Abstract
Background:
Excessive fluid administration for saving patients from hypovolemic shocks is one of the main causes of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS). The purpose of this paper is to survey the relationship between fluid resuscitation and increase intra-abdominal pressure (IAP).
Materials and Methods:
The present descriptive-analytical study recruited 100 patients with confirmed abdominal trauma and presenting to emergency departments. The cases with high IAP measured through the bladder were identified as developing ACS in case of having comorbidities involving two of the following systems: respiratory system, renal system or cardiovascular system. The volume of the fluids administered was compared in the first 24 h in subjects with and without ACS.
Results:
Of 100 patients with abdominal trauma, whose IAP was measured, 28 cases developed ACS. The mean volume of the fluids received was found to be significantly higher in the patients with ACS (8772 ml) compared to in those without (5404 ml). As a complication of excessive fluid administration, IAH can seriously threaten the patient's life.
Conclusions:
Excessive fluid resuscitation causes ACS among the critically ill or injured patients such as abdominal trauma, pelvic fracture and intra-abdominal organ injuries hence to prevent this complication in all patients requiring short-term excessive administration of fluids, great care, and sensitivity are required to constantly control IAP and adjust the fluid administration.
Key Words: Abdominal compartment syndrome, fluid resuscitation, intra-abdominal hypertension, trauma
INTRODUCTION
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are associated with increased morbidity and mortality among critically ill patients.[1,2,3,4,5] IAH/ACS not only affects the function of intra-abdominal organs but also causes physiological changes and malfunctioning of organs beyond the abdominal cavity because of the limited space and its close anatomic relationship with contiguous cavities. From the pathophysiological perspective, IAH/ACS can cause cardiovascular, respiratory and renal dysfunction and ultimately cause multiple organ failure. IAH may be acute or chronic and might turn into a fatal ACS in case of sudden functional changes in vital organs such as cardiovascular, respiratory, and renal systems.[1,2,3,4] Primary IAH/ACS is a condition associated with injury or disease in the abdominopelvic cavity[1] and mostly observed in patients with severe abdominopelvic trauma, severe pelvic fractures with hemorrhage and retroperitoneal hematoma, liver-transplant patients and in those who suffer intra-abdominal bleeding for any reasons and require radiological interventions and urgent damage control surgeries.[6] Ileus, retroperitoneal edema, ascites, mesenteric ischemia, severe pancreatitis, peritonitis, and space-occupying lesions such as tumor are examples that less involved in the creation of this syndrome.[7] Whereas IAH/ACS occurs as a result of a condition that originates outside the abdomen in a scenario lacking primary intraperitoneal injury or intervention.[1,8,9] This state appears to be related to visceral, abdominal wall, and retroperitoneal edema such as severe hypovolemic shocks, in which massive volumes of different fluids are required to be administered to resuscitate and restore patient's hemodynamic status.[9] Critically ill patients may develop a positive fluid balance because of (a) excessive fluid administration during the initial resuscitation phase of a patient who presents with hypovolemic shock,[6,7,8,9,10] (b) too little fluid removal or mobilization following the initial resuscitation phase,[8,9] (c) the type of fluids administered,[1,2,3,4,5,11,12,13] and (d) any combination of the above.[11,12,13] In the trauma setting, hemorrhagic shock requiring laparotomy for hemorrhage control is a major risk factor for IAH/ACS. Resuscitation with large amounts of crystalloids, activation of inflammatory mediators leading to capillary leakage, and reperfusion injury contribute to intestinal edema, which may increase the risk of IAH and subsequent ACS.[4,5,6,7,8] Hypervolemia coupled with severe inflammatory reactions can increase the risk of ACS in patients.[14,15,16,17,18] Rapid administration of large-volume fluids can also contribute to this condition.[9,10,13,19] Whereas fluids should be seen as drugs with indications, contra-indications and potential beneficial and adverse effects. Therefore, not only the type of fluids but also dose, timing, and speed of administration may influence the effect of the fluid.[15,16,17,18]
The volume of resuscitative fluid administered during trauma-associated shock, typically hemorrhagic shock is believed to correlate with the risk of ACS, with crystalloid resuscitation, in particular, being singled out as a major culprit.[5,6,7,8,20] Therefore, in the appropriate clinical setting, a high index of suspicion, judicious measurement of intra-abdominal pressure (IAP), and early evaluation for organ dysfunction are necessary for early identification and intervention, with the goal of reducing the associated morbidity and mortality and any measure that safely reduces the amount of fluid given without compromising resuscitation may reduce the incidence and severity of IAH/ACS and hence improve outcomes. The goal of our study was to investigate the relationship between IAH and fluid resuscitation in patients presenting to hospital with blunt abdominal trauma and to recall risk of IAH/ACS during traumatic hemorrhagic shock resuscitation.
MATERIALS AND METHODS
The present descriptive-analytical study was conducted to investigate the relationship of IAH and ACS with fluid resuscitation in patients presenting to hospital with blunt abdominal trauma in a 1-year period. The study population was patients with blunt abdominal trauma who referred to the emergency department of the hospital. The inclusion criteria include as follows: nonpenetrating abdominal trauma patient whose abdominal trauma was confirmed by an emergency physician or ultrasound or computerize tomography scanning. Patients who did not satisfy themselves or their companions for entering the study, the person who did not have a Foley catheter for any reason or had a history of the problem in urinary tract, and patients with damage to the genitalia region were excluded from the study.
IAP can be measured by direct or indirect measurement methods. The direct method that measure IAP from the peritoneal cavity is invasive and not always possible in emergency departments. Indirect IAP measurement is noninvasive and can be applied from the bladder cavity. Over the years, the indirect IAP measurement through the bladder evolved as the gold standard method for the measurement of IAP.[20] The bladder acts as a passive diaphragm whose pressure accurately reflects IAP with fluid volumes of 50–100 ml.[1,20,21] Thus, Bladder pressure measurement used in the present study.
Ethical approval
The present study was approved by Ethical Committee Medical Sciences University of Ahvaz. After selecting the eligible participant, the researcher was introduced to them, and the objectives of the study were elaborated for the participants. The informed consent was obtained from the subjects, and they were assured that their information will remain confidential.
The data collection tools comprised checklists of demographic information and other data required in the study. IAP measurement tools consisted of Foley catheters, intravenous sets, normal saline solutions, 50-ml syringes, sterile gloves, and rulers. Blunt abdominal trauma patients admitted to the emergency department who confirmed by emergency physicians, abdominal ultrasound, and computed tomography scan and had a urinary catheter was selected. Urine drainage bags were then removed from Foley catheters, and 60 ml of normal saline solution was instilled into the bladder after disinfecting the urinary catheter's tip in a fully sterile fashion (in children, the amount of liquid is injected into the bladder 1 ml/kg up to 20 ml).
The sterile intravenous set was then connected to the Foley catheter's tip at a 90° angle with the patient's hip, and the pubic symphysis was set as a baseline. The measurement was performed 30-60 s after instillation to allow bladder detrusor muscle relaxation. The clamp was then opened to let the fluid column rise through the intravenous set from the bladder. A ruler was used to measure the maximum intravenous fluid height from the baseline when the fluid level remained constant at end expiration. This was first performed upon the patient's admission to the emergency department and repeated every 4 h up to 24 h after the admission.
The World Society of ACS (WSACS) The WSACS defines IAP as the pressure within the abdominal cavity, measured at end-expiration in a relaxed, supine patient. It defines IAH as a sustained elevation of IAP >12 mmHg. The WSACS defines ACS as a sustained increase of IAP >20 mm Hg that is associated with the onset of organ dysfunction.[18,19,20,21] Currently, no standardized definitions specific for infants and children are available. Children have lower mean arterial pressures than adults do, so multiorgan failure may occur in children at lower IAP thresholds than those defined by WSACS. As a result, lower IAP cutoff values of 12 and 15 mm Hg have been used to define ACS in children.[17,18,19,20,21,22] For an individual child, the actual IAP value may be less important than the impact of the pressure on organ function. Hence, ACS in a child may be more appropriately defined as an IAP of >10 mm Hg with evidence of new organ dysfunction or failure.[21,22] Therefore, intra-bladder pressure, urine volume, blood oxygenation, and blood pressure were used to diagnose IAP and ACS.
For IAPs exceeding 20 cmH2O (15 mmHg) in adult and 13.6 cm H2O (10 mmHg), the patient was identified as an IAH sample. ACS refers to IAPs exceeding 15 mmHg (10 mmHg in children) coupled with the impairment of vital systems such as the cardiovascular, renal, and respiratory system The researcher then confirmed the ACS if two out of three symptoms, including decreased urine output (<0.5 ml/kg/h), hypotension (blood pressure <90 mm/Hg in adult and <70 mm/Hg in children), and hypoxemia (O2 saturation <80%) persist.[14,20,21]
The amount and type of the fluids administered were recorded in all the patients on their admission then the relationship between ACS and the amount of the fluids received from admission was evaluated. Descriptive and inferential statistics were used to statistically analyze the data and frequency distribution tables to classify and summarize the data. The collected and classified data were analyzed in SPSS package 18.0 for Windows (PASW Statistics for Windows Chicago: SPSS Inc.).
RESULTS
The findings revealed 28 cases (28%) with ACS in a total of 100 patients presenting to hospital emergency departments with blunt abdominal trauma, whose IAP was measured over 1 year period. The majority of the study subjects with abdominal trauma (87%) and 23 people (82.14%) of the cases diagnosed with ACS were men, while 5 (17.86%) cases with ACS were women. The Fisher's exact test found no significant relationships between gender and the prevalence of ACS [P = 0.508, Table 1].
Table 1.
Distribution of the participants in terms of developing abdominal compartment syndrome and gender

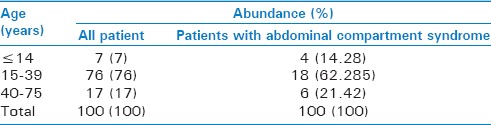
In terms of age distribution, the majority of the patients presenting with abdominal trauma (76%) and whose IAP was measured as well as those diagnosed with ACS (64.28%) were 15–39 years old. The Fisher's exact test showed no significant relationships between age and the prevalence of ACS (P = 0.599) [Table 2].
Table 2.
Distribution of the participants according to age
Based on the mean amount of fluid administration, the study patients with ACS received 6107 ml of crystalloids including Ringer's, lactated Ringer's and 0.9% saline solutions as well as 3.86 blood bags (965 ml), 5.56 fresh frozen plasma (FFP) bags (1390 ml), and 6.20 platelets bags (310 ml) within the first 24 h from hospital admission, while the subjects without ACS received on average 4493 ml of crystalloids, 0.83 blood bag (207.5 ml), 0.19 FFP bag (700 ml), and 0.07 platelets bag (3.5 ml) over the same period. The mean volumes of all four types of the fluids received are therefore found to be higher in the patients with ACS than in those without ACS. The t-test also confirmed significant differences between the patients with and without ACS in terms of the amount of the fluids received (P < 0.001) [Table 3]. The results also showed that people with pelvic fracture had a significantly higher incidence of abdominal compartment syndrome than the rest of the patient (P < 0.001) [Table 4].
Table 3.
Subjects in the amount and type of fluid intake in 24 h
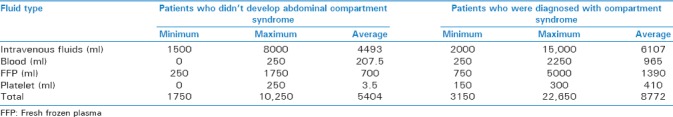
Table 4.
Distribution of the participants according specific injury

DISCUSSION
The study conducted on 1976 patients in 2016 by Hwabejire et al. titled, “ACS in trauma patients with hemorrhagic shocks” found 122 (6.2%) patients with ACS who had received significantly higher volumes of fluids compared to those without ACS.[11] This study is consistent with the present study in terms of the significant relationship observed between fluid resuscitation and IAH in the two groups with and without ACS. The present study, however, found a significantly higher prevalence of ACS in the study subjects than the figure obtained by Hwabjire et al. This difference can be justified by the fact that the present study was conducted on patients with blunt abdominal trauma, whose abdominal injuries were confirmed by the emergency department physician or clinical studies such as ultrasound, and that abdominal trauma and visceral damage can underlie IAH and ACS, while the former study was conducted on trauma subjects diagnosed with shocks.
The study conducted over a 7-year period by Cothren et al. found 54 patients with ACS, 41 (75.9%) of whom were posttraumatic and 13 (24.1%) suffered the syndrome due to internal problems. All subjects including the surgical and medical patients also received a mean volume of 16.5 ± 1.5 L fluids in the first 24 h.[22] The study conducted by Oda et al., (2006) titled “Resuscitation fluid volume and ACS in patients with major burns” found 8 (16.6%) patients in 48 cases with burns >30% TBSA to have developed ACS within a mean duration of 18.3 h after the injury. The resuscitation volume was also 0.4 ± 0.11 L/kg of body weight within the first 24 h of presentation, suggesting significant relationships between IAH and resuscitation volume. The patients with ACS also were found to have received 300 ml/kg of body weight fluids within the first 24 h.[21] Although this study was conducted on burn patients, the significantly positive relationship found between fluids volume and IAH is consistent with the present study. Moreover, the higher prevalence obtained in the present study can be a result of selecting patients with blunt abdominal trauma as the study population. The systematic review conducted by Azzopardi et al. on ACS in adults with severe burns found that high volume of fluids administered can increase the risk of ACS and that crystalloid fluids can be proposed as one of the main causes of ACS when IAP is not monitored.[13] Which is consistent with the present study.
A review of the studies conducted on ACS and its relationships with the volume of administered fluids, suggests that prompting administration of large fluids volume is one of the main factors contributing to IAH and ACS. Therefore, patients who for any reason are required to prompting administration of large fluid volumes, such as patients with extensive burns, severe abdominal trauma, retroperitoneal hematoma, unstable pelvic fractures with bleeding, hemorrhagic shock, extensive abdominal surgery, must be vigilant and with consecutive measurement of IAP, control of cardiovascular, respiratory, and renal function, and closely monitor the exact amount of administrated fluids prevent an increase in IAP and its progress to ACS. Because it is highly lethal syndrome and, as is clear from the results of this research of 28 patients diagnosed with this syndrome, 21 (75%) patients died.
CONCLUSIONS
Patients with blunt abdominal trauma and severe injury such as pelvic fracture, internal bleeding, and damage to vital organs in the abdomen that resuscitate with a large volume of liquids because of hemodynamic shock, are associated with IAH/ACS and high mortality and morbidity rate.[1,2,3,4,21,22] Thus, ACS is a clinically important problem in critically ill patients in which massive volumes of different fluids are required to be administered to resuscitate and restore their hemodynamic status.[7,8] That can be ameliorated by early recognition of IAH, optimal fluid resuscitation, and appropriate medical or surgical intervention for IAH and impending ACS. Bedside critical care and appropriate clinical setting, nurses are responsible for accurately measuring IAP and alerting physicians about important observed changes. Nurses “knowledge of IAH and ACS, awareness of the patients at risk for IAH, and recognition of IAH and progression to ACS are important. Especially control the amount of administered crystalloid fluids prevents the progression of IAP to ACS as a seriously fatal condition. A high index of suspicion, judicious measurement of IAP, active IAP surveillance for at-risk patients, and early evaluation for organ dysfunction are essential in early detection and management of ACS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis. 2011;57:159–69. doi: 10.1053/j.ajkd.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Bradley-Stevenson C, Vyas H. Intra-abdominal hypertension and the abdominal compartment syndrome. Curr Paediatr. 2004;14:191–6. [Google Scholar]
- 3.Ball C, Kirkpatrick A. Intra-abdominal hypertension and the abdominal compartment syndrome. Scan J Surg. 2007;96:197–204. doi: 10.1177/145749690709600303. [DOI] [PubMed] [Google Scholar]
- 4.Ali SR, Mohammad H, Sara S. Evaluation of the relationship between pelvic fracture and abdominal compartment syndrome in traumatic patients. J Emerg Trauma Shock. 2013;6:176–9. doi: 10.4103/0974-2700.115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balogh Z, Moore FA, Moore EE, Biffl WL. Secondary abdominal compartment syndrome: A potential threat for all trauma clinicians. Injury. 2007;38:272–9. doi: 10.1016/j.injury.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the world society of the abdominal compartment syndrome. Intensive Care Med. 2013;39:1190–206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. Definitions. Intensive Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML, De Laet IE, De Waele JJ, Kirkpatrick AW. Intra-abdominal hypertension: Definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol. 2013;27:249–70. doi: 10.1016/j.bpa.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Jansen JO, Thomas R, Loudon MA, Brooks A. Damage control resuscitation for patients with major trauma. BMJ. 2009;338:b1778. doi: 10.1136/bmj.b1778. [DOI] [PubMed] [Google Scholar]
- 10.Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–62. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 11.Hwabejire JO, Nembhard CE, Oyetunji TA, Seyoum T, Siram SM, Cornwell EE, 3rd, et al. Abdominal compartment syndrome in traumatic hemorrhagic shock: Is there a fluid resuscitation inflection point associated with increased risk? Am J Surg. 2016;211:733–8. doi: 10.1016/j.amjsurg.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Strang SG, Van Lieshout EM, Breederveld RS, Van Waes OJ. A systematic review on intra-abdominal pressure in severely burned patients. Burns. 2014;40:9–16. doi: 10.1016/j.burns.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Azzopardi EA, McWilliams B, Iyer S, Whitaker IS. Fluid resuscitation in adults with severe burns at risk of secondary abdominal compartment syndrome – An evidence based systematic review. Burns. 2009;35:911–20. doi: 10.1016/j.burns.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Kowal-Vern A, Ortegel J, Bourdon P, Chakrin A, Latenser BA, Kimball D, et al. Elevated cytokine levels in peritoneal fluid from burned patients with intra-abdominal hypertension and abdominal compartment syndrome. Burns. 2006;32:563–9. doi: 10.1016/j.burns.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Fujita T. Fluid resuscitation for burn patients at risk for abdominal complications. J Am Coll Surg. 2013;216:1027. doi: 10.1016/j.jamcollsurg.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Peng Y, Yuan Z, Cheng W, Wu J, Tang J, et al. Fluid resuscitation for major burn patients with the TMMU protocol. Burns. 2009;35:1118–23. doi: 10.1016/j.burns.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 17.McGuire MD, Heung M. Fluid as a drug: Balancing resuscitation and fluid overload in the intensive care setting. Adv Chronic Kidney Dis. 2016;23:152–9. doi: 10.1053/j.ackd.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Tan HL, Wijeweera O, Onigkeit J. Inferior vena cava guided fluid resuscitation–fact or fiction? Trends Anaesth Crit Care. 2015;5:70–5. [Google Scholar]
- 19.Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016;31:96–100. doi: 10.1016/j.jcrc.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Divarci E, Karapinar B, Yalaz M, Ergun O, Celik A. Incidence and prognosis of intraabdominal hypertension and abdominal compartment syndrome in children. J Pediatr Surg. 2016;51:503–7. doi: 10.1016/j.jpedsurg.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Oda J, Yamashita K, Inoue T, Harunari N, Ode Y, Mega K, et al. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns. 2006;32:151–4. doi: 10.1016/j.burns.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Cothren CC, Moore EE, Johnson JL, Moore JB. Outcomes in surgical versus medical patients with the secondary abdominal compartment syndrome. Am J Surg. 2007;194:804–7. doi: 10.1016/j.amjsurg.2007.08.023. [DOI] [PubMed] [Google Scholar]


