Abstract
Background:
Despite strong recommendations to use metformin as first-line therapy for type 2 diabetes (T2DM), its use has been suboptimal, likely due to concerns of lactic acidosis. This study compared the association of acidosis in patients with T2DM prescribed metformin with those prescribed other antihyperglycemic medications or no medications.
Methods:
This was a retrospective cohort study of patients with newly diagnosed T2DM utilizing an administrative database, which includes medical and prescription claims. Eligible patients had a diagnosis of T2DM, had continuous health plan enrollment 3 months prior to study enrollment and during the study period, and were at least 18 years of age. Mutually exclusive exposure groups were metformin only, other antihyperglycemic medications, and no medication. Acidosis cases were stratified by exposure group and risk factors for lactic acidosis (chronic obstructive pulmonary disease, hepatic dysfunction, alcohol abuse, heart failure, renal insufficiency, age of 80 years or older, and a history of acidosis). Degree of renal insufficiency was not available. Associations between exposure and acidosis were estimated, and risk factors evaluated.
Results:
A total of 132,780 patients met inclusion criteria: 24,936 (20%) metformin only group, 15,059 (11%) other antihyperglycemic medication group, and 92,785 (70%) no medication group. Acidosis was observed in 1.45 per 10,000 patient months (0.78 metformin, 1.59 other antihyperglycemic medication, 1.51 no medication). The unadjusted relative risk of acidosis was 0.5 for patients prescribed metformin only compared with the other exposure groups (95% confidence interval = 0.2–1.2). There was no significant difference in risk of acidosis between exposure groups, irrespective of risk factors for lactic acidosis.
Conclusions:
Risk of acidosis was similar with metformin only compared with those prescribed other antihyperglycemic medications or no medication. These results support expanded use of metformin for T2DM. Additional studies are needed to understand the impact of risk factor severity on risk of lactic acidosis.
Keywords: lactic acidosis, metformin, type 2 diabetes
Introduction
Nearly 25 million people in the Unites States (US) have type 2 diabetes mellitus (T2DM).1 To date, metformin is considered first-line therapy for T2DM3 and has demonstrated reductions in both cardiovascular morbidity and mortality.2 However, metformin prescribing remains suboptimal as only 65% of newly diagnosed patients with T2DM are initially treated with metformin.4 Reasons for suboptimal metformin use are largely unknown, but are potentially due to concerns about increased risk of lactic acidosis among certain at-risk patients.
Lactic acidosis is a type of metabolic acidosis and accounts for the majority of metabolic acidosis cases.5 The condition results from insufficient oxygenation of tissues, leading to anaerobic metabolism and increased lactate production. Risk factors for lactic acidosis can be classified as those that increase the production of lactate, prevent lactate metabolism, or impair tissue oxygenation. Potential risk factors for metformin-associated lactic acidosis include chronic obstructive pulmonary disease (COPD), hepatic dysfunction, alcohol abuse, heart failure, renal insufficiency, older age (80+ years), and a history of lactic acidosis.6 Although the presence of these risk factors is considered a precaution, and in some cases a contraindication, to metformin use, it is important to note that hyperglycemia alone is a risk factor for lactic acidosis.7–9
Previous studies have consistently demonstrated no increase in serum lactate concentrations with metformin use,10,11 suggesting the risk of lactic acidosis with metformin use is minimal and similar to the general population. Metformin has been associated with a rate of lactic acidosis of 0–9 per 100,000 patient years12–16 compared with 9.7 per 100,000 patient years in the general population.13 However, it is difficult to compare incidence of lactic acidosis across studies, given variable definitions of lactic acidosis. Further, incidence of lactic acidosis may be underestimated in retrospective studies, given it may not be documented in the face of other concurrent, acute conditions.
However, studies assessing the risk of lactic acidosis with metformin use are limited. These studies were derived from small samples and analyses often did not attempt to examine the impact of lactic acidosis risk factors. Studies that do evaluate the impact of risk factors such as heart failure and renal insufficiency have found no increased risk of lactic acidosis among metformin users.17–21 Further, past studies did not compare the risk of lactic acidosis between patients with T2DM treated with metformin with other antihyperglycemic agents or no T2DM medications. A more accurate understanding of the risk of metformin-attributable lactic acidosis is needed.
Therefore, the objective of this study was to compare the association of acidosis in patients with T2DM prescribed metformin with those prescribed other antihyperglycemic medications, or no medications, and compare the incidence among patients with and without specific risk factors for lactic acidosis.
Methods
Data source
This study utilized the PharMetrics Legacy Health Plan Claims Database, which includes paid medical, specialty, facility and pharmacy claims for more than 75 million covered lives from more than 80 health plans nationally. Patients in PharMetrics are representative of the US commercially insured population in regards to age and sex.22 Available data include service dates; ICD-9-CM diagnosis codes; medications filled (generic product index code, date filled, days supplied); provider specialty type; and demographic information such as patients’ birth year, sex and region of residence. The authors obtained a license for a 10% random sample of the entire PharMetrics database from 2001 to 2013.
Study design
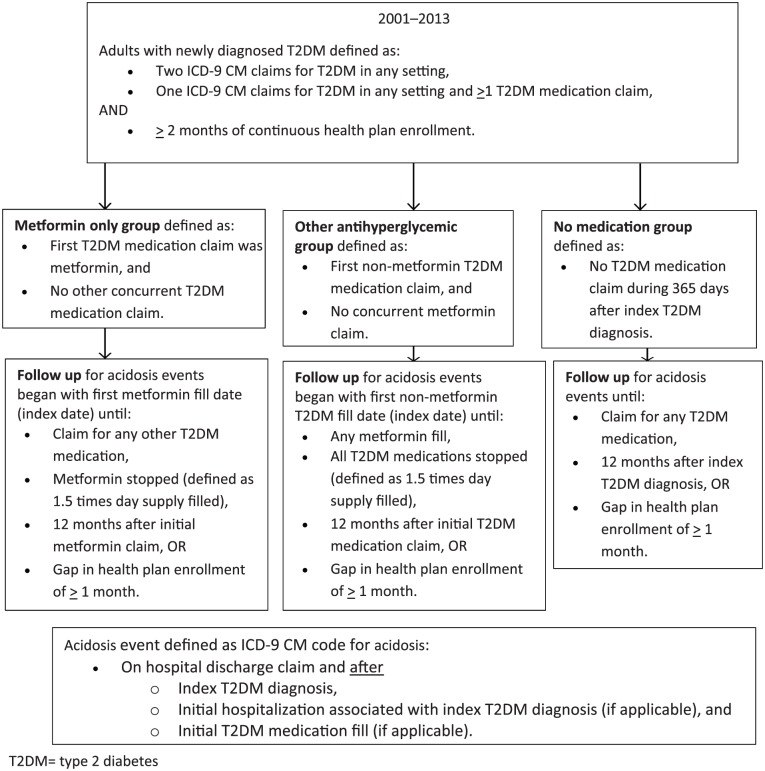
A retrospective cohort of patients with newly diagnosed T2DM was identified from the PharMetrics 10% random sample. Eligible patients met the following criteria: two or more diagnoses of diabetes (ICD-9 code 250.x) in any setting, or one diagnosis of diabetes in any setting and one or more subsequent fills of a T2DM medication (generic product index code 27xxxx); at least 3 months of continuous health plan enrollment prior to the first diagnosis of T2DM with no diabetes diagnoses or T2DM medication fills during the prior three months; at least 2 months of continuous health plan enrollment following the diabetes diagnosis; and age 18 or older at the time of their initial T2DM diagnosis. The index T2DM diagnosis date was identified as the earliest date meeting the eligibility criteria. The study cohort was evaluated for up to 1 year to identify occurrences of lactic acidosis.
Measures
Study cohorts
Three mutually exclusive exposure groups were created: the metformin group; the other antihyperglycemic medication group; and the no medication group. Cohort inclusion criteria and duration of follow up are described in Figure 1.
Figure 1.
Study design, cohort inclusion criteria and duration of follow up. T2DM, type 2 diabetes mellitus.
Outcome: acidosis
Acidosis was identified from claims records using ICD-9-CM diagnosis code 276.2 for ‘acidosis’, which includes respiratory and metabolic acidosis. Claims for code 276.2 were considered acidosis events only if they occurred during a hospital stay and the claims for code 276.2 were listed as a discharge diagnosis. To isolate lactic acidosis and exclude miscoding of diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome, claims were not considered if the 276.2 code was reported under any of the following circumstances: on a nonhospital claim; on the same day as the index T2DM medication fill; on the same day as the index T2DM diagnosis or within the same hospital stay as the index T2DM diagnosis; or during a hospital stay but not listed as one of the discharge diagnoses. The date of the first claim for acidosis was captured, allowing for the calculation of the number of days to the event. For the other antihyperglycemic medication group, we created a variable to identify which specific T2DM medications the patient was taking. The total number of acidosis events was determined during each patient’s follow-up period.
Risk factors for lactic acidosis
Risk factors for lactic acidosis included: age 80 years and over; COPD; hepatic dysfunction; history of or concurrent alcohol abuse; heart failure; renal insufficiency; and past history of acidosis. These risk factors were assessed for all patients during the baseline period (i.e. 3 months prior to their index T2DM diagnosis) using ICD-9 CM codes (see Table 1). Each risk factor was captured separately and a composite risk factor score was created by adding the total number of distinct risk factors for each patient.
Table 1.
ICD-9 CM and generic product index codes.
| Variable | Code specification |
|---|---|
| Lactic acidosis risk factors | |
| Chronic obstructive pulmonary disease | ICD-9 code: 490.x-496.x |
| Hepatic dysfunction | ICD-9 code: 571.2, 571.4-571.6, 572.2-572.8 |
| Alcohol abuse | ICD-9 code: 305.0 |
| Heart failure | ICD-9 code: 428.x |
| Renal insufficiency | ICD-9 code: 582.x, 585.x |
| History of acidosis event | ICD-9 code 276.2x on a hospital discharge claim prior to the index diagnosis |
| Medication use | |
| Sulfonylurea | GPI: 272000 |
| Glucagon-like peptide-1 agonist | GPI: 271700 |
| Thiazolidinedione | GPI: 276070 |
| Dipeptidyl peptidase-IV inhibitor | GPI: 275500 |
| Insulin | GPI4: 2710 |
| Metformin | GPI6: 272500 |
Covariates for multivariate models of acidosis risk
Comorbidities included other diagnoses observed during the baseline period, such as mental health disorders, obesity, and hypertension. All comorbidities were identified by ICD-9 CM codes. To capture overall comorbidity burden, the Charlson Comorbidity Index (CCI) and Chronic Disease Indicator (CDI) were also calculated.23,24 Concomitant medication use was measured by identifying other medications filled during the baseline period and were grouped by T2DM drug class. Demographic characteristics included patient age (in years) at index T2DM diagnosis, sex, geographic region of residence (East, South, West and Midwest), and health plan type (e.g. Medicare/Medicaid, HMO).
Statistical analyses
Descriptive statistics were used to characterize demographic and clinical characteristics of the cohort, overall and stratified by exposure group. The crude incidence of acidosis (which served as a proxy for lactic acidosis) was calculated for the total cohort and stratified by exposure group. Incidence rates were also estimated within exposure groups identified by each of the seven lactic acidosis risk factors. Specifically, incidence rates were calculated as the number of patients with acidosis at least once during their follow up divided by the total patient time at risk, accounting for unequal length of follow up. Unadjusted relative risk (RR) was calculated to compare the incidence rate among the metformin only group relative to the other exposure groups.
To estimate the more dynamic relationship between T2DM medication exposure and acidosis, multivariate modeling techniques were used. Specifically, Cox proportional hazards regression was used to estimate the risk of an acidosis event. Because patients were not randomly assigned to their T2DM medication exposure groups, the issue of nonrandom treatment allocation was addressed. A two-stage propensity analysis approach was used to estimate adjusted rate ratios for the metformin only group compared with the other exposure groups.25 First, we estimated each patient’s propensity to be in each of the three exposure groups using multinomial logistic regression. The goal of a propensity model was to mirror the provider intuition that goes into treatment decisions for specific patients. The propensity model therefore included all demographic characteristics, comorbidities, and risk factors for lactic acidosis.26 Two of the three propensity scores were then included as covariates in subsequent multivariate models.27–29
To account for immortal time bias, multivariate Cox proportional hazards regression models included time-varying indicators of exposure.30 This allowed patients in the exposed group to be included in the unexposed group until their index T2DM medication was filled, thus more accurately reflecting their exposure status over the course of the study. These multivariate Cox proportional hazards regression models included the propensity scores described above, thus estimating the propensity-adjusted risk of acidosis for the metformin only group compared with the other medication exposure groups.27,31 SAS version 9.4 was used for all data management and analyses.32
The study was approved by the University of Colorado’s Multiple Institutional Review Board and informed consent was waived.
Results
A total of 132,780 adult patients with newly diagnosed T2DM met inclusion criteria (Table 2). Nearly 20% were in the metformin only group, 11% in the other T2DM medication group, and 70% in the no medication group. The no medication group was significantly older (p < 0.0001), while the other antihyperglycemic medication group had significantly more men (58%) (p < 0.0001). Approximately 10% of the cohort had one or more risk factor for lactic acidosis during the baseline period. The other antihyperglycemic medication group had more patients with certain risk factors, specifically age 80 years and over, COPD, and heart failure (p < 0.0001). The metformin only group had more obese patients compared with the other antihyperglycemic medication group and no medication group (5.4%, 2.6%, and 3.3% in each group, respectively; p < 0.0001).
Table 2.
Demographic and clinical characteristics of the entire cohort (n = 132,780), stratified by medication group.
| Metformin only | Other antihyperglycemic medications | No T2DM medications | Total | p value | |
|---|---|---|---|---|---|
| N | 24,936 | 15,059 | 92,785 | 132,780 | . |
| Age | |||||
| Mean (SD) | 52.6 (11.27) | 53.1 (12.89) | 56.9 (12.72) | 55.6 (12.62) | <0.0001 |
| Median (interquartile range) | 53.0 (45–60) | 54.0 (45–62) | 57.0 (49–65) | 56.0 (48–64) | . |
| Min, max | 18, 84 | 18, 84 | 18, 84 | 18, 84 | . |
| Sex | |||||
| Female | 12,099 (48.5%) | 6363 (42.3%) | 46,305 (49.9%) | 64,767 (48.8%) | <0.0001 |
| Male | 12,835 (51.5%) | 8694 (57.7%) | 46,468 (50.1%) | 67,997 (51.2%) | <0.0001 |
| Unknown | 2 (0.0%) | 2 (0.0%) | 12 (0.0%) | 16 (0.0%) | . |
| Obesity | 1359 (5.4%) | 397 (2.6%) | 3066 (3.3%) | 4822 (3.6%) | <0.0001 |
| Payer source | |||||
| Commercial | 20,185 (80.9%) | 11,878 (78.9%) | 73,176 (78.9%) | 105,239 (79.3%) | <0.0001 |
| Medicaid | 604 (2.4%) | 269 (1.8%) | 1302 (1.4%) | 2175 (1.6%) | <0.0001 |
| Medicare | 1049 (4.2%) | 779 (5.2%) | 7104 (7.7%) | 8932 (6.7%) | <0.0001 |
| Unknown | 222 (0.9%) | 226 (1.5%) | 888 (1.0%) | 1336 (1.0%) | <0.0001 |
| Self insured | 2876 (11.5%) | 1907 (12.7%) | 10,315 (11.1%) | 15,098 (11.4%) | <0.0001 |
| Region | |||||
| East | 4297 (17.2%) | 2771 (18.4%) | 23,357 (25.2%) | 30,425 (22.9%) | <0.0001 |
| Midwest | 7697 (30.9%) | 4977 (33.1%) | 25,305 (27.3%) | 37,979 (28.6%) | <0.0001 |
| South | 9258 (37.1%) | 6045 (40.1%) | 33,978 (36.6%) | 49,281 (37.1%) | <0.0001 |
| West | 3684 (14.8%) | 1266 (8.4%) | 10,145 (10.9%) | 15,095 (11.4%) | <0.0001 |
| Chronic Disease Indicator score | |||||
| Mean (SD) | 1.5 (1.72) | 1.3 (1.81) | 1.0 (1.65) | 1.1 (1.69) | <0.0001 |
| Median (interquartile range) | 1.0 (0–2) | 0.0 (0–2) | 0.0 (0–2) | 0.0 (0–2) | . |
| Min, max | 0, 12 | 0, 13 | 0, 14 | 0, 14 | . |
| Charlson Comorbidity Index score | |||||
| Mean (SD) | 0.2 (0.72) | 0.4 (1.15) | 0.4 (1.04) | 0.4 (1.00) | <0.0001 |
| Median (interquartile range) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) | . |
| Min, max | 0, 11 | 0, 15 | 0, 15 | 0, 15 | . |
| Lactic acidosis risk factors | |||||
| Age ⩾80 years | 134 (0.5%) | 147 (1.0%) | 1939 (2.1%) | 2220 (1.7%) | <0.0001 |
| Chronic obstructive pulmonary disease | 1443 (5.8%) | 934 (6.2%) | 6761 (7.3%) | 9138 (6.9%) | <0.0001 |
| Hepatic dysfunction | 37 (0.1%) | 109 (0.7%) | 297 (0.3%) | 443 (0.3%) | <0.0001 |
| Alcohol abuse | 36 (0.1%) | 39 (0.3%) | 206 (0.2%) | 281 (0.2%) | 0.0261 |
| Heart failure | 275 (1.1%) | 399 (2.6%) | 2579 (2.8%) | 3253 (2.4%) | <0.0001 |
| Renal Insufficiency | 68 (0.3%) | 234 (1.6%) | 1419 (1.5%) | 1721 (1.3%) | <0.0001 |
| History of acidosis | 3 (0.0%) | 4 (0.0%) | 41 (0.0%) | 48 (0.0%) | 0.0678 |
| Composite risk score | . | ||||
| Mean (SD) | 0.1 (0.29) | 0.1 (0.39) | 0.1 (0.42) | 0.1 (0.40) | <0.0001 |
| Median (interquartile range) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) | . |
| Min, max | 0, 3 | 0, 5 | 0, 5 | 0, 5 | . |
| Composite score = 0 | 23,066 (92.5%) | 13,480 (89.5%) | 81,631 (88.0%) | 118,177 (89.0%) | <0.0001 |
| Composite score = 1 | 1752 (7.0%) | 1337 (8.9%) | 9398 (10.1%) | 12,487 (9.4%) | <0.0001 |
| Composite score = 2 | 110 (0.4%) | 203 (1.3%) | 1466 (1.6%) | 1779 (1.3%) | <0.0001 |
| Composite score = 3 | 8 (0.0%) | 34 (0.2%) | 251 (0.3%) | 293 (0.2%) | <0.0001 |
| Composite score = 4 | 0 (0.0%) | 4 (0.0%) | 36 (0.0%) | 40 (0.0%) | 0.7710 |
| Composite score = 5 | 0 (0.0%) | 1 (0.0%) | 3 (0.0%) | 4 (0.0%) | 0.8228 |
SD, standard deviation; T2DM = type 2 diabetes.
More than one third of patients (38%) in the other antihyperglycemic medication group were prescribed a sulfonylurea, one quarter were prescribed insulin, and nearly 10% were prescribed more than one T2DM medication (Table 3).
Table 3.
Diabetes medication exposure in the other diabetes medication group during follow up.
| Other diabetes medication
group (n = 15,059) |
|
|---|---|
| Sulfonylurea | 5661 (37.6%) |
| Mean days supply (SD) | 54.2 (109.27) |
| Median (interquartile range) | 0.0 (0–50) |
| Min, max days supply | 0, 930 |
| Glucagon-like peptide-1 agonist | 380 (2.5%) |
| Mean days supply (SD) | 2.7 (24.01) |
| Median (interquartile range) | 0.0 (0–0) |
| Min, max days supply | 0, 540 |
| Thiazolidinedione | 2601 (17.3%) |
| Mean days supply (SD) | 27.6 (84.21) |
| Median (interquartile range) | 0.0 (0–0) |
| Min, max days supply | 0, 900 |
| Dipeptidyl peptidase-IV inhibitor | 828 (5.5%) |
| Mean days supply (SD) | 8.7 (48.88) |
| Median (interquartile range) | 0.0 (0–0) |
| Min, max days supply | 0, 810 |
| Insulin | 3822 (25.4%) |
| Mean days supply (SD) | 28.3 (85.88) |
| Median (interquartile range) | 0.0 (0–10) |
| Min, max days supply | 0, 1049 |
| Number of diabetes medications | |
| 2 | 1198 (8.0%) |
| 3 | 94 (0.6%) |
| 4 | 9 (0.06%) |
SD, standard deviation.
A total of 187 patients (0.1% of patients) had one or more acidosis events during their follow up. There were 9 acidosis events in the metformin only group, 10 events in the antihyperglycemic agent group, and 168 events in the no medication group. Average time to an acidosis event was 153 days (median 139 days, range 1–363 days). The unadjusted relative risk of acidosis was 0.49 for the metformin only group relative to the other T2DM medication group [95% confidence interval (CI) 0.2–1.2, p = 0.12), and 0.52 for the metformin only group relative to the no medication group (95% CI 0.26–1.01, p = 0.054; Figure 2).
Figure 2.
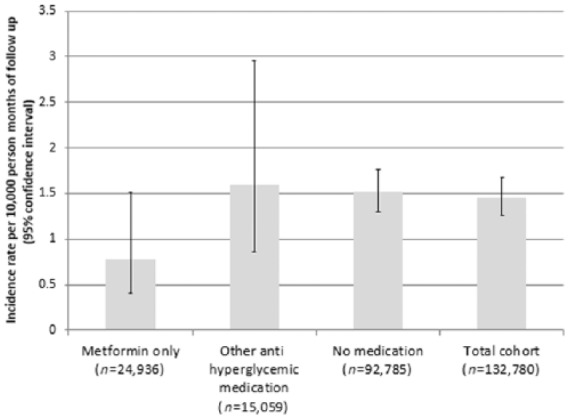
Incidence of acidosis during follow up for the entire cohort (n = 132,780), and stratified by medication group.
After adjusting for their propensity to be in one of the three exposure groups and accounting for immortal time bias, the risk of acidosis did not differ significantly across the three exposure groups. The hazard ratio (HR) of an acidosis event was 0.59 for the metformin only group relative to the no medication group (95% CI 0.3–1.16, p = 0.12) and 0.50 for the metformin only group relative to the other antihyperglycemic medication group (95% CI = 0.2–1.2, p = 0.13). The risk of an acidosis event was similar for the other antihyperglycemic medication group relative to the no medication group (HR = 1.18, 95% CI = 0.62–2.25, p = 0.61).
There were more cases of acidosis among patients with a history of acidosis and lowest among patients aged 80 years and over (Table 4). With the exception of hepatic dysfunction, each additional risk factor increased the risk of acidosis. In a Cox proportional hazards model adjusted for medication exposure group, immortal time bias and propensity scores, the number of risk factors was found to significantly increase the risk of acidosis (HR = 3.4, 95% CI = 2.9–4.1, p < 0.0001). There was no significant interaction between the number of risk factors and T2DM medication exposure group, indicating that the number of risk factors does not have a differential effect on acidosis risk across medication exposure groups. There were not enough acidosis cases within each risk factor group to estimate adjusted models. Table 5 describes the unadjusted rates of acidosis by medication exposure group and risk factor.
Table 4.
Acidosis and risk of acidosis for each risk factor for all medication exposure groups.
| Risk factor | No. patients with LA event | No. patient months | IR per 10,000 patient months (95% CI) | Relative risk (95% CI) |
|---|---|---|---|---|
| Age ⩾80 | 11 | 24,399 | 4.5 (2.5–8.1) | 3.3 (1.8–6.0) |
| Chronic obstructive pulmonary disease | 47 | 90,519 | 5.2 (3.9–6.9) | 4.4 (3.2–6.2) |
| Hepatic dysfunction | 2 | 4046 | 4.9 (1.2–19.8) | 3.4 (0.9–13.8) |
| Alcohol abuse | 2 | 2706 | 7.4 (1.8–29.7) | 5.1 (1.3–20.8) |
| Heart failure | 31 | 33,418 | 9.3 (6.5–13.2) | 7.5 (5.1–11.0) |
| Renal insufficiency | 30 | 18,109 | 16.6 (11.6–23.7) | 13.4 (9.1–19.8) |
| History of acidosis | 3 | 473 | 63.4 (20.0–200.8) | 44.3 (13.8–141.7) |
When comparing acidosis across the three exposure groups, there were no significant differences.
CI, confidence interval; IR, incidence rate; LA, lactic acidosis.
Table 5.
Rates of acidosis by risk factor and treatment group.
| Metformin only | Other antihyperglycemic medications | No T2DM medications | Total | |
|---|---|---|---|---|
| N | 9/24,936 | 10/15,059 | 168/92,785 | 187/132,780 |
| Acidosis risk factors | ||||
| Age ⩾80 years | 0/134 | 0/147 | 11/1939 | 12/2221 |
| Chronic obstructive pulmonary disease | 0/1443 | 1/934 | 46/6761 | 48/9139 |
| Hepatic dysfunction | 0/37 | 0/109 | 2/297 | 3/444 |
| Alcohol abuse | 0/36 | 0/39 | 2/206 | 2/281 |
| Heart failure | 0/275 | 0/399 | 31/2579 | 31/3253 |
| Renal insufficiency | 0/68 | 0/234 | 30/1419 | 31/1722 |
| History of acidosis | 0/3 | 0/4 | 3/41 | 3/48 |
| Composite risk score | ||||
| Composite score = 0 | 9/23,066 | 9/13,480 | 81/81,631 | 99/118,177 |
| Composite score = 1 | 0/1752 | 1/1337 | 61/9398 | 62/12,487 |
| Composite score = 2 | 0/110 | 0/203 | 16/1466 | 16/1779 |
| Composite score = 3 | 0/8 | 0/34 | 8/251 | 8/293 |
| Composite score = 4 | 0/0 | 0/4 | 2/36 | 2/40 |
| Composite score = 5 | 0/0 | 0/1 | 0/3 | 0/4 |
T2DM, type 2 diabetes mellitus.
Discussion
The results of this study suggest the risk of acidosis with metformin use is similar to that with other antihyperglycemic medications or no medications irrespective of the presence of risk factors for lactic acidosis. The similar risk of acidosis in patients treated with a T2DM medication other than metformin is especially meaningful when considering metformin is generally avoided in clinical practice in favor of another T2DM medication when there is a perceived risk of lactic acidosis. However, these findings do not account for severity of a given risk factor, such as degree of renal insufficiency.
A meta-analysis of 347 trials estimated the association between lactic acidosis and metformin use in patients with T2DM comparing metformin users to nonmetformin users.33 From the 70,490 patient years of metformin use, no cases of lactic acidosis were found in either the metformin or nonmetformin group.33 Another large prospective study assessed the composite outcome of acidosis or infection in patients with T2DM and found lower risk of the composite endpoint in patients treated with metformin compared with insulin; however, this endpoint is not limited to lactic acidosis events.34 Therefore the collective findings from the current and prior studies suggest concerns of acidosis with metformin use are largely unfounded and thought to be associated with a high risk of lactic acidosis with another biguanide medication, phenformin.35 Phenformin preceded metformin as the first biguanide and was associated with a rate of lactic acidosis of 64 per 100,000 patient years.12–16
Further, studies assessing the risk of lactic acidosis with metformin in the presence of risk factors for lactic acidosis do not support many of the precautions and contraindications listed in product labeling.6 However, the benefits of using metformin in T2DM are clearly significant.18,36–41 In fact, for heart failure and renal insufficiency, available evidence suggests there is no significant risk of lactic acidosis17–20,42 with metformin use and that metformin may actually be beneficial for patients with heart failure.36,37,43–46 Despite a lack of evidence supporting the precautions and contraindications to metformin use, and the overwhelming evidence supporting metformin’s benefits, 85% of providers do not prescribe metformin in the presence of a precaution or contraindication.47
In this study, hepatic dysfunction was the only potential risk factor for acidosis that did not significantly increase the risk of acidosis (unadjusted RR = 3.4, 95% CI = 0.9–13.8). There were 88 patients with one or more risk factors and an acidosis event; however, none of these patients were in the metformin group. When considering the prescribing patterns across patients with risk factors, metformin was significantly less likely to be prescribed for patients with risk factors for lactic acidosis, with the exceptions of patients with heart failure or history of acidosis. Further, fewer patients with lactic acidosis risk factors were prescribed metformin than other T2DM medications (8% of the metformin group compared with 12% of the other medication group). It is notable that there were fewer prescriptions for metformin in patients with risk factors for lactic acidosis and this may be the result of providers avoiding metformin for patients with risk factors. However, although metformin was less likely to be prescribed to patients with risk factors for lactic acidosis, the propensity methods we used accounted for this to the extent of information available. The propensity methods only accounted for presence of a diagnosis and not severity of a risk factor, such as degree of renal insufficiency. Providers may have been less likely to prescribe metformin to patients with more severe risk factors, which may have biased the results.
There are some limitations of this study. This was a retrospective study utilizing administrative claims data. Medication pharmacy dispensing data are not necessarily reflective of actual patient use. Coding of medical conditions may also have been inaccurate or incomplete, and variables such as glucose and creatinine levels are not available. Because of its retrospective design, causality between acidosis and medication use cannot be determined. Uncontrolled diabetes alone in addition to other factors that could have contributed to occurrences of acidosis were not able to be assessed with this study design. Considering the no medication exposure group had a higher occurrence of acidosis, it is possible that worse glycemic control was a contributing factor.7–9 It is also possible that the no medication group had better glycemic control compared with the other groups and thus did not require treatment. Severity of risk factors such as renal insufficiency and liver dysfunction were not known using this database and it is possible that more severe disease would have resulted in greater risk of lactic acidosis.48,49 A prior study evaluating patients with diabetes and higher serum creatinine values found metformin users were at increased risk of mortality compared with nonmetformin users, but this study did not estimate renal function using clinically meaningful methods such as Creatinine Clearance or Modification of Diet in Renal Disease formulas.48,50 Further, the diagnosis of acidosis defined by a nonspecific ICD-9 code that includes metabolic and respiratory acidosis may not identify all cases, and may not specifically indicate lactic acidosis; however, our rules for identifying cases decrease the chance of misclassification. Although the majority of metabolic acidosis events are due to lactic acidosis,5 it is possible that false-positive cases of lactic acidosis were identified, representing cases of respiratory acidosis or metabolic acidosis unrelated to lactic acidosis.
Conclusion
Additional studies of large sample sizes that assess causality of lactic acidosis and that assess laboratory measurements of serum lactic acid and risk factor severity, such as degree of renal insufficiency, are needed to definitively negate the fear of lactic acidosis with metformin use. In the absence of data supporting an increased risk of lactic acidosis with metformin use, our findings support expanded use of metformin for the treatment of T2DM. The association of acidosis with metformin use is similar to the risk associated with other T2DM medications or no medication in patients with newly diagnosed T2DM.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The ALSAM Foundation Skaggs Scholars Program grant at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The ALSAM Foundation had no involvement or influence in the design, conduct or reporting of the study.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Katy E. Trinkley, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences and School of Medicine, 12850 E Montview Blvd, Mail Stop C238, Aurora, CO 80045, USA.
Heather D. Anderson, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA
Kavita V. Nair, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA
Daniel C. Malone, University of Arizona College of Pharmacy, Phoenix, AZ, USA
Joseph J. Saseen, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA
References
- 1. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2011. National Diabetes Information Clearinghouse, 2011, pp.1–12. [Google Scholar]
- 2. Turner R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 3. American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care 2016; 39: S1–119.26696671 [Google Scholar]
- 4. Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med 2012; 125: 302.e1–302.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen R, Woods H. The clinical presentations and classifications of lactic acidosis. In: Clinical and biochemical aspects of lactic acidosis. Boston: Blackwell Scientific Publications, 1976. [Google Scholar]
- 6. Glucophage. Princeton, NJ: Bristol-Meyers Squibb Company; 2017. [Google Scholar]
- 7. Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf) 2011; 74: 191–196. [DOI] [PubMed] [Google Scholar]
- 8. Parsapour K, Pullela R, Raff G, et al. Type B lactic acidosis and insulin-resistant hyperglycemia in an adolescent following cardiac surgery. Pediatr Crit Care Med 2008; 9: e6–e9. [DOI] [PubMed] [Google Scholar]
- 9. English P, Williams G. Hyperglycaemic crises and lactic acidosis in diabetes mellitus. Postgrad Med J 2004; 80: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cryer DR, Nicholas SP, Henry DH, et al. Comparative outcomes study of metformin intervention versus conventional approach: the COSMIC approach study. Diabetes Care 2005; 28: 539–543. [DOI] [PubMed] [Google Scholar]
- 11. Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147: 386–399. [DOI] [PubMed] [Google Scholar]
- 12. Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care 1999; 22: 925–927. [DOI] [PubMed] [Google Scholar]
- 13. Brown JB, Pedula K, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care 1998; 21: 1659–1663. [DOI] [PubMed] [Google Scholar]
- 14. Krentz AJ, Ferner RE, Bailey CJ. Comparative tolerability profiles of oral antidiabetic agents. Epub ahead of print 1994. DOI: 10.2165/00002018-199411040-00002. [DOI] [PubMed] [Google Scholar]
- 15. Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992; 15: 755–772. [DOI] [PubMed] [Google Scholar]
- 16. Bailey C, Turner R. Metformin. N Engl J Med 1996; 334: 574–579. [DOI] [PubMed] [Google Scholar]
- 17. Lalau J-D, Arnouts P, Sharif A, et al. Metformin and other antidiabetic agents in renal failure patients. Kidney Int 2015; 87: 308–322. [DOI] [PubMed] [Google Scholar]
- 18. Emslie-Smith AM, Boyle DI, Evans JM, et al. Contraindications to metformin therapy in patients with type 2 diabetes–a population-based study of adherence to prescribing guidelines. Diabet Med 2001; 18: 483–488. [DOI] [PubMed] [Google Scholar]
- 19. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013; 6: 395–402. [DOI] [PubMed] [Google Scholar]
- 20. Holstein A, Nahrwold D, Hinze S, et al. Contra-indications to metformin therapy are largely disregarded. Diabet Med 1999; 16: 692–696. [DOI] [PubMed] [Google Scholar]
- 21. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. J Am Med Assoc 2014; 312: 2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. IMS Health Incorporated. Pharmetrics health plan claims data user guide and data dictionary. [Google Scholar]
- 23. Malone DC, Billups SJ, Valuck RJ, et al. Development of a Chronic Disease Indicator score using a veterans affairs medical center medication database. J Clin Epidemiol 1999; 52: 551–557. [DOI] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 25. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 757–763. [DOI] [PubMed] [Google Scholar]
- 26. Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163: 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 28. Vansteelandt S, Daniel RM. On regression adjustment for the propensity score. Stat Med 2014; 33: 4053–4072. [DOI] [PubMed] [Google Scholar]
- 29. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 30. Shintani AK, Girard TD, Eden SK, et al. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med 2009; 37: 2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubin DB. On the limitations of comparative effectiveness research. Stat Med 2010; 29: 1991–1995. [DOI] [PubMed] [Google Scholar]
- 32. SAS Language, version 9.4. SAS Institute, Cary, NC. [Google Scholar]
- 33. Salpeter SR, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010; CD002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ekström N, Schiöler L, Svensson A-M, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open; 2 Epub ahead of print January 2012. DOI: 10.1136/bmjopen-2012-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lalau J-D. Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf 2010; 33: 727–740. [DOI] [PubMed] [Google Scholar]
- 36. Tahrani AA, Varughese GI, Scarpello JH, et al. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ Br Med J 2007; 335: 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khurana R, Malik IS. Metformin: safety in cardiac patients. Postgrad Med J 2010; 86: 371–373. [DOI] [PubMed] [Google Scholar]
- 38. McCormack J, Johns K, Tildesley H. Metformin’s contraindications should be contraindicated. CMAJ 2005; 173: 502–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111: 583–590. [DOI] [PubMed] [Google Scholar]
- 40. Roussel R, Travert F, Pasquet B, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010; 170: 1892–1899. [DOI] [PubMed] [Google Scholar]
- 41. Scheen AJ, Paquot N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab 2013; 39: 179–190. [DOI] [PubMed] [Google Scholar]
- 42. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease. J Am Med Assoc 2014; 312: 2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aguilar D, Chan W, Bozkurt B, et al. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Hear Fail 2011; 4: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacDonald MR, Eurich DT, Majumdar SR, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. general practice research database. Diabetes Care 2010; 33: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eurich DT, Majumdar SR, McAlister FA, et al. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care 2005; 28: 2345–2351. [DOI] [PubMed] [Google Scholar]
- 46. Andersson C, Olesen JB, Hansen PR, et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia 2010; 53: 2546–2553. [DOI] [PubMed] [Google Scholar]
- 47. Ricci JR, Coulen C, Berger JE, et al. Prescriber compliance with black box warnings in older adult patients. Am J Manag Care 2009; 15: e103–e108. [PubMed] [Google Scholar]
- 48. Hung S-C, Chang Y-K, Liu J-S, et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol 2015; 3: 605–614. [DOI] [PubMed] [Google Scholar]
- 49. Huang W, Castelino RL, Peterson GM. Adverse event notifications implicating metformin with lactic acidosis in Australia. J Diabetes Complications 2015; 29: 1261–1265. [DOI] [PubMed] [Google Scholar]
- 50. Trinkley KE, Nikels SM, Ii RLP, et al. Automating and estimating glomerular filtration rate for dosing medications and staging chronic kidney disease. Int J Gen Med 2014; 7: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]