Abstract
The demonstration that the neurosteroid pregnenolone sulfate (PREGS) is active on memory function at both the physiological and pharmacological levels led to us examining in detail the effects of the steroid on spatial working memory by using a two-trial recognition task in a Y-maze, a paradigm based on the natural drive in rodents to explore a novel environment. Dose–response studies in young male adult Sprague–Dawley rats and Swiss mice, after the postacquisition intracerebroventricular injection of steroid, showed an U-inverted curve for memory performance and indicated a greater responsiveness in rats compared with mice. Remarkably, the synthetic (−) enantiomer of PREGS not only also displayed promnesiant activity, but its potency was 10 times higher than that of the natural steroid. Intracerebroventricular coadministration experiments with dl-2-amino-5-phosphonovaleric acid, a competitive selective antagonist of the N-methyl-d-aspartate receptor, abolished the memory-enhancing effect of PREGS, but not that of the PREGS enantiomer, evoking enantiomeric selectivity at the N-methyl-d-aspartate receptor and/or different mechanisms for the promnestic function of the two enantiomers.
Keywords: spatial memory‖Y-maze‖N-methyl-d-aspartate receptor‖ 2-amino-5-phosphovaleric acid‖rodents
Steroids found in the brain, of peripheral origin or locally synthesized (neurosteroids), are known to exert modulatory actions on several functions in the central nervous system. Among them, the neurosteroid pregnenolone sulfate (3β-hydroxy-5-pregnen-20–one sulfate, PREGS) has been well described as a potent steroidal enhancer of learning and memory processes in rodents (1–6). Of particular interest is the physiological role of PREGS in the hippocampus, which was recently demonstrated for the preservation of age-related spatial memory loss (4). With regards to the mechanism(s) underlying the promnestic action of this endogenous steroid, it is most likely occurring by regulating neurotransmitter receptor function [particularly γ-aminobutyric acid type A (GABAA) and N-methyl-d-aspartate (NMDA) receptor activities] in several brain regions (2, 3, 7–13).
Particularly relevant to the work here on enantiomeric steroids are the structure-activity data that have demonstrated a marked stereoselectivity for steroid regulation of neurotransmitter receptor function. For example, the endogenous 3α-hydroxy ring A-reduced steroids, such as the neurosteroid allopregnanolone (3α-hydroxy-5α-pregnan-20–one), display potent positive-allosteric modulation of GABAA receptors (14–16) that may explain their anesthetic, anxiolytic, hypnotic, and anticonvulsant effects in animals (17). Conversely, epiallopregnanolone and pregnanolone, the respective 3β- and 5β-hydroxy- diastereomers of allopregnanolone (diastereomers having the opposite configuration at only one of multiple chiral centers, in this case at C-3 or C-5, also are known as epimers) are ineffective in potentiating GABAA receptors (18). Cognate evidence for a direct interaction between steroids and GABAA receptors has recently been provided with enantiomeric pairs of steroids. As opposed to diastereomers, enantiomers (absolute mirror-symmetric, nonsuperimposable images) have identical physical properties, but differ in shape (hence have different rotation of plane-polarized light) and act dissimilarly only in a chiral environment (dissimilar binding to protein receptor sites). For example, the non-natural enantiomer (−)3α-hydroxy-5α-pregnan-20–one has been shown to display greatly reduced GABAA receptors' modulatory and anesthetic potency in tadpoles and mice (19), and this finding strongly supports the action of the steroid at a unique chiral site on protein regions of GABAA receptors (20, 21). Moreover, the synthetic (−) enantiomers of dehydroepiandrosterone sulfate, but not of pregnanolone sulfate (3α-hydroxy-5β-pregnan-20-one sulfate) and PREGS, showed weaker electrophysiological inhibitory effects on GABA-mediated chloride ion currents in cultured hippocampal neurons, as compared with the (+) natural enantiomers (21). The extension of this strategy, exploring the enantioselectivity of neuroactive steroids to other neurotransmitter receptors such as NMDA receptors, and importantly to any central nervous system function in vivo such as memory, has not been reported.
The present study was thus designed to examine the influence of the administration of the synthetic (−) enantiomer of PREGS (ent-PREGS) in comparison with that of the (+) natural PREGS on short-term memory performances of rats and mice tested in a Y-maze two-trial recognition task, a paradigm based on the innate tendency of rodents to explore novel environments (22). Particular attention was made toward the detailed quantitative investigation of steroid potency after intracerebroventricular (ICV) injection. Moreover, in an attempt to elucidate the enantioselectivity of the steroids' action at the NMDA receptor in vivo, ICV coadministration of either PREGS or ent-PREGS with the competitive NMDA receptor antagonist dl-2-amino-5-phosphonovaleric acid (AP5) were performed.
Materials and Methods
Animal Housing and Surgery.
Male Sprague–Dawley rats and Swiss mice (Janvier, Le Genest St-Isle, France), 2 months of age and weighing 250–300 g for rats and 30–32 g for mice at the time of surgery, were used. They were grouped-housed in a temperature-controlled (22°C ± 1°C) and humidity-controlled (50% ± 5%) room. They were maintained on a 12-h light/12-h dark (08.00–20.00) schedule and had access to food and water ad libitum. All animals were treated as approved by the local committee and in accordance with the European Communities Council guidelines for care and use of laboratory animals.
Rats were anesthetized with a mixture of 5% ketamine (Imalgène 1000, Merial, France) and 0.1% acepromazine (Vetranquil 1%, Sanofi, Libourne, France) in saline, administered i.p. at 1.5 ml/kg. Mice were anesthetized with a mixture of 0.5% ketamine and 0.1% xylazine, under the volume of 15 ml/kg. Animals were mounted in a stereotaxic apparatus (model 900, Kopf Instruments, Tujunga, CA) and implanted with unilateral stainless steel cannula guides (23 gauge, 7 mm long) above the lateral ventricle, according to the following coordinates from the atlas of Paxinos and Watson (23) for rats: −0.8 mm posterior from bregma, ± 1.6 mm lateral to the midline, and −2.7 mm below the skull surface and from the atlas of Franklin and Paxinos (24) for mice: −0.5 mm posterior from bregma, ± 1.0 mm lateral to the midline, and −1.5 mm below the skull surface. Cannulas were secured to the skull with stainless steel screws and dental cement. Animals were used for experiments after a 7-day postrecovery period. Each animal was handled daily for 3 days before the experiments, and on the day before each experiment, cannula function was confirmed by gravity flow of isotonic saline.
Treatment and Injection Procedures.
PREGS was obtained from Steraloids (Newport, RI), ent-PREGS was synthesized as described (20), and AP5 was provided by Sigma. All compounds were dissolved in 0.30% saline (vehicle). For dose–response studies, PREGS was administered ICV at doses of 0 (vehicle), 0.05, 0.25, 0.5, 2.5, 5.0, and 12.5 nmol in rats and mice; ent-PREGS was injected ICV at doses of 0 (vehicle), 0.025, 0.05, 0.25, and 0.5 nmol in rats and 0 (vehicle), 0.025, 0.05, 0.25, 0.5, and 2.5 nmol in mice. For ICV coadministration studies, 0.5 nmol PREGS or 0.05 nmol ent-PREGS was given in conjunction with 10 nmol AP5 in rats and 0.5 nmol PREGS or 0.05 nmol ent-PREGS in conjunction with 2 nmol AP5 in mice. Drug and control-vehicle solutions were injected unilaterally through the cannula guide. A total volume of 5 μl in rats and 2.5 μl in mice was infused by using a KDS pump (Fisher Scientific), at a constant rate of 1 μl/min, through a 30-gauge stainless steel injector attached to a 10-μl microsyringe (Hamilton) by polyethylene tubing. The injectors were 1.5 mm and 0.8 mm longer in rats and mice, respectively, than the cannula guides and were left in place an additional 40 s to allow diffusion of solutions away from the tip.
Behavioral Apparatus and Testing.
The two-trial memory task based on spontaneous exploration of novelty was adapted from the ones described by Dellu et al. (22) in rats and Ladurelle et al. (6) in mice. Experiments were performed in a gray polyvinychloride Y-maze. The size of each arm was 40 cm long, 15 cm wide, and 35 cm high for rats and 30 cm long, 10 cm wide, and 17 cm high for mice. The floor of the maze was covered with odor-saturated sawdust that was mixed after each trial. The maze was placed in a sound-attenuated room under dim illumination. Numerous visual cues were placed on the walls of the testing room and kept constant during the entire behavioral testing.
The test consisted of two trials, separated by a 6-h intertrial interval. During the first trial (acquisition phase), one arm of the Y-maze (subsequently called novel arm) was closed with a guillotine door, thus allowing the animal to explore only the other two arms, for 5 min and 3 min for rats and mice, respectively. During the second trial (retention phase), the animals had access to the three arms for 5 min. The time spent in each arm of the maze was recorded manually every minute. Recognition was assumed to have occurred when the animal spent more time in the novel arm compared with the familiar ones. The total number of arm visits provided a measure of general motor activity.
Drugs were administered immediately after the acquisition phase, which thus was not affected, and retention was then tested after a long intertrial interval (6 h), the time at which vehicle-control animals did not show any discrimination in the exploration duration of the three arms of the Y-maze (chance level is 33%). The influence of steroids on retention performance are thus interpreted as being the result of changes in memory processing and neurotransmitter receptor activity occurring shortly after acquisition and during the consolidation phase as demonstrated earlier (3, 8).
Data Analysis.
A between-subjects design was used where each observation was made for a separate animal. Data were reported as the amount of time spent in the novel arm relative to the total duration of visits in the three arms (novel/total × 100) during the first 3 min. They were expressed as mean ± SEM and analyzed by using one-way ANOVA followed by Newmann–Keuls posthoc test. Significance was defined as P < 0.05 or P < 0.01.
The dose–effect curves of steroids and the coadministration studies required 2–3 experiments. The data were pooled between the different experiments because the variation was low for the values of control or same-drug doses-treated animals. When the Z score test was run there were at the most two outlying values per group.
Results
Animals were injected ICV with drug and vehicle solutions immediately after the acquisition trial of the test, and their memory recognition performances were evaluated after a 6-h intertrial interval, as described above. The retention test score was defined as the % time spent in the novel arm relative to that in the three arms of the Y-maze. The total number of visits in the three arms of the maze during the retention phase did not differ between controls and drug-injected groups of rats and mice in any experiment (data not shown).
Spatial Memory Performances of Rats and Mice After ICV Administration of PREGS.
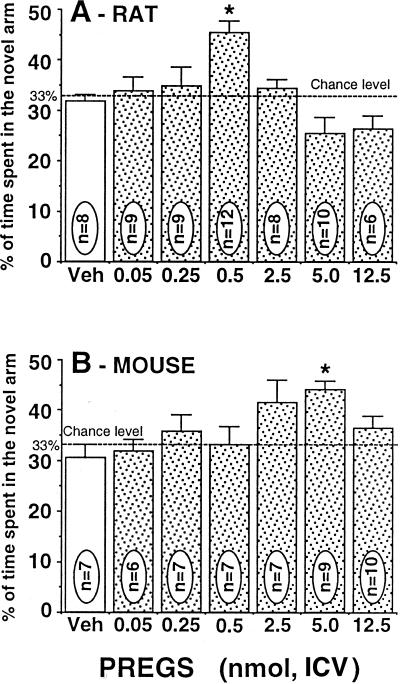
The effect of the neurosteroid PREGS on spatial memory recognition was analyzed by using several doses of the steroid (0.025–12.5 nmol, ICV), and U-inverted dose–response curves were obtained in both rats and mice (Fig. 1). Overall group comparison indicated that PREGS had a significant treatment effect in the two species [one-way ANOVA, F(6,56) = 6.34; P = 0.0001 for rats and F(7,53) = 3.66; P = 0.0027 for mice]. Detailed analysis using the Newman–Keuls test revealed that the % time spent in the novel arm was significantly higher in the steroid-injected groups at the PREGS doses of 0.5 nmol in rats (Fig. 1A) and 5.0 nmol in mice (Fig. 1B) relative to vehicle-control groups (P < 0.05). The other doses of PREGS did not significantly improve performance compared with the control groups.
Figure 1.
Dose–response curves of PREGS administered ICV on the retention performances of rats (A) and mice (B) tested in the Y-maze. Data were expressed as the percentage of time spent in the novel arm relative to the total duration of visits in the three arms during the first 3 min of the test (mean ± SEM). *, P < 0.05 vs. vehicle group, Newman–Keuls test. Veh, vehicle.
Spatial Memory Performances of Rats and Mice After ICV Administration of ent-PREGS.
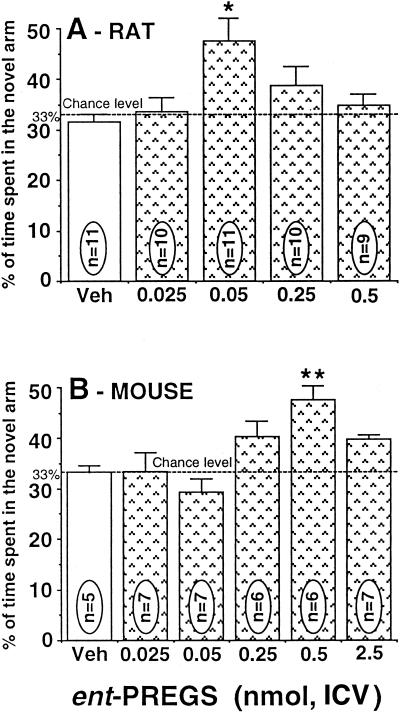
The effect of the synthetic ent-PREGS on spatial memory retention was examined at doses of 0.025–2.5 nmol ICV. ent-PREGS was found to affect spatial recognition performances of rats and mice in a dose-dependent and bell-shaped manner (Fig. 2). One-way ANOVA showed a significant effect of treatment, F(4,52) = 6.34, P = 0.0073 for rats and F(6,38) = 4.97, P = 0.0008 for mice. Posthoc Newman–Keuls test indicated that performance was significantly increased in groups given ent-PREGS at the doses of 0.05 nmol in rats (P < 0.05; Fig. 2A) and 0.5 nmol in mice (P < 0.01; Fig. 2B) compared with vehicle-control groups. The other doses of ent-PREGS did not significantly improve performance compared with the control groups.
Figure 2.
Dose–response curves of ent-PREGS administered ICV on the retention performances of rats (A) and mice (B) tested in the Y-maze. Data were expressed as the percentage of time spent in the novel arm relative to the total duration of visits in the three arms during the first 3 min of the test (mean ± SEM). *, P < 0.05; **, P < 0.01 vs. vehicle-control group, Newman–Keuls test. Veh, vehicle.
ICV Coadministration of Steroids with AP5 and Cognitive Performance.
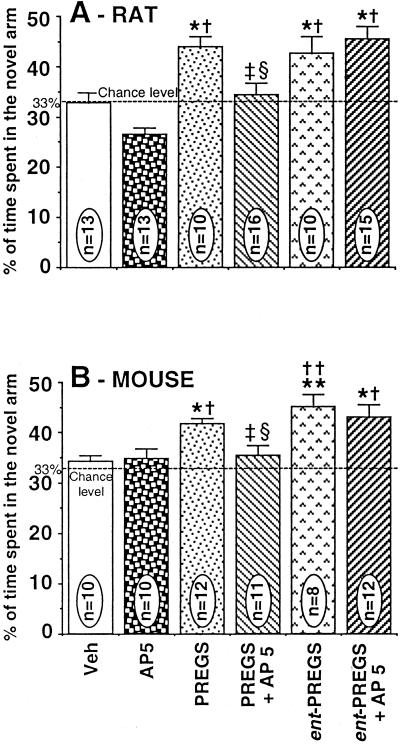
Fig. 3 shows the effects of the ICV injection of the competitive and selective NMDA receptors antagonist AP5, at ICV doses of 2 and 10 nmol in mice and rats, respectively, on the memory-enhancing effects of PREGS and ent-PREGS.
Figure 3.
Effects of ICV administration of AP5 on promnestic responses induced by PREGS or ent-PREGS in rats (A) and mice (B) tested in the Y-maze. The following doses were used for rats: AP5, 10 nmol; PREGS, 0.5 nmol; ent-PREGS, 0.05 nmol; and for mice: AP5, 2 nmol; PREGS, 5.0 nmol; ent-PREGS, 0.5 nmol. Data were expressed as the percentage of time spent in the novel arm relative to the total duration of visits in the three arms during the first 3 min of the test (mean ± SEM). *, P < 0.05 vs. vehicle group; **, P < 0.01 vs. vehicle group; †, P < 0.05 vs. AP5 group; ††, P < 0.01 vs. AP5 group; ‡, P < 0.05 vs. PREGS group; §, P < 0.05 vs. ent-PREGS group; Newman–Keuls test. Veh, vehicle.
Comparison between the recognition performances of all drug-treated groups (AP5, PREGS, ent-PREGS, PREGS + AP5, ent-PREGS + AP5) revealed a significant effect of treatment [F(5,71) = 9,39; P = 0.0001 in rats; F(5,57) = 6,14; P = 0.0001 in mice]. Detailed posthoc Newman–Keuls test analysis indicated that performance did not significantly differ between vehicle-control and AP5-treated groups of rats and mice (P > 0.05). As observed previously, the ICV effective doses of PREGS and ent-PREGS alone significantly increased recognition performances in rats (Fig. 3A) and mice (Fig. 3B) compared with vehicle-controls (P < 0.05).
When AP5 (rat: 10 nmol; mice: 2 nmol) was administered together with PREGS, the memory performances were significantly reduced in rats (Fig. 3A) as well as in mice (Fig. 3B) compared with the groups treated with the neurosteroid alone (P < 0.05). However, the animals coinjected with ent-PREGS and AP5 did not perform differently from those receiving ent-PREGS alone (P > 0.05). Preliminary results indicated that a higher dose of AP5 (25 nmol) was also ineffective on the promnestic effect of ent-PREGS (0.05 nmol) in rats (data not shown).
Discussion
The important finding of this study refers to the activity on memory of the non-natural enantiomer of the neurosteroid PREGS. Our findings demonstrate a positive action of a non-natural enantiomer of a natural steroid on memory.
Physiological PREGS and Spatial Memory.
Whereas the preventive effect of ICV-injected PREGS on experimental amnesia has been reported in several studies (25), only one report, to the best of our knowledge, has mentioned a promnestic effect of the neurosteroid after ICV administration in rats tested in a spatial recognition task (5). In the present study, results are supportive of a direct improvement by ICV injection of PREGS on memory retention. The effect of PREGS displays U-inverted shape dose–response relationship with the most robust effect at 0.5 nmol and 5 nmol for rats and mice, respectively. Although much of the research has pointed to the relevant role played by PREGS in enhancing learning and memory functions (see references in the Introduction), there are some discrepancies mostly related to the dose of the steroid, its route of administration (peripheral vs. central), and the behavioral paradigm tested. Results may, indeed, be difficult to interpret in the case of peripheral administration (9, 26) because hydrophilic-acidic compounds such as sulfated steroids poorly cross the blood-brain barrier (27, 28). In addition, in studies using foot-shock avoidance-based paradigms, results may not only reflect the specific modulation of memory processes, but also interfering emotional factors: for instance, the ICV administration of PREGS at 0.84–1680 pmol in rats and 1–100 pmol in mice has been found ineffective on retention performances in avoidance learning tasks (7, 8), whereas doses of the steroid between 2.4 × 10−18 mol and 10−14 mol infused into mice amygdala and hippocampus increased retention in the same paradigm (2). Our study extends previous work by showing that the natural PREGS directly improves short-term memory retention when injected centrally, in a task that exploits the innate tendency of rodents to explore a novel environment.
The NMDA receptor function is known to play a crucial role in information processing during spatial learning and memory (29, 30). This receptor complex has received most attention in behavioral pharmacology caused, in part, by availability of selective competitive antagonists, such as the most potent and widely used AP5 (31–33). Under our behavioral conditions, which specifically addressed drug effects on memory retention phase (and not on learning), the injection of AP5 alone did not significantly affect spatial memory performances because the values of AP5-treated animals did not significantly differ from the vehicle-control ones. In contrast, PREGS stimulated spatial memory retention, a result that was antagonized by the coadministration of AP5. Thus, evidence is shown here for an improvement of spatial recognition by PREGS (rather than reversal of amnesia) that is consistent with the known interaction of this neurosteroid with NMDA receptors.
Nonphysiological ent-PREGS and Spatial Memory.
Results presented here indicate that ent-PREGS is not only very active on spatial memory performances, but it is even more active than PREGS, the efficient ICV doses being roughly 10 times lower than that of PREGS, in both rats and mice, respectively. Interestingly, rats are more sensitive than mice to the action of ent-PREGS, as also observed for PREGS. More importantly, AP5 does not obliterate this effect under our behavioral conditions. These data suggest strongly the implication of a steroid binding site at the NMDA receptor that can discriminate between the molecular shapes of PREGS enantiomers. Thus, enantioselectivity is demonstrated for the in vivo action of PREGS at the NMDA receptors. In recent electrophysiological studies, ent-PREGS and PREGS (both at doses of 30 mM and 100 mM) similarly augmented the currents gated by NMDA (20 mM) in cultured neonatal rat hippocampal neurons, suggesting no enantioselectivity at NMDA receptors in vitro (Charles F. Zorumski, personal communication). These results may not be easily extrapolated to in vivo behavioral action and a complex pharmacological picture might be envisaged to explain the mechanism(s) underlying improvement of memory by ent-PREGS.
Because the memory-enhancing effect of ent-PREGS, in contrast to that of PREGS, does not involve the AP5 antagonist recognition site of the NMDA receptor complex, one can hypothesize that ent-PREGS and PREGS may act by distinct mechanisms. The activity of ent-PREGS may implicate, among other possibilities, either (i) a binding site specific for steroid enantiomer on the same or independent class of NMDA receptor or (ii) a steroid binding site on another class of glutamate receptors, such as α−amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and kainate receptors, or other neurotransmitter receptors, in particular Sigma1 and GABAA receptors. However, recent electrophysiological studies with cultured rat hippocampal neurons have shown that ent-PREGS and PREGS bind to the same site on GABAA receptor, suggesting that a chiral recognition site does not exist for these steroids (21). Besides different affinities for glutamate receptors, the larger effect of ent-PREGS on memory may result as well from another mechanism related to differences in brain metabolism between ent-PREGS and PREGS.
In summary, the work described here demonstrates the positive effects of ent-PREGS on memory function in rat and mouse, and thus proposes the enantioselective behavioral properties of PREGS. It is also a valuable step toward elucidating the molecular interactions between enantiomeric steroids and NMDA receptors for which a critical role in memory function and synaptic plasticity associated with long-term potentiation has been established (34–36). Further characterization of the nature of PREGS and ent-PREGS binding site(s) on these receptors is required, as well as the brain structures and neuronal pathways involved in the memory-improvement effect of the enantiomers. Moreover, it would be of great interest to address the pathophysiological significance and potential therapeutic use of such potent steroid memory enhancers in humans having mild cognitive impairment or neurodegenerative diseases such as Alzheimer's disease and other dementias.
Acknowledgments
This work was supported by an Artemis grant (to E.-E.B.), National Institutes of Health Grant GM 47969 (to D.F.C.), and the Institut National de la Santé et de la Recherche Médicale.
Abbreviations
- PREGS
pregnenolone sulfate
- ent-PREGS
PREGS enantiomer
- ICV
intracerebroventricular
- NMDA
N-methyl-d-aspartate
- AP5
dl-2-amino-5-phosphonovaleric acid
- GABAA
γ-aminobutyric acid type A
References
- 1.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu E E, Simon H. Brain Res. 1993;607:324–328. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- 4.Vallée M, Mayo W, Darnaudéry M, Corpéchot C, Young J, Koehl M, Le Moal M, Baulieu E E, Robel P, Simon H. Proc Natl Acad Sci USA. 1997;94:14865–14867. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnaudéry M, Koehl M, Piazza P V, Le Moal M, Mayo W. Brain Res. 2000;852:173–179. doi: 10.1016/s0006-8993(99)01964-2. [DOI] [PubMed] [Google Scholar]
- 6.Ladurelle N, Eychenne B, Denton D, Blair-West J, Schumacher M, Robel P, Baulieu E. Brain Res. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- 7.Mathis C, Paul S M, Crawley J N. Psychopharmacology. 1994;116:201–206. doi: 10.1007/BF02245063. [DOI] [PubMed] [Google Scholar]
- 8.Mathis C, Vogel E, Cagniard B, Criscuolo F, Ungerer A. Neuropharmacology. 1996;35:1057–1064. doi: 10.1016/s0028-3908(96)00041-x. [DOI] [PubMed] [Google Scholar]
- 9.Melchior C L, Ritzmann R F. Pharmacol Biochem Behav. 1996;53:51–56. doi: 10.1016/0091-3057(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 10.Meziane H, Mathis C, Paul S M, Ungerer A. Psychopharmacology. 1996;126:323–330. doi: 10.1007/BF02247383. [DOI] [PubMed] [Google Scholar]
- 11.Maurice T, Su T P, Privat A. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- 12.Reddy D S, Kulkarni S K. Brain Res. 1998;799:215–229. doi: 10.1016/s0006-8993(98)00419-3. [DOI] [PubMed] [Google Scholar]
- 13.Pallarès M, Darnaudéry M, Day J, Le Moal M, Mayo W. Neuroscience. 1998;87:551–558. doi: 10.1016/s0306-4522(98)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Paul S M, Purdy R H. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 15.Lambert J J, Belelli D, Hill-Venning C, Peters J A. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 16.Lambert J J, Belleli D, Shepherd S E, Pistis M, Peters J A. In: Neurosteroids: A New Regulatory Function in the Nervous System. Baulieu E E, Robel P, Schumacher M, editors. Totowa, NJ: Humana; 1999. pp. 125–142. [Google Scholar]
- 17.Olsen R W, Sapp D W. Adv Biochem Psychopharmacol. 1995;48:57–74. [PubMed] [Google Scholar]
- 18.Gee K W, Bolger M B, Brinton R E, Coirini H, McEwen B S. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- 19.Wittmer L L, Hu Y, Kalkbrenner M, Evers A S, Zorumski C F, Covey D F. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- 20.Covey D F, Nathan D, Kalkbrenner M, Nilsson K R, Hu Y, Zorumski C F, Evers A S. J Pharmacol Exp Ther. 2000;293:1009–1016. [PubMed] [Google Scholar]
- 21.Nilsson K R, Zorumski C F, Covey D F. J Med Chem. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- 22.Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. New York: Academic; 1986. [Google Scholar]
- 24.Franklin R B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 25.Mathis C, Meziane H, Ungerer A. J Soc Biol. 1999;193:299–306. [PubMed] [Google Scholar]
- 26.Cheney D L, Uzunov D, Guidotti A. NeuroReport. 1995;6:1697–1700. doi: 10.1097/00001756-199508000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Knapstein P, David A, Wu C-H, Archer D F, Flickinger G L, Touchstone J C. Steroids. 1968;11:885–896. doi: 10.1016/s0039-128x(68)80102-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang M D, Wahlstrom G, Backstrom T. J Steroid Biochem Mol Biol. 1997;62:299–306. doi: 10.1016/s0960-0760(97)00041-1. [DOI] [PubMed] [Google Scholar]
- 29.Morris R G. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannerman D M, Good M A, Butcher S P, Ramsay M, Morris R G. Nature (London) 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 31.Davies J, Francis A A, Jones A W, Watkins J C. Neurosci Lett. 1981;21:77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- 32.Butcher S P, Davis S, Morris R G. Eur Neuropsychopharmacol. 1990;1:15–20. doi: 10.1016/0924-977x(90)90005-u. [DOI] [PubMed] [Google Scholar]
- 33.Olverman H J, Jones A W, Watkins J C. Neuroscience. 1988;26:1–15. doi: 10.1016/0306-4522(88)90123-6. [DOI] [PubMed] [Google Scholar]
- 34.Collingridge G. Nature (London) 1987;330:604–605. doi: 10.1038/330604a0. [DOI] [PubMed] [Google Scholar]
- 35.Collingridge G L, Singer W. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 36.Tsien J Z, Huerta P T, Tonegawa S. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]