Abstract
Background
Endovascular treatment (EVT) of brain arteriovenous malformations has evolved from cyanoacrylate derivatives such as N-butyl cyanoacrylate, an adhesive glue, to ethylene vinyl copolymer-based liquid embolics such as Onyx® and SQUID® dissolved in dimethyl sulfoxide. Although these agents offer several advantages, their rapidly decreasing radiopacity, as a result of the sedimentation of tantalum powder, compromises visual control during EVT. This study aims to quantify and compare tantalum sedimentation rates of several liquid embolic agents, and determine their effects on radiopacity.
Methods
The rate of sedimentation of liquid embolics Onyx 18®, SQUID 12®, and SQUID 18® was measured after preparation by single x-ray exposures for a period of 30 minutes. The signal-to-noise ratios (SNRs) of the suspension of each liquid embolic was calculated at various time points as tantalum settled out of the suspension. Precipitating Hydrophobic Injectable Liquid (PHIL®) was imaged as a control.
Results
Onyx 18® demonstrated the fastest sedimentation rate of the liquid embolics analyzed and demonstrated a threefold faster drop in SNR compared to SQUID 18® over 30 minutes. Onyx 18® demonstrated a one and a half times faster drop in SNR compared to SQUID 12®. Although PHIL 25® maintained constant SNR over the same time, it was lower at baseline immediately after preparation compared to tantalum-based liquids.
Conclusion
Caution during long injections using tantalum-based agents is advised. Onyx 18® has a significantly faster drop in radiopacity compared to SQUID 12® and SQUID 18®. Covalently bonded iodine-based embolics like PHIL® demonstrate constant radiopacity over time.
Keywords: Liquid embolics, tantalum, sedimentation, Onyx 18®, SQUID 12®, SQUID 18®, PHIL 25®, brain arteriovenous malformations, image quality
Introduction
Cyanoacrylate derivatives such as N-butyl cyanoacrylate (NBCA) have been used in the past to treat brain arteriovenous malformations (bAVMs) alone or in combination with radiosurgery or surgical removal.1 Inherent limitations of NBCA, such as risk of intravascular catheter adhesion and significant learning curve, led to the development of newer agents. Onyx® (Medtronic, Dublin, Ireland) and SQUID® (Emboflu, Fribourg, Switzerland) consist both of ethylene vinyl copolymer (EVOH) and dimethyl sulfoxide (DMSO), which are combined with tantalum powder for radiopacity. They offer several advantages over cyanoacrylates, specifically the possibility of multiple, repeated, and longer injections through the same catheter, which according to some studies have led to higher endovascular cure rates for bAVMs.2,3
The added tantalum powder requires Onyx® and SQUID® to be prepared as a suspension in a Vortex Genie shaker (Scientific Industries) prior to use in order to suspend the tantalum homogenously and provide uniform radiopacity. However, as soon as the vial is taken out of the shaker, the tantalum particles begin to settle out of the suspension and as this occurs, the liquid consequently starts becoming less radiopaque.4–6 SQUID® has been developed to address these limitations, and is advocated as having a smaller “micronized” tantalum powder grain compared to Onyx®. This in turn should provide a “high homogeneity in radiopacity” and “long visibility and long injection times.”7 Although the manufacturer states that the sedimentation of tantalum in other liquid embolic agents occurs at a rate twice as fast as in SQUID®, these claims have not been quantified thus far.
More recently, a different type of liquid embolic Precipitating Hydrophobic Injectable Liquid (PHIL®, MicroVention, Tustin, CA, USA) was introduced to overcome the disadvantages of tantalum powder. This liquid embolic is similar to Onyx® and SQUID® in that it is composed of a nonadhesive copolymer (specifically, polylactide-co-glycolide and polyhydroxyethylmethacrylate) and also uses DMSO as a solvent. However, its stable radiopacity throughout prolonged injections is based on covalently bonded iodine, which requires no preparation prior to use.8–10 PHIL® also offers several other advantages over EVOH-based embolics such as faster plug formation and fewer artifacts on postembolization imaging with computed tomography (CT) or DynaCT.10 On the other hand, the disadvantages of PHIL® include its inferior visibility within the microcatheter and during initial cast formation of an AVM when compared to Onyx®.10 Its lower radiopacity may also be disadvantageous when used in high-flow shunts, such as in direct AV fistulas.
It can be assumed that some of the complications reported with the use of tantalum-based liquid embolic agents may be due to inconsistent and or impaired visualization during the injection under fluoroscopy (Road Map). Rapid sedimentation of tantalum powder can lead to clogging of the microcatheter and has been identified by some authors as a potential cause of contrast inhomogeneity, specifically during long injections.4
Although the issue of tantalum powder sedimentation in EVOH-based embolics and its impairment of image quality has been known for some time, it has never been thoroughly evaluated.5 To our knowledge, no study has been undertaken to compare tantalum sedimentation rates in different liquid embolic agents. We sought to objectively evaluate the visualization of various liquid embolic agents by measuring the sedimentation rates of tantalum powder after preparation through signal-to-noise ratio (SNR) calculations. We also directly compared the SNR of a new iodine-based embolic liquid with the existing tantalum-containing liquids.
Methods
Baseline single-shot acquisition images were acquired of 1.5 ml vials filled with Onyx 18®, SQUID 18®, and SQUID 12®, and of a 1 ml syringe filled with PHIL 25®, all of which were placed on the angiographic table directly out of the packaging. Then, Onyx 18®, SQUID 12®, and SQUID 18® were prepared with the Vortex Genie shaker on a setting of 8 for 20 minutes per instructions per use of the manufacturers. One minute after preparation, the vials were placed again on the angiographic table in the exact same positions and single-shot acquisitions were performed at five-minute intervals for a period of 30 minutes after preparation. Images were performed on a Philips Allura with Clarity (software: Allura Xper, 8.1.17.2, using single-shot acquisitions and high-dose mode fluoroscopy mode (“Mode 3, “Glue”)). The setup consisted of a source-to-image distance of 117 cm, table height of 0 and the flat panel detector size of 27 cm, kVp = 70.5. Images were exported in digital imaging and communications in medicine format to a dedicated workstation for post-processing and calculation of SNR.11–14 The region of interest (ROI) tool in OsiriX MD (version 7.0.4) was used to measure mean pixel values using a previously described method.11–14 Briefly, the ROI tool can extract average pixel values that are a surrogate for photon attenuation. When compared to a region of background pixel value, this information can be used to create an SNR. ROI size was selected in order to measure only the liquid portion of the embolic, i.e. to avoid the edge of the vial and the tantalum sediment.
Care was taken to place the ROIs on top of the liquid section of the vials, such that they measured only the embolic agents. The size of the ROI (15.2 × 1.1 mm2) was selected in order to avoid the edge of the vial and tantalum sediment. The baseline measurement was taken one minute after preparation (shaking). The vials were not moved during subsequent imaging, so that the ROI could be placed within the exact same location in subsequent images using the copy/paste function. Images were acquired at five-minute intervals for a total of 30 minutes. No registration tools were applied.
The following formula was used to calculate SNR:
BG = Mean background value for pixel data. Background information was taken as a baseline ROI within the vial at one minute after preparation.
X = ROI within a liquid embolic agent imaged at five-minute intervals until 30 minutes after preparation.
STD (X) = standard deviation of pixel data for a particular embolic agent.
STD (BG) = standard deviation of pixel data for background data.
Results
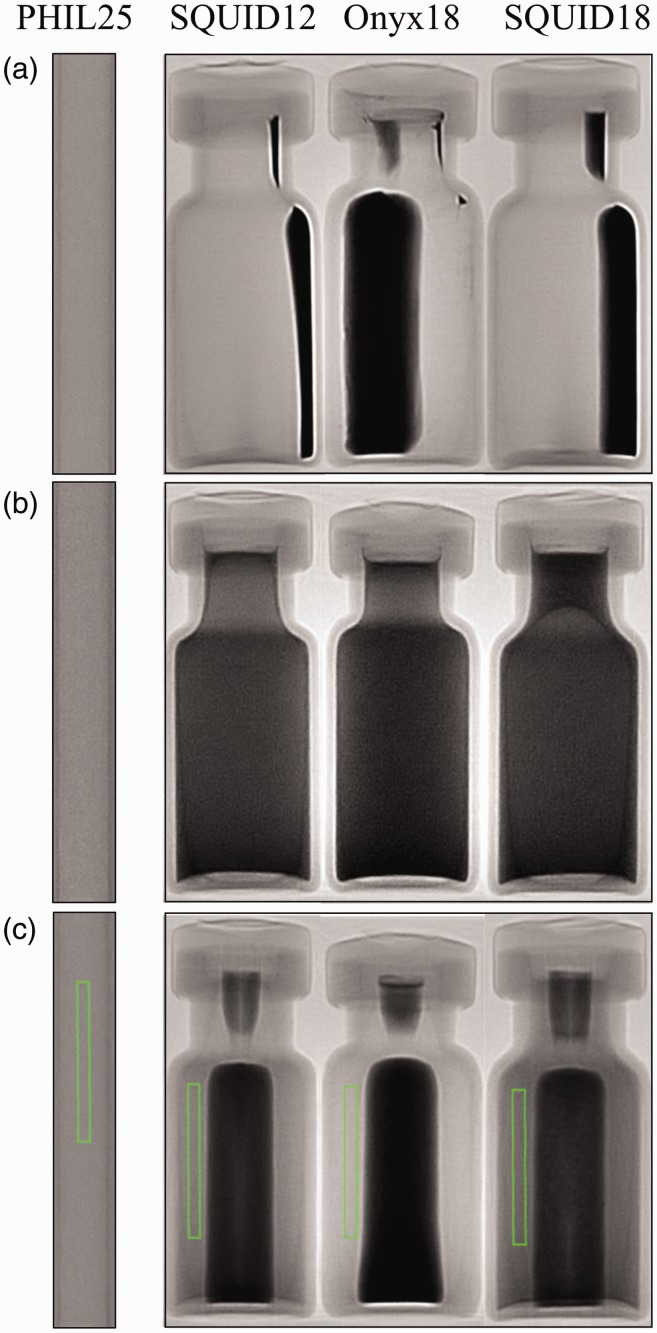
Prior to preparation, the tantalum sedimentation in the Onyx 18®, SQUID 18®, and SQUID 12® was observed under single acquisition x-ray whereas PHIL 25®, as expected, demonstrated uniform radiopacity (Figure 1(a)). The liquid portion of Onyx 18®, SQUID 18®, and SQUID 12® containing the EVOH and DMSO is radiolucent.
Figure 1.
(a) Onyx 18®, SQUID 12®, SQUID 18®, and PHIL 25® prior to preparation. Tantalum sediment is clearly visible in the vials of Onyx 18®, SQUID 12®, and SQUID 18®. (b) Studied embolic agents one minute after preparation: Tantalum is uniformly distributed. (c) After 30 minutes, tantalum can be seen settling within the middle of each vial. Additionally, regions of interest (ROIs) used to measure pixel data seen are placed within each liquid embolic. These ROIs were copied into the exact same position for all measurements.
Immediately after preparation, Onyx 18®, SQUID 18®, and SQUID 12® demonstrate homogeneity due to the uniform distribution of tantalum (Figure 1(b)). The previously radiolucent DMSO and EVOH portions are now radiopaque because of the dispersion of tantalum particles throughout the suspension. As expected, PHIL 25® demonstrates no changes in radiopacity.
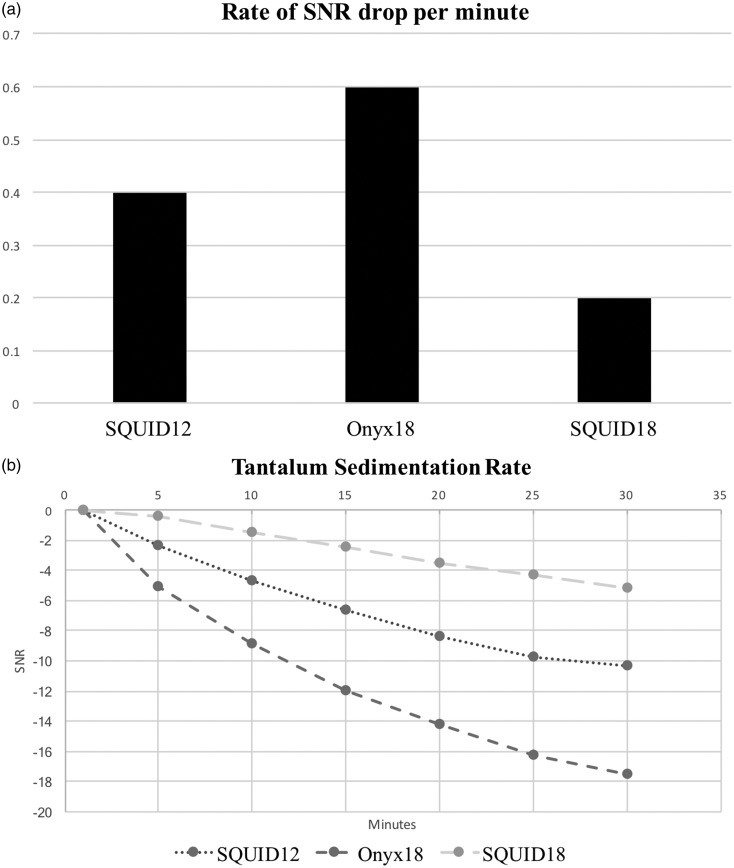
Thirty minutes after initial imaging all embolic liquids except PHIL 25® demonstrated increasing sedimentation of tantalum within the middle portion of each vial (Figure 1(c)). Quantitatively, Onyx 18® showed the greatest drop in SNR over 30 minutes (Figure 2(a) and (b)), suggesting that the tantalum settled out of suspension much faster than in SQUID 12® and SQUID 18®. Onyx 18® had a nearly three times faster rate of drop in SNR compared to SQUID 18®and a 1.5 times faster rate of drop in SNR compared to SQUID 12® over a period of 30 minutes. Based on our calculations, the Onyx® SNR dropped at a rate of 0.6 per minute, compared to 0.4 per minute with SQUID 12® and 0.2 per minute with SQUID 18®. As expected, PHIL 25® demonstrated no change in SNR over 30 minutes. Conversely, it can be clearly observed from Figure 1(b) that of all liquid embolics, PHIL 25® had the lowest radiopacity at baseline after preparation of the tantalum-containing embolics.
Figure 2.
(a) Drop in signal-to-noise ratio (SNR) of the liquid embolic agents per minute. Onyx 18® = 0.6 per minute. SQUID12® = 0.4 per minute. SQUID 18® = 0.2 per minute. (b) Drop in SNR for Onyx 18®, SQUID 12®, and SQUID 18® at five-minute increments from minute 1 to minute 30 after preparation.
Discussion
Cyanoacrylate derivatives such as NBCA have been used in endovascular treatment of AVMs and various other vascular disorders for more than 30 years. They are usually mixed with Lipiodol (Ethiodol), which acts as a retardant for polymerization while not only changing viscosity, but also adding radiopacity at the same time.15 Prior attempts to characterize the visualization of NBCA looked at density on CT by measuring the Hounsfield units of different concentrations of NBCA in tubes with inside diameters of 1 mm.16 It was noted that the addition of metallic powders, such as tungsten or tantalum, was necessary despite the limitation of sedimentation.1 That can particularly be the case in high-concentration mixtures, e.g. >50% NBCA.
When first developed, EVOH and DMSO were combined with metrizadmide to provide radiopacity,2 but eventually tantalum powder was added to make the embolic radiopaque. The use of this agent may have led to higher cure rates for endovascular embolization of bAVMs compared to NBCA.17 One of the key advantages of this agent was the ability to perform multiple injections through the same catheter. Reported injection times for Onyx® are as high as 135 minutes, and an average of 43 minutes in some studies;17 however, long injection times have raised the concern that the tantalum will settle out of suspension,4,9 leading to a liquid embolic agent with lower and inconsistent radiopacity.
As a widely used technique, the injection of Onyx® requires a small amount of reflux along the catheter’s tip to create a “plug” that allows eventually antegrade flow (“push”) of the embolic.18 Onyx® with significantly lower amounts of tantalum is more difficult to visualize on fluoroscopy, and can easily result in greater amounts of reflux than intended. Excessive reflux may lead to microcatheter retention19 or non-target vessel occlusion.5,18,20 Additionally, longer injection times have also been associated with microcatheter retention.21 Although this has been somewhat mitigated by the introduction of detachable-tip catheters,22 unnoted reflux beyond the detachable tip markers can make the detachment unexpectedly more difficult.
Recent attempts to quantify the visualization of Onyx® in an in vitro study by Jiang et al. demonstrated differences in Onyx® radiopacity due to sedimentation,5 suggesting significant inhomogeneity of Onyx®. They showed that pauses during Onyx® injections allowed tantalum powder to settle out of suspension, with resulting measurable decreases in the radiopacity of Onyx®.5 Beyond this study, the issue of sedimentation and its effect on visualization of tantalum-based embolic agents has not been quantified or thoroughly evaluated.5
Our study specifically quantifies how rapidly the SNR drops after preparation of liquid embolic agents that utilize tantalum for radiopacity. The more time passes after preparation, the lower the SNR of the liquid embolic agent (Figure 2(b)). The consequences of the sedimentation of tantalum powder from suspension in liquid embolics include: (1) a constant measurable decrease in SNR over time, (2) decreased SNR resulting in decreased visibility, and (3) ever decreasing homogeneity in visual quality, which likely contributes to clinical complications, such as inadvertent vessel occlusion and more reflux than necessary. These points illustrate the need to reduce the effects of sedimentation. Other methods could be used to increase SNR, such as altering kVp to maximize visualization of the tantalum.
To the best of our knowledge, this is the first investigation that studies and compares sedimentation rates of different tantalum-containing embolic agents, and quantifies how it affects digital radiographic visualization (DR, single-shot acquisitions) and Road Map (fluoroscopy). We have demonstrated that there are measurable differences in sedimentation rates for the tantalum-based embolics Onyx 18®, SQUID 12®, and SQUID 18®. Onyx 18® demonstrated the fastest rate of sedimentation, up to three times faster compared to SQUID 18® over a period of 30 minutes (Figure 2(b)). Our results likely reflect that micronized tantalum powder in SQUID 12® and SQUID 18® allows particles to remain in suspension longer than standard-grain tantalum powder in Onyx 18®. Future studies should aim to make more direct visualization comparisons between tantalum embolics and newer liquid embolic agents that are covalently bonded to iodine and require no preparation, like PHIL® and Easyx®.4,8
Limitations
There are several limitations to our current study: (1) We imaged Onyx® and SQUID® in their original bottles, and not in syringes, which would be closer to clinical use; (2) there are other formulas and concentrations of liquid embolics that we did not analyze, for example, SQUID LD®, Onyx 34®, PHIL 35®, and NBCA formulas such as Glubran® or Trufill®; (3) we made no attempt to simulate anatomical structures, and did not have any mechanism in place to simulate tissue absorption—which may have altered kVp and mA; (4) no injections were performed through a 150 cm microcatheter as used in clinical scenarios; (5) as in any in vitro study, physiological conditions potentially influencing opacification during injections were not created; (6) we did not use electron microscropy or other techniques to study and measure the actual grain size of tantalum powder in Onyx® and SQUID®; and (7) our study evaluated single vials for each separate embolic agent; variability among these different formulations could account for some or all of the changes we have measured, and therefore future investigations could repeat the analysis using multiple versions of the same formulation in order to confirm consistency among the samples.
Conclusions
Sedimentation of tantalum particles results in significant loss of radiopacity for EVOH-based liquid embolic agents such as Onyx® and SQUID®. Caution is advised during long injections using tantalum-based embolic agents because of rapid decrease in SNR. Onyx 18® demonstrates the fastest rate of sedimentation of tantalum powder, and therefore lost radiopacity faster, compared to SQUID 12®and SQUID 18®. PHIL® demonstrated constant SNR thanks to its covalently bonded iodine property.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Pollak JS, White RI. The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol 2001; 12: 907–913. [DOI] [PubMed] [Google Scholar]
- 2.Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. Am J Neuroradiol 1990; 11: 163–168. [PMC free article] [PubMed] [Google Scholar]
- 3.Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer. Report of three cases. J Neurosurg 1991; 75: 655–660. [DOI] [PubMed] [Google Scholar]
- 4.Kulcsár Z, Karol A, Kronen PW, et al. A novel, non-adhesive, precipitating liquid embolic implant with intrinsic radiopacity: Feasibility and safety animal study. Eur Radiol 2016; 27: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 5.Jiang YY, Jo YE, Woo JM, et al. In vitro quantification of the radiopacity of Onyx during embolization. Neurointervention 2017; 12: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loffroy R, Guiu B, Cercueil J, et al. Endovascular therapeutic embolisation: An overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009; 7: 250–263. [DOI] [PubMed] [Google Scholar]
- 7.Embflu. SQUID: The new liquid embolic device [brochure], 2012.
- 8.Leyon JJ, Chavda S, Thomas A, et al. Preliminary experience with the liquid embolic material agent PHIL (Precipitating Hydrophobic Injectable Liquid) in treating cranial and spinal dural arteriovenous fistulas: Technical note. J Neurointerv Surg 2016; 8: 596–602. [DOI] [PubMed] [Google Scholar]
- 9.Samaniego EA, Kalousek V, Abdo G, et al. Preliminary experience with Precipitating Hydrophobic Injectable Liquid (PHIL) in treating cerebral AVMs. J Neurointerv Surg 2016; 8: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 10.Koçer N, Hanımoğlu H, Batur Ş, et al. Preliminary experience with precipitating hydrophobic injectable liquid in brain arteriovenous malformations. Diagn Interv Radiol 2016; 22: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vano E, Ubeda C, Leyton F, et al. Radiation dose and image quality for paediatric interventional cardiology. Phys Med Biol 2008; 53: 4049–4062. [DOI] [PubMed] [Google Scholar]
- 12.Mason JR and Benndorf G. Silent migration of Onyx® (EVOH) injections examined using a calibrated vascular model. Presented at the Texas Radiological Society meeting, San Antonio, USA, April 2016.
- 13.Mason JR and Benndorf G. Resolution limits of angiography—Fluoroscopic visualization thresholds in neuro-biplane systems: A comparison of Philips Allura with Clarity and Siemens Artis Zee. Presented at the American Society of Neuroradiology meeting, Long Beach, USA, April 2017.
- 14.Mason JR, Gal G and Benndorf G. Evaluation of visual control during fluoroscopic Onyx® (EVOH) injections using a novel calibrated vascular model. Presented at the American Society of Neuroradiology meeting, Chicago, USA, May 2015.
- 15.Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008; 25: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bracard S, Macho-Fernández J, Wang X, et al. Influence of temperature on embolisation with cyanoacrylate. Interv Neuroradiol 1998; 4: 301–305. [DOI] [PubMed] [Google Scholar]
- 17.Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: Long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 2011; 115: 78–88. [DOI] [PubMed] [Google Scholar]
- 18.Nogueira RG, Dabus G, Rabinov JD, et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. Am J Neuroradiol 2008; 29: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walcott BP, Gerrard JL, Nogueira RG, et al. Microsurgical retrieval of an endovascular microcatheter trapped during Onyx embolization of a cerebral arteriovenous malformation. J Neurointerv Surg 2011; 3: 77–79. [DOI] [PubMed] [Google Scholar]
- 20.Ayad M, Eskioglu E, Mericle RA. Onyx: A unique neuroembolic agent. Expert Rev Med Devices 2006; 3: 705–715. [DOI] [PubMed] [Google Scholar]
- 21.Vu PD, Grigorian AA. Microcatheter entrapment retrieval from Onyx embolization in brain arteriovenous malformations: A technical note. Interv Neuroradiol 2015; 21: 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maimon S, Strauss I, Frolov V, et al. Brain arteriovenous malformation treatment using a combination of Onyx and a new detachable tip microcatheter, SONIC: Short-term results. Am J Neuroradiol 2010; 31: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]