., Chen T.C., Lee A.S. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 35.Firczuk M., Gabrysiak M., Barankiewicz J., Domagala A., Nowis D., Kujawa M., Jankowska-Steifer E., Wachowska M., Glodkowska-Mrowka E., Korsak B. GRP78-targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis. 2013;4:e741. doi: 10.1038/cddis.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wey S., Luo B., Tseng C.-C., Ni M., Zhou H., Fu Y., Bhojwani D., Carroll W.L., Lee A.S. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito S., Furuno A., Sakurai J., Sakamoto A., Park H.-R., Shin-ya K., Tsuruo T., Tomida A. Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Res. 2009;69:4225–4234. doi: 10.1158/0008-5472.CAN-08-2689. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y., Wey S., Wang M., Ye R., Liao C.-P., Roy-Burman P., Lee A.S. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of er chaperone GRP78/BiP in prostate epithelium. Proc. Natl. Acad. Sci. USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X.Z., Harding H.P., Zhang Y., Jolicoeur E.M., Kuroda M., Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirasophon W., Welihinda A.A., Kaufman R.J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (IRE1P) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Adachi M., Zhao S., Hareyama M., Koong A., Luo D., Rando T., Imai K., Shinomura Y. Preventing oxidative stress: A new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A.-H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koong A.C., Chauhan V., Romero-Ramirez L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol. Ther. 2006;5:756–759. doi: 10.4161/cbt.5.7.2973. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Feldman D.E., Deng C., Brown J.A., De Giacomo A.F., Gaw A.F., Shi G., Le Q.T., Brown J.M., Koong A.C. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in saccharomyces cerevisiae. Mol. Cancer Res. 2005;3:669–677. doi: 10.1158/1541-7786.MCR-05-0181. [DOI] [PubMed] [Google Scholar]
- 45.Romero-Ramirez L., Cao H., Regalado M.P., Kambham N., Siemann D., Kim J.J., Le Q.T., Koong A.C. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl. Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C., Lennon C.J., Kluger Y., Dynlacht B.D. XBP1 controls diverse cell type-and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y., Zhang X., Liang Y., Yu H., Chen X., Zheng T., Zheng B., Wang L., Zhao L., Shi C. Targeting x box-binding protein-1 (XBP1) enhances sensitivity of glioma cells to oxidative stress. Neuropathol. Appl. Neurobiol. 2011;37:395–405. doi: 10.1111/j.1365-2990.2010.01155.x. [DOI] [PubMed] [Google Scholar]
- 48.Papandreou I., Denko N.C., Olson M., Van Melckebeke H., Lust S., Tam A., Solow-Cordero D.E., Bouley D.M., Offner F., Niwa M. Identification of an ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marciniak S.J., Garcia-Bonilla L., Hu J., Harding H.P., Ron D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase perk. J Cell Biol. 2006;172:201–209. doi: 10.1083/jcb.200508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DuRose J.B., Scheuner D., Kaufman R.J., Rothblum L.I., Niwa M. Phosphorylation of eukaryotic translation initiation factor 2α coordinates rrna transcription and translation inhibition during endoplasmic reticulum stress. Mol. Cell. Biol. 2009;29:4295–4307. doi: 10.1128/MCB.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y., Vattem K.M., Sood R., An J., Liang J., Stramm L., Wek R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/MCB.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 53.Verfaillie T., Garg A.D., Agostinis P. Targeting er stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 55.Rouschop K.M., Dubois L.J., Keulers T.G., van den Beucken T., Lambin P., Bussink J., van der Kogel A.J., Koritzinsky M., Wouters B.G. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ros. Proc. Natl. Acad. Sci. USA. 2013;110:4622–4627. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koumenis C., Naczki C., Koritzinsky M., Rastani S., Diehl A., Sonenberg N., Koromilas A., Wouters B.G. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase perk and phosphorylation of the translation initiation factor eIF2α. Mol. Cell. Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blais J.D., Addison C.L., Edge R., Falls T., Zhao H., Wary K., Koumenis C., Harding H.P., Ron D., Holcik M. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell. Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferri K.F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 59.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cullinan S.B., Diehl J.A. Coordination of er and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z.-W., Zhu H.-T., Chen K.-L., Dong X., Wei J., Qiu C., Xue J.-H. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2013;12:158. doi: 10.1186/1475-2840-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokouchi M., Hiramatsu N., Hayakawa K., Okamura M., Du S., Kasai A., Takano Y., Shitamura A., Shimada T., Yao J. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J. Biol. Chem. 2008;283:4252–4260. doi: 10.1074/jbc.M705951200. [DOI] [PubMed] [Google Scholar]
- 63.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 64.Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li G., Mongillo M., Chin K.-T., Harding H., Ron D., Marks A.R., Tabas I. Role of ero1-α–mediated stimulation of inositol 1, 4, 5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozcan L., Tabas I. Pivotal Role of Calcium/Calmodulin-Dependent Protein Kinase II in ER Stress-Induced Apoptosis. Cell cycle. 2010;9:223–224. doi: 10.4161/cc.9.2.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamaguchi H., Wang H.-G. Chop is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 68.Li H., Zhu H., Xu C.-J., Yuan J. Cleavage of bid by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 69.Puthalakath H., O’Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Reimertz C., Kögel D., Rami A., Chittenden T., Prehn J.H. Gene expression during ER stress–induced apoptosis in neurons: Induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J.Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huber A.-L., Lebeau J., Guillaumot P., Pétrilli V., Malek M., Chilloux J., Fauvet F., Payen L., Kfoury A., Renno T. P58 IPK-mediated attenuation of the proapoptotic perk-chop pathway allows malignant progression upon low glucose. Mol. Cell. 2013;49:1049–1059. doi: 10.1016/j.molcel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Nakagawa H., Umemura A., Taniguchi K., Font-Burgada J., Dhar D., Ogata H., Zhong Z., Valasek M.A., Seki E., Hidalgo J. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous hcc development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsukawa J., Matsuzawa A., Takeda K., Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J. Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 74.Fischer H., Koenig U., Eckhart L., Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophy. Res. Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 75.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoneda T., Imaizumi K., Oono K., Yui D., Gomi F., Katayama T., Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the er stress. J. Biol. Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 77.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris C.A., Johnson E.M. BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J. Biol. Chem. 2001;276:37754–37760. doi: 10.1074/jbc.M104073200. [DOI] [PubMed] [Google Scholar]
- 79.Yu C., Minemoto Y., Zhang J., Liu J., Tang F., Bui T.N., Xiang J., Lin A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein bad. Mol. Cell. 2004;13:329–340. doi: 10.1016/S1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 80.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 81.Li J., Ni M., Lee B., Barron E., Hinton D., Lee A. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Guo S.-H., Shang X.-J., Yu L.-S., Zhu J.-W., Zhao A., Zhou Y.-F., An G.-H., Zhang Q., Ma B. Triptolide induces sertoli cell apoptosis in mice via ROS/JNK-dependent activation of the mitochondrial pathway and inhibition of Nrf2-mediated antioxidant response. Acta Pharmacol. Sin. 2018;39:311–327. doi: 10.1038/aps.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y., Chen F. Reactive oxygen species (ROS), troublemakers between nuclear factor-κb (NF-κb) and c-Jun NH2-terminal kinase (JNK) Cancer Res. 2004;64:1902–1905. doi: 10.1158/0008-5472.CAN-03-3361. [DOI] [PubMed] [Google Scholar]
- 84.Kowaltowski A.J., Naia-da-Silva E.S., Castilho R.F., Vercesi A.E. Ca2+-stimulated mitochondrial reactive oxygen species generation and permeability transition are inhibited by dibucaine or Mg2+ Arch. Biochem. Biophys. 1998;359:77–81. doi: 10.1006/abbi.1998.0870. [DOI] [PubMed] [Google Scholar]
- 85.Tan Y., Dourdin N., Wu C., De Veyra T., Elce J.S., Greer P.A. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2006;281:16016–16024. doi: 10.1074/jbc.M601299200. [DOI] [PubMed] [Google Scholar]
- 86.Duchen M.R. Contributions of mitochondria to animal physiology: From homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuwana T., Bouchier-Hayes L., Chipuk J.E., Bonzon C., Sullivan B.A., Green D.R., Newmeyer D.D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Hao Z., Duncan G.S., Chang C.-C., Elia A., Fang M., Wakeham A., Okada H., Calzascia T., Jang Y., You-Ten A. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 89.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and datp-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 90.Li L.Y., Luo X., Wang X. Endonuclease G is an apoptotic dnase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 91.Bobrovnikova-Marjon E., Grigoriadou C., Pytel D., Zhang F., Ye J., Koumenis C., Cavener D., Diehl J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie J., Xu Y., Huang X., Chen Y., Fu J., Xi M., Wang L. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumor Biol. 2015;36:1279–1288. doi: 10.1007/s13277-014-2754-7. [DOI] [PubMed] [Google Scholar]
- 93.Huang T.-C., Chen J.-Y. Proteomic analysis reveals that pardaxin triggers apoptotic signaling pathways in human cervical carcinoma hela cells: Cross talk among the UPR, c-Jun and ROS. Carcinogenesis. 2013;34:1833–1842. doi: 10.1093/carcin/bgt130. [DOI] [PubMed] [Google Scholar]
- 94.Gutiérrez-Rodríguez A.G., Juárez-Portilla C., Olivares-Bañuelos T., Zepeda R.C. Anticancer activity of seaweeds. Drug Discov. Today. 2018;23:434–437. doi: 10.1016/j.drudis.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 95.Cragg G.M., Grothaus P.G., Newman D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 96.Kelloff G.J., Crowell J.A., Steele V.E., Lubet R.A., Malone W.A., Boone C.W., Kopelovich L., Hawk E.T., Lieberman R., Lawrence J.A. Progress in cancer chemoprevention: Development of diet-derived chemopreventive agents. J. Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 97.Sharma S., Stutzman J.D., Kelloff G.J., Steele V.E. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. 1994;54:5848–5855. [PubMed] [Google Scholar]
- 98.Mann J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 99.Kim C., Song H.S., Park H., Kim B. Activation of ER stress-dependent miR-216b has a critical role in salviamiltiorrhiza ethanol-extract-induced apoptosis in U266 and U937 cells. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cha J.A., Song H.-S., Kang B., Park M.N., Park K.S., Kim S.-H., Shim B.-S., Kim B. Mir-211 plays a critical role in cnidium officinale makino extract-induced, ROS/ER stress-mediated apoptosis in U937 and U266 cells. Int. J. Mol. Sci. 2018;19:865. doi: 10.3390/ijms19030865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park S.K., Sanders B.G., Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010;124:361–375. doi: 10.1007/s10549-010-0786-2. [DOI] [PubMed] [Google Scholar]
- 102.Al Husaini H., Wheatley-Price P., Clemons M., Shepherd F.A. Prevention and management of bone metastases in lung cancer: A review. J. Thorac. Oncol. 2009;4:251–259. doi: 10.1097/JTO.0b013e31819518fc. [DOI] [PubMed] [Google Scholar]
- 103.Qazi A.K., Siddiqui J.A., Jahan R., Chaudhary S., Walker L.A., Sayed Z., Jones D.T., Batra S.K., Macha M.A. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis. 2018;39:522–533. doi: 10.1093/carcin/bgy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marciniak S.J. Endoplasmic reticulum stress in lung disease. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0018-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siu F.M., Ma D.L., Cheung Y.W., Lok C.N., Yan K., Yang Z., Yang M., Xu S., Ko B.C., He Q.Y., et al. Proteomic and transcriptomic study on the action of a cytotoxic saponin (polyphyllin D): Induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics. 2008;8:3105–3117. doi: 10.1002/pmic.200700829. [DOI] [PubMed] [Google Scholar]
- 106.Hung J.Y., Hsu Y.L., Ni W.C., Tsai Y.M., Yang C.J., Kuo P.L., Huang M.S. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer. 2010;68:355–365. doi: 10.1016/j.lungcan.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 107.Moon J.L., Kim S.Y., Shin S.W., Park J.-W. Regulation of brefeldin A-induced ER stress and apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. Biochem. Biophys. Res. Commun. 2012;417:760–764. doi: 10.1016/j.bbrc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 108.Yang K.M., Kim B.M., Park J.B. Omega-hydroxyundec-9-enoic acid induces apoptosis through ROS-mediated endoplasmic reticulum stress in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2014;448:267–273. doi: 10.1016/j.bbrc.2014.04.111. [DOI] [PubMed] [Google Scholar]
- 109.Wu S.-H., Hang L.-W., Yang J.-S., Chen H.-Y., Lin H.-Y., Chiang J.-H., Lu C.-C., Yang J.-L., Lai T.-Y., Ko Y.-C. Curcumin induces apoptosis in human non-small cell lung cancer NCI-h460 cells through er stress and caspase cascade-and mitochondria-dependent pathways. Anticancer Res. 2010;30:2125–2133. [PubMed] [Google Scholar]
- 110.Hsia T.C., Yu C.C., Hsu S.C., Tang N.Y., Lu H.F., Huang Y.P., Wu S.H., Lin J.G., Chung J.G. Cantharidin induces apoptosis of h460 human lung cancer cells through mitochondria-dependent pathways. Int. J. Oncol. 2014;45:245–254. doi: 10.3892/ijo.2014.2428. [DOI] [PubMed] [Google Scholar]
- 111.Xu W.S., Dang Y.Y., Guo J.J., Wu G.S., Lu J.J., Chen X.P., Wang Y.T. Furanodiene induces endoplasmic reticulum stress and presents antiproliferative activities in lung cancer cells. Evid.-Based Complement. Altern. Med. ECAM. 2012;2012:426521. doi: 10.1155/2012/426521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao X., Liu X., Su L. Parthenolide induces apoptosis via tnfrsf10b and pmaip1 pathways in human lung cancer cells. J. Exp. Clin. Cancer Res. 2014;33:3. doi: 10.1186/1756-9966-33-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seong Y.A., Shin P.G., Yoon J.S., Yadunandam A.K., Kim G.D. Induction of the endoplasmic reticulum stress and autophagy in human lung carcinoma a549 cells by anacardic acid. Cell Biochem. Biophys. 2014;68:369–377. doi: 10.1007/s12013-013-9717-2. [DOI] [PubMed] [Google Scholar]
- 114.Clarke R., Cook K.L., Hu R., Facey C.O., Tavassoly I., Schwartz J.L., Baumann W.T., Tyson J.J., Xuan J., Wang Y., et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72:1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ko E.-Y., Moon A. Natural products for chemoprevention of breast cancer. J. Cancer Prev. 2015;20:223–231. doi: 10.15430/JCP.2015.20.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tseng C.N., Hong Y.R., Chang H.W., Yu T.J., Hung T.W., Hou M.F., Yuan S.S., Cho C.L., Liu C.T., Chiu C.C., et al. Brefeldin a reduces anchorage-independent survival, cancer stem cell potential and migration of MDA-MB-231 human breast cancer cells. Molecules. 2014;19:17464–17477. doi: 10.3390/molecules191117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park I.J., Kim M.J., Park O.J., Choe W., Kang I., Kim S.S., Ha J. Cryptotanshinone induces ER stress-mediated apoptosis in HEPG2 and MCF7 cells. Apoptosis. 2012;17:248–257. doi: 10.1007/s10495-011-0680-3. [DOI] [PubMed] [Google Scholar]
- 118.Shi J.M., Bai L.L., Zhang D.M., Yiu A., Yin Z.Q., Han W.L., Liu J.S., Li Y., Fu D.Y., Ye W.C. Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochem. Pharmacol. 2013;85:913–926. doi: 10.1016/j.bcp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 119.Pan M.Y., Shen Y.C., Lu C.H., Yang S.Y., Ho T.F., Peng Y.T., Chang C.C. Prodigiosin activates endoplasmic reticulum stress cell death pathway in human breast carcinoma cell lines. Toxicol. Appl. Pharmacol. 2012;265:325–334. doi: 10.1016/j.taap.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 120.Chen S., Zhao Y., Zhang Y., Zhang D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE. 2014;9:e108157. doi: 10.1371/journal.pone.0108157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kamiya T., Nishihara H., Hara H., Adachi T. Ethanol extract of brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. J. Agric. Food Chem. 2012;60:11065–11070. doi: 10.1021/jf303004n. [DOI] [PubMed] [Google Scholar]
- 122.Zhou Y., Shu F., Liang X., Chang H., Shi L., Peng X., Zhu J., Mi M. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS ONE. 2014;9:e89021. doi: 10.1371/journal.pone.0089021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qazi A.K., Hussain A., Aga M.A., Ali S., Taneja S.C., Sharma P.R., Saxena A.K., Mondhe D.M., Hamid A. Cell specific apoptosis by RLX is mediated by NFκB in human colon carcinoma HCT-116 cells. BMC Cell Biol. 2014;15:36. doi: 10.1186/1471-2121-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wielenga M.C.B., Colak S., Heijmans J., van Lidth de Jeude J.F., Rodermond H.M., Paton J.C., Paton A.W., Vermeulen L., Medema J.P., van den Brink G.R. ER-stress-induced differentiation sensitizes colon cancer stem cells to chemotherapy. Cell Rep. 2015;13:489–494. doi: 10.1016/j.celrep.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 125.Cao A., Li Q., Yin P., Dong Y., Shi H., Wang L., Ji G., Xie J., Wu D. Curcumin induces apoptosis in human gastric carcinoma ags cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis. 2013;18:1391–1402. doi: 10.1007/s10495-013-0871-1. [DOI] [PubMed] [Google Scholar]
- 126.Guichard C., Pedruzzi E., Fay M., Marie J.C., Braut-Boucher F., Daniel F., Grodet A., Gougerot-Pocidalo M.A., Chastre E., Kotelevets L., et al. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis. 2006;27:1812–1827. doi: 10.1093/carcin/bgl009. [DOI] [PubMed] [Google Scholar]
- 127.Tseng C.N., Huang C.F., Cho C.L., Chang H.W., Huang C.W., Chiu C.C., Chang Y.F. Brefeldin a effectively inhibits cancer stem cell-like properties and mmp-9 activity in human colorectal cancer colo 205 cells. Molecules. 2013;18:10242–10253. doi: 10.3390/molecules180910242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park J.-W., Woo K.J., Lee J.-T., Lim J.H., Lee T.-J., Kim S.H., Choi Y.H., Kwon T.K. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol. Rep. 2007;18:1269–1273. doi: 10.3892/or.18.5.1269. [DOI] [PubMed] [Google Scholar]
- 129.Edagawa M., Kawauchi J., Hirata M., Goshima H., Inoue M., Okamoto T., Murakami A., Maehara Y., Kitajima S. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib. J. Biol. Chem. 2014;289:21544–21561. doi: 10.1074/jbc.M114.558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Protiva P., Hopkins M.E., Baggett S., Yang H., Lipkin M., Holt P.R., Kennelly E.J., Bernard W.I. Growth inhibition of colon cancer cells by polyisoprenylated benzophenones is associated with induction of the endoplasmic reticulum response. Int. J. Cancer. 2008;123:687–694. doi: 10.1002/ijc.23515. [DOI] [PubMed] [Google Scholar]
- 131.Yaffe P.B., Power Coombs M.R., Doucette C.D., Walsh M., Hoskin D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinogenesis. 2015;54:1070–1085. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 132.Kuo Y.F., Su Y.Z., Tseng Y.H., Wang S.Y., Wang H.M., Chueh P.J. Flavokawain B, a novel chalcone from alpinia pricei Hayata with potent apoptotic activity: Involvement of ros and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010;49:214–226. doi: 10.1016/j.freeradbiomed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 133.Mullen J.T., Ryan D.P. Neoadjuvant chemotherapy for gastric cancer: What are we trying to accomplish? Ann. Surg. Oncol. 2014;21:13–15. doi: 10.1245/s10434-013-3250-9. [DOI] [PubMed] [Google Scholar]
- 134.Boelens J., Lust S., Offner F., Bracke M.E., Vanhoecke B.W. The endoplasmic reticulum: A target for new anticancer drugs. In Vivo. 2007;21:215–226. [PubMed] [Google Scholar]
- 135.Ahn J., Lee J.S., Yang K.M. Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress. Arch. Pharm. Res. 2014;37:783–792. doi: 10.1007/s12272-013-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sheu M.L., Liu S.H., Lan K.H. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS ONE. 2007;2:e1096. doi: 10.1371/journal.pone.0001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang X., Zhang Z., Jia L., Zhao Y., Zhang X., Wu K. Endoplasmic reticulum stress contributes to vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. Cancer Lett. 2010;296:123–131. doi: 10.1016/j.canlet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 138.Huang X., Li L., Zhang L., Zhang Z., Wang X., Zhang X., Hou L., Wu K. Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by alpha-tocopheryl succinate in human gastric carcinoma cells. Br. J. Nutr. 2013;109:727–735. doi: 10.1017/S0007114512001882. [DOI] [PubMed] [Google Scholar]
- 139.Zhou Y., Tian L., Long L., Quan M., Liu F., Cao J. Casticin potentiates trail-induced apoptosis of gastric cancer cells through endoplasmic reticulum stress. PLoS ONE. 2013;8:e58855. doi: 10.1371/journal.pone.0058855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zou P., Zhang J., Xia Y., Kanchana K., Guo G., Chen W., Huang Y., Wang Z., Yang S., Liang G. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget. 2015;6:5860. doi: 10.18632/oncotarget.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kallifatidis G., Hoy J.J., Lokeshwar B.L. Bioactive Natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016:160–169. doi: 10.1016/j.semcancer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyake H., Hara I., Arakawa S., Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J. Cell. Biochem. 2000;77:396–408. doi: 10.1002/(SICI)1097-4644(20000601)77:3<396::AID-JCB5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 143.Yu C.C., Wu P.J., Hsu J.L., Ho Y.F., Hsu L.C., Chang Y.J., Chang H.S., Chen I.S., Guh J.H. Ardisianone, a natural benzoquinone, efficiently induces apoptosis in human hormone-refractory prostate cancers through mitochondrial damage stress and survivin downregulation. Prostate. 2013;73:133–145. doi: 10.1002/pros.22548. [DOI] [PubMed] [Google Scholar]
- 144.Rizzi F., Naponelli V., Silva A., Modernelli A., Ramazzina I., Bonacini M., Tardito S., Gatti R., Uggeri J., Bettuzzi S. Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized pnt1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35:828–839. doi: 10.1093/carcin/bgt481. [DOI] [PubMed] [Google Scholar]
- 145.Li G., Petiwala S.M., Pierce D.R., Nonn L., Johnson J.J. Selective modulation of endoplasmic reticulum stress markers in prostate cancer cells by a standardized mangosteen fruit extract. PLoS ONE. 2013;8:e81572. doi: 10.1371/journal.pone.0081572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang H., Sun J., Xu Q., Liu Y., Wei J., Young C.Y., Yuan H., Lou H. Marchantin M: A novel inhibitor of proteasome induces autophagic cell death in prostate cancer cells. Cell Death Dis. 2013;4:e761. doi: 10.1038/cddis.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chiu H.W., Fang W.H., Chen Y.L., Wu M.D., Yuan G.F., Ho S.Y., Wang Y.J. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS ONE. 2012;7:e40462. doi: 10.1371/journal.pone.0040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu K.C., Yen C.Y., Wu R.S., Yang J.S., Lu H.F., Lu K.W., Lo C., Chen H.Y., Tang N.Y., Wu C.C., et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014;29:428–439. doi: 10.1002/tox.21769. [DOI] [PubMed] [Google Scholar]
- 149.Chan M.L., Liang J.W., Hsu L.C., Chang W.L., Lee S.S., Guh J.H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:1223–1236. doi: 10.1007/s00210-015-1152-z. [DOI] [PubMed] [Google Scholar]
- 150.Spangenberg H.C., Thimme R., Blum H.E. Targeted therapy for hepatocellular carcinoma. Nature Reviews Gastroenterol. Hepatol. 2009;6:423–432. doi: 10.1038/nrgastro.2009.86. [DOI] [PubMed] [Google Scholar]
- 151.Carr B.I. Hepatocellular carcinoma: Current management and future trends. Gastroenterology. 2004;127:S218–S224. doi: 10.1053/j.gastro.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 152.Malhi H., Kaufman R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi A.Y., Choi J.H., Hwang K.Y., Jeong Y.J., Choe W., Yoon K.S., Ha J., Kim S.S., Youn J.H., Yeo E.J., et al. Licochalcone a induces apoptosis through endoplasmic reticulum stress via a phospholipase cgamma1-, Ca(2+)-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis. 2014;19:682–697. doi: 10.1007/s10495-013-0955-y. [DOI] [PubMed] [Google Scholar]
- 154.Moon D.O., Park S.Y., Choi Y.H., Ahn J.S., Kim G.Y. Guggulsterone sensitizes hepatoma cells to trail-induced apoptosis through the induction of chop-dependent DR5: Involvement of ros-dependent er-stress. Biochem. Pharmacol. 2011;82:1641–1650. doi: 10.1016/j.bcp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 155.Moon D.O., Asami Y., Long H., Jang J.H., Bae E.Y., Kim B.Y., Choi Y.H., Kang C.H., Ahn J.S., Kim G.Y. Verrucarin a sensitizes trail-induced apoptosis via the upregulation of DR5 in an eIF2alpha/CHOP-dependent manner. Toxicol. In Vitro. 2013;27:257–263. doi: 10.1016/j.tiv.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Yang J.F., Cao J.G., Tian L., Liu F. 5, 7-dimethoxyflavone sensitizes trail-induced apoptosis through DR5 upregulation in hepatocellular carcinoma cells. Cancer Chemother. Pharmacol. 2012;69:195–206. doi: 10.1007/s00280-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 157.Yoon J.S., Kim H.M., Yadunandam A.K., Kim N.H., Jung H.A., Choi J.S., Kim C.Y., Kim G.D. Neferine isolated from nelumbo nucifera enhances anti-cancer activities in Hep3B cells: Molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine. 2013;20:1013–1022. doi: 10.1016/j.phymed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 158.Fan L., Song B., Sun G., Ma T., Zhong F., Wei W. Endoplasmic reticulum stress–induced resistance to doxorubicin is reversed by paeonol treatment in human hepatocellular carcinoma cells. PLoS ONE. 2013;8:e62627. doi: 10.1371/journal.pone.0062627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu R., Zhou P., Peng Y.-B., Xu X., Ma J., Liu Q., Zhang L., Wen X.-D., Qi L.-W., Gao N. 6-shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress. PLoS ONE. 2012;7:e39664. doi: 10.1371/journal.pone.0039664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yeh T.C., Chiang P.C., Li T.K., Hsu J.L., Lin C.J., Wang S.W., Peng C.Y., Guh J.H. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem. Pharmacol. 2007;73:782–792. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 161.Richardson J.L., Marks G., Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J. Clin. Oncol. 1988;6:1746–1752. doi: 10.1200/JCO.1988.6.11.1746. [DOI] [PubMed] [Google Scholar]
- 162.Coates A., Abraham S., Kaye S.B., Sowerbutts T., Frewin C., Fox R., Tattersall M. On the receiving end—Patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 163.Tsuruo T., Naito M., Tomida A., Fujita N., Mashima T., Sakamoto H., Haga N. Molecular targeting therapy of cancer: Drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shuda M., Kondoh N., Imazeki N., Tanaka K., Okada T., Mori K., Hada A., Arai M., Wakatsuki T., Matsubara O. Activation of the ATF6, XBP1 and GRP78 genes in human hepatocellular carcinoma: A possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 2003;38:605–614. doi: 10.1016/S0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 165.Corazzari M., Lovat P.E., Armstrong J.L., Fimia G.M., Hill D.S., Birch-Machin M., Redfern C.P.F., Piacentini M. Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: The role of stress proteins ERDJ5 and ERP57. Br. J. Cancer. 2007;96:1062–1071. doi: 10.1038/sj.bjc.6603672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gray M.D., Mann M., Nitiss J.L., Hendershot L.M. Activation of the unfolded protein response is necessary and sufficient for reducing topoisomerase IIΑ protein levels and decreasing sensitivity to topoisomerase-targeted drugs. Mol. Pharmacol. 2005;68:1699–1707. doi: 10.1124/mol.105.014753. [DOI] [PubMed] [Google Scholar]
- 167.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 168.Da Rocha A.B., Lopes R.M., Schwartsmann G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001;1:364–369. doi: 10.1016/S1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 169.Gordaliza M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 170.López-Antón N., Rudy A., Barth N., Schmitz L.M., Pettit G.R., Schulze-Osthoff K., Dirsch V.M., Vollmar A.M. The marine product cephalostatin 1 activates an endoplasmic reticulum stress-specific and apoptosome-independent apoptotic signaling pathway. J. Biol. Chem. 2006;281:33078–33086. doi: 10.1074/jbc.M607904200. [DOI] [PubMed] [Google Scholar]
- 171.Law B.Y., Wang M., Ma D.-L., Al-Mousa F., Michelangeli F., Cheng S.-H., Ng M.H., To K.-F., Mok A.Y., Ko R.Y. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ atpase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol. Cancer Ther. 2010;9:718–730. doi: 10.1158/1535-7163.MCT-09-0700. [DOI] [PubMed] [Google Scholar]
- 172.Carew J.S., Nawrocki S.T., Krupnik Y.V., Dunner K., McConkey D.J., Keating M.J., Huang P. Targeting endoplasmic reticulum protein transport: A novel strategy to kill malignant B cells and overcome fludarabine resistance in CLL. Blood. 2006;107:222–231. doi: 10.1182/blood-2005-05-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen T.C., Wang W., Golden E.B., Thomas S., Sivakumar W., Hofman F.M., Louie S.G., Schonthal A.H. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302:100–108. doi: 10.1016/j.canlet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 174.Lu T., Gu M., Zhao Y., Zheng X., Xing C. Autophagy contributes to falcarindiol-induced cell death in breast cancer cells with enhanced endoplasmic reticulum stress. PLoS ONE. 2017;12:e0176348. doi: 10.1371/journal.pone.0176348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dolan L.C., Matulka R.A., Burdock G.A. Naturally occurring food toxins. Toxins. 2010;2:2289–2332. doi: 10.3390/toxins2092289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ibrahim M.Y., Abdul A., Ibrahim T.A.T., Abdelwahab S.I., Elhassan M.M., Syam M. Evaluation of acute toxicity and the effect of single injected doses of zerumbone on the kidney and liver functions in sprague dawley rats. Afr. J. Biotechnol. 2010;9:4442–4450. [Google Scholar]
- 177.Chang P.Y., Mirsalis J., Riccio E.S., Bakke J.P., Lee P.S., Shimon J., Phillips S., Fairchild D., Hara Y., Crowell J.A. Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon e. Environ. Mol. Mutagenesis. 2003;41:43–54. doi: 10.1002/em.10129. [DOI] [PubMed] [Google Scholar]
- 178.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 179.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and alzheimer’s pathology. J. Neuroinflamm. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]