Abstract
Objective
Embolism due to coagulopathy might be the main pathomechanism underlying cancer-related stroke (CRS). CRS patients with a large artery occlusion could be candidates for endovascular recanalization therapy (ERT), although its procedural and clinical outcomes are not well known. This study aimed to investigate the procedural and clinical outcomes of ERT in CRS patients and the characteristics associated with outcomes compared with those of conventional stroke patients.
Methods
A registry of consecutive acute ischemic stroke patients who underwent ERT between January 2011 and October 2015 was retrospectively reviewed. CRS patients are described as those who had (a) cryptogenic stroke with advanced or metastatic cancer; (b) no other possible causes of stroke such as cardioembolism (CE) and large artery atherosclerosis (LAA); and (c) elevated D-dimer levels or diffusion-restricted lesions in multiple vascular territories. We compared procedural and clinical outcomes at discharge among CRS, CE, and LAA patients.
Results
A total of 329 patients were finally enrolled in this study; of these, 19 were CRS patients. The rate of successful recanalization, defined as modified treatment in cerebral infarction grade 2b or 3, was lower in the CRS group than in the LAA and CE groups (63% versus 84% versus 84%, p = .06). CRS subtype was an independent predictor for successful recanalization after ERT in the multivariate analysis (odds ratio, 0.317; 95% confidence interval, 0.116–0.867; p < .001). No significant difference in the rate of good clinical outcomes at discharge was observed among groups.
Conclusions
Although clinical outcomes at discharge were similar for CE and LAA patients, complete recanalization seemed more difficult to achieve in CRS patients than in conventional stroke patients.
Keywords: Cancer, stroke, thrombectomy, recanalization
Introduction
Ischemic stroke commonly occurs in the elderly with systemic cancer. About 15% of all cancer patients have been known to have cerebrovascular disease during their clinical course.1 The pathomechanism of ischemic stroke can be heterogeneous, and ischemic strokes with conventional stroke mechanisms commonly occur in cancer patients. Cancer-specific stroke mechanisms are related to coagulopathy by tumor cell-derived cytokines or microparticles. Elevated D-dimer levels (>1.11 µg/mL) and multiple lesions in multiple vascular territories are known to be associated with cancer-related coagulopathy.2
A previous population study showed that intravenous thrombolysis for acute ischemic stroke (AIS) in cancer patients was not associated with an increased risk of intracerebral hemorrhage or in-hospital mortality.3 However, it is often contraindicated in this population because of thrombocytopenia, coagulopathy, or recent surgery. Endovascular recanalization therapy (ERT) using stent retrievers is a pivotal option for stroke patients with a large artery occlusion, and its efficacy has been proven in several clinical trials and a meta-analysis.4 ERT could be a suitable treatment for AIS patients with cancer-specific mechanisms. However, because most clinical trials excluded these patients, the procedural and clinical outcomes of ERT using stent retrievers in patients with a large artery occlusion due to cancer-specific mechanisms are unknown.
This study aimed to investigate the procedural and clinical outcomes of ERT for cancer-related stroke (CRS) and the characteristics associated with outcomes in comparison with those for conventional stroke mechanisms such as large artery atherosclerosis (LAA) and cardioembolism (CE).
Methods
Study population
This study was retrospectively performed with the approval of our institutional review board (approval no. B-1704-390-112), and the need for obtaining informed consent from patients or their legal representatives was waived.
We screened consecutive AIS patients who underwent ERT at Seoul National University Bundang Hospital from January 2011 to October 2015. A total of 401 patients were treated by ERT in a medical center. Clinical and radiologic data were reviewed. Clinical data included demographic information, time of stroke onset or last normal time since hospital arrival, and baseline National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores at discharge and 3 months after discharge. Computed tomography (CT) and/or magnetic resonance imaging (MRI) data acquired before ERT were also scrutinized. All patients underwent MRI to select subjects eligible for ERT, with the exception of those with MRI contraindications who instead underwent multimodal CT scans (noncontrast CT, CT angiography, and perfusion CT). Angiograms were analyzed to identify the occlusion’s location and reperfusion status after ERT.
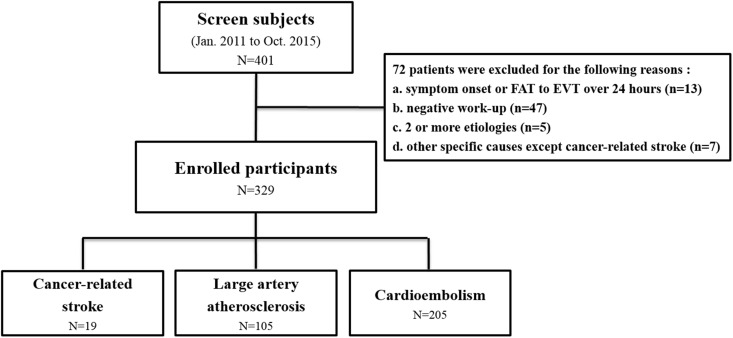
Patients were excluded from the analysis if they had (a) time of symptom onset or first abnormal time since hospital arrival of >24 h (n = 13); (b) an undetermined negative workup (n = 47); (c) two or more undetermined etiologies (n = 5); or (d) other specific etiologies except for CRS (n = 7) (Figure 1). Stroke subtypes were categorized using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification.5 CRS patients referred to those who had (a) cryptogenic stroke with advanced or metastatic cancer at the time of stroke onset; and (b) elevated D-dimer levels (>1.11 µg/mL) and/or diffusion-restricted lesions in multiple vascular territories. The involvement of multiple vascular territories was defined as multiple ischemic lesions in the unilateral anterior and posterior circulation, bilateral anterior circulation, or bilateral anterior and posterior circulation.6 A representative case with multiple acute ischemic lesions in multiple vascular territories is shown in Figure 2.
Figure 1.
Flow sheet showing the study design and patient exclusion criteria.
EVT: emergency room visit time; FAT: first abnormal time.
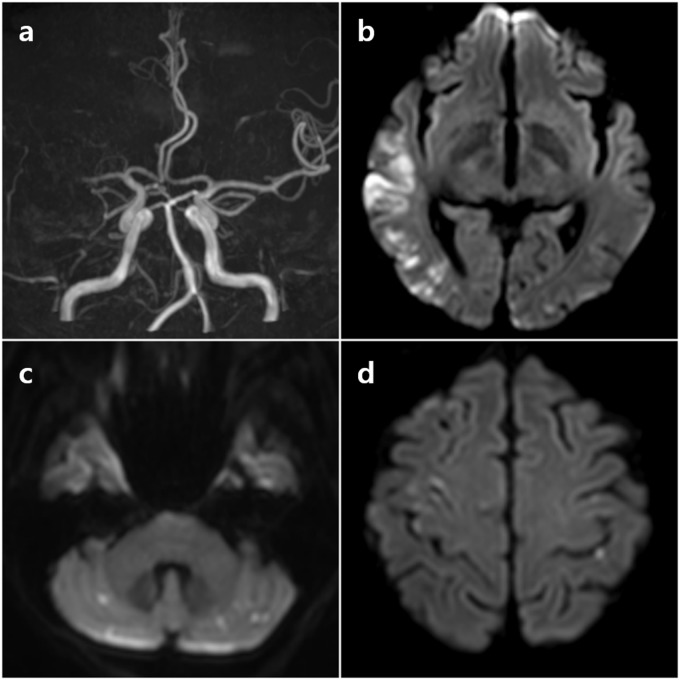
Figure 2.
Representative imaging findings of patient with cancer-related stroke. Intracranial magnetic resonance angiography showed an occlusion in the right middle cerebral artery (a). Diffusion-weighted images showed diffusion-restricted lesions in the multiple vascular territories (b–d).
Intra-arterial thrombolysis and technical strategy
All procedures were performed using a femoral artery approach under local anesthesia. A 6-Fr coaxial guiding system (Shuttle SL Flexor, Cook Medical, Bloomington, Indiana, USA, and Envoy 5-Fr/6-Fr, Cordis, Miami Lakes, Florida, USA) or a 9-Fr balloon guide catheter (Optimo, Tokai Medical Products Inc., Japan, or Cello, Medtronic, Irvine, California, USA) was introduced through a femoral sheath into the affected extracranial artery. A balloon guide catheter was routinely used unless technically unfeasible, and a heparinized saline solution was continuously perfused through the catheter during the procedure.
Mechanical thrombectomy using stent retrievers (Solitaire FR, ev3 Inc., Irvine, California, USA, or Trevo, Concentric Medical Inc., Mountain View, California, USA) was usually attempted as the first-line treatment. Forced-suction thrombectomy using a Penumbra System (PS) reperfusion catheter (Penumbra Inc., Alameda, California, USA) was sometimes attempted initially at the discretion of the treating neurointerventionalist or secondarily when the initial therapy failed. Forced-suction thrombectomy using a PS reperfusion catheter without a separator was performed by employing the technique described by Kang et al.7 If mechanical thrombectomy failed, intra-arterial (IA) fibrinolysis, mechanical disruption, and permanent stenting were additionally considered as rescue techniques.
Imaging analysis
Acute stroke MRI was performed for all ERT candidates using 1.5-T or 3.0-T MRI units (Gyroscan, Achieva, or Ingenia, Philips Medical Systems, Best, Netherlands), and the protocol consisted of diffusion-weighted imaging (DWI), T2*-weighted gradient echo (GRE) imaging, three-dimensional time-of-flight magnetic resonance angiography, and perfusion imaging.
Susceptibility vessel sign (SVS) was defined as a blooming hypointense signal on GRE imaging within the corresponding symptomatic occluded artery. The hypointense signal diameter should exceed the contralateral vessel diameter.8,9
Outcome measurement
Clinical outcome measurement at discharge included assessment of mortality and disability according to the mRS score. We dichotomized patients into groups with good (mRS score ≤ 2) and poor (mRS score > 2) clinical outcomes at discharge and 3 months after stroke. The rate of successful recanalization was defined as modified treatment in cerebral infarction (mTICI) grade 2b or 3.10 Recanalization time was defined as the interval between groin puncture and the final reperfusion with mTICI ≥ grade 2a.
Statistical analysis
The baseline characteristics of CRS, LAA, and CE groups were compared using the Kruskal–Wallis H test for continuous variables and Pearson chi-squared test for categorical variables. When a significant difference was detected by the Kruskal–Wallis H test, multiple comparisons among the groups with different etiologies using the Mann–Whitney U test for continuous variables and chi-squared test or Fisher’s exact test for categorical variables were used to identify the source of the difference. Multivariate analyses were performed to identify predictors for successful recanalization and good clinical outcomes at discharge. Statistical significance was set at 2-tailed p < .05. We presented the values as frequencies (percentages), means (standard deviations), or medians (interquartile ranges), as deemed appropriate. All statistical analyses were performed using Stata/SE 13.0 (StataCorp, College Station, Texas, USA).
Results
Excluding the 72 patients who met the exclusion criteria, 329 patients were finally enrolled in this study. Of these patients, 19 comprised the CRS group, and 105 and 205 belonged to the LAA and CE groups, respectively, according to the TOAST classification (Figure 1). All patients in the CRS group had a large artery occlusion in the anterior circulation. Diffusion-restricted lesions in multiple vascular territories were observed in 10 patients (53%). D-dimer levels were elevated in all patients in the CRS group except for two patients who were not examined and had diffusion-restricted lesions in multiple vascular territories on DWI. The most common location for primary cancer was the lung (37%) in the CRS group, followed by the gallbladder (26%). Histologically, 16 patients (84%) had adenocarcinoma. No patient in the CRS group interestingly displayed an SVS on T2*-weighted GRE imaging. Seventeen patients in the CRS group had stage IV cancer at the time of stroke onset. Five patients underwent chemotherapy within a month, with four patients treated with drugs that could increase the risk of ischemic stroke (cisplatin in two patients, fluorouracil in two patients).
There were significant differences in baseline characteristics between the CRS and conventional stroke groups. Median age, male gender, baseline NIHSS score, history of hypertension, atrial fibrillation, and current smoking were significantly different among the three groups (Table 1). The proportion of males and patients with a history of hypertension was significantly lower in the CRS group than in the LAA group. The proportion of patients treated with intravenous recombinant tissue plasminogen activator was significantly lower in the CRS group than in the CE group (Table 1 and Supplementary Table 1). All patients in the CRS group had an occlusion in the anterior circulation, whereas 84% and 90% of patients in the LAA and CE groups, respectively, had an occlusion in the anterior circulation. The detailed occlusion sites in the anterior circulation are summarized in Supplementary Table 2. Occlusions in the proximal M2 segment were more prevalent in the CRS group than in the conventional stroke groups.
Table 1.
Baseline characteristics of participants grouped by stroke etiologies.
| CRS (n = 19) | LAA (n = 105) | CE (n = 205) | p value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 69 (58–75)a,b | 69 (64–76)a | 73 (65–79)b | 0.003 |
| Male, n (%) | 9 (47)a | 76 (72)b | 96 (47)a | <0.001 |
| Initial NIHSS, median (IQR) | 16 (6–20)a,b | 12 (7–17)a | 15 (10–20)b | 0.004 |
| Occlusion in the anterior circulation, n (%) | 19 (100) | 88 (84) | 184 (90) | 0.081 |
| Risk factors, n (%) | ||||
| Hypertension | 9 (47)a | 81 (77)b | 123 (60)a | 0.003 |
| Diabetes | 3 (16) | 36 (34) | 56 (27) | 0.190 |
| Dyslipidemia | 4 (21) | 22 (21) | 39 (19) | 0.912 |
| Ischemic heart disease | 1 (5) | 13 (12) | 31 (15) | 0.438 |
| Previous stroke | 4 (21) | 16 (15) | 44 (22) | 0.417 |
| Atrial fibrillation | 0a | 2 (1.9)a | 182 (89)b | <0.001 |
| Current smoking | 5 (26)a,b | 35 (33)a | 25 (12)b | <0.001 |
| Time from LNT to hospital arrival (hours), median (IQR) | 3.5 (1–7)a,b | 4.8 (1.5–11)a | 1.9 (0.9–5.7)b | <0.001 |
| Clear onset, n (%) | 12 (63) | 68 (65) | 149 (73) | 0.293 |
| Time from hospital arrival to groin puncture (hours), median (IQR) | 1.5 (1.2–1.9) | 1.6 (1.2–2) | 1.5 (1.2–1.8) | 0.292 |
| Use of IV fibrinolysis, n (%) | 3 (17)a | 40 (38)a | 114 (56)b | <0.001 |
Note: Between-group comparisons were performed with Kruskal–Wallis H test for continuous variables and with Pearson chi-squared test for categorical variables. When a significant difference was detected by the Kruskal–Wallis H test, multiple comparisons between the groups with different etiology using a Mann–Whitney U test for continuous variables and a chi-squared test or a Fisher’s exact test for categorical variables were used to identify the source of the difference. Values not sharing the same superscripts a, b, and c are significantly different at p < 0.05.
CE: cardioembolism; CRS: cancer-related stroke; IQR: interquartile range; IV: intravenous; LAA: large artery atherosclerosis; LNT: last normal time; NIHSS: National Institutes of Health Stroke Scale.
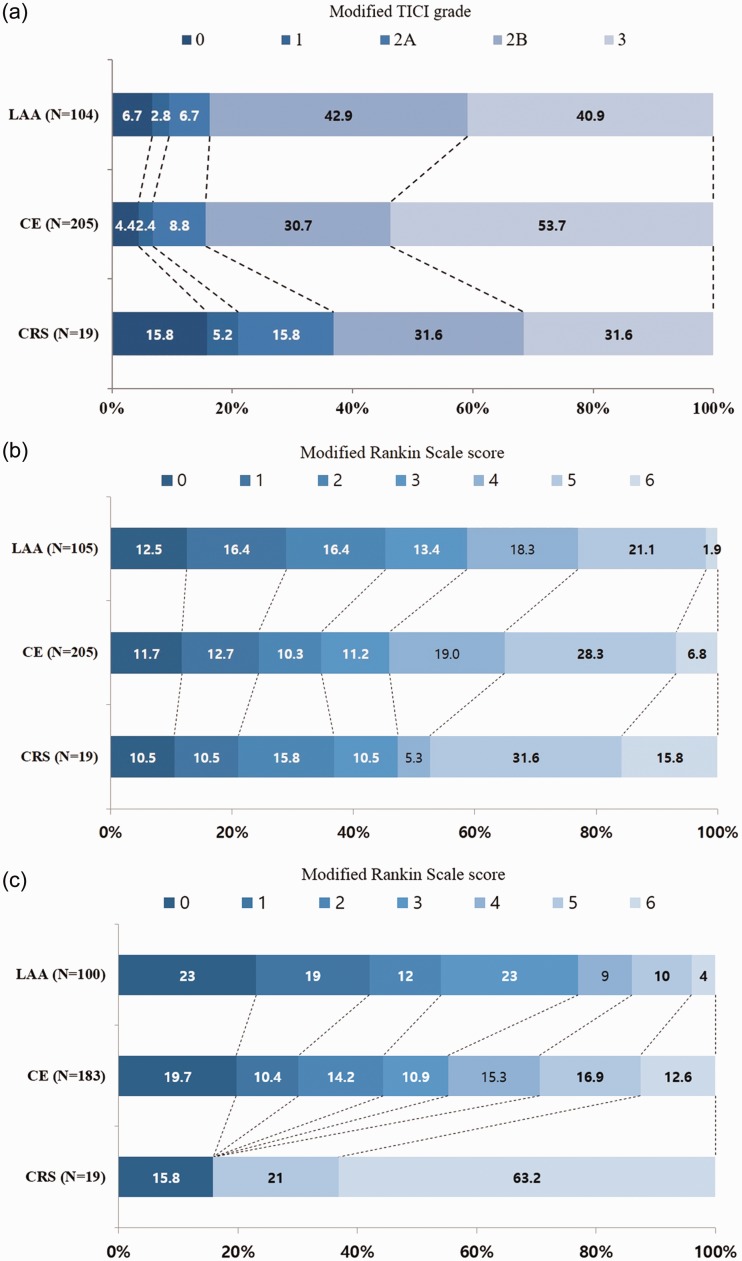
The interval between groin puncture and recanalization after ERT did not significantly differ between the CRS and conventional stroke groups. In ERT, the use of IA fibrinolysis was more frequent in the LAA group than in the CRS and CE groups (54% versus 21% versus 28%, p < .001). Rescue techniques except for IA fibrinolysis were more frequently used in the LAA group than in the CE and CRS groups (23% versus 11% versus 5.3%, p = .06), although with no statistical difference between the LAA and CRS groups (Table 2 and Supplementary Table 1). The rate of successful recanalization was lower in the CRS group than in the LAA and CE groups (63% versus 84% versus 84%, p = .060) (Figure 3(a)). The differences in the rate of successful recanalization were especially significant when comparing CRS and each conventional stroke mechanism (CRS versus LAA, 63% versus 84%, p = .036; CRS versus CE, 63% versus 84%, p = .02) (Supplementary Table 1). No statistically significant difference in the rate of good clinical outcomes (mRS score 0–2) at discharge was observed among the groups (CRS versus LAA versus CE, 37% versus 45% versus 35%, p = .194) (Figure 3(b)). However, the mortality rate at discharge was higher in the CRS group than in the LAA and CE groups (16% versus 1.9% versus 6.8%, p = .033). Three patients in the CRS group died at discharge. The causes of death were symptomatic hemorrhagic transformation or malignant infarction in two patients and sepsis in one patient. The rate of good clinical outcomes at 3 months after stroke was extremely low in the CRS group, with only three patients (16%) having good clinical outcomes at 3 months after stroke (Table 2 and Figure 3(c)). Twelve patients (63%) in the CRS group died at 3 months after stroke. Except for three patients who died at discharge, six patients died of nonvascular causes (two, sepsis; three, pneumonia; and one, acute renal failure). We did not confirm the causes of death for the remaining three patients. Four patients exhibited hemorrhagic transformation on follow-up imaging after ERT, but only one patient had a symptomatic intracranial hemorrhage (5.3%) in the CRS group.
Table 2.
Results of endovascular recanalization therapy in participants grouped by stroke etiologies.
| CRS (n = 19) | LAA (n = 105) | CE (n = 205) | p value | |
|---|---|---|---|---|
| Time from groin puncture to recanalization (hours), median (IQR) | 0.5 (0.3–1.1) | 0.8 (0.3–1.0) | 0.5 (0.3–0.9) | 0.098 |
| ERT strategies, n (%) | ||||
| Mechanical thrombectomy | 19 (100) | 99 (94) | 197 (96) | 0.483 |
| IA fibrinolysis | 4 (21)a | 57 (54)b | 58 (28)a | <0.001 |
| Rescue techniques except fibrinolysis | 1 (5.3)a,b | 24 (23)a | 23 (11)b | 0.011 |
| TICI 2b or 3, n (%) | 12 (63)a | 88 (84)b | 173 (84)b | 0.060 |
| mRS 0–2 at discharge, n (%) | 7 (37) | 47 (45) | 71 (35) | 0.194 |
| Death at discharge, n (%) | 3 (16)a | 2 (1.9)b | 14 (6.8)a,b | 0.033 |
| Outcomes at 3 months after stroke | n = 19 | n = 100 | n = 183 | |
| mRS 0–2 at 3 months, n (%) | 3 (16)a | 54 (54)b | 81 (44)b | 0.008 |
| Death at 3 months, n (%) | 12 (63)a | 4 (4.0)b | 23 (13)c | <0.001 |
Note: Rescue techniques except fibrinolysis included mechanical disruption using wire or balloon, balloon angioplasty and/or permanent stenting. Between-group comparisons were performed with Kruskal–Wallis H test for continuous variables and with Pearson chi-squared test for categorical variables. When a significant difference was detected by the Kruskal–Wallis H test, multiple comparisons between the groups with different etiology using a Mann–Whitney U test for continuous variables and a chi-squared test or a Fisher’s exact test for categorical variables were used to identify the source of the difference. Values not sharing the same superscripts a, b, and c are significantly different at p < 0.05.
CE: cardioembolism; CRS: cancer-related stroke; ERT: endovascular recanalization therapy; IA: intra-arterial; IQR: interquartile range; IV: intravenous; LAA: large artery atherosclerosis; mRS: modified Rankin Scale score; TICI: treatment in cerebral infarction.
Figure 3.
Differences in the modified TICI grade (a) and modified Rankin Scale Score at discharge (b) and 3 months after stroke onset (c) according to the stroke etiologies.
CE: cardioembolism; CRS: cancer-related stroke; LAA: large artery atherosclerosis; TICI: treatment in cerebral infarction.
CRS subtype (adjusted odds ratio (aOR), 0.238; 95% confidence interval (95% CI), 0.082–0.692; p = .008) was an independent predictor for successful recanalization after ERT in the multivariate analysis compared with CE stroke as the reference and current smoking (aOR, 3.492; 95% CI, 1.130–10.795; p = .03). Baseline NIHSS score (aOR, 0.809; 95% CI, 0.765–0.857; p < .001), current smoking (aOR, 3.832; 95% CI, 1.655–8.875; p = .002), time of stroke onset or last normal time since hospital arrival (aOR, 0.999; 95% CI, 0.998–0.9999; p = .042), and interval between groin puncture and recanalization (aOR, 0.985; 95% CI, 0.975–0.995; p = .004) were independent predictors for good clinical outcomes at discharge. Stroke subtypes were not significantly associated with clinical outcomes at discharge (Table 3 and Supplementary Table 3).
Table 3.
Predictors for successful recanalization with modified TICI 2b or 3 and good functional outcome at discharge.
| Dichotomized outcome analysis |
||||
|---|---|---|---|---|
| Crude OR (95% CI) | p | Adjusted OR | p | |
| Predictors for successful recanalization | ||||
| Age, per 1 year increase | 0.979 (0.953–1.001) | 0.124 | 0.981 (0.953–1.010) | 0.205 |
| Male gender | 1.805 (1.009–3.227) | 0.046 | 1.333 (0.704–2.524) | 0.377 |
| Current smoking | 3.741 (1.301–10.755) | 0.014 | 3.492 (1.130–10.795) | 0.030 |
| Stroke subtypes | ||||
| Cardioembolism | Reference | Reference | ||
| Large artery atherosclerosis | 0.957 (0.504–1.819) | 0.894 | 0.685 (0.344–1.361) | 0.280 |
| Cancer-related stroke | 0.317 (0.116–0.867) | <0.001 | 0.238 (0.082–0.692) | 0.008 |
| Predictors for good clinical outcome at discharge | ||||
| Age, per 1 year increase | 0.948 (0.927–0.969) | <0.001 | 0.979 (0.951–1.009) | 0.172 |
| Male gender | 2.043 (1.289–3.238) | 0.002 | 0.982 (0.508–1.898) | 0.957 |
| History of hypertension | 0.578 (0.364–0.919) | 0.020 | 0.695 (0.361–1.341) | 0.277 |
| Current smoking | 4.163 (2.337–7.418) | <0.001 | 3.832 (1.655–8.875) | 0.002 |
| Baseline NIHSS, per 1 point increase | 0.828 (0.791–0.867) | <0.001 | 0.809 (0.765–0.857) | <0.001 |
| Time from stroke onset or LNT to hospital arrival | 0.999 (0.998–0.9999) | 0.024 | 0.999 (0.998–0.9999) | 0.042 |
| Time from groin puncture to recanalization | 0.988 (0.987–0.995) | 0.001 | 0.985 (0.975–0.995) | 0.004 |
| Stroke subtypes | ||||
| Cardioembolism | Reference | Reference | ||
| Large artery atherosclerosis | 1.556 (0.961–2.519) | 0.072 | 1.125 (0.553–2.289) | 0.745 |
| Cancer-related stroke | 1.101 (0.415–2.921) | 0.847 | 0.839 (0.189–3.734) | 0.819 |
CI: confidence interval; LNT: last normal time; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; TICI: treatment in cerebral infarction.
Discussion
To our knowledge, this is the largest series that assessed the efficacy of ERT in CRS patients compared with that in conventional stroke patients. Two AIS patients with active systemic cancer who were successfully treated with ERT using stent retrievers were reported.11 However, the reports did not describe the efficacy of ERT in CRS patients with that in conventional stroke patients. In our series, the rate of successful recanalization (mTICI grade 2b or 3) in the CRS group was 63% and was lower than that in the LAA and CE groups (84% and 84%, respectively). The difference in procedural outcome was also significant in the multivariate analysis with CE stroke as the reference (aOR, 0.238; 95% CI, 0.082–0.692; p = .008). The rate of good clinical outcomes at discharge in the CRS group was similar to that in the CE and LAA groups despite their high mortality rate at 3 months after stroke.
CRS stroke mechanism
Stroke in cancer patients can be either related or not related to cancer-specific mechanisms. CRS patients exhibited a lower prevalence of some important vascular risk factors such as hypertension, diabetes, and atrial fibrillation than conventional stroke patients (Table 1), suggesting that CRS has cancer-specific mechanisms that differ from conventional ones. Ischemic stroke in cancer patients could occur as a result of various etiologies such as coagulation disorders induced by cancer or cancer therapy, direct spread of tumor emboli, vascular invasion, and radiation-induced vascular injury.9 Previous studies have shown that embolism due to cancer-related coagulopathy could be the main pathomechanism in stroke development.12,13 There was no evidence of direct spread of tumor emboli, direct vascular invasion, or radiation-induced vascular injury in our series. Some chemotherapeutic drugs (most commonly cisplatin but also methotrexate, fluorouracil, and L-asparaginase) increase the risk of stroke. In a retrospective study that followed up 10,963 cancer patients for 1 month after chemotherapy, the prevalence rate of ischemic stroke was 0.137%. Most strokes occur within 10 days after chemotherapy.14 In our study, five patients in the CRS group underwent chemotherapy within a month, and four of them were treated with drugs that could increase the risk of ischemic stroke.
Identification of CRS
The DWI patterns of ischemic lesions in multiple vascular territories were more frequently observed in cancer patients without a conventional stroke mechanism, whereas those of single or multiple lesions in one arterial territory were more frequently observed in cancer patients with a conventional stroke mechanism.2 A previous study has shown that concealed cancer should be considered in patients with ischemic lesions in multiple vascular territories on DWI.15 Laboratory findings suggesting coagulopathy may also predict cancer-specific stroke mechanisms. The level of D-dimer, a specific derivative of cross-linked fibrin, has been used in many previous studies as a measure of hypercoagulability.16 The DWI pattern of ischemic lesions in multiple vascular territories and elevated D-dimer level (>1.11 µg/mL) were independently associated with the possibility of a CRS mechanism.2 Thus, these parameters may predict a possible CRS mechanism. In our study, 17 patients had an elevated D-dimer level, and 10 patients had multiple territorial infarctions on DWI. The remaining two patients in which the D-dimer level was not examined had multiple ischemic lesions in multiple vascular territories on DWI. Although the possibility of chemotherapy-related ischemic stroke in four patients could not be ruled out, all patients had an elevated D-dimer level, and two of them had lesions in multiple vascular territories, suggesting hypercoagulability as the stroke mechanism. The main sources of embolism in cancer patients with coagulopathy were presumed to be intravascular clot formation or nonbacterial thrombotic endocarditis (NBTE).13 We could not confirm the main sources of embolism because there was no case in which transesophageal echocardiography or cardiac CT indicated the presence of vegetation in the cardiac valve; therefore, it was not possible to identify the main sources of large clots.
Clot composition in CRS
In our series, no patient in the CRS group had an SVS in the occluded vessels on GRE imaging. An SVS is a blooming hypointense signal on GRE imaging within an occluded vessel and is helpful to predict CE stroke and subsequent recanalization; the thrombi in the occluded vessels may be observed as hypointense because of the paramagnetic property of deoxygenated hemoglobin components in trapped red blood cells.9 Clot composition could be important to predict recanalization subsequent to medical and interventional treatment. Bourcier et al. reported more favorable clinical outcomes in the positive SVS group and a trend of reduced rate of recanalization in the negative SVS group.17 The negative SVS on GRE imaging in the CRS group may be due to different clot compositions compared with that in the CE group. A previous study reported the surgical pathology of NBTE and showed that the clots were dominantly composed of platelets and fibrin in all patients. In contrast, the substantial red blood cell component was detected in only 9% of patients.18 The clots in CRS patients usually form in the arterial systems or cardiac valves, which are areas of high shear stress. The fibrin- and platelet-rich clots in CRS patients may be regarded as negative SVS. However, the lack of a histologic examination in our study did not permit the correlation of SVS on GRE imaging with clot composition. It has been suggested that SVS can potentially affect the rate of recanalization of ERT. The lower rate of successful recanalization in the CRS group than in the CE group might be related to the different clot characteristics possessed by the fibrin-dominant thrombus, as suggested by the negative SVS. The causes of different procedural outcomes after ERT between CRS and LAA patients were not clear. Thrombectomy using stent retrievers is known to be effective for intracranial artery stenotic lesions in in situ thrombosis, although rescue techniques such as IA fibrinolysis and angioplasty are often required because of re-occlusion or flow insufficiency.19 The retrieved thrombi are generally small in occlusions related to intracranial atherosclerotic disease.
The location of occlusion is well known to affect the degree of recanalization after ERT.20 For example, the internal carotid artery terminus and middle cerebral artery have different responses to thrombectomy because of the difference in clot burden and degree of collateral flow. Although middle cerebral artery occlusions, especially in the M2 segment, indicating small clot burden, were more prevalent in the CRS group than in conventional stroke groups, the rate of successful recanalization was lower in the CRS group than in the conventional stroke groups. This means that clot characteristics were also very important to achieve successful recanalization.
Pros and cons of performing ERT for CRS
Although 37% of CRS patients who underwent mechanical thrombectomy had favorable outcomes at discharge, the outcomes at 3 months were extremely poor. Except for three patients with good clinical outcomes at 3 months, the rest were bed-ridden (n = 4, 21.1%) or died (n = 12, 63.2%) at 3 months. This extremely poor prognosis in CRS patients might question the need for ERT. However, the majority of the causes of death (58%) were nonvascular diseases. Despite the lower rate of recanalization in CRS patients, all three patients with mRS score of 0 at 3 months after stroke and six of the seven patients with good clinical outcomes at discharge had complete recanalization after ERT. Thus, we think that ERT in CRS patients could improve the quality of life because those with advanced cancer and limited life expectancies also feel better if they are not hospitalized, not staying in the intensive care unit, or not bed-ridden. Additionally, it is more suitable than intravenous thrombolysis in CRS patients considering that this population usually has contraindications for intravenous thrombolysis.
Study limitations
Our study has limitations, particularly due to its retrospective design. First, given the small sample size, the patients who were admitted to a single center in this study cannot represent all CRS patients. Second, a major obstacle for correlating clot composition with SVS on GRE imaging was that histologic examination of the retrieved clots was not performed and controls in the conventional stroke groups were lacking. However, SVS on GRE imaging is reportedly related to clot composition, and this seems sufficient to conjecture that CRS clots with negative SVS are fibrin-dominant. Third, we could not entirely exclude the possibility of coincident conventional stroke in cancer patients. We routinely performed angiography, electrocardiography, and transthoracic echocardiography in all ischemic stroke patients to determine the etiologies of stroke. In CRS patients, there was no evidence of other sources of stroke during the routine examination. However, we did not perform transesophageal echocardiography and 24-h Holter monitoring in all patients.
In conclusion, ERT could be considered in CRS patients. To achieve successful recanalization, we have to keep in mind the comparative characteristics of this population when performing ERT. It is more difficult to recanalize the occluded vessels in CRS patients than in CE patients because the clots might be dominantly composed of fibrin, as suggested by SVS on GRE imaging. Therefore, it seems that targeting the fibrin-dominant embolus as a technical strategy for IA thrombolysis including fibrinolysis could be possible to increase the rate of successful recanalization in CRS patients. Further studies are required to confirm the effect of IA fibrinolysis when performing IA thrombolysis in CRS patients.
Supplemental Material
Supplemental material, Supplementary Tables for Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke by Seunguk Jung, Cheolkyu Jung, Jae Hyoung Kim, Byung Se Choi, Yun Jung Bae, Leonard Sunwoo, Ho Geol Woo, Jun Young Chang, Beom Joon Kim, Moon-Ku Han and Hee-Joon Bae in Interventional Neuroradiology
Ethical approval
For this type of study formal consent is not required.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985; 64: 16–35. [DOI] [PubMed] [Google Scholar]
- 2.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010; 41: 798–801. [DOI] [PubMed] [Google Scholar]
- 3.Murthy SB, Karanth S, Shah S, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 2013; 44: 3573–3576. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Park JH, Lee M-J, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One 2012; 7: e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang D-H, Hwang Y-H, Kim Y-S, et al. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol 2011; 32: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flacke S, Urbach H, Keller E, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology 2000; 215: 476–482. [DOI] [PubMed] [Google Scholar]
- 9.Cho K-H, Kim JS, Kwon SU, et al. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke 2005; 36: 2379–2383. [DOI] [PubMed] [Google Scholar]
- 10.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkler AE, Marcus JR, Gupta A, et al. Endovascular therapy for acute stroke in patients with cancer. Neurohospitalist 2014; 4: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012; 43: 3029–3034. [DOI] [PubMed] [Google Scholar]
- 13.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010; 68: 213–219. [DOI] [PubMed] [Google Scholar]
- 14.Li S-H, Chen W-H, Tang Y, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clinical Neurol Neurosurg 2006; 108: 150–156. [DOI] [PubMed] [Google Scholar]
- 15.Kwon H-M, Kang BS, Yoon B-W. Stroke as the first manifestation of concealed cancer. J Neurol Sci 2007; 258: 80–83. [DOI] [PubMed] [Google Scholar]
- 16.Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand 2009; 119: 1–16. [DOI] [PubMed] [Google Scholar]
- 17.Bourcier R, Volpi S, Guyomarch B, et al. Susceptibility vessel sign on MRI predicts favorable clinical outcome in patients with anterior circulation acute stroke treated with mechanical thrombectomy. AJNR Am J Neuroradiol 2015; 36: 2346–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiken PW, Edwards WD, Tazelaar HD, et al. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc 2001; 76: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Hong JM, Lee KS, et al. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke 2016; 18: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telischak NA, Wintermark M. Imaging predictors of procedural and clinical outcome in endovascular acute stroke therapy. Neurovasc Imaging 2015; 1: 4–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Tables for Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke by Seunguk Jung, Cheolkyu Jung, Jae Hyoung Kim, Byung Se Choi, Yun Jung Bae, Leonard Sunwoo, Ho Geol Woo, Jun Young Chang, Beom Joon Kim, Moon-Ku Han and Hee-Joon Bae in Interventional Neuroradiology