Abstract
Background:
Rechallenge with imatinib is an option in advanced gastrointestinal stromal tumor (GIST) patients following progression with standard tyrosine-kinase inhibitors (TKIs), imatinib, sunitinib and regorafenib. We retrospectively collected data from metastatic Italian GIST patients treated with imatinib resumption after progression to conventional TKIs.
Methods:
A total of 104 eligible advanced GIST patients, previously treated with imatinib, sunitinib and regorafenib, were collected from six referral Italian institutions. Mutational analysis was recorded and correlated with survival and response according to RECIST 1.1 or CHOI criteria.
Results:
Overall, 71 patients treated with imatinib 400 mg as rechallenge were included. Mutational status was available in all patients. The median follow up was 13 months. In patients who received a rechallenge therapy, the median time to progression (TTP) was 5.4 months [95% confidence interval (CI) 1.9–13.5] and overall survival (OS) was 10.6 months (95% CI 2.8–26.9). A correlation between mutational status, response rate, TTP and OS was not found but comparing deleted versus nondeleted KIT exon 11 patients, a significant difference was identified in terms of TTP and OS (p = 0.04 and p = 0.02, respectively).
Conclusions:
Our retrospective data confirm that imatinib rechallenge is a reasonable option in advanced GIST. The prognostic value of the specific KIT mutations was confirmed in our series.
Keywords: exon 11 KIT mutation, GIST, imatinib, rechallenge, TKI
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors deriving from interstitial cells of Cajal in the gastrointestinal tract, mainly located in the stomach (60%), and small intestine.1 Since 2000, GIST became targetable by new tyrosine-kinase inhibitors (TKIs), given the role played by KIT and PDGFRA in its pathogenesis.2–4 In fact, around 85% of GISTs contain oncogenic mutations in one of the two tyrosine-kinase receptor genes KIT or PDGFRA, and constitutive activation of either of these receptors has a central role in the pathogenesis of disease. Roughly 10–15% of GISTs, previously designated as wild-type GISTs, have no detectable mutations in KIT or PDGFRA, but might have genomic changes in other genes, such as SDH, NF1, or BRAF.5,6 Surgery is the mainstay of initial treatment and adjuvant imatinib is suggested in patients with a high risk of relapse.7–9 Upon relapse, response and survival depend on the specific sensitivity to targeted treatment based on driver mutations. TKIs improved the prognosis of KIT-mutated patients and became the standard treatment in this cohort of patients. In this setting, the standard first-line therapy is imatinib mesylate, an oral multiple TKI, active against KIT, PDGFRA, ABL, and DDR.10 The recommended starting dose is 400 mg/day. Resistance to imatinib appears after a median time of 20–24 months in large series11,12 while in a substantial proportion of patients it is observed at 4 years, due to the acquisition of additional mutations resulting in resistant KIT proteins.13,14 When resistance occurs, physicians may choose to either escalate imatinib up to 800 mg/day or start a second-line treatment.15 The standard second-line treatment after imatinib failure is sunitinib, although its benefit over placebo in terms of overall survival (OS) is relatively short, with numerous potentially serious side effects.9,16,17 In the setting of imatinib failure, the phase III trial of sunitinib resulted in a median time to progression (TTP) of about 7 months, leading to the approval of sunitinib as the standard second-line therapy for GISTs.16 After the evidence of progressive disease with imatinib and sunitinib, regorafenib represents the subsequent effective treatment, which demonstrated a better progression-free survival (PFS) compared with placebo. Regorafenib has been approved as third-line therapy based on the results of an international phase III trial, which documented significant improvement in PFS with regorafenib compared with placebo (4.8 versus 0.9 months) after prior failure of at least imatinib and sunitinib.18 No further validated treatment options are available. A small randomized trial (RIGHT trial) showed that imatinib rechallenge after other TKIs, can improve PFS compared with placebo.19 This result can be explained by the fact that keeping on with a continuous kinase inhibition blocks tumor cells still sensitive to imatinib, until new resistant clones come out.
Currently, data on the use of imatinib rechallenge in daily clinical practice in metastatic GIST patients are not available and little is known about its impact on patients’ outcome.
Thus, we retrospectively collected data about metastatic GIST patients treated with imatinib rechallenge after progression with conventional third or fourth line therapy in the Italian real-life experience.
Patients and methods
Patients enrolment
A total of 71 eligible advanced GIST patients, previously treated with imatinib, sunitinib and regorafenib, at six Italian referral cancer centers (Campus Bio-Medico, Rome; Fondazione IRCCS Istituto Nazionale dei Tumori, Milan; IRCCS Candiolo-Fondazione del Piemonte per l’Oncologia, Candiolo; University of Bologna, Bologna; Azienda Ospedaliera Universitaria Careggi, Firenze; University of Palermo, Palermo) were included in the present analysis. All collected patients were referred to these centers from October 2015 to October 2017. Our data were not reported in previous publications and there was no overlap between this population and those of other studies of our groups. All patients received all the three standard kinase inhibitors. Double dose of imatinib as active second line or as first line in exon 9 mutant GISTs was allowed. Mutational status was available in all patients; it was performed at the beginning of medical therapy, therefore before starting imatinib (imatinib was the first therapy in all patients) and in 68 patients, details about the type of mutation were available. Disease status was assessed according to standard practice every 12 weeks. Patients with oligo-progressing disease who had undergone surgical debulking in order to delay change of therapy, were included in the present analysis. Patients treated within clinical trials with new experimental therapies were excluded. Chemotherapy was not used in any patient. The population of patients was much selected and patients who received other agents before rechallenge were excluded from the analysis.
The study protocol was approved by the ethics committee of Sant’Orsola Hospital, Bologna, Italy (No. 164/2017/O/Oss) as part of a large retrospective analysis of patients with rare tumors. All patients provided written informed consent for inclusion in the study.
Statistical analysis
Descriptive analysis was made using median values and range. Differences between groups were assessed using the Chi-square test. TTP was calculated as the period from the treatment start to the first evidence of disease progression. OS was calculated from the date of rechallenge until the date of death or the last documented time the patient was known to be alive. Patients with no evidence of progression were censored at the date of last tumor assessment.
Death was considered an event regardless of the cause. Patients alive or lost to follow up were censored at the last contact. Survival analysis was performed by the Kaplan–Meier product-limit method and the differences in term of TTP and OS according to the treatment received or the type of mutation detected were evaluated by the log-rank test. SPSS software (version 17.00, SPSS, Chicago, IL, USA) was used for statistical analysis. A p-value < 0.05 was considered to indicate statistical significance.
Results
Patients’ population
A total of 104 metastatic or advanced GIST patients collected from six referral Italian institutions were included in the present retrospective analysis. A total of 10 of them were excluded because follow-up data were incomplete. Overall, six patients were excluded because of various reasons (i.e. inclusion in clinical trial or treated with other off-label drugs). Therefore, 71 patients were considered fully evaluable. Patients characteristics are summarized in Table 1. The median age was 63 years (range: 29–85). 39 patients (54.9%) were male and 32 (45.1%) were female.
Table 1.
Patients’ features.
| Number of patients | % of patients | |
|---|---|---|
| Sex (male) | 39 | 54.9% |
| Sex (female) | 32 | 45.1% |
| Age, years | ||
| Median | 63 | – |
| Range | 29–85 | – |
| PS (ECOG 0 −1) | 63 | 88.7% |
| Primary tumor | ||
| Stomach | 37 | 52.1% |
| Small bowel | 23 | 32.4% |
| Colon rectum | 11 | 15.5% |
| Primary tumors not resected at diagnosis | 29 | 40.8% |
| Liver involvement | 59 | 83.1% |
| >2 disease sites | 42 | 59.2% |
| Peritoneal involvement | 31 | 43.7% |
| Adjuvant imatinib | 32 | 45.1% |
| Second line with imatinib (800 mg) | 19 | 26.8% |
| Patients with exon 11 mutation detected | 62 | 87.3% |
| Patients with full mutational status details available | 59 | 83.1% |
| Exon 11 mutated patients with full mutational status available | 54 | |
| Deletion in exon 11 | 24 | 44.4% |
| Other exon 11 mutation | 30 | 55.6% |
ECOG, Eastern Cooperative Oncology Group; PS, performance score.
A total of 63 of 72 patients had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1. The site of the primary GIST was the stomach in 37 patients (52.1%), the small bowel in 23 (32.4%), and colon or rectum in 11 (15.5%). The primary tumor was not deemed surgically amenable at the beginning of the treatment course in 29 patients (40.8%). Overall, 59 (83.1%) patients showed liver involvement, 42 patients (59.2%) had more than two metastatic organs involved (liver and local infiltration of other organs or peritoneum), and 31 (43.7%) patients showed peritoneal involvement. A total of 32 patients (45.1%) had received adjuvant imatinib and 19 patients (26.8%) had received imatinib (800 mg) as a second-line therapy.
All patients had received all the three standard kinase inhibitors active in this setting (imatinib, sunitinib and regorafenib). A double dose of imatinib as active second-line or as first-line treatment in exon 9 mutant GISTs was allowed.
Response to imatinib rechallenge
Among all 71 patients treated with rechallenge therapy, imatinib was administered at the standard dose of 400 mg daily in 59 (83 %) patients, of which 19 (27 %) patients changed to a personalized schedule, such as reduced dose or discontinuous schedule. 12 (17 %) patients received imatinib at a lower dose from the beginning.
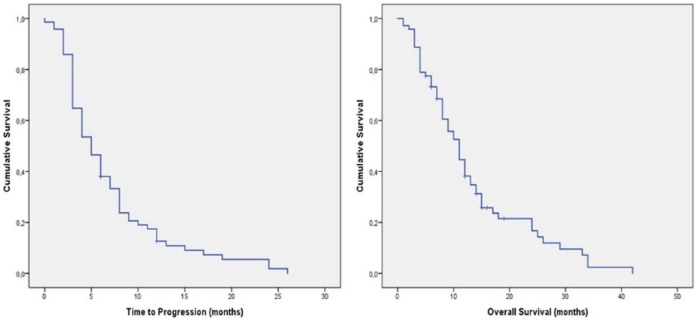
The median follow up was 13 months (range 1–42 months). The median TTP in this population was 5.5 months [95% confidence interval (CI) 4.4–6.69]. OS was 11 months (95% CI 6.83–16.71; Table 2 and Figure 1). The best response in our patients was partial response (PR), achieved in 5 (7%) patients, 32 (45%) patients had stable disease and 34 (48%) had disease progression (PD). Overall, 37 (52%) achieved a tumor control rate.
Table 2.
Outcome in patients who received imatinib rechallenge.
| Survival data |
p-value | ||
|---|---|---|---|
| Survival time | 95% CI (months) | ||
| TTP | 5.5 months | 4.40–6.96 months | |
| OS | 11 months | 6.83–16.71 months | |
| Radiological response rate | |||
| Partial response | 5 pts | 7 % | - |
| Stable disease | 32 pts | 45% | - |
| Disease progression | 34 pts | 48 % | - |
CI, confidence interval; OS, overall survival; TTP, time to progression.
Figure 1.
Time to progression in patients treated with imatinib rechallenge.
After progression to imatinib rechallenge, 18 patients received further therapies: 9 (50%) patients were treated again with sunitinib (most of them with personalized schedules) and 9 (24%) patients were maintained on imatinib beyond documented progression.
Between patients who received post-imatinib rechallenge, two patients out of nine treated with sunitinb achieved a PR and four patients a disease stabilization lasting more than 3 months. In addition, three patients treated with imatinib beyond progression showed a disease stabilization lasting at least 3 months; one of these is still on therapy 18 months after the documented radiological progression without any additional locoregional therapy.
Mutational status and response to imatinib rechallenge
Exon 11 KIT-mutant GISTs were 62 (87.3%), but whole sequence details were available in 59 patients (83.1%). Among the remaining nine patients, three carried an exon 9 KIT mutation, four patients were D842V PDGFRA mutant and two were KIT/PDGFRA wild-type. Of the latter, one patient was succinate dehydrogenase complex iron sulfur subunit B (SDHB) deficient by immunohistochemistry while the other patients were SDHB positive.
The specific subtype of KIT exon 11 mutation was available in 54 patients: 24 (44.4%) harbored a KIT exon 11 deletion, while in 30 patients (56.6%) different exon 11 genomic aberrations were detected.
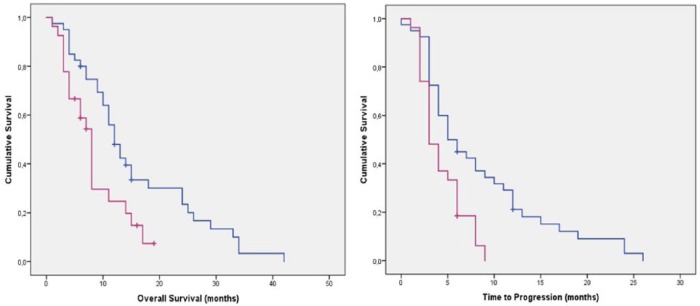
According to the type of KIT exon 11 mutations, patients with exon 11 deletion showed a median TTP of 3.2 months (95% CI 1.98–4.02 months) versus 5.2 months (95% CI 2.9–8.3 months) of patients with other exon 11 mutations (p = 0.04). In terms of OS, patients with an exon 11 KIT deletion showed a median OS of 8 months (95% CI 4.61–9.33) versus 12 months (95% CI 9.67–14.32 months; Table 3 and Figure 2).
Table 3.
TTP and OS according to type of KIT exon 11 mutation.
| Exon 11 deletions | Exon 11 other mutations | p-value | |
|---|---|---|---|
| Median TTP (months; 95% CI) |
3.2 months (1.98–4.02 months) |
5.2 months (2.9–8.3 months) |
0.04 |
| Median OS (months; 95% CI) | 8 months (4.61–9.33 months) |
12 months (9.67–14.32 months) |
0.09 |
CI, confidence interval; OS, overall survival; TTP, time to progression.
Figure 2.
Time to progression and overall survival in patients with exon 11 deletions (purple) and exon 11 other mutation (blue).
Median TTP associated with the previous anticancer therapy (regorafenib) was 7.1 months in our patients’ population, inferior but not far from the one recorded with imatinib rechallenge.
In addition, focusing on the PDGFRA mutant patients, three patients showed a prolonged disease stabilization (7, 15 and 20 months). In particular, one patient after 20 months is still on therapy with imatinib rechallenge without any evidence of disease progression. Another patient after developing disease progression (TTP 15 months) was maintained on therapy with imatinib and is still alive with a cumulative OS of 28 months.
Discussion
In metastatic KIT-mutant GIST patients, treatment is mainly based on imatinib, to be continued until progression. The standard daily dosage of imatinib is 400 mg. This drug is usually well tolerated. Secondary resistance is the limiting factor to imatinib therapy long-term efficacy. This mechanism can be mainly due to the acquisition/appearance of new molecular abnormalities associated with the KIT and PDGFRA receptor signalling pathway, such as the acquisition of several different receptor mutations, the loss of KIT expression, the genomic amplification of KIT, the activation of an alternative downstream signalling pathways or to other mechanisms not related to KIT/PDGFRA receptors.20
Standard second-line therapy is sunitinib, a TKI-inhibiting vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3 as well as KIT and PDGFRA, which showed to improve PFS in a randomized trial versus placebo in patients failing imatinib treatment.21 Regorafenib, another TKI with activity on KIT, PDGFRA and VEGFR 1, 2 and 3, showed to be effective in third-line therapy in patients, who underwent treatment with imatinib and sunitinib, as demonstrated in the GRID trial.18 After these standard therapies, imatinib rechallenge was proposed as a valid option.
The hypothesis that some tumors were slowed down continuing the same TKI beyond progression came out from the fact that some patients in the GRID trial, going on with regorafenib, reached a post-progression PFS similar to the first one.
The suggestion that imatinib rechallenge could be effective was based on two observations. The first one is the finding, even if anecdotal, that imatinib interruption in patients with an imatinib-refractory GIST-induced acute exacerbation or appearance of symptoms. The second one is the ‘flare up’ phenomenon on 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging, consisting in a rapid upregulation of metabolic activity even within previously dormant tumor lesions.22,23 This phenomenon was observed in the context of a phase I/II clinical trial investigating the multi-targeted receptor TKI sunitinib. In this trial, patients with imatinib-refractory GISTs stopped imatinib therapy prior to starting sunitinib. Several of these patients had a 18FDG-PET flair up few days following imatinib cessation. This finding implies that imatinib responsive tumor cell populations persist in these patients and that secondary resistance and disease progression most likely occurred in a distinctive clone with another mutation, which confers imatinib resistance.
These observations led to the hypothesis that even if progressive disease during other TKI treatments can be due to clones resistant to imatinib, other clones could be still sensitive, supporting the rationale for imatinib rechallenge.
Results of the RIGHT trial confirmed these observations, showing an advantage in term of PFS with imatinib rechallenge compared with placebo (1.8 versus 0.9 months, respectively).19
As expected, our multicenter retrospective analysis confirmed that imatinib rechallenge is widely used in Italian clinical practice. Of interest, TTP and OS reached in our series were longer than those observed in previous studies.
This new approach can be considered as a turning point with the traditional cancer treatment strategy, which consists in the succession of different anticancer treatment options changed after the progression of the tumor without rechallenge to previous ones. With the spreading use of TKIs and the increasing recourse to surgery, the concept of disease progression as well as that of the time to treatment-switch is changing and the rechallenge strategy can be located on this path.
In our series, a correlation between mutational status and response rate, TTP and OS was not found. On the contrary, exon 11 KIT-mutant GIST showed to be a prognostic factor also in our population. In particular, patients carrying a deletion of KIT exon 11 displayed a shorter OS and TTP than patients carrying other KIT exon 11 mutations.
These data confirmed that exon 11 KIT deletion is a negative prognostic factor. It was firstly described by Martin and colleagues as an independent prognostic factor in primary localized GISTs and then confirmed by Kontogianni and colleagues.24–26 Moreover it was demonstrated by our group too in a retrospective analysis comparing imatinib and sunitinib in second-line setting, where 11 KIT deletion compared with other exon mutations was identified as a negative prognostic factor for OS, in both treatment arms and to be associated with a shorter TTP.15
In conclusion, our retrospective data confirm that imatinib rechallenge is widely used in Italian clinical practice and that it positively affects patient outcome, with an OS (10.6 months) and TTP (5.4 months) advantage superior to that observed in other studies. Therefore, imatinib rechallenge should be offered to all patients after failure of previous treatments.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Marco Stellato  https://orcid.org/0000-0002-0993-7540
https://orcid.org/0000-0002-0993-7540
Margherita Nannini  https://orcid.org/0000-0002-2103-1960
https://orcid.org/0000-0002-2103-1960
Contributor Information
Bruno Vincenzi, Associate Professor in Medical Oncology, University Campus Bio-Medico, Via Alvaro del Portillo 200, 00128, Rome, Italy.
Margherita Nannini, Department of Specialized, Experimental and Diagnostic Medicine, Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna.
Giuseppe Badalamenti, Department of Surgical, Oncological and Oral Science, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Giovanni Grignani, Candiolo Cancer Institute, FPO - IRCCS, Candiolo, Turin, Italy.
Elena Fumagalli, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Silvia Gasperoni, Azienda Ospedaliera Universitaria Careggi, Florence, Italy.
Lorenzo D’Ambrosio, Candiolo Cancer Institute, FPO - IRCCS, Candiolo, Turin, Italy.
Lorena Incorvaia, Department of Surgical, Oncological and Oral Science, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Marco Stellato, Medical Oncology Department, University Campus Bio-Medico of Rome, Rome, Italy.
Mariella Spalato Ceruso, Medical Oncology Department, University Campus Bio-Medico of Rome, Rome, Italy.
Andrea Napolitano, Medical Oncology Department, University Campus Bio-Medico of Rome, Rome, Italy.
Sergio Valeri, Department of General Surgery, University Campus Bio-Medico of Rome, Rome, Italy.
Daniele Santini, Medical Oncology Department, University Campus Bio-Medico of Rome, Rome, Italy.
Giuseppe Tonini, Medical Oncology Department, University Campus Bio-Medico of Rome, Rome, Italy.
Paolo Giovanni Casali, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Angelo Paolo Dei Tos, Azienda ULSS 9 Treviso, Treviso, Italy.
Maria Abbondanza Pantaleo, Department of Specialized, Experimental and Diagnostic Medicine, Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna.
References
- 1. Judson I, Demetri G. Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol Off J Eur Soc Med Oncol 2007; 18(Suppl. 1): 20–24. [DOI] [PubMed] [Google Scholar]
- 2. Nakahara M, Isozaki K, Hirota S, et al. A novel gain-of-function mutation of c-KIT gene in gastrointestinal stromal tumors. Gastroenterology 1998; 115: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 3. Corless CL, Ballman K V, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014; 32: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnett CM, Corless CL, Heinrich MC. Gastrointestinal stromal tumors: molecular markers and genetic subtypes. Hematol Oncol Clin North Am 2013; 27: 871–888. [DOI] [PubMed] [Google Scholar]
- 5. Nishida T, Blay J-Y, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016; 19: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nannini M, Urbini M, Astolfi A, et al. The progressive fragmentation of the KIT/PDGFRA wild-type (WT) gastrointestinal stromal tumors (GIST). J Transl Med 2017; 15: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015; 33: 634–642. [DOI] [PubMed] [Google Scholar]
- 8. Wozniak A, Rutkowski P, Schöffski P, et al. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a european multicenter analysis based on ConticaGIST. Clin Cancer Res 2014; 20: 6105–6116. [DOI] [PubMed] [Google Scholar]
- 9. Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev 2011; 37: 75–88. [DOI] [PubMed] [Google Scholar]
- 10. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–480. [DOI] [PubMed] [Google Scholar]
- 11. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008; 26: 620–625. [DOI] [PubMed] [Google Scholar]
- 12. Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006; 42: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 13. Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006; 24: 4764–4774. [DOI] [PubMed] [Google Scholar]
- 14. Bauer S, Duensing A, Demetri GD, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene 2007; 26: 7560–7568. [DOI] [PubMed] [Google Scholar]
- 15. Vincenzi B, Nannini M, Fumagalli E, et al. Imatinib dose escalation versus sunitinib as a second line treatment in KIT exon 11 mutated GIST: a retrospective analysis. Oncotarget 2016; 7: 69412–69419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demetri GD, Garrett CR, Schöffski P, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 2012; 18: 3170–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwandt A, Wood LS, Rini B, et al. Management of side effects associated with sunitinib therapy for patients with renal cell carcinoma. Onco Targets Ther 2009; 2: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013; 14: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maleddu A, Pantaleo MA, Nannini M, et al. Mechanisms of secondary resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumours (Review). Oncol Rep 2009; 21: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 21. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006; 368: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 22. Kang Y-K, Kang HJ, Kim K-M, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat 2012; 44: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Italiano A, Cioffi A, Coco P, et al. Patterns of care, prognosis, and survival in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. Ann Surg Oncol 2012; 19: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 24. Martin-Broto J, Gutierrez A, Garcia-del-Muro X, et al. Prognostic time dependence of deletions affecting codons 557 and/or 558 of KIT gene for relapse-free survival (RFS) in localized GIST: a Spanish Group for Sarcoma Research (GEIS) Study. Ann Oncol Off J Eur Soc Med Oncol 2010; 21: 1552–1557. [DOI] [PubMed] [Google Scholar]
- 25. Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005; 23: 6190–6198. [DOI] [PubMed] [Google Scholar]
- 26. Kontogianni-Katsarou K, Dimitriadis E, Lariou C, et al. KIT exon 11 codon 557/558 deletion/insertion mutations define a subset of gastrointestinal stromal tumors with malignant potential. World J Gastroenterol 2008; 14: 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]