Abstract
Background
The relationship between bridging thrombolysis and femoral access site complications after mechanical thrombectomy remains contested. Use of a closure device could minimise bleeding complications. This study aimed to elucidate the rate of access site complications in a cohort of patients treated using an 8F groin sheath with subsequent closure using the Angio-Seal to assess safety and the impact of bridging thrombolysis on access site complication rate.
Methods
All patients with large vessel occlusive stroke treated between 2014 and 2017 with thrombectomy with or without bridging thrombolysis were reviewed. A prospectively acquired departmental database was used to obtain baseline data, and the radiology information and haematology reporting systems were used to record imaging or transfusion relating to subsequent access site complications.
Results
Seventy-five patients treated with thrombectomy alone were compared to 70 patients treated with prior intravenous thrombolysis. All had an 8F femoral sheath placed for arterial access, and all underwent attempted haemostasis with an 8F Angio-Seal. Two patients (1.14%) suffered Angio-Seal device failure necessitating manual pressure. One patient (0.6%) suffered a small femoral pseudo-aneurysm. No retroperitoneal haemorrhage, haematoma requiring transfusion, ipsilateral deep-vein thrombosis or ipsilateral acute limb ischaemia was encountered. There was no significant difference in the rate of haemorrhagic, ischaemic or infective complications between those treated with bridging thrombolysis or thrombectomy alone.
Conclusion
Use of the Angio-Seal closure device for 8F femoral access is safe in acute stroke patients. Intravenous thrombolysis prior to endovascular thrombectomy does not significantly alter femoral access site complication rate if this approach is used.
Keywords: Stroke, thrombectomy, access complication, haemorrhage, thrombolysis
Introduction
Following publication of multiple positive trials, endovascular mechanical thrombectomy (MT) is now the standard of care for treatment of proximal occlusive ischemic stroke.1–8 Use of MT after intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) in eligible patients is accepted proven practice, but increasingly, the additional benefit of thrombolysis is being questioned.9 Theoretically, rtPA could soften thrombus, aiding re-canalisation, or could treat distal emboli. However, proponents of a thrombectomy-only approach cite the potential increased risks of thrombus fragmentation and, importantly, increased risks of intracranial and systemic haemorrhage.9 Essentially, bridging thrombolysis may not add to efficacy and could compromise safety by increasing bleeding risks.
A cause of some debate is whether thrombolytics specifically increase femoral access site complications, with the potential for increased morbidity and mortality. Use of larger bore groin sheaths, including 8F and 9F sizes, is increasingly common to facilitate neuro-interventional procedures. This allows use of balloon guide catheters or long guide sheaths to support wide bore distal aspiration catheters. Both thrombolytics10 and larger sheaths11 theoretically increase the risk of access site bleeding. Use of a closure device could minimise bleeding complications, though this is controversial, and some studies suggest that use of such a device could potentiate the risk.12–14 Furthermore, closure devices can be complicated by infection or limb ischaemia.15,16
A number of recent trials have reported on haemorrhagic access site complications (though definitions varied) in 2–10.7% of cases.1,3,7,8 However, the impact of intravenous rtPA cannot be gleaned from these data. This study aimed to elucidate the overall rate of significant access site complications in a cohort of patients treated using an 8F groin sheath with subsequent closure using an 8F Angio-Seal closure device (Terumo) in the setting of acute stroke. The study set out to compare rates of access site complications for patients treated with thrombectomy alone versus combined or bridging intravenous thrombolysis and thrombectomy.
Methods
This study was approved by the local audit and clinical effectiveness committee. This was a single-centre, retrospective, observational study of patients with large vessel occlusive stroke treated at the authors’ institution between 2014 and 2017 with thrombectomy with or without rtPA. The investigation included a review of a prospectively acquired database of baseline clinical variables, including age, sex, stroke severity, prior use of anticoagulants, documented hypertension, diabetes mellitus, mode of anaesthesia and use of intravenous thrombolytics. In addition, a search of the institutional radiology information system was undertaken for each patient in order to record the use of ultrasound-guided femoral puncture and to confirm that no abdomino-pelvic computed tomography, ultrasound or angiographic procedures had been performed post thrombectomy. The admission haematocrit (% of patients with level <36%)17 and platelet levels <100,000/mm3 were also recorded,18 and the haematology reporting system was analysed to ensure that no blood products had been released for transfusion in the immediate post-procedure period.
The decision to withhold intravenous thrombolysis was made on an individual patient basis by the referring stroke neurologist following recognised ‘relative’ and ‘absolute’ contraindications, including time parameters, history of recent trauma/surgery, recent stroke or anticoagulant therapy.
All patients were treated in the acute setting for anterior or posterior circulation proximal occlusive stroke either directly or under supervision by a consultant interventional neuroradiologist. An 8F groin sheath (Merit Medical/Cordis USA) was used in all cases to facilitate use of a long guide sheath (6F 90 cm NeuronMax, Penumbra, Inc.) or balloon guide catheter (8F 95 cm FlowGate, Stryker/8F 95 cm Cello, Medtronic). Use of ultrasound for femoral access was principally based on operator preference. It is departmental policy not to use a heparin bolus, but all flush bags are loaded with heparin at 1000 IU/1000 mL. Intra-arterial rtPA was not used routinely to minimise risk of significant intracranial haemorrhage, particularly on the background of intravenous rtPA use.19 The decision to use general anaesthesia was based upon immediate preoperative assessment of patient agitation, the need for airway protection and whether challenging target vessel access and/or distal intracranial occlusion were suspected. If patients were compliant, procedures were performed under local anaesthetic. Conscious sedation was rarely used as an intermediate. All patients included in the primary study cohort were treated with an Angio-Seal closure device. These were inserted in line with the manufacturer’s instructions.20 Manual pressure was employed if the common femoral artery was assessed to be significantly diseased or if the puncture was at or below the common femoral bifurcation on an angiographic run of the common femoral artery at the end of the procedure. Antibiotics were not used specifically to cover the interventional procedure.
The population was dichotomised on the basis of prior treatment with intravenous rtPA. Rates of device failure necessitating manual pressure, femoral pseudo-aneurysm formation, retroperitoneal haemorrhage, groin haematoma requiring transfusion, acute limb ischaemia, localised abscess formation and ipsilateral iliofemoral deep-vein thrombosis (DVT) were recorded on the basis of subsequent follow-up imaging, as described above, or analysis of the haematology reporting system. Parametric data were compared using the t-test and non-parametric data using Fisher’s exact test employing GraphPad Prism statistical software (GraphPad Software, Inc.).
Results
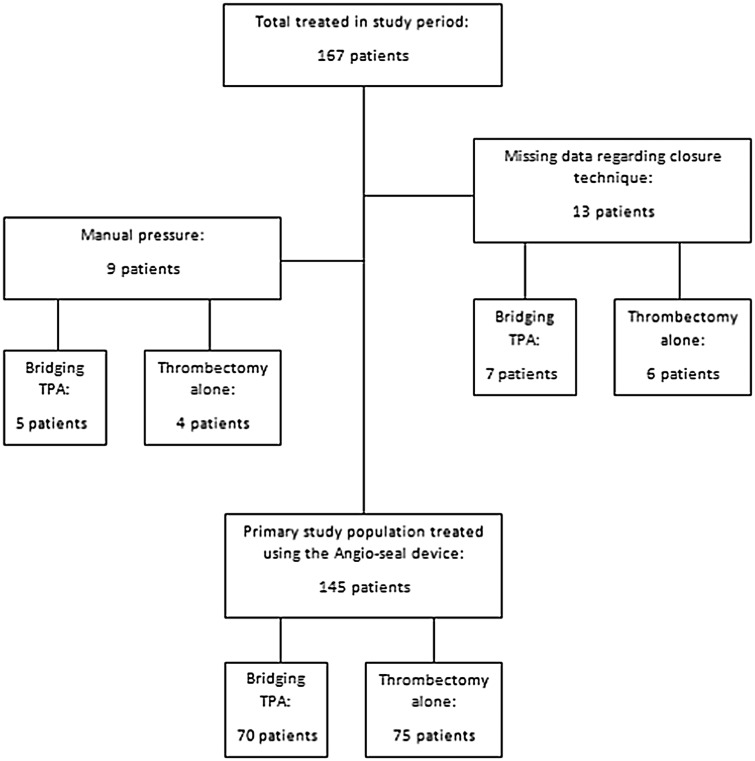
A total of 167 patients were treated during the study period. All had an 8F femoral sheath placed for arterial access. Data on closure device use were missing in six patients who underwent thrombectomy alone and seven patients who received bridging thrombolysis. No femoral complication was identified in this group. Five patients receiving bridging thrombolysis and four patients undergoing thrombectomy alone were treated with manual pressure following sheath removal and were not included from the primary study population, as they had not been treated with a closure device. One of these patients suffered a significant superficial haematoma with complicating hypotension during the procedure that was investigated and controlled intra-procedurally with pressure and required no further action. Seventy-five patients treated with thrombectomy alone were therefore compared to 70 patients treated with prior intravenous thrombolysis. A flow chart of treatment for all patients and the primary study population is provided in Figure 1. Baseline characteristics are displayed in Table 1.
Figure 1.
Patient selection for inclusion in the study.
Table 1.
Baseline characteristics (%, unless stated).
| Variable | Bridging therapy (n = 70) | Thrombectomy alone (n = 75) | p |
|---|---|---|---|
| Males | 33 (47.1) | 38 (50.7) | 0.740 |
| Age, mean (SD) | 69.4 (15.0) | 68.8 (13.8) | 0.845 |
| General anaesthesia | 23 (32.9) | 33 (44.0) | 0.173 |
| NIHSS | 18.32 (6.5) | 18.11 (7.6) | 0.805 |
| US-guided puncture | 43 (61.4) | 42 (56.0) | 0.613 |
| Plt < 100,000/mm3 | 0 | 0 | 1.000 |
| HCT < 36.0 | 19 (27.1) | 18 (24.0) | 0.706 |
| Anticoagulation | 1(1.4) | 16 (21.3) | <0.001 |
| Anti-platelets | 3 (4.2) | 4 (5.3) | 1.000 |
| Hypertension | 23 (32.9) | 25 (33.3) | 1.000 |
| Diabetes mellitus | 6 (8.6) | 5 (6.7) | 0.759 |
| Congestive heart failure | 4 (5.7) | 3 (4.0) | 0.712 |
| Atrial fibrillation | 8 (11.4) | 22 (29.3) | 0.004 |
NIHSS: National Institutes of Health Stroke Scale; US: ultrasound; Plt: platelets; HCT: haemocrit.
There was no significant difference between groups for age, sex, presentation National Institutes of Health Stroke Scale, use of ultrasound for femoral access, use of general anaesthesia, low haematocrit or low platelets. There were significantly more patients with a prior diagnosis of atrial fibrillation and anticoagulant use in the thrombectomy-only group. This is unsurprising, since patients with atrial fibrillation are routinely treated with anticoagulation, and anticoagulation is a contraindication to the use of intravenous thrombolysis.
Complications are summarised in Table 2. Two patients suffered Angio-Seal device failure necessitating manual pressure. One patient who had received bridging rtPA suffered a small femoral pseudo-aneurysm treated with thrombin injection. No retroperitoneal haemorrhage, haematoma requiring transfusion, ipsilateral DVT or ipsilateral acute limb ischaemia was encountered. One patient (who had undergone thrombectomy alone) developed a groin infection necessitating antibiotics and percutaneous drainage of a small collection. There was no significant difference in the rate of complications encountered at the puncture site for patients treated with thrombectomy alone versus those treated with combined intravenous thrombolysis and endovascular procedures. The overall incidence of clinically apparent pseudo-aneurysm formation in patients treated with a closure device in this setting was 0.6%.
Table 2.
Complications of femoral access.
| Complication | Bridging therapy (70) | Thrombectomy alone (75) | p |
|---|---|---|---|
| Device failure requiring manual pressure | 1 (1.4) | 1 (1.3) | 1.000 |
| Pseudo-aneurysm formation | 1 (1.4) | 0 | 0.483 |
| Infection | 0 | 1 (1.3) | 1.000 |
| Haematoma requiring transfusion | 0 | 0 | 1.000 |
| Acute limb ischaemia | 0 | 0 | 1.000 |
| Ipsilateral ileofemoral DVT | 0 | 0 | 1.000 |
DVT: deep-vein thrombosis.
Discussion
Femoral access site complications after endovascular procedures may significantly contribute to patient morbidity and mortality. The reported rate of access site complications encountered in prospective thrombectomy trials ranged from 2% to 10.7%, but definitions as to what constituted a complication varied.1,3,7,8 Use of thrombolytic agents, heparin or glycoprotein IIb/IIIa inhibitors, older age, obesity, low haematocrit, hypertension, multiple punctures, larger sheath size and use of arterial closure devices have been shown to increase risk of access site complications in the peripheral vascular and cardiology literature.10–12,21–25 Many of these risk factors are commonplace in the acute stroke population. Use of intravenous thrombolysis is routine pre thrombectomy, and this practice is supported by the results of multiple randomised trials.1–4 Use of intra-arterial thrombolytics is more controversial and could be associated with higher rates of symptomatic intracranial haemorrhage, especially if used in conjunction with intravenous thrombolysis.19 Peripheral bleeding complications could also be potentiated.26 Neuro-interventional practice is moving towards the use of wider bore groin sheaths (8F or even 9F) to facilitate use of balloon guide catheters or long sheaths with distal aspiration catheters in order to improve re-canalisation rates, but in this context, there is a potential trade-off, as some of the benefit could theoretically be counteracted by procedural complications.
This retrospective observational study suggests that use of intravenous thrombolysis prior to endovascular thrombectomy via a large bore (8F) femoral sheath with subsequent closure using the Angio-Seal device does not significantly alter haemorrhagic complications. The overall rate of pseudo-aneurysm formation was 0.6%, despite high rates of thrombolysis use and anticoagulation. No retroperitoneal haematoma was encountered. This benefit is not offset by complicating ischaemia, though one infection was encountered (0.6%). These results are of significance, as there is little evidence for a standardised approach to managing femoral access sites in the context of acute stroke post thrombolysis when using an 8F femoral sheath, and the low complication rate would suggest that this is a safe approach for managing femoral access in acute stroke. Similarly, in post hoc analysis of STAR and SWIFT trials, Coutinho et al.27 demonstrated similar rates of groin haematoma between thrombectomy-only and combined thrombolysis and thrombectomy groups (1.9 vs. 1.5%). In a retrospective single-centre study, Weber et al. also noted no significant difference in the rate of groin haematoma formation (1.9% vs. 1.4%).28 However, it is not clear how the access sites were managed in these studies. In a large series of 472 patients, Shah et al.29 managed the femoral puncture site in a similar way to that seen in this study, with an 8F or 9F sheath site closed using a collagen or suture-based device in 98%. Only two definite groin complications were identified. Of this group, 156 patients were administered intravenous rtPA, and none of these patients was found to have a significant groin complication.
In an attempt to circumnavigate the risk of bleeding complications, it is practice in some institutions to leave the femoral sheath in situ for a protracted time period prior to removal in order to allow rtPA decay and minimise bleeding complications.30 This approach could conceivably be complicated by femoral clot formation and limb ischaemia, infection and longer periods of patient immobility. In one study, a femoral sheath clot was seen to form in 78% of cases where the sheath was left in situ overnight, despite use of a heparinised flush.31
Use of manual compression is another approach, but evidence for this approach following thrombolytic administration and use of a large bore sheath is lacking. Within the current study, 9/167 patients were treated in this way because of a perceived increased risk of complicating limb ischemia if employing a closure device due to puncture location or vessel disease. One patient was specifically managed with pressure because of intra-procedural haematoma formation around the sheath, but no other complications were recognised. The inability to use a closure device due to too low a puncture does, however, highlight the utility of ultrasound guidance in this setting.
Specific to stroke patients undergoing general anaesthesia, extubation can lead to Valsalva and patient agitation, which can result in further haemorrhage. The use of a closure device also obviates the need for a prolonged period of bed rest and graded mobilisation which can be difficult to achieve in patients who may be confused and disoriented secondary to their recent neurological insult. A direct comparison of manual compression with an Angio-Seal closure device in neuro-interventional practice was undertaken by Wong et al. who found that patients treated with Angio-Seal more rapidly achieved haemostasis than those treated with manual compression, with less time needed for haemostasis, less local haematoma formation (8.6% vs. 25.7%) and an acceptably low level of complications in patients with prolonged elevated activated clotting time (range 250–500 seconds).16 For the manual compression group, the mean time to haemostasis was 44.7 minutes. The evidence for closure device over manual compression in the cardiology literature is conflicting, with smaller studies often reporting higher complication rates with closure devices.12–14 However, in a very large multi-centre analysis commissioned by the Food and Drug Administration32 of >166,000 patients from the American College of Cardiology’s national cardiovascular data registry, both collagen- and suture-based devices showed a decrease in total complications of 21% and 15%, respectively. To the authors’ knowledge, there has been no comparison of manual compression with a closure device in the setting of bridging thrombolysis therapy with large bore access sheath use. Conceivably, the risk of bleeding in this population is higher with manual compression alone.
Closure devices are, however, not without complications, and this study is too small to describe fully the rates of potential problems encountered specifically using the Angio-Seal. Applegate et al.33 summarised the complications of this device in 3898 patients. This retrospective study demonstrated a minor complication (haematoma, arteriovenous fistula or pseudo-aneurysm) rate of 0.8% and a major complication (vascular death, vascular repair, vessel occlusion, or bleeding with >3 g haemoglobin drop) rate of 0.6%. Recently, Angio-Seal failure has been found to be associated with low body mass index (<21 kg/m2) or a skin to artery depth of <11 mm.34
An alternative approach that has been employed in the setting of acute stroke is trans-radial access.35 In addition to providing access in cases with challenging vascular anatomy when the trans-femoral approach is not feasible, a potential benefit of the trans-radial approach is a reduction in haemorrhagic and ischemic complications associated with femoral arteriotomy closure. It is commonplace for a short 6F sheath to be inserted into the radial artery after testing for ulnar collateral flow.35 However, this does limit the size of the guide systems that can be used and does not facilitate easy use of distal aspiration catheters or the current generation of balloon guide catheters. The latter are likely to improve first pass re-canalisation36 which significantly impacts on favourable clinical outcome,37 suggesting that for most cases, at least for the time being, the femoral route will be the first line in acute stroke therapy.
This was a relatively small retrospective single-centre study. Complications were defined based on re-imaging or blood transfusion which should capture those lesions that were symptomatic but could have missed smaller less clinically identifiable lesions, specifically small haematomas. However, the relevance of identifying these is debatable. The study is of insufficient power to elucidate fully the impact of rtPA on femoral access site complications, but the results do infer that the approach to treating post-thrombolysis patients with large bore sheaths and Angio-Seal closure is safe.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, et al. A randomised trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 371: 11–20. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 9.Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke 2017; 48: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 10.Ouriel K, Gray B, Clair DG, et al. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol 2000; 11: 295–298. [DOI] [PubMed] [Google Scholar]
- 11.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1: 202–209. [DOI] [PubMed] [Google Scholar]
- 12.Dangas G, Mehran R, Kokolis S, et al. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J Am Coll Cardiol 2001; 38: 638–641. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoch F, Neumann FJ, Theiss W, et al. Efficacy and safety of collagen implants for haemostasis of the vascular access site after coronary balloon angioplasty and coronary stent implantation: a randomized study. Eur Heart J 1995; 16: 640–646. [DOI] [PubMed] [Google Scholar]
- 14.Shrake KL. Comparison of major complication rates associated with four methods of arterial closure. Am J Cardiol 2000; 85: 1024–1025. [DOI] [PubMed] [Google Scholar]
- 15.Johanning JM, Franklin DP, Elmore JR, et al. Femoral artery infections associated with percutaneous arterial closure devices. J Vasc Surg 2001; 34: 983–985. [DOI] [PubMed] [Google Scholar]
- 16.Wong H-F, Lee C-W, Chen Y-L, et al. Prospective comparison of Angio-Seal versus manual compression for hemostasis after neurointerventional procedures under systemic heparinization. Am J Neuroradiol 2013; 34: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non–ST-segment elevation myocardial infarction: the CRUSADE bleeding score. Circulation 2009; 119: 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mowla A, Kamal H, Lail NS, et al. Intravenous thrombolysis for acute ischemic stroke in patients with thrombocytopenia. J Stroke Cerebrovasc Dis 2017; 26: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 19.Singer OC, Berkefeld J, Lorenz MW, et al. Risk of symptomatic intracerebral hemorrhage in patients treated with intra-arterial thrombolysis. Cerebrovasc Dis 2009; 27: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angio-Seal™ VIP vascular closure device, www.terumois.com/products/closure/angio-seal-vascular-closure-devices/angio-seal.html (accessed 1 March 2018).
- 21.Andersen K, Bregendahl M, Kaestel H, et al. Haematoma after coronary angiography and percutaneous coronary intervention via the femoral artery frequency and risk factors. Eur J Cardiovasc Nurs 2005; 4: 123–127. [DOI] [PubMed] [Google Scholar]
- 22.Piper WD, Malenka DJ, Ryan TJ, et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J 2003; 145: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 23.Berry C, Kelly J, Cobbe SM, et al. Comparison of femoral bleeding complications after coronary angiography versus percutaneous coronary intervention. Am J Cardiol 2004; 94: 361–363. [DOI] [PubMed] [Google Scholar]
- 24.Hamner JB, Dubois EJ, Rice TP. Predictors of complications associated with closure devices after transfemoral percutaneous coronary procedures. Crit Care Nurse 2005; 25: 30–32, 34–37. [PubMed] [Google Scholar]
- 25.Waksman R, King SB, Douglas JS, et al. Predictors of groin complications after balloon and new-device coronary intervention. Am J Cardiol 1995; 75: 886–889. [DOI] [PubMed] [Google Scholar]
- 26.The IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007; 38: 2127–2135. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho JM, Liebeskind DS, Slater L-A, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR studies. JAMA Neurol 2017; 74: 268–274. [DOI] [PubMed] [Google Scholar]
- 28.Weber R, Nordmeyer H, Hadisurya J, et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerventional Surg 2017; 9: 229–233. [DOI] [PubMed] [Google Scholar]
- 29.Shah VA, Martin CO, Hawkins AM, et al. Groin complications in endovascular mechanical thrombectomy for acute ischemic stroke: a 10-year single center experience. J Neurointerventional Surg 2016; 8: 568–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierot L, Herbreteau D, Bracard S, et al. An evaluation of immediate sheath removal and use of the Angio-Seal vascular closure device in neuroradiological interventions. Neuroradiology 2006; 48: 45–49. [DOI] [PubMed] [Google Scholar]
- 31.Koenigsberg RA, Wysoki M, Weiss J, et al. Risk of clot formation in femoral arterial sheaths maintained overnight for neuroangiographic procedures. AJNR Am J Neuroradiol 1999; 20: 297–299. [PMC free article] [PubMed] [Google Scholar]
- 32.Tavris DR, Gallauresi BA, Lin B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol 2004; 16: 459–464. [PubMed] [Google Scholar]
- 33.Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Intervent Cardiol 2006; 19: 67–74. [DOI] [PubMed] [Google Scholar]
- 34.Aida Y, Misaki K, Kamide T, et al. Physical risk factors of hemorrhagic complications associated with Angio-Seal closure device use in neurointerventional procedures. World Neurosurg 2018; 111: e850–855. [DOI] [PubMed] [Google Scholar]
- 35.Sur S, Snelling B, Khandelwal P, et al. Transradial approach for mechanical thrombectomy in anterior circulation large-vessel occlusion. Neurosurg Focus 2017; 42: e13. [DOI] [PubMed] [Google Scholar]
- 36.Brinjikji W, Starke RM, Murad MH, et al. Impact of balloon guide catheter on technical and clinical outcomes: a systematic review and meta-analysis. J Neurointerv Surg 2018; 10: 335–339. [DOI] [PubMed] [Google Scholar]
- 37.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]