Abstract
Endogenous circadian clocks are robust regulators of physiology and behavior. Synchronization or entrainment of biological clocks to environmental time is adaptive and important for physiological homeostasis and for the proper timing of species-specific behaviors. We studied subjects in the laboratory for up to 55 days each to determine the ability to entrain the human clock to a weak circadian synchronizing stimulus [scheduled activity–rest cycle in very dim (≈1.5 lux in the angle of gaze) light–dark cycle] at three ≈24-h periods: 23.5, 24.0, and 24.6 h. These studies allowed us to test two competing hypotheses as to whether the period of the human circadian pacemaker is near to or much longer than 24 h. We report here that imposition of a sleep–wake schedule with exposure to the equivalent of candlelight during wakefulness and darkness during sleep is usually sufficient to maintain circadian entrainment to the 24-h day but not to a 23.5- or 24.6-h day. Our results demonstrate functionally that, in normally entrained sighted adults, the average intrinsic circadian period of the human biological clock is very close to 24 h. Either exposure to very dim light and/or the scheduled sleep–wake cycle itself can entrain this near-24-h intrinsic period of the human circadian pacemaker to the 24-h day.
A central circadian pacemaker, located in the suprachiasmatic nucleus, drives rhythms in multiple behavioral, physiologic, and endocrine variables (1–3). Regulatory feedback loops of gene expression have been reported to underlie the self-sustained near-24-h periodicity of biological clocks (4–8). Furthermore, these clock gene regulatory mechanisms within the suprachiasmatic nuclei can be reset by exposure to environmental synchronizers (e.g., refs. 5, 9–11). However, the strength of the synchronizer necessary to adjust for the daily drift due to the on-average longer-than-24-h period (12) of the human circadian pacemaker is unknown. There is also disagreement as to the average amount of daily shift required for entrainment of the human clock to the 24-h day (13, 14) as this is functionally dependent on the intrinsic circadian period of the pacemaker during entrainment. We define intrinsic circadian period (τ̂) as the actual period emanating from within the circadian pacemaker at a given time, as distinct from observed circadian periods (τOBS), which are influenced by extrinsic resetting stimuli acting on the pacemaker during the time of observation (14, 15). In practice it is virtually impossible to completely remove all factors that reset or affect the period of the pacemaker. However, the forced desynchrony protocol has been reported to reveal a much closer estimate of the actual period emanating from within the circadian pacemaker than the free running protocol (12, 16). It has thus recently been reported that the intrinsic period of the human circadian pacemaker is much closer to 24 than 25 h (reviewed in ref. 12). However, this conclusion has subsequently been challenged, with the argument that the near-24-h circadian period observed on the forced desynchrony protocol may no more accurately reflect the intrinsic period of the human circadian clock than the near-25-h circadian period estimate observed from the traditional free-running paradigm (13, 14). The fact that the imposed periods to which a pacemaker can be entrained to a weak synchronizer are constrained to a limited range around the intrinsic period provides an alternative functional method for the assessment of intrinsic circadian period.
Methods
Subject Screening and Prelaboratory Conditions.
We studied 12 healthy men and three healthy women (Table 1). Participants gave written informed consent, and the Brigham and Women's Hospital/Partners Health Care Human Research Committee approved the procedures for the protocol. The investigation was conducted according to the principles expressed in the Declaration of Helsinki. All participants passed a rigorous health screening, including medical history, physical examination, electrocardiogram, blood and urine chemistries, toxicology screen for drug use, psychological tests, and an interview with a clinical psychologist. None reported regular night work or rotating shift work within the past 3 years or crossing more than one time zone in the previous 3 months. Participants maintained a regular routine of 8 h of scheduled sleep and 16 h of scheduled wakefulness for a minimum of 3 weeks while living at home before the in-laboratory protocol, as verified by sleep logs and call-in times to a time-stamped voice recorder. Actigraphy recordings occurred for at least 1 week before laboratory admission.
Table 1.
Circadian period estimates
| Subject | Age, years | Gender | τOBSm, hr ± SD | (95% CI), hr | τm, hr ± SD | τt, hr ± SD |
|---|---|---|---|---|---|---|
| T cycle = 24.0 h | ||||||
| 18G6 | 20 | M | 23.97 ± 0.03 | (23.92–24.02) | 23.88 ± 0.01 | 23.88 ± 0.04 |
| 1814 | 24 | M | 23.96 ± 0.02 | (23.91–24.01) | 23.92 ± 0.01 | 23.91 ± 0.05 |
| 1842 | 38 | M | 23.98 ± 0.04 | (23.89–24.06) | — | — |
| 1983 | 38 | F | 24.02 ± 0.05 | (23.92–24.12) | 24.06 ± 0.03 | 24.08 ± 0.07 |
| 19A4 | 24 | M | 24.06 ± 0.04 | (23.98–24.14) | 24.12 ± 0.02 | 24.14 ± 0.04 |
| 18G1 | 31 | M | 24.27 ± 0.03 | (24.21–24.32) | 24.36 ± 0.01 | 24.48 ± 0.05 |
| T cycle = 23.5 h* | ||||||
| 2029 | 28 | M | 23.89 ± 0.09 | (22.79–24.99) | — | — |
| 19F8 | 44 | M | 24.14 ± 0.02 | (24.05–24.22) | — | — |
| 2028 | 26 | M | 24.19 ± 0.03 | (23.76–24.62) | — | — |
| T cycle = 24.6 h | ||||||
| 1916 | 37 | M | 23.87 ± 0.03 | (23.82–23.92) | 23.77 ± 0.01 | 23.77 ± 0.04 |
| 18J5 | 31 | M | 24.02 ± 0.03 | (23.96–24.07) | 23.93 ± 0.01 | 23.95 ± 0.07 |
| 1715 | 27 | M | 24.35 ± 0.01 | (24.33–24.37) | — | — |
| 1922 | 33 | M | 24.34 ± 0.03 | (24.28–24.39) | 24.16 ± 0.01 | 24.07 ± 0.05 |
| 19A9 | 40 | F | 24.34 ± 0.03 | (24.29–24.39) | 24.20 ± 0.03 | 24.16 ± 0.08 |
| 1947 | 41 | F | 24.40 ± 0.03 | (24.35–24.45) | 24.23 ± 0.01 | 24.22 ± 0.09 |
| Range 20–44 | 23.77–24.36 | 23.77–24.48 | ||||
Observed plasma melatonin rhythm (τOBSm) during imposed 24.0-, 23.5-, and 24.6-h days and 95% confidence intervals; intrinsic circadian period as estimated by melatonin (τm) and core body temperature (τt) rhythms during forced desynchrony (T = 28 h). For each subject, the periods of the (τm) versus the (τt) rhythms during forced desynchrony were strongly correlated (Pearson correlation: r = 0.97; P < 0.0001). Period estimates were not available in five subjects because the forced desynchrony protocol was not conducted.
Because of the few data points collected, observed periods estimates and 95% CI for T = 23.5 h were derived from linear fits of melatonin onsets during CRs and day 14 (Fig. 1a).
In-Laboratory Conditions.
Subjects were tested individually in an environment free of time cues. Ambient light, room temperature, sleep–wake opportunities, activity, and nutrition intake (breakfast, lunch, dinner, and a snack) were strictly controlled. Exercise and napping were proscribed. Performance testing began on awakening and ≈30-min performance sessions occurred every 2 h thereafter. Subjects engaged in leisure activities between performance batteries. Participants were maintained on a 24.0-h schedule for 3–6 days, followed by a 40.0-h constant routine (CR) protocol that was used to assess circadian phase (1, 12). After this first CR, individuals were scheduled for several weeks to a 23.5-, 24.0-, or 24.6-h day. A second CR was used to assess circadian phase after exposure to these scheduled day lengths. Women with consistent regular menstrual cycles of 25–32 days in length began the study during the week of menses so that the assessment of circadian rhythms for CR1 and CR2 would occur during the follicular phase of their menstrual cycle.
Subjects in the 24.0- and 24.6-h conditions were then scheduled to a forced desynchrony protocol by using a 28.0-h dim light–dark activity–rest cycle, which was known to be outside the range of entrainment of the human circadian pacemaker under such conditions (12). This forced desynchrony protocol was used to estimate the intrinsic circadian period of individuals by assessing their plasma melatonin and core body temperature rhythms.
Ceiling-mounted fluorescent lamps [Phillips (Eindhoven, The Netherlands) T8 and T80] with a 4,100 K color temperature produced a spectrum of white light. Clear polycarbonate lenses filtered 99.9% of the light in the UV range. Light–dark cycles of very dim light (≈1.5 lux in the angle of gaze) during scheduled wakefulness and darkness during scheduled sleep were 15.83:7.66 h for the 23.5-h day, 16:8 h for the 24.0-h day, and 16.4:8.2 h for the 24.6-h day. Ambient light intensity, as measured with an IL-1400 photometer (International Light, Newburyport, MA) with the sensor on a table top at ≈76 cm aimed in the direction of the light fixtures was <3 lux; the maximum light intensity in the room at ≈183 cm with the sensor aimed toward the light fixtures was <8 lux. Lighting conditions for subjects in the 24.0- and 24.6-h conditions for baseline day 1 during scheduled wakefulness were ≈3.0 lux in the angle of gaze (<5 lux ambient; <15 lux maximum). Between baseline days 2 and 6, these participants were exposed to bright indoor light (≈450 lux in the angle of gaze; <1,100 lux ambient; <1,500 lux maximum). Subjects in the 23.5-h day were exposed to normal indoor light (≈110 lux in the angle of gaze) during their 3 baseline days. Light levels during scheduled wakefulness of the constant routine and of the forced desynchrony protocols were ≈1.5 lux in the angle of gaze.
Circadian Entrainment Analyses.
Plasma melatonin levels (sampled every 30–60 min for ≈3-day sampling windows weekly) were assayed by using RIA 125I (Elias USA, Osceola, WI). The sensitivity of the assay was 2.5 pg/ml. Interassay coefficients of variation for low and high controls were 7.9 and 8.6%, respectively, and the average intraassay coefficient of variation was 6.0%. Given the large interindividual differences in melatonin levels, melatonin onset (17) was defined as the linear interpolated point in time at which melatonin levels reached 25% of the fitted peak-to-trough amplitude of the 3-harmonic of each individual's data from the first constant routine. This 25% melatonin onset threshold (DLMO25%), as determined on the first constant routine, was applied to data at all other portions of the protocol. Circadian melatonin (sampled every 60 min daily across the forced desynchrony) and temperature (sampled every minute) period estimates were computed by a nonorthogonal spectral analysis technique with an exact maximum likelihood fitting procedure (12).
Results and Discussion
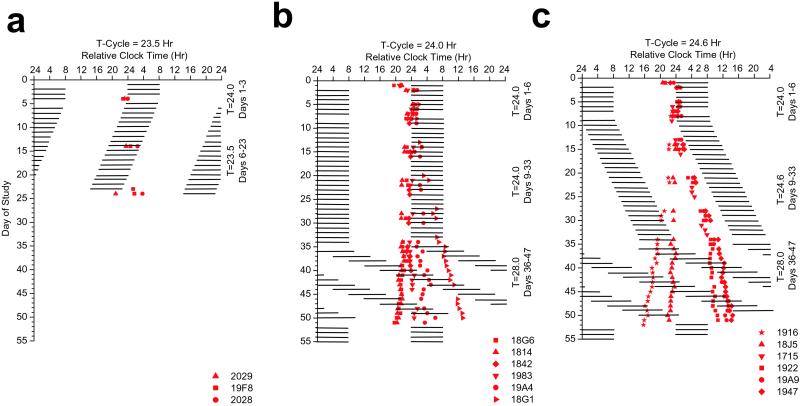
Five of six subjects studied on the 24.0-h day (T = 24.0 h) in ≈1.5 lux were classified as entrained to the 24-h day, because the phase relationships between the timing of their melatonin and core body temperature rhythms and the timing of the scheduled sleep–wake cycle were maintained within the normal range (18), and the 95% confidence intervals (95% CI) of the observed circadian period estimates for these five subjects included 24.0 h (Table 1). Fig. 1b shows that melatonin onset (DLMO25%) occurred near habitual sleep time during baseline and throughout exposure to the 24.0-h schedule for these five subjects. One of the subjects (18G1) was classified as not entrained to the T = 24-h schedule, because his DLMO25% progressively drifted to a later hour outside the normal range (18), and the 95% CI for his observed circadian period did not include 24.0 h. By the end of the T = 24-h segment of the protocol, his melatonin levels were abnormally high during the scheduled waking day and low during the scheduled night. The forced desynchrony segment of the protocol revealed that this subject had an intrinsic circadian period furthest from 24.0 h in this group (Table 1).
Figure 1.
Melatonin onset (DLMO25%) times. (a) Subjects scheduled to the 23.5-h day. Data are plotted to a relative clock time with lights out assigned a value of 2400 h on baseline day 1. Black bars represent scheduled sleep. During the imposed 23.5-h segment, lights out and lights on are advanced by 30 min each day. Melatonin onset occurred near to, but advanced relative to, lights out for the subjects during baseline days, whereas during T = 23.5-h days, melatonin onset progressively phase delays relative to lights out. These data demonstrate failure to entrain the circadian pacemaker to the scheduled 23.5-h wakefulness–sleep light–dark cycle. (b) Subjects scheduled to the 24.0-h day. During the imposed 24.0-h segment, melatonin onset appears stable and occurs near to, but advanced relative to, lights out for half of the subjects and delayed for the other half. These data demonstrate that most subjects entrained to the 24.0-h day with a new phase angle, as would be expected to occur in response to the weak environmental synchronizer. A greater dispersion of melatonin onsets can be observed during forced desynchrony, reflecting individual differences in intrinsic circadian period. In two of six subjects, circadian period advanced and in the remaining subjects circadian period delayed during forced desynchrony (Table 1). Subject 18G1 failed to entrain to the 24.0-h day. (c) Subjects scheduled to the 24.6-h day. During the imposed 24.6-h segment, lights out and lights on are delayed by 36 min each day. Melatonin onset occurs near to, but advanced relative to, lights out for half of the subjects and delayed for the other half during baseline days, whereas during the 24.6-h segment, melatonin onset is progressively phase advanced relative to lights out. These data demonstrate a failure to entrain the human circadian pacemaker to the scheduled 24.6-h day. Individual differences in intrinsic circadian period result in a large dispersion of melatonin onsets during the T = 24.6- and T = 28.0-h forced desynchrony protocols.
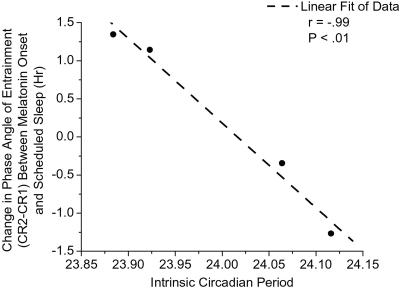
Fig. 2 shows that the direction and magnitude of change in phase angle between the onset of the melatonin rhythm and scheduled sleep time were strongly related to intrinsic circadian period, as later assessed during forced desynchrony. Consistent with the latter, the observed circadian period during the T = 24.0-h schedule (τOBSm) was closer to 24.0 h compared with the estimate of the intrinsic circadian period (τm), as assessed in the same individuals during the T = 28.0-h schedule (forced desynchrony) (Fig. 3a). However, a robust relationship between the observed circadian period during the T = 24.0-h and T = 28.0-h schedules (Fig. 3a) still remained. Our finding—that the phase angle of entrainment to a 24.0-h environmental cycle of weak entraining strength is strongly associated with intrinsic circadian period—is in accordance with classic entrainment theory and demonstrates a fundamental property of the circadian pacemaker in humans. Indeed, this could explain the mechanism underlying the reported association between circadian period and morning–evening behavioral preferences in humans (19).
Figure 2.
Association between phase angle of melatonin onset and scheduled sleep time during the T = 24.0-h segment and intrinsic circadian period during forced desynchrony (T = 28.0 h) for entrained subjects. Symbols represent individual subjects for whom phase angle and intrinsic period data were available (four of five entrained subjects). The change in the phase angle of entrainment between melatonin onset and the scheduled 24.0-h day, as determined on CR1 and CR2, is negatively and robustly related to intrinsic circadian period (τm) with a slope of −11.12. Subjects with a circadian period shorter than 24.0 h advanced, and subjects with a period longer than 24.0 h delayed, such that for every 0.1-h change in circadian period, there was a 1.11-h change in the phase angle of entrainment.
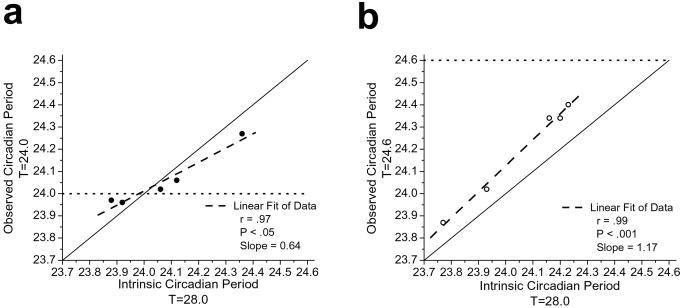
Figure 3.
Observed circadian periods during forced desynchrony T = 28.0 h and during (a) T = 24.0 h and (b) T = 24.6 h protocols. Symbols represent individual subjects for whom observed and intrinsic period data were available. The solid black line represents the line of equality, the dashed line represents a linear fit of the data, and the dotted lines represent the target period to entrain to. The average intrinsic circadian period was similar for both groups of subjects during the forced desynchrony [average ± SD (a) 24.07 ± 0.19 h vs. (b) 24.06 ± 0.20 h]. The circadian period lengthened closer to 24.0 h in two subjects and shortened closer to 24.0 h in three subjects during T = 24.0 h compared with their intrinsic circadian period (τm), as assessed during T = 28.0 h. The absolute change in period was significantly greater than 0 (one sample t test: P < 0.05). Subjects scheduled to the 24.6-h day showed a significantly longer observed period during T = 24.6 h (24.19 ± 0.23 h) compared with T = 28.0 h (t test: P < 0.01). These data demonstrate coupling between the endogenous circadian pacemaker and the weak environmental time cues, showing that environmental and/or behavioral periodicity can significantly influence the observed period of the pacemaker when the day length is close to the pacemakers intrinsic period regardless of whether the pacemaker entrains to the environmental day length.
Given the day-to-day variability in their phase assessments and the proximity of subjects' intrinsic circadian periods (range: 23.88–24.12 h) to the imposed period (T = 24.0 h), a much longer study (>6–12 months) would be required to distinguish definitively between relative coordination and entrainment. We have therefore used a classification method that is consistent with those used to classify blind subjects as entrained or not entrained (20–22).
None of the subjects studied on the T = 23.5-h schedule or the T = 24.6-h schedule were able to achieve the daily phase shift required to entrain to those schedules in dim light (Table 1). Instead, all subjects tested on the T = 23.5-h schedule exhibited an abnormally delayed phase angle of melatonin onset relative to the timing of the scheduled sleep episode (Fig. 1a). Subjects on the T = 24.6-h schedule showed abnormally advanced phase angles of melatonin onset relative to the timing of the scheduled sleep episode (Fig. 1c). Melatonin levels during baseline conditions were high during scheduled sleep. However, during the imposed T = 24.6-h schedule, the wakefulness–sleep cycle was insufficient to synchronize their circadian pacemaker in ≈1.5 lux and, as a consequence, there were high levels of melatonin during the scheduled waking day and low levels during scheduled sleep episodes in darkness. The 95% confidence interval for the observed melatonin period during the imposed T = 24.6-h schedule did not include 24.6 h for any subject. On the basis of the latter and the abnormal phase angles observed, no subjects were classified as being entrained to the 24.6-h day. The observed period (τOBSm) during the T = 24.6-h schedule was strongly related to each subject's intrinsic circadian period (τm), as assessed during forced desynchrony (Fig. 3b); however, the average observed circadian period was significantly longer during the T = 24.6-h schedule, as compared with the average intrinsic circadian period during the T = 28.0-h schedule. This suggests that the imposed 24.6-h schedule exerted an effect on the oscillator but was of insufficient strength to entrain it (i.e., relative coordination). The lengthening in the observed melatonin period during the T = 24.6-h schedule presumably occurred because the imposed period of the synchronizer was close enough to the intrinsic period of the pacemaker to exert an influence on it, consistent with predictions based on model simulations (16). It is also possible that this relative coordination may have induced aftereffects on the intrinsic period during the subsequent forced desynchrony. However, any such aftereffects of relative coordination would appear to be small, because the average circadian period during the forced desynchrony was indistinguishable after T = 24.0- and T = 24.6-day lengths (see Fig. 3 legend).
Examining circadian entrainment to 24.0- and 24.6-h schedules allowed us to test two competing hypotheses as to the intrinsic period of the human circadian pacemaker. If the average intrinsic circadian period in humans were close to 24.0 h (reviewed in ref. 12), then more subjects would be entrained to the imposed 24.0-h schedule than to the imposed 24.6-h schedule when exposed to environmental cycles of weak entraining strength. If, on the other hand, the average intrinsic circadian period in sighted humans were closer to 24.5 or 25.0 h (23–26), then more subjects would have entrained to the 24.6-h day. We hypothesized that a subject's ability to entrain to either day length would depend on her/his circadian period and that only those subjects with intrinsic circadian periods within a small range close to that of the imposed schedule could be entrained by an environmental cycle of weak strength. Our results demonstrating that a weak synchronizer is able to maintain entrainment of the human circadian pacemaker to the T = 24.0-h schedule in most individuals, but not to a T = 23.5- or 24.6-h schedule (Yates corrected χ2: 24.0 vs. 24.6 day, P < 0.05), resolve this controversy as to whether the intrinsic period is near 24 h, near 25 h, or simply dependent on study conditions (12–14). These results demonstrate functionally that the intrinsic period in humans is thus in fact near 24 h, consistent with the period derived theoretically from forced desynchrony studies (reviewed in ref. 12). Thus, the amount by which the human circadian pacemaker must be reset each day to maintain entrainment depends on the intrinsic circadian period of the individual, which in healthy sighted humans is on average near 24.0 h. This validates the use of the forced desynchrony protocol (reviewed in ref. 12), rather than the classical free running protocol (13, 14, 23–25, 27), as an accurate method of assessing intrinsic circadian period of the pacemaker driving the rhythm of plasma melatonin in humans upon release from entrainment. It is this estimate of the intrinsic circadian period—immediately on release from entrainment to the 24-h day—that is most relevant in understanding the strength of the synchronizing cues required for entrainment (28), because it is this period, in combination with the phase-response curve and, perhaps, the τ-response curve to light (29), that determines the stability of entrainment. As noted earlier (12), it is possible that the observed near-24.0-h intrinsic period of the human circadian pacemaker is influenced by prior entrainment to the 24.0-h day. Aftereffects of circadian entrainment and of light exposure history on circadian period have been described in many species but have not yet been demonstrated conclusively in humans (28, 30).
On the basis of the distribution of intrinsic circadian periods observed in the current study and those previously published by our laboratory (12, 31), we estimate that the ≈1.5:0 lux light–dark/activity–rest cycle would be capable of maintaining entrainment in nearly half of the healthy young adult sighted population. However, additional research is necessary to determine the upper and lower range of periods that are capable of being entrained to T = 24 h by this weak synchronizer. Furthermore, the strength of the synchronizer necessary to capture or phase shift the circadian system in humans remains to be determined. Nevertheless, the unanticipated finding that these human subjects remained entrained to the 24.0-h day in candlelight is remarkable given that this illuminance level is less than one-thousandth of the intensity once thought to be necessary for entrainment (23). Failure of subjects studied in ≈1.5 lux to entrain to the scheduled activity–rest/dim light–dark cycle of both 23.5 h, as reported earlier (32), and 24.6 h demonstrates that the range of entrainment of the circadian pacemaker in sighted humans is centered near 24.0 h. Stronger environmental synchronizers would be necessary to expand the range of entrainment to include these day lengths. There is increasing evidence that exposure to indoor artificial lighting can alter the phase of the circadian pacemaker in humans (33, 34). Taken together, these findings support the hypothesis that exposure to inappropriately timed artificial light may underlie or exacerbate circadian sleep disorders in humans. Stronger synchronizers used to treat such sleep disorders, such as exposure to bright light (>1,770 lux), have been reported to increase the range of entrainment of the human circadian pacemaker (35, 36). Our result showing entrainment to the 24.0-h day in a very dim light environment appears to depend on strict scheduling of the light–dark/activity–rest cycle, because the circadian rhythms of subjects studied in a similar illuminance level, whose light–dark/activity–rest cycles were not scheduled, were not entrained to the 24.0-h day (37). Thus, it is impossible to determine whether the observed entrainment is a function of the strict schedule of nonphotic stimuli, the timing of light and darkness, or a combination of the two. The potential importance of nonphotic synchronizers, such as periodic scheduling of sleep and/or activity, is illustrated by the reported entrainment to a 23.8-h day of a circadian blind human subject living in near darkness (<0.03 lux) (22). Pharmacological doses of melatonin have also been reported to capture and maintain circadian entrainment to the 24-h day in blind humans (20, 21). In sighted humans, nonphotic stimuli such as exercise have also been reported to shift the timing of the circadian system (38, 39). Because exercise and pharmacological agents were restricted in the current studies, these results suggest that periodic events such as scheduled sleep and wakefulness (40), meals, or other recurrent daily activities (41) may have greater effects on the human pacemaker than previously recognized.
The entrainment limits of the near-24-h human circadian pacemaker have important implications for the entrainment of astronauts during Earth orbit (42) and during exploration-class space missions such as a mission to Mars (43), for submariners and other Navy personnel who are scheduled to work on an 18-h day (44), for blind persons (20–22), for shift workers, and for transmeridian travelers. The relationship between the phase angle of entrainment and the genetically determined circadian period has important implications for understanding the mechanisms underlying circadian rhythm sleep disorders, as recently demonstrated by the identification of the gene responsible for the period abnormality underlying a familial form of advanced sleep phase syndrome (45).
Acknowledgments
We thank the subject volunteers, research staff, and subject recruiters (S.Ma, N. Gonzalez) who participated in this study. We also thank M. E. Jewett, E. B. Klerman, S. W. Lockley, and J. M. Ronda for scientific discussions and comments on the manuscript. This work was supported by National Aeronautics and Space Administration (NASA) Cooperative Agreement NCC9–58 with the National Space Biomedical Research Institute and by NASA Grant NAG69–1035. The studies were performed in a General Clinical Research Center supported by National Institutes of Health (NIH) Grant MO1-RR02635. K.P.W. was supported by fellowships from NIH Grant T32-DK07529, the Medical Foundation, and the Harold Whitworth Pierce Charitable Trust.
Abbreviation
- CR
constant routine
References
- 1.Czeisler C A, Wright K P., Jr . In: Regulation of Sleep and Circadian Rhythms. Turek F W, Zee P C, editors. New York: Dekker; 1999. pp. 149–180. [Google Scholar]
- 2.Moore R Y. In: Circadian Clocks and Their Adjustment, Ciba Foundation Symposium no. 183. Waterhouse J M, editor. Chichester, U.K.: Wiley; 1994. pp. 88–99. [Google Scholar]
- 3.Aronin N, Sagar S M, Sharp F R, Schwartz W J. Proc Natl Acad Sci USA. 1990;87:5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Nature (London) 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 5.Zylka M J, Shearman L P, Weaver D R, Reppert S M. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 6.Gekakis N, Staknis D, Nguyen H B, Davis F C, Wilsbacher L D, King D P, Takahashi J S, Weitz C J. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 7.Shearman L P, Sriram S, Weaver D R, Maywood E S, Chaves I, Zheng B, Kume K, Lee C C, van der Horst G T J, Hastings M H, et al. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 8.Lowrey P L, Shimomura K, Antoch M P, Yamazaki S, Zemenides P D, Ralph M R, Menaker M, Takahashi J S. Science. 2000;288:483–491. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers M P, Wager-Smith K, Rothenfluh-Hilfiker A, Young M W. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 10.Maywood E S, Mrosovsky N, Field M D, Hastings M H. Proc Natl Acad Sci USA. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceriani M F, Darlington T K, Staknis D, Más P, Petti A A, Weitz C J, Kay S A. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler C A, Duffy J F, Shanahan T L, Brown E N, Mitchell J F, Rimmer D W, Ronda J M, Silva E J, Allan J S, Emens J S, et al. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 13.Campbell S. Science. 2000;288:1174–1175. [PubMed] [Google Scholar]
- 14.Czeisler C A, Dijk D-J, Kronauer R E, Brown E M, Duffy J F, Allan J S, Shanahan T L, Rimmer D W, Ronda J M, Mitchell J F, et al. Science. 2000;288:1174–1175. [PubMed] [Google Scholar]
- 15.Kronauer R E, Czeisler C A, Pilato S F, Moore-Ede M C, Weitzman E D. Am J Physiol. 1982;242:R3–R17. doi: 10.1152/ajpregu.1982.242.1.R3. [DOI] [PubMed] [Google Scholar]
- 16.Klerman E B, Dijk D-J, Kronauer R E, Czeisler C A. Am J Physiol. 1996;270:R271–R282. doi: 10.1152/ajpregu.1996.270.1.R271. [DOI] [PubMed] [Google Scholar]
- 17.Hughes R J, Sack R L, Lewy A J. Sleep. 1998;21:52–68. [PubMed] [Google Scholar]
- 18.Lewy A J, Cutler N L, Sack R L. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 19.Duffy J F, Rimmer D W, Czeisler C A. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 20.Sack R L, Brandes R W, Kendall A R, Lewy A J. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 21.Lockley S W, Skene D J, James K, Thapan K, Wright J, Arendt J. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 22.Klerman E B, Rimmer D W, Dijk D-J, Kronauer R E, Rizzo J F, III, Czeisler C A. Am J Physiol. 1998;274:R991–R996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 23.Wever R A. The Circadian System of Man: Results of Experiments Under Temporal Isolation. New York: Springer; 1979. [Google Scholar]
- 24.Weitzman E D, Czeisler C A, Zimmerman J C, Moore-Ede M C. In: Advanced Biochemistry and Psychopharmacology. Martin J B, Reichlin S, Bick K L, editors. New York: Raven; 1981. pp. 475–499. [Google Scholar]
- 25.Aschoff J, Wever R. Naturwissenschaften. 1962;49:337–342. [Google Scholar]
- 26.Campbell S S, Dawson D, Zulley J. Sleep. 1993;16:638–640. [PubMed] [Google Scholar]
- 27.Jones C R, Campbell S S, Zone S E, Cooper F, DeSano A, Murphy P J, Jones B, Czajkowski L, Ptác̆ek L J. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 28.Pittendrigh C S, Daan S. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- 29.Beersma D G M, Spoelstra K, Daan S. J Biol Rhythms. 1999;14:524–531. doi: 10.1177/074873099129000858. [DOI] [PubMed] [Google Scholar]
- 30.Pittendrigh C S, Daan S. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 31.Wyatt J K, Ritz-De Cecco A, Czeisler C A, Dijk D-J. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K. Hokkaido J Med Sci. 1996;71:403–422. [PubMed] [Google Scholar]
- 33.Boivin D B, Duffy J F, Kronauer R E, Czeisler C A. Nature (London) 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 34.Zeitzer J M, Dijk D-J, Kronauer R E, Brown E N, Czeisler C A. J Physiol (London) 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eastman C I, Miescke K J. Am J Physiol. 1990;259:R1189–R1197. doi: 10.1152/ajpregu.1990.259.6.R1189. [DOI] [PubMed] [Google Scholar]
- 36.Wever R A. J Biol Rhythms. 1989;4:161–185. [PubMed] [Google Scholar]
- 37.Middleton B, Arendt J, Stone B M. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 38.Buxton O M, Frank S A, L'Hermite-Balériaux M, Leproult R, Turek F W, Van Cauter E. Am J Physiol. 1997;273:E536–E542. doi: 10.1152/ajpendo.1997.273.3.E536. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki T, Hashimoto S, Masubuchi S, Honma S, Honma K-I. Am J Physiol. 2001;281:R197–R205. doi: 10.1152/ajpregu.2001.281.1.R197. [DOI] [PubMed] [Google Scholar]
- 40.Grossman G H, Mistlberger R E, Antle M C, Ehlen J C, Glass J D. NeuroReport. 2000;11:1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- 41.Antle M C, Mistlberger R E. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dijk D-J, Neri D F, Wyatt J K, Ronda J M, Riel E, Ritz-De Cecco A, Hughes R J, Elliott A R, Prisk G K, West J B, et al. Am J Physiol. 2001;281:R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 43.White R J, Averner M. Nature (London) 2001;409:1115–1118. doi: 10.1038/35059243. [DOI] [PubMed] [Google Scholar]
- 44.Kelly T L, Neri D F, Grill J T, Ryman D, Hunt P D, Dijk D-J, Shanahan T L, Czeisler C A. J Biol Rhythms. 1999;14:190–196. doi: 10.1177/074873099129000597. [DOI] [PubMed] [Google Scholar]
- 45.Toh K L, Jones C R, He Y, Eide E J, Hinz W A, Virshup D M, Ptác̆ek L J, Fu Y-H. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]