Abstract
Cellulite remains an obstinate clinical and cosmetic problem. In this study, we adapted the Thai traditional noninvasive treatment formulated with 5 additional herbals to improve blood flow, edema, and lipolysis, thereby augmenting cellulite treatment. This was a double-blind, randomized placebo-controlled paired trial. Twenty-one women (20-55 years) having cellulite (grade ≥2) were treated with steamed placebo or herbal compresses randomly assigned to one or other thigh twice weekly for 8 weeks with 2 weeks washout. Cellulite reduction was assessed from standardized photographs by 3 blinded evaluators at baseline and every 2 weeks; also assessed were thigh circumferences and cutaneous skin-fold thicknesses, trial diaries, and participant feedback. After 8 weeks, herbal compress treatment reduced Nürnberger-Müller cellulite scores from 12.6 ± 2.0 to 9.9 ± 2.4 compared with 12.5 ± 2.1 to 12.1 ± 2.0 (means ± SEM) for contralateral placebo-treated thighs (P < .0001; effect size [ES] = 1.16, confidence interval [CI] = 0.48-1.83). Thigh circumferences diminished by 2.2 ± 0.9 cm (herbal) and 1.4 ± 0.7 cm (placebo) (ES = 0.96, CI = 0.30-1.61) and correspondingly skin-folds by 5.6 ± 2.2 and 2.4 ± 1.3 mm (ES = 1.72, CI = 0.99-2.45). No adverse actions were reported, and there were no dropouts, no missing data, and 100% adherence. Herbal compresses were efficacious against cellulite and thigh sizes. The herbal formula might be adapted to other delivery options, and rationally added herbals may increase effectiveness of traditional therapies and more sustainable actions.
Keywords: cellulite, herbal medicine, compress, clinical trial
Cellulite describes an aesthetically unpleasant appearance of excessive and dystrophic gynoido-femoral fat of postpubescent females. It has numerous risks,1,2 but there is little consensus about its root cause.3 The tissue pathology is characterized by edema, vasoconstriction, inflammation, and especially the thick unyielding fibrosis,4 which is difficult to treat. Estrogen effectiveness on inflated gynoidal adipocytes remains unconfirmed, while the role of dermal adipocytes in cellulite awaits exploration. Thus, treatments are symptomatically directed at more readily amenable pathologies through pharmacological restoration by improving blood flow, relieving tissue pressurization, and improving adipocyte function.
Cellulite treatments are diverse, often rational, but their mechanisms of action elusive,5 and efficacy testing commonly lacks key elements of unbiased study design.6 Topically applied herbal remedies are popular because of perceived safety, avoidance of animal testing, or traditional usages. Many previous formulations were target-based and some alleviated cellulite6,7 while some contained too many components to permit mechanistic interpretation.2
In this study, we developed a treatment incrementally by beginning with a traditional herbal mixture (Trikatu) containing 6-gingerol and piperine, which reduced thigh diameter by ∼1.2 cm (N Waranuch et al, unpublished data). To this, we added another commonly used anti-cellulite ingredient, caffeine (tea/coffee). Thai hot compresses were traditionally used against aches and pains of daily drudgery.8 They are applied with heat, massaging motions, and herbs, all of which are appropriate to cellulite treatment.
Accordingly, an herbal compress was formulated (Table 1) and tested in a double-blind, single-arm, placebo-controlled trial on a cohort of 21 women with cellulite using its appearance as the primary end point. Compresses containing either active or inactive ingredients were randomly allocated to either thigh. The study demonstrates that evolution from Thai traditional wisdom into a clinical setting provided an effective cellulite treatment.
Table 1.
Ingredients of the Herbal Compressa.
| Ingredient | ||||
|---|---|---|---|---|
| Classification | Botanical Name (Common Name) | Amount (% w/w) | Part Used | Active Constituent (mg/g ± SD) |
| Constituents of traditional compresses (50% w/w of whole recipe). Herbs reducing inflammation or showing other benefits to skin | ||||
| Curcuma longa L. (Turmeric)24 | 5.0 | Rhizome | ND | |
| Zingiber cassumunar Roxb. (Plai)25 | 5.0 | Rhizome | ND | |
| Cymbopogon citratus (DC.) Stapf. (Lemon grass)26 | 10.0 | Stalk | ND | |
| Citrus hystrix DC. (Kaffir lime)27 | 10.0 | Peel of fruit | ND | |
| Cinnamomum camphora (L.) J.Presl (Camphor)28 | 13.0 | Bark | Camphor (106.0 ± 2.3) | |
| NaCl (salt) | 7.0 | NA | NA | |
| Herbal drugs selected for potential anti-cellulite action (50% of whole recipe). Herbs reducing inflammation, increasing microvascular and lymphatic flow, and/or stimulating lipolysis and reducing lipogenesis | ||||
| Zingiber officinale Roscoe (Ginger)29 | 20.0 | Rhizome | 6-Gingerol (4.11 ± 0.2) | |
| Piper nigrum L. (Black pepper)30 | 7.5 | Fruit | Piperine (10.3 ± 0.3) | |
| Piper retrofractum Vahl (Java long pepper)31 | 7.5 | Fruit | Piperine (10.0 ± 0.3) | |
| Camellia sinensis (L.) Kuntze (Tea)32 | 7.5 | Leaf | Caffeine (19.7 ± 0.2) | |
| Coffea arabica L. (Coffee)33 | 7.5 | Seed | Caffeine (6.4 ± 0.5) | |
Abbreviations: ND, not determined; NA, not analyzed; HPLC-QTOF-MS, high performance liquid chromatography-quantitative time-of-flight mass spectrometry.
aThe active ingredients were determined by HPLC-QTOF-MS.10
Materials and Methods
Study Design
This was a double-blind, randomized placebo-controlled paired trial where every participant received both herbal and placebo compress treatments to one or other leg.
Cohort Size
With repeated measures of 2 treatments (test vs placebo) and 6 measurements (6 time points), the power was calculated as 84% for total sample size equal to 42 legs. Other studies, summarized by Turati et al7 and our previous data (N Waranuch et al, unpublished data) also suggest a minimum of 21 participants (42 legs).
Outcomes
The primary endpoint was cellulite reduction.
The secondary endpoints were thigh circumference, skin-fold thickness, and participant comments.
Participant Procurement
Advertisements requesting female volunteers, aged 20 to 55 years, with upper leg cellulite, were placed around Naresuan University campus.
Inclusion Criteria
Women; aged 20 to 55 years; and having thigh cellulite, grade ≥2.0 by Nürnberger and Müller.9
Exclusion Criteria
Pregnancy; lactation; coagulation disorders; scars, local infections, or marks obscuring cellulite over the thighs; systemic diseases; history of allergic contact dermatitis including herbs; neuropathy; disorders of skin or its vascularity; use of chemical contraceptives; antihistamines, steroids, or nonsteroidal anti-inflammatories within 3 days before study participation; major surgery within the past year; and anti-cellulite treatment within the past 3 months.
Setting
Participants were recruited from an area within 5 km of Naresuan University, and all data were collected within the “Asom Sa-lao Clinic,” Applied Thai Traditional Medicine Center, Faculty of Public Health, Naresuan University. Potential recruits visited the clinic and thighs photographed to determine cellulite scored by NN as described below and their exclusion criteria verified by a checklist questionnaire. The testing laboratory comprised a changing room, interview room/office, a testing room, a photography cubicle, a preparation area, and 5 curtained off treatment areas equipped with couches, all at 25± 2°C.
Randomization
Since legs and cellulite are normally bilaterally symmetrical, allocation to either left or right thighs with simple randomization was performed using the ID codes; the herbal compress treatment “arm” was randomized by lottery (coin throwing) and the contralateral thigh allocated placebo compress treatment.
Blinding and Allocation Concealment
IDs and allocations were determined and securely stored by the principal investigator (KI) who had no role in compress production, storage, compress application, measurements, or data analysis. NN also kept the allocation table needed with appropriate compress to the practitioner applying the treatments. Compresses were steamed by a technician in the treatment laboratory and treatments given in 5 cubicles off the laboratory by 5 practitioners on Mondays and Thursdays for 8 weeks. Testing (thigh circumference, skin-fold thicknesses, thigh photography) were conducted on different days by a different technician. All practitioners and technicians were paid, were told that the trial compared different treatments, were not authors, nor had other conflicts. All data analyses were deferred until every participant had completed the trial.
Baseline Characteristics
All participants were ethnic Thais (rather than Han Chinese, etc), had similar lifestyles, and were living within a 5 km radius of the testing clinic (Table 2). After reading the participant information sheets, their cellulite assessed, and a ∼15 minute briefing, volunteers meeting the selection criteria signed the informed consent form, enrolled, and were given a study diary. All were told that 2 compresses would be tested, without stating whether one was a placebo.
Table 2.
Baseline Data.
| Parameter | Placebo Compress | Herbal Compress |
|---|---|---|
| Persons answering advertisements, n | 48 | |
| Participants enrolled, n | 21 | |
| Participants completing the trial, n | 21 | |
| Female | 100% | |
| Age (years), mean ± SD (range) | 38.0 ± 8.3 (24-53) | |
| Body weight (kg), mean ± SD | 60.2 ± 9.0 | |
| Body mass index (kg/m2), mean ± SD | 24.4 ± 3.3 | |
| Cellulite grade, mean ± SD | 3 ± 0a | 3 ± 0a |
| Cellulite Severity Score (CSS), mean ± SD | 12.54 ± 2.02* | 12.63 ± 2.00* |
| Thigh circumference (cm), mean ± SD | ||
| At 15 cm (lower) | 55.4 ± 4.0 | 55.3 ± 4.0 |
| At 25 cm (upper) | 61.4 ± 3.5 | 61.4 ± 3.6 |
| Cutaneous fold thickness (mm), mean ± SD | ||
| Anterior | 40.7 ± 7.2 | 40.6 ± 7.1 |
| Posterior | 43.5 ± 7.3 | 44.1 ± 6.7 |
aAll participants corresponding CSS = 11-15 (3 on the Nürnberger and Müller scale).
*P = .8, paired t test.
Monitoring
Treatments were regarded as very low risk since they were topically applied, have been used for many generations, and most ingredients are food products. Nevertheless, after every visit, each participant was queried about the treatment, and their diaries examined, problems discussed, and acted upon when needed.
Interventions
Herbal Compress
The ingredients (Table 1) were all sourced and formulated in the Herbal Production Unit, Bangkratum Hospital (Bangkratum, Phitsanulok, Thailand), except roasted coffee beans (Arabica 100% Coffman brand) and tea (Three Horses brand), which were purchased from the local market. The materials were verified by the hospital as being similar to the traditional specification.
Specimens of all 9 herbs were collected and authenticated by comparing voucher lots in the Biological Sciences Herbarium, Naresuan University, Phitsanulok, or botanical illustrations, and deposited in the same herbarium.
Materials were dried, powdered, sieved, and standardized as described elsewhere.10 In brief, methods were developed, systematically characterized for sensitivity, linearity, and so on, and validated for analyses of key ingredients given in Table 1 using HPLC-QTOF-MS (high performance liquid chromatography–quantitative time-of-flight mass spectrometry). Total ion chromatograms showing peaks for these ingredients are shown for standards and Methanol extracts of the herbal compress mixture.10
The Placebo
These compresses contained rice hulls from paddy (Oryza sativa L.) combined with broken rice to have a similar texture to the herbal compresses.
Components of herbal compresses produced odor, and after steaming and use, they had a yellowish coloration while the placebos had a lighter, dirty yellow color. Trying to replicate or disguise such smells and coloration in placebo compresses during use is problematic and introduction of any substances risks influencing the placebo status.
Preparation of Compresses
Using the Thai traditional method, a 450 × 450 mm cotton cloth was laid flat and ∼150 g of powdered herbal mix or placebo placed in the middle. The cloth was folded with the 4 corners meeting and cord tied at the top of the entrapped ingredients. The remaining cloth underwent further folds forming a handle consolidated by cord wound up the cloth handle (please search the Internet with “Make a Thai herbal compress ball” to find YouTube demonstrations), and used within 2 weeks.
Application of Compresses
The 5 practitioners were trained by NN (Bachelor of Applied Thai Traditional Medicine) to ensure consistency of compress application. On treatment days, compresses were placed in water for 15 minutes, then steamed for 20 minutes. After cooling, each compress was re-steamed for 10 minutes and then cooled to ∼45°C (tested by application to practitioner’s forearm); the appropriate compress applied to the participant’s left leg with its handle making a 365° conical motion for ∼10 seconds per revolution about a fixed axis perpendicular to the skin surface, with the handle tilted at 45° to the skin surface, and pressing with ∼2 kg force. This was repeated at 100 mm intervals from the inguinal fold to the patella, thus covering a 100 × 500 mm skin area depending on leg length. After 5 minutes, the compress was exchanged with a re-steamed compress and the process continued so that the lateral and inner thigh surfaces were treated each for 5 minutes, and anterior and posterior aspects for 10 minutes each. The right leg was then treated with the compress determined by the randomization. Each compress was used for 30 minutes in total over 2 consecutive treatments before being discarded.
Instructions to Participants
Participants wore shorts at visits. During the 11-week study, participants were asked to maintain their normal routines and diets, and refrain from anti-cellulite products. The participants were asked not to shower, wash, or rub their thighs for 30 minutes. Participants were paid 150 Thai Baht per attendance (∼US$5).
Measurement Methods
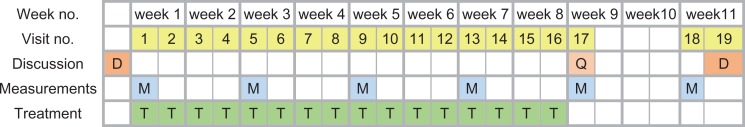
All the tests were conducted at baseline, and at 2-week intervals 3 days after the preceding treatment (Figure 1).
Figure 1.
Protocol for each participant for the 11-week trial. Before starting, participants were interviewed, recruited if they fit the selection criteria, and entered into the randomization table. At the first visit, recruited participants underwent their baseline measurements followed by the first treatment. Throughout 8 weeks, treatments (T) were applied twice per week at 3- to 4-day intervals at the same time of day. Full sets of measurements (M) were conducted at 2-week intervals and always preceded treatment on those days. During weeks 10 and 11, there was no treatment and the final visit was for debriefing and diary collection (D). Questionnaires (Q) were presented at week 9.
Cellulite Photography and Grading
Each participant stood with feet placed on fixed floor-markings at a fixed camera distance (Nikon D50/100 mm macro lens) and a semicircular (150° arc) white LED illuminating strip. Optimal illumination, camera height, and camera distance for each participant determined at baseline was used thereafter.11 Muscles of the test leg were relaxed by participants supporting their weight on the contralateral leg to ensure reproducibility.
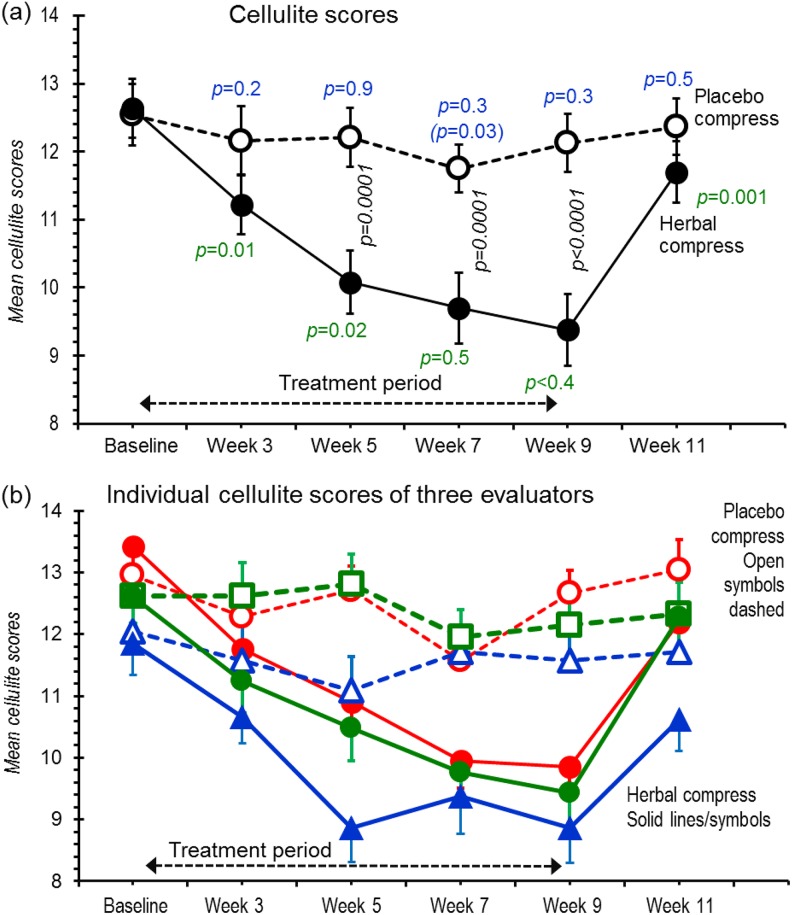
Three blinded, paid, independent master’s-level evaluators were trained by NN in grading and each tested 3 times for consistency of 20 graded monochrome cellulite photographs that we had validated using the Cellulite Severity Scale12 based on 5 key morphologies: (1) depression numbers; (2) depression depths; (3) clinical appearance of evident raised lesions; (4) grade of laxity, flaccidity, or sagging skin; and (5) cellulite grade according to Nürnberger-Müller9 classification. Each aspect was graded 0 to 3 yielding summed scores (0-15) and 3 classifications: mild (1-5), moderate (6-10), and severe (11-15). Photographs were presented to evaluators in random order after trial completion (Figure 2b indicates concordance between evaluators).
Figure 2.
Cellulite scores obtained during the 11-week trial. Scores were assessed from photographs taken under standardized conditions and given to 3 blinded evaluators in random order. (a) Decoded data averaged from the 3 scorers; (b) Separate plots from each evaluator (red, green, and blue) for placebo compresses (open symbols, dashed lines) and herbal compresses (filled symbols, solid lines). Points are means ± SEMs for all participants. In (a), P values (black, italics) positioned between the same placebo and herbal compress time points compare respective values by ANOVA with repeated measures and Bonferroni’s post hoc test. P values under the herbal compress values (green) compare the corresponding data time point with previous time point using paired t test for individual legs. In the same fashion, the top P values (in blue) compare the corresponding data time point of placebo with previous time point using paired t test (the value in parenthesis compares baseline). An absent value indicates P > .05.
Thigh Circumferences
Two circumferences were measured at (1) 15 cm (lower thigh) and (2) 25 cm (upper thigh) using the superior border of the proximal patella as the reference and one designated tape measure and conducted in triplicate by the same blinded operator.
Skin-Fold Thicknesses
Measurements were made by 2 blinded personnel, one manually forming a skin-fold and the other measuring the cutaneous fold thicknesses midway between the proximal patella and inguinal fold on the anterior and posterior aspects of each thigh. Each skin-fold was formed by the thumb and index finger gripping the skin 1 cm away from the measurement points and then skin-fold thickness measured with a plicometer. Participants were supine with the leg raised, supported, and relaxed.
Diary and (Dis)Satisfaction Questionnaire
To monitor side effects, participants were given 22-page diaries into which they spontaneously entered specific comments about treatments to each leg, any adverse effects, the current date, as well as scheduled appointments, and trial information. After the last treatment, participants at week 9 were given a satisfaction questionnaire based on similar studies13,14 (Table 4) but translated into Thai.
Table 4.
Responses to the Questionnaire on the Test Herbal and the Placebo Compressesa.
| Topic (The Question Appearing on the Questionnaire—Translated From Thai) | Placebo Compress Leg | Herbal Compress Treated Leg | P b |
|---|---|---|---|
| 1. Physical appearance of the compress | |||
| 1.1. Appearance looks inviting to use. | 4.38 | 4.57 | .6 |
| 1.2. I liked its shape. | 4.57 | 4.62 | .8 |
| 1.3. I liked its size. | 4.62 | 4.62 | 1 |
| 2. Properties of the compress | |||
| 2.1. Compress liquids do not ooze out. | 4.14 | 4.43 | .4 |
| 2.2. I felt that compress herbs were absorbed by the skin. | 4.25 | 4.24 | .9 |
| 2.3. Comfortable sense of warmth. | 4.05 | 4.33 | .2 |
| 2.4. Contented with length of treatment session. | 4.38 | 4.38 | — |
| 3. Performance of compress | |||
| 3.1. Left no stickiness on skin. | 3.86 | 4.14 | 1 |
| 3.2. Left no skin staining. | 4.05 | 3.76 | .6 |
| 3.3. I felt relaxed following treatment. | 4.76 | 4.81 | .3 |
| 3.4. My thigh looked thinner after treatment. | 3.95 | 4.24 | .01 |
| 3.5. Treatment did not cause itching or irritation. | 4.85 | 4.67 | .3 |
| 4. The smell of the compress | |||
| 4.1. I liked the smell. | 3.75 | 3.62 | .5 |
| 5. Overall satisfaction | |||
| 5.1. Happy with number of steps for each treatment session. | 4.67 | 4.67 | — |
| 5.2. Treatment was up to expectation. | 4.38 | 4.38 | — |
| 6. Overall treatment (score out of 10) | |||
| 6.1. Grade the compress for each thigh. | 8.2 | 9.0 | .002 |
a The responses were given a numerical value of 1 to 5, where 1 = Strongly disagree, 5 = Strongly agree, and 3 = Neutral.
b P value determined using Wilcoxon signed rank test (2-tailed).
Protocol
Treatment and testing schedules for all participants are shown in Figure 1. The participants laid on beds for 30 minutes before treatment to facilitate muscle and cardiovascular relaxation. Participants were treated twice weekly for 8 weeks (Mondays/Thursdays or Tuesdays/Fridays for some participants) always using the same leg for test or control, 30 minutes each leg. The measurements (at weeks 3-11) were 3 days after the last preceding treatment.
Statistical Analyses
For continuous outcomes, means ± SD were calculated and analysis of variance with repeated measures comparing effects of placebo and herbal compresses over time. Differences between individual time points were assessed with paired t test after testing for normality. Thigh circumferences and skin-fold/fat-fold thicknesses were compared with baseline using Bonferroni’s procedure using R.15 Effect sizes were calculated by Cohen’s d (using R). Questionnaire satisfaction scores were compared by Wilcoxon signed-rank test.
Results
Of 48 women answering the advertisement, 21 fitting the selection criteria were enrolled. After the first treatment, one participant could not commit to further treatments because of modified work-related schedules. Another woman fitting the specification was found, who was allocated the withdrawn participant ID and began treatment the next day. Including this participant and all other participants, baseline thigh characteristics showed close matching for outcome parameters (Table 2). There were no further withdrawals/dropouts, no exclusions, and protocol variations; adherence was 100%; and data analyzed as intention to treat for 21 participants for all time points. Body mass index showed no change throughout the study (baseline, 24.4 ± 3.3; week 9, 24.4 ± 3.4; week 11, 24.4 ± 3.4 kg/m2). Participants were overweight and 6 were obese as defined by the Asian rating scale.16 Cellulite Severity Scale scores indicated that 16 had severe cellulite in the test thighs and 17 of the placebos. Baseline cellulite and thigh circumference and skin-fold measurements showed remarkable concordance between placebo and herbal compresses (Figures 2a, 4, and 5).
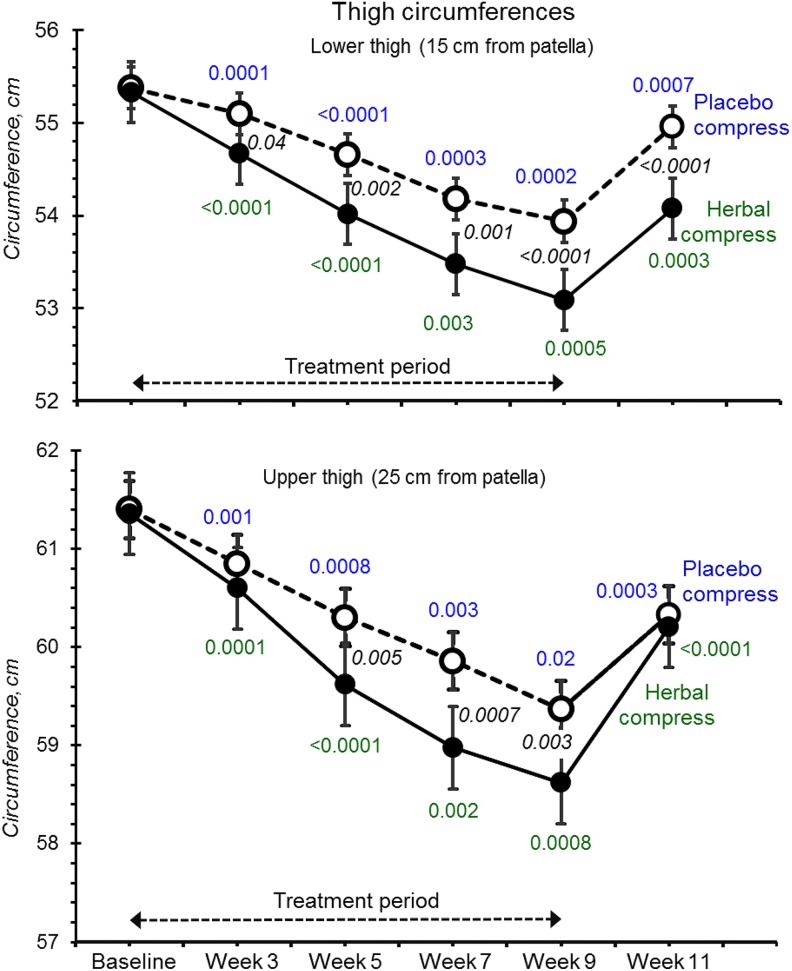
Figure 4.
Lower and upper thigh circumferences at baseline, with 8 weeks of treatment with placebo (open circles) and herbal compresses (filled circles) followed by 2 weeks without treatment (Figure 1). All P values have the same meaning as that stated in Figure 2. Effect sizes are listed in Supplemental Table S1.
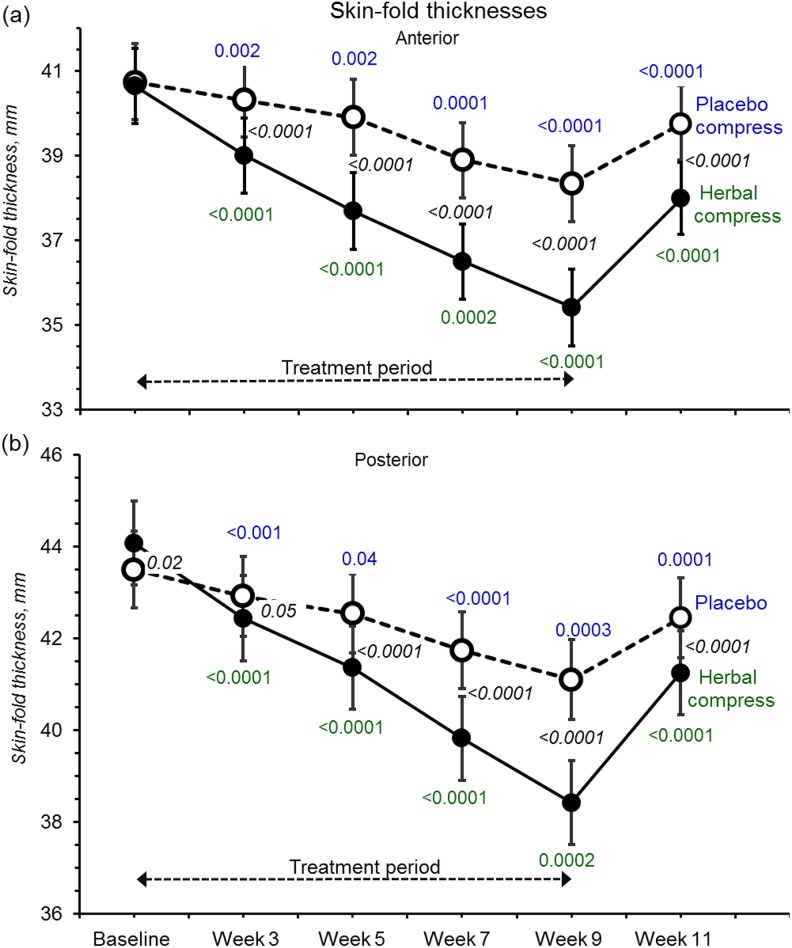
Figure 5.
Anterior and posterior skin-fold thicknesses at baseline with all symbols and P values having the same meaning as in Figure 2. Effect sizes are listed in Supplemental Table S1.
Herbal Compresses Reduced Cellulite
At most time points, placebo-filled compresses had no detectable effect on cellulite (Figure 2a), whereas at every measurement point after treatment commenced, herbal compresses robustly reduced cellulite scores, progressively up to 5 weeks (between 1-3 and 3-5 week measurement times). The Cohen d effect size at the 9-week time point was 1.16 (confidence interval [CI] = 0.48 to 1.83; full list in Supplemental Table S1; available in the online version of the article). But within the 2-week posttreatment period (“washout”), the treatment effect had dissipated.
Cellulite assessments are subjective so we plotted data from each evaluator separately (Figure 2b). Although variation was substantial, every evaluator detected similar general trends.
For week 9 and using herbal/placebo differences, each participant showed reduced cellulite except 2 who had increased cellulite scores (+0.3 and +2.3) albeit values compatible with errors (SD = 2.2). Figure 3 shows a set of standardized photographs from one participant. Cellulite reduction did not correlate with age (P = .6) or body mass index (P = .9).
Figure 3.
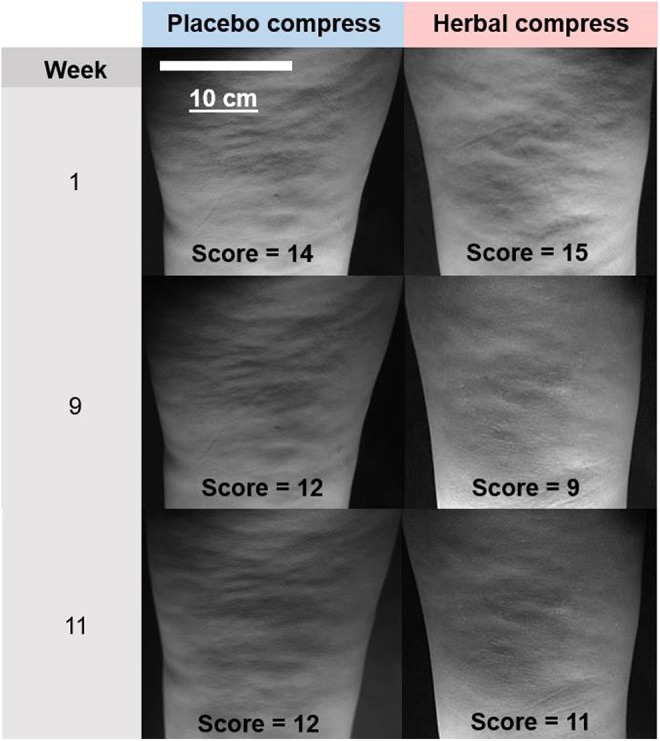
Representative photographs showing rear views of the upper legs given to the cellulite evaluators. They are all of the same participant taken at baseline (week 1), during treatment (week 9), and after cessation of treatment (week 11). The cellulite scores are the average values estimated by the 3 evaluators.
Thigh Circumference and Skin-Fold Thicknesses Decreased
Throughout the 8 week treatment, both lower and upper thighs became progressively thinner with both placebo and herbal compresses (Figure 4). However, reductions were clearly and consistently greater for herbal compresses treated legs at both measurement positions (by ∼0.8 cm at week 9) (effect sizes were 0.96, CI = 0.31 to 1.61 at 15 cm above the knee, and 0.55, CI = −0.18 to 1.19 at 25 cm; see also Supplemental Table S1; available in the online version of the article).
Similarly, skin-fold thicknesses were decreased by herbal compresses (Figure 5). The effect was proportionately greater (85% to 90% baseline) compared to circumference as expected for changes confined to skin and subcutaneous fat (effect sizes were 1.37, CI = 0.67 to 2.06 for front thigh, and 1.72, CI = −0.99 to 2.45, rear thigh).
At week 9, 3 participants showed increased upper thigh circumferences compared with placebo, 2 participants at the lower measurement position, and 1 participant for posterior skin-fold thickness but not the same participants. Only 1 participant showed consistent increases for all 4 treatment time points, comparing baseline, in the upper thigh, while her other 3 metrics (lower thigh and skin-folds) all decreased. These data suggest increases were spurious rather than opposite pharmacological outcomes in these women.
All 4 metrics indicated progressive declines and had not plateaued by week 9, suggesting that skin-fold and thigh thinning may have been greater had treatment continued (Figures 4 and 5). On ceasing treatments, both test and placebo legs clearly regained most of their skin-fold thicknesses and circumferences within the 2-week “washouts.”
Placebo actions, however, were strikingly different: substantial on thigh girth and skin-fold yet little impact on cellulite scores. This fortifies views of some commentators that cellulite appearance is the only reliable end point whereas surrogates including thigh mass can be misleading. This observation also questions the value of massage and heat that is provided by our placebo compress but not directly reducing cellulite. Nevertheless, a permissive effect on other anti-cellulite components is possible.
Diary Record and Self-Assessment
Participant diary entries about perceptions throughout the trial favored treated over placebo legs (Table 3); for example, firmer legs and looser fitting pants while relaxed feelings were commented on. The satisfaction questionnaire sought overall participant perceptions of test versus placebo compresses (Table 4) and gave high scores but “perceived leg-size” discriminated test/placebo. Compress appeal, treatment satisfaction, and freedom from irritation attracted highest ratings, while smell and skin stickiness and staining were less favorable.
Table 3.
Summaries of Spontaneous Comments Entered into the Diaries During the Triala.
| Generalized Comment | About Placebo- Treated Leg | About Herbal- Treated Leg |
|---|---|---|
| My skin seems firmer. | 3 | 6 |
| My skin seems smoother. | 1 | 2 |
| I feel relaxed. | 8 | 9 |
| My thighs seem to be smaller. | 4 | 5 |
| Work related aches and pains were relieved. | 2 | 2 |
| My pants seem to looser at the thighs. | 1 | 4 |
| Total number of diary entries | 19 | 28 |
aThe participants are grouped by the general sense of statements falling into the categories noted below as “Generalized Comments” about the leg treated with placebo or herb-containing compresses. They were translated into English by a blinded assessor.
Adverse Effects
No adverse effects were reported via questionnaires, diaries, or verbal communication, and no reddening, swelling, or irritation were observed around treatment areas.
Discussion
Mechanisms of Action
The mechanisms are harder to define but improved hemodynamics, edema clearance, and reduced inflammation probably play a major role.17 Adipocyte function may also improve through stimulating triglyceride mobility and antioxidation, which is attenuated in obesity18 and helps switch adipose tissue from predominantly inflammatory to an anti-inflammatory phenotype. Thus, reduced subcutaneous perivascular fat in particular can improve vascular function, even without weight loss.19
Pharmacokinetics
Hemodynamic and immune improvements should respond within hours/days of treatment, while onset was slow, particularly clear for thigh morphology. This suggests complex actions. On ceasing treatment, cellulite reappeared rapidly, suggesting the underlying pathology had been untreated. The characteristic cellulite fibrosis4 and fibroblasts unable to remodel extracellular matrix in a nonfacilitative environment are undoubtedly major contributors to this recalcitrance along with lost elastic fibers.20 Thus, while our knowledge of cellulite pathophysiology remains vacuous, efficacious treatment will remain symptomatic.
Multiple Drugs
Confining treatment to one bioactive is unlikely to provide optimal efficacy. Our hybrid was based on traditional wisdom and pharmacological rationale acting on multiple targets likely to relieve cellulite. Thus, integration of effects, interactions, and other unidentified constituents may explain the overall efficacy observed here. This approach has been criticized,2 but testing each separately needs large cohorts to achieve meaningful effect sizes, and biopsies to assess molecular actions. This approach is clearly unfeasible. Instead, additions of, for example, β-3 receptor, adenylate cyclase-3, and AMPK agonists, and adipocyte browning will provide useful adjunct actions. The holy grail of fibrosis reversal by stimulating extracellular matric remodeling will also depend on polypharmacy21 supplemented by anti-fibrotic herbals.
Protocol Limitations
Two factors could compromise blinding, compress color and odor. Nevertheless, participants were probably impartial to ambient odors because inhaled influences absorbed into the systemic circulation and vasomotor action show bilateral symmetry.22 More crucially and surprisingly, the practitioners had decided among themselves that the placebo compresses were “new ones” being tested because they had less odor and color than in their past experience. Furthermore, they noticed that placebo treatment left the skin slightly whiter, a desirable outcome for brown Asia women, compared with their dislike of the skin staining of herbal compresses. Thus, the practitioners voiced preference for the placebo and had a potential bias against the intervention. While participant homogeneity helped study precision, it sacrificed generalizability. Nevertheless, the predominantly rural catchment has succumbed to the global obesity epidemic, with high cellulite incidences, and whose acquired lifestyles typify >50% of the global population. In common with all topical cellulite treatments, the body area affected can be extensive, making complete treatment impractical. Notwithstanding, the low intrinsic herbal costs could also find application as hot herbal baths, thereby treating the whole body including otherwise inaccessible areas and greatly reducing professional fees.
This study was the first using Thai herbal compresses to counteract cellulite. When combined with several traditional herbs and other anti-cellulite compounds (Table 1), our composite had clear-cut anti-cellulite efficacy. At the same time, thighs and skin-folds thinned comparably or greater than previous chemical treatments7 while having no dropouts and full compliance with minimum requirements for unbiased clinical trials.23
Conclusions
Our preliminary study shows that rationally designed, steamed hot herbal compresses provide useful cellulite reduction without detectable side effects. Nevertheless, further work, particularly enhancing the sustainability of treatment outcomes, is needed. But this is hampered by the subjective, time-consuming metrics and the inability to dissect out individual pathophysiological processes that could underpin future rationally targeted multidrug treatments. However, lifestyle changes that reduce systemic inflammation associated with obesity and metabolic disease should supplement these, or we should use more advanced polypharmaceutical approaches to ensure enduring cellulite amelioration.
Supplemental Material
Supplementary_File_794158 for Cellulite Reduction by Modified Thai Herbal Compresses; A Randomized Double-Blind Trial by Ngamrayu Ngamdokmai, Neti Waranuch, Krongkarn Chootip, Katechan Jampachaisri, C. Norman Scholfield, and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine
Acknowledgments
We thank the independent evaluators who graded cellulite; the clinical coordinators at the Applied Thai Traditional Medicine Center and the practitioners in the “Asom Sa-lao Clinic,” both located in the Faculty of Public Health, Naresuan University; and the participants for their enthusiastic cooperation.
Authors’ Note: Following the study completion (June 24, 2016), the university filed an application with the Thai Patent Office for the herbal compress formula, which also cited IK, NW, KC, NN, and KJ.
Author Contributions: The work presented in this article was carried out through collaboration between all authors. NN was involved with study planning and performance. KI, NW, and KC were involved with study planning and made the initial hypothesis. All authors participated in defining the research theme and provided the proposal. KJ performed the statistical analysis. CNS discussed the data and corrected and reviewed the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand, which provided a studentship to NN. Other funding came from Naresuan University, Thailand (Grant Number R2558B011). Neither funding agency influenced or restricted any part of the trial, its data, or publication.
ORCID iD: Krongkarn Chootip  http://orcid.org/0000-0001-6973-158X
http://orcid.org/0000-0001-6973-158X
Supplemental Material: Supplemental material for this article is available online.
Ethical Approval: The protocol was approved by the Institutional Review Board and Ethics Committee, Naresuan University, in accordance with the Helsinki Declaration (2008) and Good Clinical Practices (IRB No. 161/2013, dated October 24, 2013) and registered with the Thai Clinical Trials Registry #TCTR20160302001. The recruitment and clinical study ran during January to March 2015 at the Clinic of Applied Thai Traditional Medicine, the Faculty of Public Health, Naresuan University, Phitsanulok, Thailand.
References
- 1. Bacci PA, Leibaschoff GC. Pathophysiology of cellulite In: Goldman MP, Bacci PA, Leibaschoff GC, Hexcel D, Angelini F, eds. Cellulite: Pathophysiology and Treatment. 1st ed. New York, NY: Taylor & Francis; 2006:41–74. [Google Scholar]
- 2. Emanuele E. Cellulite: advances in treatment: facts and controversies. Clin Dermatol. 2013;31:725–730. [DOI] [PubMed] [Google Scholar]
- 3. de la Casa Almeida M, Serrano SC, Roldán RJ, Rejano JJ. Cellulite’s aetiology: a review. J Eur Acad Dermatol Venereol. 2013;27:273–278. [DOI] [PubMed] [Google Scholar]
- 4. Hexsel DM, Abreu M, Rodrigues TC, Soirefmann M, do Prado DZ, Gamboa MML. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35:1471–1477. [DOI] [PubMed] [Google Scholar]
- 5. Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luebberding S, Krueger N, Sadick NS. Cellulite: an evidence-based review. Am J Clin Dermatol. 2015;16:243–256. [DOI] [PubMed] [Google Scholar]
- 7. Turati F, Pelucchi C, Marzatico F, et al. Efficacy of cosmetic products in cellulite reduction: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2014;28:1–15. [DOI] [PubMed] [Google Scholar]
- 8. Institute of Thai Traditional Medicine. Thai Traditional Medicine Training Guide. Bangkok, Thailand: The War Veterans Organization of Thailand under Royal Patronage of His Majesty the King Publishing; 1995. [Google Scholar]
- 9. Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221–229. [DOI] [PubMed] [Google Scholar]
- 10. Ngamdokmai N, Waranuch N, Chootip K, Neungchamnong N, Ingkaninan K. HPLC-QTOF-MS method for quantitative determination of active compounds in an anti-cellulite herbal compress. Songklanakarin J Sci Technol. 2017;39:463–470. [Google Scholar]
- 11. Bielfeldt S, Buttgereit P, Brandt M, Springmann G, Wilhelm KP. Non-invasive evaluation techniques to quantify the efficacy of cosmetic anti-cellulite products. Skin Res Technol. 2008;14:336–346. [DOI] [PubMed] [Google Scholar]
- 12. Hexsel DM, Dal’forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23:523–528. [DOI] [PubMed] [Google Scholar]
- 13. Dupont E, Journet M, Oula ML, et al. An integral topical gel for cellulite reduction: results from a double-blind, randomized, placebo-controlled evaluation of efficacy. Clin Cosmet Invest Dermatol. 2014;7:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bacci PA, Allegra C, Albergati F, Brambilla E, Botta G, Mancini S. Randomized, placebo-controlled double-blind clinical study of the efficacy of a multifunctional plant complex in the treatment of so-called “cellulite.” Intertl J Cosmet Surg Aesthet Dermatol. 2003;5:53. [Google Scholar]
- 15. R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 16. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 17. Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng B, Zhang T, Xu H. Human adipose dynamics and metabolic health. Ann N Y Acad Sci. 2013;1281:160–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aghamohammadzadeh R, Greenstein AS, Yadav R, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ezure T, Amano S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp Dermatol. 2015;24:924–929. [DOI] [PubMed] [Google Scholar]
- 21. Nanthakumar CB, Hatley RJD, Lemma S, Gauldie J, Marshall RP, Macdonald SJF. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat Rev Drug Discov. 2015;14:693–720. [DOI] [PubMed] [Google Scholar]
- 22. Billhult A, Lindholm C, Gunnarsson R, Stener-Victorin E. The effect of massage on cellular immunity, endocrine and psychological factors in women with breast cancer—a randomized controlled clinical trial. Auton Neurosci. 2008;140:88–95. [DOI] [PubMed] [Google Scholar]
- 23. Turner L, Shamseer L, Altman DG, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012;(11):MR000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9:161–168. [DOI] [PubMed] [Google Scholar]
- 25. Pithayanukul P, Tubprasert J, Wuthi-Udomlert M. In vitro antimicrobial activity of Zingiber cassumunar (Plai) oil and a 5% Plai oil gel. Phytotherap Res. 2007;21:164–169. [DOI] [PubMed] [Google Scholar]
- 26. Ekpenyong CE, Akpan E, Nyoh A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin J Nat Med. 2015;13:321–337. [DOI] [PubMed] [Google Scholar]
- 27. Chueahongthong F, Ampasavate C, Okonogi S, Tima S, Anuchapreeda S. . Cytotoxic effects of crude kaffir lime (Citrus hystrix, DC.) leaf fractional extracts on leukemic cell lines. J Med Plants Res. 2011;5:3097–3105. [Google Scholar]
- 28. Kotaka T, Kimura S, Kashiwayanagi M, Iwamoto J. Camphor induces cold and warm sensations with increases in skin and muscle blood flow in human. Biol Pharm Bull. 2014;37:1913–1918. [DOI] [PubMed] [Google Scholar]
- 29. Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. [DOI] [PubMed] [Google Scholar]
- 30. Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L. (black pepper): a review. Asian Pac J Trop Med. 2012;2:S1945–S1953. [Google Scholar]
- 31. Choudhary G. Mast cell stabilizing activity of Piper longum Linn. Indian J Allergy Asthma Immunol. 2006;20:112–116. [Google Scholar]
- 32. Ramalho SA, Nigam N, Oliveira GB, et al. Effect of infusion time on phenolic compounds and caffeine content in black tea. Food Res Int. 2013;51:155–161. [Google Scholar]
- 33. Al-Bader T, Byrne A, Gillbro J, et al. Effect of cosmetic ingredients as anticellulite agents: synergistic action of actives with in vitro and in vivo efficacy. J Cosmet Dermatol. 2012;11:17–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_File_794158 for Cellulite Reduction by Modified Thai Herbal Compresses; A Randomized Double-Blind Trial by Ngamrayu Ngamdokmai, Neti Waranuch, Krongkarn Chootip, Katechan Jampachaisri, C. Norman Scholfield, and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine