 Terpinolene induces apoptotic cell death via oxidative stress and mitochondrial impairment.
Terpinolene induces apoptotic cell death via oxidative stress and mitochondrial impairment.
Abstract
Terpinolene is one of the most abundant monoterpenes used as a food supplement or odorant in cosmetics and the pharmaceutical industry. In this study, we aimed to assess apoptotic, oxidative and cytotoxic effects of terpinolene. We used the fission yeast (Schizosaccharomyces pombe) as a promising uni-cellular model organism in molecular toxicology and cell death research, due to its resemblance to mammalian cells at the molecular level. After terpinolene exposure (200–800 mg L–1), the IC50 and LC50 were calculated as 349.17 mg L–1 and 593.87 mg L–1. Cells, stained with acridine orange/ethidium bromide and DAPI, showed apoptotic nuclear morphology, chromatin condensation and fragmentation. 2,7-Dichlorodihydrofluorescein diacetate (DCFDA) fluorescence gradually increased (1.5–2-fold increase) in correlation with increasing concentrations of terpinolene (200–800 mg L–1). Mitochondrial impairment at higher concentrations of terpinolene (400–800 mg L–1) was shown by Rhodamine 123 staining. Real-time PCR experiments showed significant increases (1.5–3-fold) in SOD1 and GPx1 levels (p < 0.05) as well as 2–2.5-fold increases (p < 0.05) in pro-apoptotic factors, Pca1 and Sprad9. The potential effects of terpinolene on programmed cell death and the underlying mechanisms were clarified in unicellular model fungi, Schizosaccharomyces pombe.
1. Introduction
Monoterpenic essential oils (also known as monoterpene hydrocarbons) extracted from glandular trichomes and resin ducts of aromatic and medicinal plants, such as the Pinaceae family, are secondary metabolites that play a defensive role against herbivores and some pathogens.1 Hamamelis water and sage leaf extracts, containing monoterpenes, sesquiterpenes and phenolic acids, are known to display anti-inflammatory and antibacterial activity in vitro and in vivo.2–4 Terpinolene, one of monoterpenes used widely as a flavoring additive and odorant, is extracted by fractional distillation from turpentine, which is collected from pine balsam5 as well as Citrus, Juniperus and Myristica species, or produced by the alcoholic sulfuric acid treatment of pinene.6
The potential toxicity of terpinolene has been evaluated in vivo7 and in vitro.8 0.025% and higher terpinolene concentrations were reported to have a protective role against the oxidation of lipophilic and proteinaceous parts of LDL molecules when terpinolene was incubated with human blood plasma.9 On the other hand, terpinolene increased total antioxidant capacity and total oxidative stress without a genotoxic effect in both primary rat neurons and N2a neuroblastoma cell lines in a dose dependent manner.10 Besides, inhibition of cell proliferation via down-regulating Akt1 expression in K562 cells even in 50 μg ml–1 terpinolene concentration was reported.11 Given the significance of terpinolene in the future of medicine, cytotoxic, inhibitory and cancer fighting potentials together with the underlying mechanisms should be unraveled. Research for terpinolene toxicity mainly focused on laboratory experiments with rats,7 some plants,12 plant pests13 and plant pathogenic fungus.1 However, the undefined mechanisms of terpinolene toxicity in model fungi are currently limited.
The fission yeast Schizosaccharomyces pombe is one of the most studied unicellular model organisms in molecular toxicology, biochemistry and cell biology.14–16 In laboratory experiments, the fission yeast constitutes a valuable model organism with its characteristics including short generation time,17 relatively and easily manipulated small genome (approx. 5000 genes),18 cell-cycle control16,19 and mitochondrial biogenesis analogous to mammals,20,21 and also conserved different programmed cell death (PCD) subroutines.22 Furthermore, the rapid proliferation of yeast cells with metabolic treatments, resembling the Warburg effect in cancer cells, provides opportunities in cancer research.23,24 Therefore, drug candidates for cancer and other human diseases can be evaluated using yeast cells to understand cytotoxic, apoptotic and genotoxic effects not only for risk assessment, but also for underlying mechanisms that are expected to be deciphered.25–27
In this study, the potential cytotoxic effects of terpinolene and underlying mechanisms were investigated in experimental unicellular eukaryotic model S. pombe. We aimed to assess the effects of terpinolene on the proliferation index, cell viability and programmed cell death; i.e. apoptosis. Besides, oxidative stress, mitochondrial impairment or DNA damage induced by terpinolene were evaluated in molecular levels as accounting mechanisms. The study contributes to both understanding terpinolene cytotoxicity and managing risk assessment for future studies.
2. Materials and methods
2.1. Reagents
Methylene blue was obtained from Merck (Istanbul, Turkey). Components of culture media were from BD Difco (Fisher Scientific, Turkey). Glucose was from Emboy (Istanbul, Turkey). Taq polymerase, SYBR Green I Master Mix and cDNA synthesis kit were from Roche (Elips, Turkey). All other chemicals, terpinolene, arsenic(iii) oxide, acridine orange, ethidium bromide, DCFDA (2,7-dichlorodihydrofluorescein diacetate) and Rhodamine 123 were purchased from Sigma (Istanbul, Turkey). NBT (p-nitro-blue tetrazolium chloride) and DAPI (4′,6-diamidino-2-phenylindole) were kind gifts from S. Tuncer-Gurbanov (Bilecik SEU) and E. Yoruk (Istanbul YYU).
2.2. Yeast strain, media and growth conditions
S. pombe wild type strain (Sp972h–) was a kind gift from A. T. Sarikaya (Istanbul YYU). Yeast was grown in a standard YEL medium (1% yeast extract, 2% glucose) on a rotary shaker at 150 rpm at 30 °C in all of the experiments. 1 × 106 cells per ml cultures from overnight incubation (14 h) were used for experiments.
2.3. Terpinolene exposure and toxicity testing
Yeast cells from overnight culture (OD600 ≈ 1) in YEL media were washed with 100 mM phosphate buffer (PBS) with pH 7.4, counted under an optical microscope (Karl-Zeiss) and dispensed to conical flasks at a final concentration of 1 × 106 cells per ml. Cells were exposed to a graded concentration of terpinolene (200–3000 mg L–1 in Tween-20) for 3 h. All concentrations were lethal for cells except 200 mg L–1 exposure. Therefore, the concentration grade was changed to a range of 0–800 mg L–1 (solvent control, 200, 400, 600 and 800 mg L–1 of terpinolene). Arsenic(iii) oxide was used as a positive control for testing apoptosis.28 Inhibition of proliferation was assessed using a hemocytometer. Cells were suspended in PBS and a sample (100 μl) of the cell suspension was stained with 100 μl methylene blue (0.1 mg ml–1 in 2% sodium citrate buffer) for mortality evaluation. After 5 min incubation at room temperature, mortality was examined under a microscope from at least 500 cells in one biological replicate. Dead cells were blue, and viable cells were colorless. The mortality rate was calculated as a ratio of stained cells to total cells.
2.4. Detection of apoptosis by acridine orange/ethidium bromide (AO/EB) and DAPI
Acridine orange/ethidium bromide dual staining was carried out to detect apoptotic morphology. The AO/EB dual staining assay was performed as previously described.29 After washing with PBS (pH: 7.4), 100 μl of cells were mixed with 5 μl of acridine orange/ethidium bromide solution (60 μg ml–1 of AO: 100 μg ml–1 of EB, dissolved in PBS). After 5 min incubation at room temperature, cells were washed with PBS and examined under a fluorescence microscope (Karl-Zeiss, Colibri 7) at λex = 500 nm and λem = 530 nm for acridine orange, and λex = 510 nm and λem = 595 nm for ethidium bromide. While acridine orange permeates into the nucleus of all cells and stains green, ethidium bromide is only taken up by dead cells and stains orange-red when membrane integrity is lost. The cell nucleus was also stained with 1 μg ml–1 DAPI (4′,6-diamidino-2-phenylindole) to visualize apoptotic DNA fragmentation and chromatin condensation after fixation with 3.7% formaldehyde for 1 h. The staining assay was performed as previously described.30 Cells were washed with PBS and examined under a fluorescence microscope (Karl-Zeiss, Colibri 7) at λex = 358 nm and λem = 461 nm.
2.5. Intracellular ROS detection by DCFDA staining and colorimetric NBT assay
Intracellular ROS analysis was performed as indicated previously.31 Cells were incubated with 10 μM DCFDA in culture media for 1 h before harvesting at 30 °C. Cells were washed twice in ice-cold PBS and examined under a fluorescent microscope (Karl-Zeiss, Colibri 7) at λex = 495 nm and λem = 529 nm. Increasing green fluorescence was associated with ROS production. Intensity analysis was made using a Karl-Zeis Zen 2.3 Blue Edition instrument. The colorimetric NBT assay was performed as previously described.32 NBT stock solution was prepared at 10 mg ml–1 in distilled water. After washing with PBS, cells were incubated with NBT (a final concentration of 0.1%) for 1 h at 1 × 106 cells per ml concentration. The supernatants were removed and the cells were fixed by the addition of 200 μl of absolute methanol and washed twice with 70% methanol, then dried. The final dry pellet was solubilized in 120 μl of 2 M KOH and 140 μl DMSO, and was read at 620 nm in a microplate spectrophotometer (Thermo Scientific, Multiskan Go).
2.6. Detection of mitochondrial transmembrane potential (MTP) by Rhodamine 123 assay
Mitochondria were stained with Rhodamine 123, which is readily sequestered by mitochondria depending on mitochondrial membrane potential, as indicated previously.33 Cells were suspended in a final concentration of 50 mM sodium citrate (pH 5.0), 2% glucose and 25 μM Rhodamine 123, and incubated 15 min at room temperature. After washing with PBS, cells were visualized by fluorescence microscopy at λex = 505 nm and λem = 534 nm.
2.7. Evaluation of antioxidant enzymes and apoptosis-related genes by mRNA expression assay
The mRNA expression assay for antioxidant enzymes and apoptosis-related genes is described in the ESI.† Gene specific primers were designed using Primer3Plus (Version: 2.3.6) software and declared in Table S1.†
2.8. Statistical analysis
The data were expressed as mean ± standard error of the mean (SEM) and at least three independent experiments were performed. The IC50 and LC50 values were calculated by the Probit method. Differences between groups were analyzed by One-way ANOVA with Tukey's multiple comparison test using GraphPad Prism (California, USA).
3. Results and discussion
3.1. Toxicity testing, cell proliferation and viability
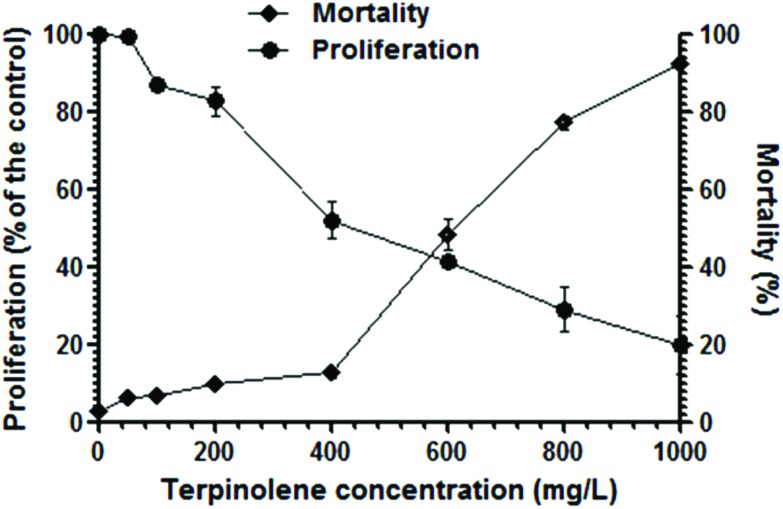
Growth inhibition of cells significantly increased (p < 0.01) after exposure to terpinolene at 200–1000 mg L–1 (Fig. 1). The IC50 value (inhibition concentration 50%) was 349 mg L–1. On the other hand, cell survival notably decreased (p < 0.01) gradually after exposure to terpinolene at 200–1000 mg L–1. The LC50 value (lethal concentration 50%) was 593 mg L–1. Turkez et al. (2015) reported that terpinolene induced cytotoxic cell death even at lower doses (150–200 mg L–1) in human peripheral lymphocyte culture.34 The difference in mortality rates is probably due to the rigid and relatively impermeable yeast cell wall enforced by crosslinkages between chitin, mannoproteins and β-glucan,35 although terpinolene, as well as other monoterpenes, is a membrane permeable lipophilic compound.10 The inhibitory and anti-fungal potentials of terpinolene were found less effective in comparison to other monoterpenes, α-terpineol and 1,8-cineole.36 Necrosis following changes in cell-membrane fluidity was proposed to explain the anti-fungal action of some monoterpenes.36 Pontin et al. (2015) suggested that alteration in fungal cell membrane permeability was a possible explanation for the anti-fungal activity of terpinolene.37 In addition, significant increases in lipid peroxidation, ROS levels and total oxidative stress were reported after terpinolene exposure in a variety of species.10,38 It is well known that excessive ROS production could promote mitochondrial disruption- and oxidative DNA damage-mediated cell death signaling.39,40
Fig. 1. Cell proliferation and viability after exposure to 0–1000 mg L–1 terpinolene solutions for 3 h. Cell proliferation was assessed by a hemocytometer. Mortality of cells was assessed by the Methylene Blue assay. Values are presented as mean ± SEM. Calculations were made from at least five independent biological replica (n = 5).
3.2. Detection of apoptosis
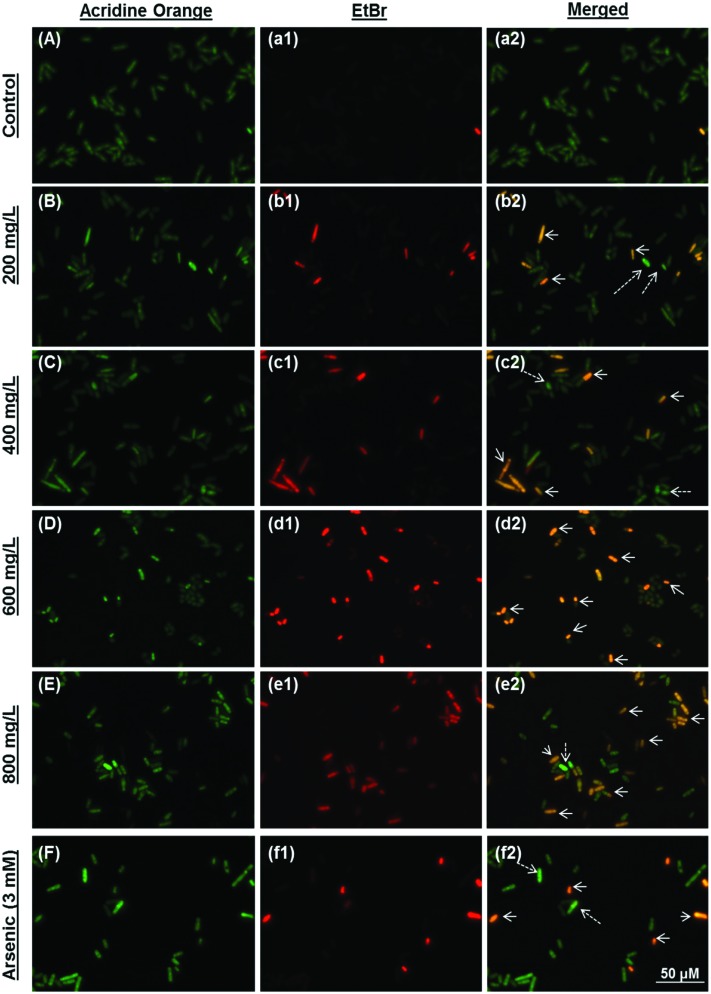
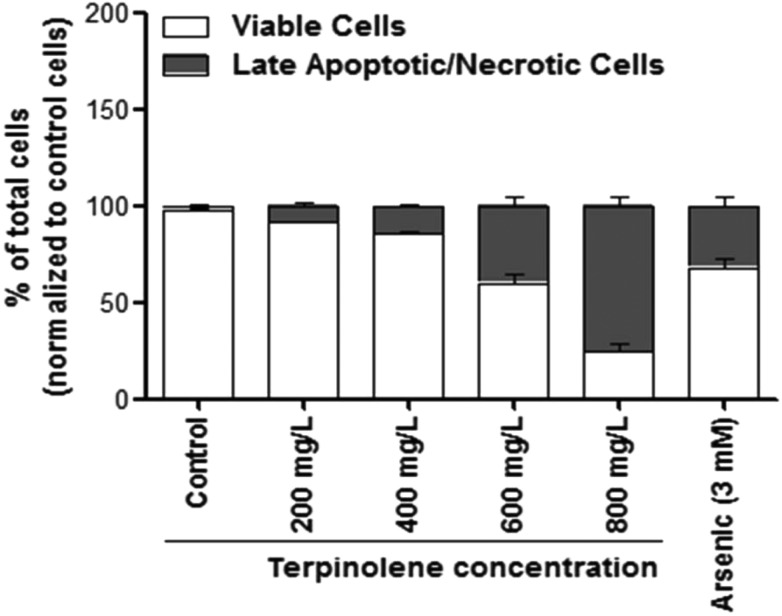
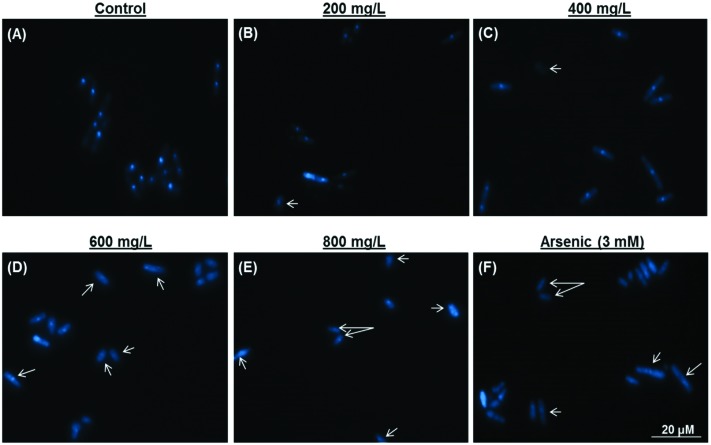
Late apoptotic/necrotic and early apoptotic cells were observed with acridine orange–ethidium bromide dual staining (Fig. 2). Apoptotic and late apoptotic cells were stained bright green and orange-red due to nuclear conformation and cell-membrane status. Apoptotic cells display fragmented and condensed orange-red chromatin, and live cells display a regular green nucleus. Apoptotic cells were indicated by arrows in Fig. 2 (Fig. 2, b2: 200 mg L–1; c2: 400 mg L–1; d2: 600 mg L–1; e2: 800 mg L–1 terpinolene and f2: 3 mM arsenic trioxide). As shown in Fig. 3, percentages of the late apoptotic/necrotic cells and viable cells were significantly different in experimental groups (200–800 mg L–1) compared with the control (p < 0.001). While apoptotic cells were shown in all concentrations of terpinolene, apoptosis was markedly increased at 600 mg L–1 (39.85%), which was close to the mortality rate (47.53%), similar to arsenic induced apoptosis (31.89% at 3 mM). At 800 mg L–1, apoptotic cell death and mortality rates were very similar (75.52% and 77.12%). In order to confirm apoptosis following exposure to terpinolene, cells were fixed with formaldehyde and stained with DAPI. Chromatin condensation and DNA fragmentation, as typical markers of apoptosis, were observed in experimental groups (200–800 mg L–1 terpinolene) as shown in Fig. 4. While the nuclei of control cells were seen intact, terpinolene- and arsenic-treated cell nuclei were condensed dot-shaped and crescent-shaped masses, which were previously reported as apoptotic markers.41–43 Similar results were declared by Okumura et al., (2012), who demonstrated that terpinolene induced cell apoptosis in K562 cells.11
Fig. 2. Apoptosis of S. pombe cells was evaluated using acridine orange–ethidium bromide dual staining. Viable, early and late apoptotic/necrotic cells were visualized using a fluorescent microscope after exposure to 0 (A, a1 and a2), 200 (B, b1 and b2), 400 (C, c1 and c2), 600 (D, d1 and d2), 800 (E, e1 and e2) mg L–1 terpinolene and 3 mM arsenic(iii) (F, f1, f2) solutions. Arsenic(iii) was used as a positive control. Arrows: Late apoptotic/necrotic cells; dashed arrows: early apoptotic cells. At least 200 cells were counted in each biological replica (n = 5).
Fig. 3. Percentage of viable and late apoptotic/necrotic cells after exposure to 0–800 mg L–1 terpinolene and 3 mM arsenic solutions. At least 200 cells were counted in each biological replica. A small number of early apoptotic cells were ignored. Values are presented as mean ± SEM. Calculations were made from at least five independent biological replicas (n = 5).
Fig. 4. Nuclear morphology was evaluated using 4,6-diamidino-2-phenylindole (DAPI) staining after exposure to 0 (A), 200 (B), 400 (C), 600 (D), 800 (E) mg L–1 terpinolene and 3 mM arsenic(iii) (F) solutions. Arsenic(iii) was used as a positive control. Arrows: Degraded and fragmented DNA. Images were taken using a fluorescent microscope from at least three independent biological replica (n = 3).
3.3. Quantification of ROS and antioxidant enzyme mRNA levels
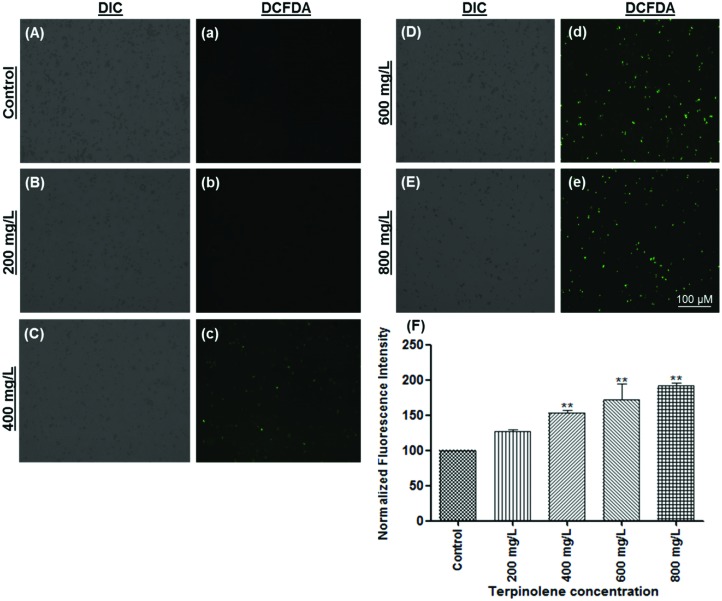
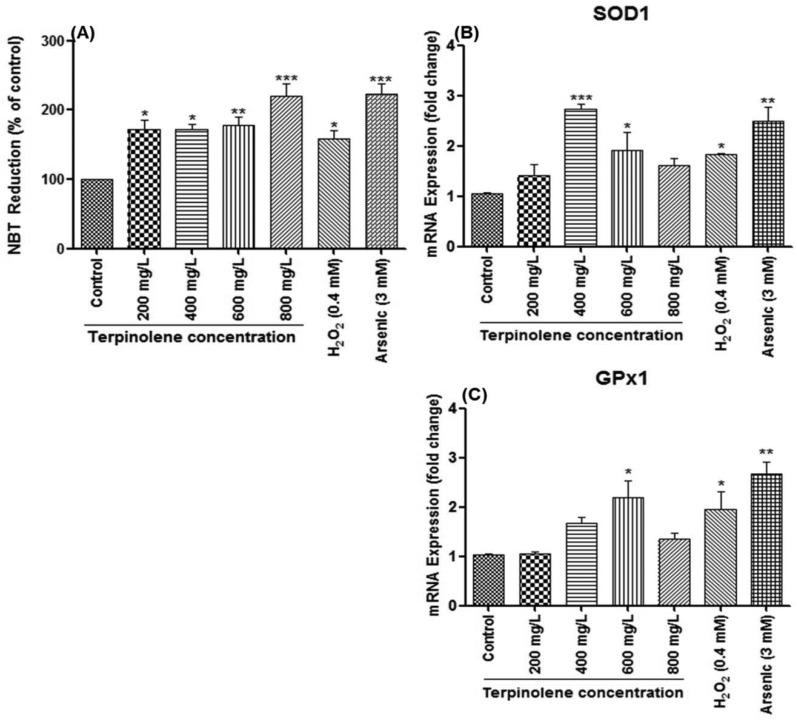
Intracellular ROS levels were shown using the DCFDA fluorescence assay. DCFDA is a membrane permeable dye and reacts with ROS to transform into an oxidized fluorescent form, DCF. As shown in Fig. 5, ROS levels significantly increased (p < 0.01) after exposure to terpinolene in a dose-dependent manner (200–800 mg L–1) as indicated by increasing green fluorescence (Fig. 5A and a: control; Fig. 5B and b: 200 mg L–1; Fig. 5C and c: 400 mg L–1; Fig. 5D and d: 600mg L–1; Fig. 5E and e: 800 mg L–1; Fig. 5F: normalized fluorescence intensity). To confirm the results, ROS levels were measured by the NBT assay. The reduction of NBT to insoluble blue formazan is a marker for superoxide generation.32 Similar to the DCFDA assay, as shown in Fig. 6A, NBT reduction dramatically increased (p < 0.05) at 200–600 mg L–1 terpinolene exposure analogous with H2O2 exposure (0.4 mM). Interestingly, NBT reduction markedly increased more than two-fold (p < 0.001) at 800 mg L–1 terpinolene exposure similar to arsenic(iii) exposure (3 mM). This dramatic increase at 800 mg L–1 was consistent with increase in mortality rates (77.21%) and apoptotic cell death (75.52%) at 800 mg L–1 (see Fig. 1 and 3). As shown in Fig. 6B and C, SOD1 and GPx1 mRNA levels firstly increased 2–3-fold between 400 and 600 mg L–1 terpinolene, however, followed by decreases at 800 mg L–1 terpinolene. Upregulation of antioxidant enzyme gene expression generally correlates with increased ROS levels44,45 in order to recover the scavenging activity of antioxidant enzymes inhibited by the elevated ROS level.46 On the other side, recovered gene expression levels at 800 mg L–1 may be due to high concentrations of ROS and related increases of mortality rates. Turkez et al. (2015) reported a two-fold increase in total oxidative stress levels and mortality rates in cultured human blood cells after exposure to 200 mg L–1 terpinolene.34 Similar results were declared above 200 mg L–1 terpinolene in rat brain cells10 in contrast to other monoterpenes, α-pinene, cineol and myrtenol.47,48
Fig. 5. Measurement of ROS levels using DCFDA (2′,7′-dichlorofluorescin diacetate) staining. ROS generation of cells exposed to 0 (A and a), 200 (B and b), 400 (C and c), 600 (D and d) and 800 (E and e) mg L–1 was visualized and measured by a fluorescence microscope. (F) ROS generation of cells exposed to 0–800 mg L–1 terpinolene was calculated as normalized fluorescence intensity. Values are presented as mean ± SEM. Statistical analysis was made to compare experimental groups and the control group. Significantly different values are indicated by asterisks (One-way ANOVA, **p < 0.01). Images were taken from at least three independent biological replicas (n = 3).
Fig. 6. Cellular ROS levels and gene expression levels of antioxidant enzymes. (A) ROS levels in cells exposed to 0–800 mg L–1 terpinolene were measured by the NBT (3,3′-(3,3′-dimethoxy-4,4′-biphenylene)bis[2-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride) assay. ROS generation of cells was determined as absorbance of reduced NBT at 620 nm in a microplate reader and expressed as normalized NBT reduction compared to the control group. (B and C) mRNA levels of SOD1 and GPx1 in cells exposed to 0–800 mg L–1 were measured by RT-PCR. Values are presented as mean ± SEM. Statistical analysis was made to compare experimental groups and the control group. Significantly different values are indicated by asterisks (One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001). Values were taken from at least three independent biological replicas (n = 3).
3.4. Measuring the mitochondrial transmembrane potential (MTP)
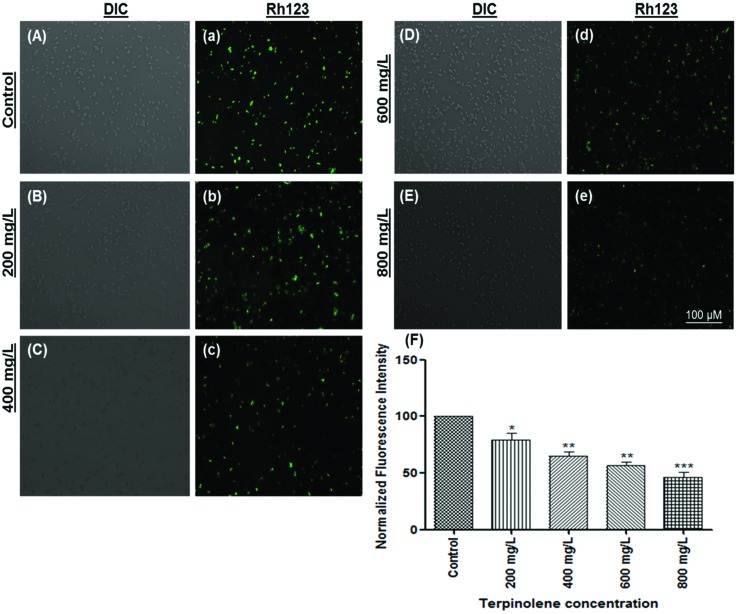
Measuring the mitochondrial transmembrane potential (ΔΨm) is a reliable method to evaluate the vitality of mitochondria.49 Loss of MTP, generally accepted as a marker for early apoptosis, is monitored using the Rhodamine 123 stain, which is sequestered by intact mitochondria.50 As shown in Fig. 7, Rhodamine fluorescence was markedly decreased in a dose-dependent manner (200–800 mg L–1) (Fig. 7A, a: control; Fig. 7B, b: 200 mg L–1; Fig. 7C, c: 400 mg L–1; Fig. 7D, d: 600 mg L–1 and Fig. 7E, e: 800 mg L–1). Calculated fluorescence intensities dramatically decreased at least 2-fold (p < 0.01) at 600 and 800 mg L–1 compared with the control (Fig. 7F). Mitochondrial impairment may be due to highly elevated ROS concentrations. It is well known that oxidative stress can result in altered mitochondrial membrane permeability and mitochondrial DNA damage.51,52 However, mitochondrial dysfunction can stimulate ROS production.53,54 Our results indicate that apoptosis following terpinolene exposure was related to the dissipation of mitochondrial membrane potential. Similarly, terpinen-4-ol, one of the most known monoterpenes, was shown to induce apoptosis via disruption of MTP in human lung cancer cells.55
Fig. 7. The mitochondrial transmembrane potential (MTP) of S. pombe cells was evaluated using Rhodamine123. The MTP of cells exposed to 0 (A and a), 200 (B and b), 400 (C and c), 600 (D and d) and 800 (E and e) mg L–1 terpinolene solutions was visualized and measured by a fluorescence microscope. (F) Dose-dependent decline in the fluorescent intensity of cells exposed to increasing concentrations of terpinolene (0–800 mg L–1) was measured as normalized fluorescence intensity. Values are presented as mean ± SEM. Statistical analysis was made to compare experimental groups and the control group. Significantly different values are indicated by asterisks (One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001). Images were taken from at least three independent biological replicas (n = 3).
3.5. Evaluation of apoptotic gene expression
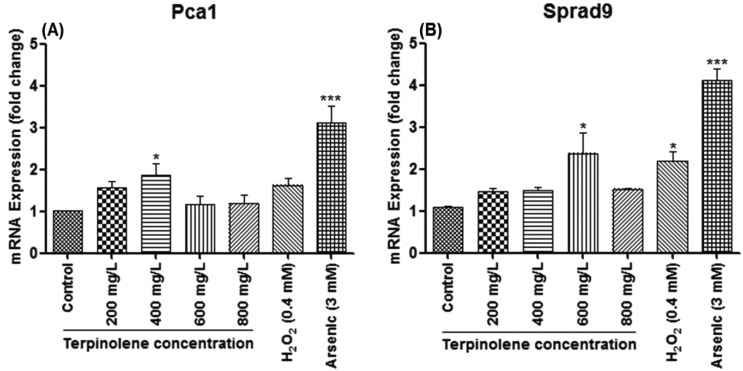
S. pombe caspase 1 (Pca1), homologous to Yca1 of S. cerevisiae, is encoded in the S. pombe genome and predicted to play a role in apoptosis,56 whereas Lim et al. (2007) suggested that Pca1 can also act as an anti-apoptotic factor in response to chemical stress and relevant toxicity.57 The precise function of Pca1 in apoptosis still remains unclear.22 However, as shown in Fig. 8A, Pca1 expression significantly (p < 0.05) increased (2-fold) at 400 mg L–1 correlating with mortality rates and apoptotic cell death (see Fig. 1 and 3). Interestingly, the obvious decline in Pca1 mRNA levels at 600 and 800 mg L–1, which are close to mRNA levels of untreated cells, may be due to rapid increase in mortality. Another pro-apoptotic gene, Sprad9 (S. pombe rad9), which is homologous to human Rad9, functions in DNA damage response.58 Sprad9 was suggested to have a dual and opposing role in the regulation of apoptosis caused by nutritional and nitrosative stress.59,60 As illustrated in Fig. 8B, Sprad9 expression dramatically increased (p < 0.05) at 600 mg L–1 terpinolene and 0.4 mM H2O2 concentrations indicating severe DNA damage. The dose-dependent decline of Sprad9 mRNA levels was quite similar to that of Pca1. However, Pca1 and Sprad9 expression were induced up to 4–5-fold in response to 3 mM arsenic trioxide (p < 0.001), which reveals the importance of these pro-apoptotic genes in the apoptotic process.
Fig. 8. Apoptosis-related mRNA expression in S. pombe cells exposed to 0–800 mg L–1 terpinolene. mRNA levels of Pca1 and Sprad9 were measured by RT-PCR. Values are presented as mean ± SEM. Statistical analysis was made to compare experimental groups and the control group. Significantly different values are indicated by asterisks (One-way ANOVA, *p < 0.05, ***p < 0.001). Values were taken from at least three independent biological replicas (n = 3).
4. Conclusion
In this study, S. pombe was assessed as a unicellular eukaryotic model organism to shed light on the potential toxic effects of terpinolene and the underlying mechanism. Cell proliferation and viability significantly decreased after terpinolene exposure. Besides, apoptosis in relation to terpinolene toxicity was shown to be mediated by oxidative stress and reduction of the mitochondrial transmembrane potential. This is the first complete mechanistic study for providing experimental data on terpinolene-induced apoptosis in S. pombe. However, experimental data for other programmed cell death mechanisms, autophagy and lipotoxic cell death, are still lacking and warrant further study.
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
This study was supported by the board of trustees of Istanbul Yeni Yuzyil University. We wish to thank Aysegul Topal-Sarikaya, Emre Yoruk and Sinem Tunçer-Gurbanov for consumables and chemicals, Cenk Kig for his advice and for sharing experiences, and Gulsen Uz for providing S. pombe.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tx00100f
References
- Farooq A., Choudhary M. I., Rahman A. U., Tahara S., Başer K. H. C., Demirci F. Z. Naturforsch., C: J. Biosci. 2002;57:863–866. doi: 10.1515/znc-2002-9-1018. [DOI] [PubMed] [Google Scholar]
- Dawid-Pać R. Postep Derm. Alergol. 2013;30:170–177. doi: 10.5114/pdia.2013.35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M. G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Antimicrob. Agents Chemother. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueca B., Pagán R., García-Gonzalo D. Int. J. Food Microbiol. 2014;189:126–131. doi: 10.1016/j.ijfoodmicro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Masten S. and Haneke K. E., Toxicological Summary For Turpentine [8006-64-2], https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/turpentine_508.pdf.
- Masten S. and Tice R., Toxicological Summary for Terpinolene, http://www.jonnsaromatherapy.com/pdf/Tice_Toxicological_Summary_for_Terpinolene_1999.pdf.
- U.S. National Library of Medicine, TOXNET Toxicology Data Network, https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+586-62-9.
- Siani A. C., Ramos M. F., Menezes-de-Lima O., Ribeiro-dos-Santos R., Fernadez-Ferreira E., Soares R. O., Rosas E. C., Susunaga G. S., Guimarães A. C., Zoghbi M. G., Henriques M. G. J. Ethnopharmacol. 1999;66:57–69. doi: 10.1016/s0378-8741(98)00148-2. [DOI] [PubMed] [Google Scholar]
- Graßmann J., Hippeli S., Spitzenberger R., Elstner E. F. Phytomedicine. 2005;12:416–423. doi: 10.1016/j.phymed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Aydin E., Türkez H., Taşdemir Ş. Arh. Hig. Rada Toksikol. 2013;64:415–424. doi: 10.2478/10004-1254-64-2013-2365. [DOI] [PubMed] [Google Scholar]
- Okumura N., Yoshida H., Nishimura Y., Kitagishi Y., Matsuda S. Oncol. Lett. 2012;3:321–324. doi: 10.3892/ol.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. F., Southwell I. A. Phytochemistry. 2003;62:683–689. doi: 10.1016/s0031-9422(02)00607-6. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Li Y., Lei C. L. Nat. Prod. Res. 2009;23:1080–1088. doi: 10.1080/14786410802267759. [DOI] [PubMed] [Google Scholar]
- Laliberté J., Whitson L. J., Beaudoin J., Holloway S. P., Hart P. J., Labbé S. J. Biol. Chem. 2004;279:28744–28755. doi: 10.1074/jbc.M403426200. [DOI] [PubMed] [Google Scholar]
- Liu M., Huang Y., Wen H., Qiu G. Huanjing Kexue. 2015;36:3943–3951. [PubMed] [Google Scholar]
- Hagan I. M., Grallert A., Simanis V., Cold Spring Harbor. Protoc., 2016, 20169 , , pdb.top082800 . [DOI] [PubMed] [Google Scholar]
- Eisenberg T., Carmona-Gutierrez D., Büttner S., Tavernarakis N., Madeo F. Apoptosis. 2010;15:257–268. doi: 10.1007/s10495-009-0453-4. [DOI] [PubMed] [Google Scholar]
- Sajiki K., Hatanaka M., Nakamura T., Takeda K., Shimanuki M., Yoshida T., Hanyu Y., Hayashi T., Nakaseko Y., Yanagida M. J. Cell Sci. 2009;122:1418–1429. doi: 10.1242/jcs.046466. [DOI] [PubMed] [Google Scholar]
- Hartmuth S., Petersen J. J. Cell Sci. 2009;122:1737–1746. doi: 10.1242/jcs.049387. [DOI] [PubMed] [Google Scholar]
- Schafer B. Curr. Genet. 2003;43:311–326. doi: 10.1007/s00294-003-0404-5. [DOI] [PubMed] [Google Scholar]
- Koyama M., Nagakura W., Tanaka H., Kujirai T., Chikashige Y., Haraguchi T., Hiraoka Y., Kurumizaka H. Biochem. Biophys. Res. Commun. 2017;482:896–901. doi: 10.1016/j.bbrc.2016.11.130. [DOI] [PubMed] [Google Scholar]
- Lin S. J., Austriaco N. FEMS Yeast Res. 2014;14:119–135. doi: 10.1111/1567-1364.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D., Reisenbichler A., Heimbucher P., Bauer M. A., Braun R. J., Ruckenstuhl C., Büttner S., Eisenberg T., Rockenfeller P., Fröhlich K.-U., Kroemer G., Madeo F. Cell Cycle. 2011;10:3973–3978. doi: 10.4161/cc.10.22.18212. [DOI] [PubMed] [Google Scholar]
- Madeo F., Fröhlich E., Fröhlich K. U. J. Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Mori A., Yanagida M. PLoS One. 2011;6:e22021. doi: 10.1371/journal.pone.0022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villahermosa D., Fleck O. Sci. Rep. 2017;7:7225. doi: 10.1038/s41598-017-07647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng W. K., Faucette L., Johnson R. K., Sternglanz R. Mol. Pharmacol. 1988;34:755–760. [PubMed] [Google Scholar]
- Du L., Yu Y., Chen J., Liu Y., Xia Y., Chen Q., Liu X. FEMS Yeast Res. 2007;7:860–865. doi: 10.1111/j.1567-1364.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Pajaniradje S., Mohankumar K., Pamidimukkala R., Subramanian S., Rajagopalan R. Biomed. Res. Int. 2014;2014:474953. doi: 10.1155/2014/474953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazotte B., Cold Spring Harbor Protoc., 2011, 20111 , , pdb.prot5556 . [DOI] [PubMed] [Google Scholar]
- Azad G. K., Singh V., Mandal P., Singh P., Golla U., Baranwal S., Chauhan S., Tomar R. S. FEBS Open Biol. 2014;4:77–89. doi: 10.1016/j.fob.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Cedeño R., Rodríguez J., Van Der Knaap W. P. W., Mialhe E., Bachère E. Aquaculture. 2000;191:89–107. [Google Scholar]
- Kwolek-Mirek M., Zadrag-Tecza R. FEMS Yeast Res. 2014;14:1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Turkez H., Aydın E., Geyikoglu F., Cetin D. Cytotechnology. 2015;67:409–418. doi: 10.1007/s10616-014-9698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Uscanga B., Franç J. M. Lett. Appl. Microbiol. 2003;37:268–274. doi: 10.1046/j.1472-765x.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- Yu D., Wang J., Shao X., Xu F., Wang H., Shao C. X. J. Appl. Microbiol. 2015;119:1253–1262. doi: 10.1111/jam.12939. [DOI] [PubMed] [Google Scholar]
- Pontin M., Bottini R., Burba J. L., Piccoli P. Phytochemistry. 2015;115:152–160. doi: 10.1016/j.phytochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Montanari R. M., Barbosa L. C. A., Demuner A. J., Silva C. J., Andrade N. J., Ismail F. M. D., Barbosa M. C. A. Molecules. 2012;17:9728–9740. doi: 10.3390/molecules17089728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Lai S.-H., Yang H.-H., Xing D.-G., Zeng C.-C., Tang B., Wan D., Liu Y.-J. RSC Adv. 2017;7:17752–17762. [Google Scholar]
- Xiong X., Gan L., Liu Y., Zhang C., Yong T., Wang Z., Xu H., Yang X. Nanoscale. 2015;7:5217–5229. doi: 10.1039/c4nr07248k. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Kitajima S., Ichihara S. Biosci., Biotechnol., Biochem. 2011;75:1113–1118. doi: 10.1271/bbb.110019. [DOI] [PubMed] [Google Scholar]
- Munoz A. J., Wanichthanarak K., Meza E., Petranovic D. FEMS Yeast Res. 2012;12:249–265. doi: 10.1111/j.1567-1364.2011.00781.x. [DOI] [PubMed] [Google Scholar]
- Salucci S., Burattini S., Falcieri E., Gobbi P. Eur. J. Histochem. 2015;59:2539. doi: 10.4081/ejh.2015.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Chen L., Su Y., Yin H., Pang Y., Zhuang Z. Toxicol. Res. 2015;4:1389–1399. [Google Scholar]
- Zhu S., Luo F., Zhu B., Wang G.-X. Toxicol. Res. 2017;6:719–728. doi: 10.1039/c7tx00123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel D., Salas-Vidal E., Narváez V., del Rayo Sánchez-Carbente M., Hernández-García D., Cuervo R., Covarrubias L. Dev. Biol. 2006;291:291–299. doi: 10.1016/j.ydbio.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Porres-Martínez M., González-Burgos E., Carretero M. E., Gómez-Serranillos M. P. Z. Naturforsch., C: J. Biosci. 2016;71:191–199. doi: 10.1515/znc-2014-4135. [DOI] [PubMed] [Google Scholar]
- Gomes B. S., Neto B. P. S., Lopes E. M., Cunha F. V. M., Araújo A. R., Wanderley C. W. S., Wong D. V. T., Júnior R. C. P. L., Ribeiro R. A., Sousa D. P., Venes R Medeiros J., Oliveira R. C. M., Oliveira F. A. Chem.-Biol. Interact. 2017;273:73–81. doi: 10.1016/j.cbi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Zorova L. D., Popkov V. A., Plotnikov E. Y., Silachev D. N., Pevzner I. B., Jankauskas S. S., Babenko V. A., Zorov S. D., Balakireva A. V., Juhaszova M., Sollott S. J., Zorov D. B., Anal. Biochem., 2017. , S0003-2697(17)30293-2 10.1016/j.ab.2017.07.009 , [Epub ahead of print] . [Google Scholar]
- Baracca A., Sgarbi G., Solaini G., Lenaz G. Biochim. Biophys. Acta. 2003;1606:137–146. doi: 10.1016/s0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- Guo C., Sun L., Chen X., Zhang D. Neural Regener Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., Ganie S. A. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Zuin A., Gabrielli N., Calvo I. A., García-Santamarina S., Hoe K.-L., Kim D. U., Park H.-O., Hayles J., Ayté J., Hidalgo E. PLoS One. 2008;3:e2842. doi: 10.1371/journal.pone.0002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu X., Zhang Y., Tian F., Zhao G., Yu Q., Jiang F., Liu Y. Toxicol. Res. 2012;1:137. [Google Scholar]
- Wu C.-S., Chen Y.-J., Chen J. J. W., Shieh J.-J., Huang C.-H., Lin P.-S., Chang G.-C., Chang J.-T., Lin C.-C. Evid. Based Complement. Altern. Med. 2012;2012:818261. doi: 10.1155/2012/818261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. P., Yang H. Biochim. Biophys. Acta, Mol. Cell Res. 2008;1783:1335–1349. doi: 10.1016/j.bbamcr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lim H.-W., Kim S.-J., Park E.-H., Lim C.-J. Can. J. Microbiol. 2007;53:1016–1023. doi: 10.1139/W07-067. [DOI] [PubMed] [Google Scholar]
- Wakida T., Ikura M., Kuriya K., Ito S., Shiroiwa Y., Habu T., Kawamoto T., Okumura K., Ikura T., Furuya K. eLife. 2017;6:e29953. doi: 10.7554/eLife.29953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.-H., Park E.-H., Lim C.-J. FEMS Microbiol. Lett. 2007;275:270–277. doi: 10.1111/j.1574-6968.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Low C. P., Shui G., Liew L. P., Buttner S., Madeo F., Dawes I. W., Wenk M. R., Yang H. J. Cell Sci. 2008;121:2671–2684. doi: 10.1242/jcs.028977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.