Abstract
D-aspartate, a natural and endogenous amino acid, widely exists in animal tissues and can be synthesized through aspartate racemase and transformed by D-aspartate oxidase (DDO). D-aspartate mainly serves as a neurotransmitter and has been demonstrated to exhibit various physiological functions, including nutritional potential, regulation on reproduction and hormone biology, and neuron protection. This article mainly reviews the synthesis, racemization, and physiological functions of D-aspartate with emphasis on the potential in diseases.
Keywords: D-aspartate, Biosynthesis, Racemization, Nutrition, Functions
1. Introduction
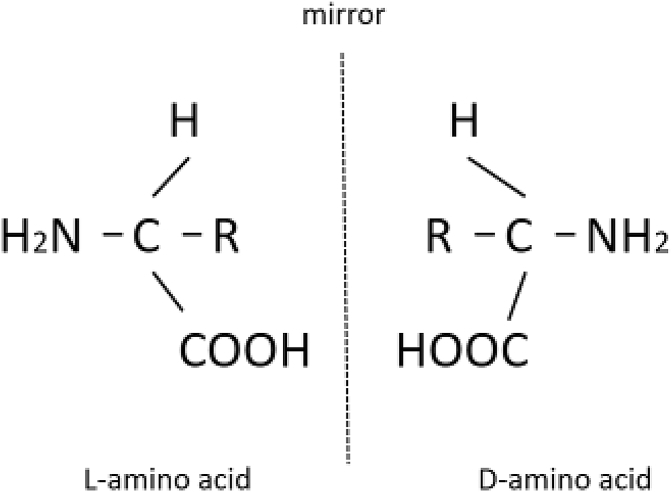
Most amino acids include asymmetric-carbon atoms and exhibit 2 stereoisomers: left-handed (L-forms) and right-handed (D-forms) (Fig. 1) (Katane and Homma, 2011, Fujii et al., 2011), while only L-forms occupy the protein-building residues (Chung and Reed, 2015). Thus, some researchers speculated that only L-amino acids play functional roles in the body (Katane and Homma, 2011).
Fig. 1.
D- and L-forms of amino acid (Chung and Reed, 2015).
Aspartate widely exists in animal tissues with D-enantiomers, especially in nervous and reproductive systems (Ota et al., 2012, Di Fiore et al., 2014). In 1977, high concentration of D-aspartate has been firstly found in the brain of Octopus vulgaris Lam (D'Aniello and Giuditta, 1977) and D-aspartate in other tissues has been also detected subsequently, including heart, lung, stomach, intestine (Motoie et al., 2009), salivary glands (Masuda et al., 2003), seminal plasma and spermatozoa (D'Aniello et al., 2005), neural system (D'Aniello and Garcia-Fernàndez, 2006), knee cartilage (Fisher et al., 2006), testis (Lamanna et al., 2006), pre-ovulatory ovarian follicle (D'Aniello et al., 2007), and retinae (Opere et al., 2009). Wide existence of D-aspartate in body tissues suggests a potential physiological function in animals. Indeed, various studies focused on the regulatory effects of D-aspartate on the nervous (D'Aniello, 2007), endocrine, and reproduction (Lamanna et al., 2007). For example, D-aspartate contributes to the synthesis and release of glucocorticoids, prolactin, oxytocin, and steroids (D'Aniello et al., 2017). Currently, nutritional potential of D-aspartate has been investigated and the results found that low-dosage of D-aspartate improved growth performance and stress-resistance in animal industries. Here, we reviewed the biosynthesis, racemization and functional roles of D-aspartate in animals with emphasis on D-aspartate related signaling pathways.
2. D-aspartate in biosynthesis and racemization
D-aspartate can be synthesized in body tissues, which was firstly found in rat pheochromocytoma PC-12 cells (Koyama et al., 2005). In the body, D-aspartate racemase mainly contributes to the biosynthesis of D-aspartate by converting L-aspartate to D-aspartate (D'Aniello et al., 2011, Kim et al., 2010). Mouse glutamic-oxaloacetic transaminase 1-like 1 has been reported to encode Asp racemase and synthesize substantially D-aspartate from L-aspartate in adult neurogenesis (Tanaka-Hayashi et al., 2015, Kim et al., 2010, Matsuda et al., 2015). Synthesized D-aspartate can be oxidized by D-aspartate oxidase (DDO) and tissue D-aspartate concentration is mainly controlled by DDO activity, as DDO is the only enzyme known to selectively degrade D-aspartate (Van Veldhoven and Mannaerts, 1991, D'Aniello and Petrucelli, 1993, Cristino et al., 2015). For example, D-aspartate is abundant during the animal embryo and parturient of the brain and markedly reduced in the maturity due to the change of DDO activity (Punzo et al., 2016, Han et al., 2015). Furthermore, inhibition of DDO activity shows a sustained overflow of extracellular D-aspartate (Punzo et al., 2016). In DDO−/− mice (lacking DDO gene), the amounts of D-aspartate drastically increase compared with the wild-type mice (DDO+/+) (Han et al., 2015).
3. Functions of D-aspartate
3.1. Potential nutrition
Lower-dosage D-aspartate improves growth performance, indicating a potential nutritional function in animal nutrition. For example, D-amino acid mixed with L-amino acid improve mouse growth performance, while excess D-amino acid inhibit growth (Berg, 1953). Similarly, oral administration of D-aspartate (0, 3.75, 7.5 and 15 mmol/kg body weight) linearly decreased feed intake (Erwan et al., 2013). Zhang et al. (2015) reported that excess D-aspartate acts as a potent damaging agent for DNA, vitamin C and E, and thus inhibits cell growth. The possible mechanism of D-aspartate in the body may be N-methyl-D-aspartate receptors (NMDAR) and L-Glu/L-aspartate transporter signals. D-aspartate can activate metabotropic glutamate receptors 5 (mGlu5) (Molinaro et al., 2010) and stimulate NMDAR-mediated neurotransmission by directly acting at the glutamate-binding site (GluN1 and GluN2B subunits) of NMDAR, thus functioning as an endogenous agonist for this subclass of glutamate receptors (Errico et al., 2013, Cristino et al., 2015). Moreover, when intracellular uptake the D-aspartate, D-aspartate can be released by L-Glu/L-aspartate transporter that makes use of the Ca2+-mediated manner after electrochemical stimulation (Savage et al., 2001, Muzzolini et al., 1997) and its uptake by Na+/K+ electrical or chemical manner to against their concentration gradient (Adachi et al., 2004, Koyama et al., 2005, Errico et al., 2015). Therefore, we speculate that the proper dose of D-aspartate may play a potential value in nutrition and excess D-aspartate blocks the synthesis and metabolism of other important substances in the body.
3.2. D-aspartate in reproduction
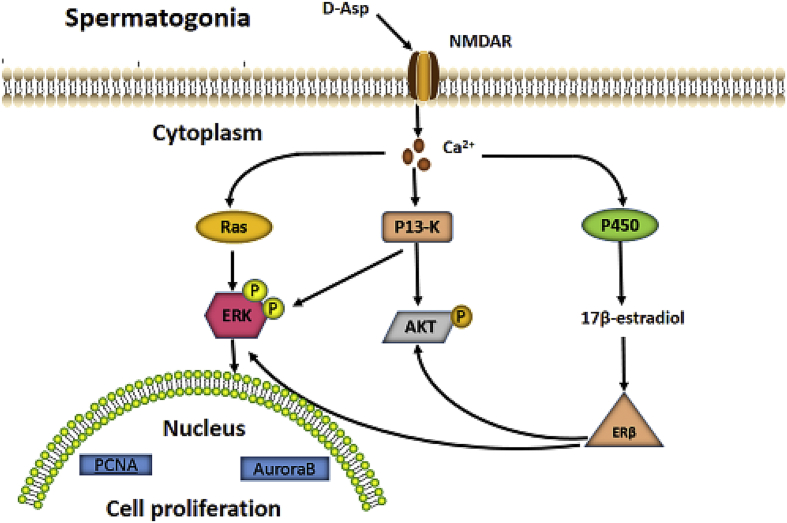
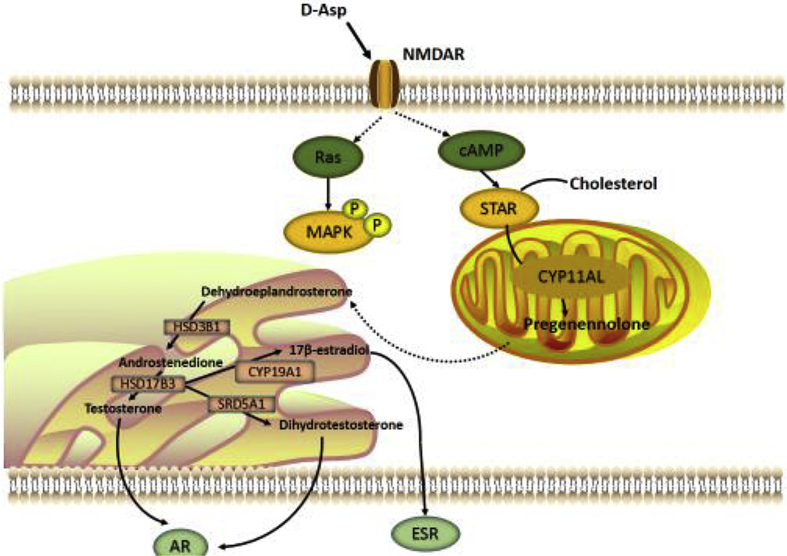
D-aspartate has a positive effect on reproduction in animals by improving sperm quality, testosterone synthesis, spermatogenesis, and steroidogenesis proliferation (Fiore et al., 2017). For example, dietary 200 mg/kg per day BW D-aspartate can improve fresh and post-thawed sperm quality and post-thawed sperm fertility in male broiler breed, especially for sperm cryopreservation (Ansari et al., 2017). Giacone et al. (2017) reported that the effect of D-aspartate on reproduction may be due to the antioxidant function and protecting DNA integrity, which may further improve sperm motility and embryo development (Barbato et al., 2017). Santillo et al. (2016) also reported that D-aspartate activates proliferative pathways in GC-1 cells (mitotic germ cell). Meanwhile, D-aspartate in rats improves testosterone synthesis and activates testicular NMDAR, extracellular signal-regulated kinase (ERK) pathway, and androgen receptor (Santillo et al., 2014). Similarly, D-aspartate stimulates spermatogonia proliferation through the NMDAR-mediated ERK and ser-ine-threonine protein kinase (AKT) pathways (Palazzo et al., 2016), which further activates the P450 aromatase/ERb pathway (Santillo et al., 2016) (Fig. 2). D-aspartate also either stimulates steroidogenesis and spermatogenesis proliferation by binding to the NMDAR and thereby increasing the expression of the steroidogenic cascade enzymes by cAMP or stimulates mitogen-activated protein kinase (MAPK) and AKT pathways and then increased the expression of androgen receptor (Fig. 3) (Di Fiore et al., 2016).
Fig. 2.
Schematic representation of molecular pathways activated by D-aspartate in spermatogonia (Santillo et al., 2016). NMDAR = N-methyl-D-aspartate receptor; Ras = renin-angiotensin system; PI3-K = phosphatidylinositol-4,5-bisphosphate 3-kinase; ERK = extracellular signal-regulated kinase; AKT = serine-threonine kinase; ERβ = estrogen receptor β; PCNA = proliferation cell nuclear antigen.
Fig. 3.
Schematic representation of molecular pathways activated by D-aspartete in Leydig cell (Di Fiore et al., 2016). cAMP = cyclic adenosine monophosphate; MAPK = mitogen-activated protein kinase; STAR = steroidogenic acute regulatory protein; CYP11AL = P450 cholesterol side-chain cleavage; HSD3B1 = 3β-hydroxysteroid dehydrogenase; HSD17B3 = 17β-hydroxysteroid dehydrogenase; CYP19A1 = cytochrome P450 aromatase; SRD5A1 = 5α-reductase; AR = androgen receptor; ESR = estrogen receptor.
3.3. Serving as endogenous neurotransmitter
D-aspartate is founded in the multitude brain regions (Palazzo et al., 2016), such as hippocampus and prefrontal. In mammals, D-aspartate is found in synaptic vesicles of nerve endings (D'Aniello et al., 2011) and maintains long-term potentiation and regulatory effect on the synaptic plasticity decay in the hippocampus of rodents (Errico et al., 2011a, Errico et al., 2011b). D-aspartate excites dopamine neurons and exerts a protective uptake mechanism by stimulating NMDA receptor and metabotropic glutamate receptors to prevent the neuronal degeneration (Krashia et al., 2016). Also, DDO with the change of D-aspartate concentration has been reported to regulate the homeostasis of glutamatergic system and, thus, suppresses neurodegenerative processes (Cristino et al., 2015). Moreover, D-aspartate plays important roles in plasticity, physiology, neuronal dendritic morphology, gray matter volume, and brain activity in mammals (Errico et al., 2014). For example, treatment with D-aspartate (20 mmol/L) in drinking water markedly alleviates mechanical allodynia through glutamate neurotransmission normalization after spared nerve injury in neuropathic mice (Palazzo et al., 2016).
3.4. Regulation of hormone release
D-aspartate plays an important role in the synthesis and release of hormones, such as glucocorticoids, prolactin, oxytocin, growth hormone (D’Aniello et al., 2000), and steroids in the prefrontal cortex and hippocampus (D'Aniello et al., 2017). D-aspartate was reported to promote the release of gonadotropin-releasing hormone by stimulating luteinizing hormone secretion in the pituitary gland (D'Aniello et al., 2000). Likewise, D-aspartate is positively associated with serum luteinizing hormone, androstenedione, and testosterone concentrations through increasing steroidogenic acute regulatory protein, P450 cholesterol side-chain cleavage enzyme, and 3b-hydroxysteroid dehydrogenase/D5-D4 isomerases gene expressions in rat testis and Leydig cells (Raucci et al., 2014).
4. D-aspartate in diseases
Due to antioxidant potential, D-aspartate plays an important role in the endocrine and nervous system to prevent or treat neurological diseases and reproductive diseases (Afraei et al., 2017, Boccella et al., 2015, D'Aniello et al., 2017). D-aspartate regulates nociceptive specific neuron electrophysiological activity and behavior to reflect the pain conditions (Boccella et al., 2015). Meanwhile, D-aspartate also serves as a pharmacological tool in chronic pain to relieve related psychiatric condition, including Alzheimer's disease (D'Aniello et al., 2017). Injection of D-aspartate has a sedative effect with and without a hypnotic effect (Erwan et al., 2014). The potential mechanism may be that D-aspartate can activate NMDAR-mediated neurotransmission in the core of the postsynaptic density and then enhances glutamate signaling in the postsynaptic site, which is responsible to antipsychotic and schizophrenia treatment (de Bartolomeis et al., 2015).
5. Conclusion
D-aspartate is widely detected in animals and human with D-enantiomers and can be metabolized by D-aspartate racemase and DDO. D-aspartate mainly serves as a neurotransmitter and plays important roles in growth performance, reproduction, nervous, and endocrine mediatory functions, whereas the effects of D-aspartate on intestinal absorption and mechanism of feed intake need further studies.
Conflicts of interest
No potential conflicts of interest were reported by the authors.
Acknowledgments
This study was supported by Hunan Province key research and development projects (2017NK2321), National Basic Research Program of China (973) (2013CB127301), National Natural Science Foundation of China (31472106), and China Agriculture Research System (CARS-35).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Tiejun Li, Email: tjli@isa.ac.cn.
Yulong Yin, Email: yinyulong@isa.ac.cn.
References
- Adachi M., Koyama H., Long Z., Sekine M., Furuchi T., Imai K. L-Glutamate in the extracellular space regulates endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Arch Biochem Biophys. 2004;424(1):89–96. doi: 10.1016/j.abb.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Afraei S., D'Aniello A., Sedaghat R., Ekhtiari P., Azizi G., Tabrizian N. Therapeutic effects of D-aspartate in a mouse model of multiple sclerosis. J Food Drug Anal. 2017;25(3):699–708. doi: 10.1016/j.jfda.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Sharafi M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology. 2017;92:69–74. doi: 10.1016/j.theriogenology.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Barbato V., Talevi R., Braun S., Merolla A., Sudhakaran S., Longobardi S. Supplementation of sperm media with zinc, D-aspartate and co-enzyme Q10 protects bull sperm against exogenous oxidative stress and improves their ability to support embryo development. Zygote. 2017;25(2):168–175. doi: 10.1017/S0967199416000459. [DOI] [PubMed] [Google Scholar]
- Berg C.P. Physiology of the D-amino acids. Ark Patol. 1953;33(2):145–189. doi: 10.1152/physrev.1953.33.2.145. [DOI] [PubMed] [Google Scholar]
- Boccella S., Vacca V., Errico F., Marinelli S., Squillace M., Guida F. D-aspartate modulates nociceptive-specific neuron activity and pain threshold in inflammatory and neuropathic pain condition in mice. BioMed Res Int. 2015;2015:905906. doi: 10.1155/2015/905906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.Y., Reed S. IgE binding to peanut allergens is inhibited by combined D-aspartic and D-glutamic acids. Food Chem. 2015;166:248–253. doi: 10.1016/j.foodchem.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Cristino L., Luongo L., Squillace M., Paolone G., Mango D., Piccinin S. d-Aspartate oxidase influences glutamatergic system homeostasis in mammalian brain. Neurobiol Aging. 2015;36(5):1890–1902. doi: 10.1016/j.neurobiolaging.2015.02.003. [DOI] [PubMed] [Google Scholar]
- D'Aniello A. D-Aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Rev. 2007;53(2):215–234. doi: 10.1016/j.brainresrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- D'Aniello A., Di Fiore M.M., Fisher G.H., Milone A., Seleni A., D'Aniello S. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. Faseb J Official Publ Fed Am Soc Exp Biol. 2000;14(5):699–714. doi: 10.1096/fasebj.14.5.699. [DOI] [PubMed] [Google Scholar]
- D'Aniello A., Giuditta A. Identification of D-aspartic acid in the brain of Octopus vulgaris Lam. J Neurochem. 1977;29(6):1053–1057. doi: 10.1111/j.1471-4159.1977.tb06508.x. [DOI] [PubMed] [Google Scholar]
- D'Aniello A., Luongo L., Romano R., Iannotta M., Marabese I., Boccella S. D-Aspartic acid ameliorates painful and neuropsychiatric changes and reduces beta-amyloid Abeta1-42 peptide in a long lasting model of neuropathic pain. Neurosci Lett. 2017 doi: 10.1016/j.neulet.2017.04.041. [DOI] [PubMed] [Google Scholar]
- D'Aniello A., Vetere A., Petrucelli L. Further study on the specificity of D-amino acid oxidase and D-aspartate oxidase and time course for complete oxidation of D-amino acids. Comp Biochem Physiol B. 1993;105(3-4):731–734. doi: 10.1016/0305-0491(93)90113-j. [DOI] [PubMed] [Google Scholar]
- D'Aniello G., Grieco N., Di Filippo M.A., Cappiello F., Topo E., D'Aniello E. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum Reprod. 2007;22(12):3178–3183. doi: 10.1093/humrep/dem328. [DOI] [PubMed] [Google Scholar]
- D'Aniello G., Ronsini S., Guida F., Spinelli P., D'Aniello A. Occurrence of D-aspartic acid in human seminal plasma and spermatozoa: possible role in reproduction. Fertil steril. 2005;84(5):1444–1449. doi: 10.1016/j.fertnstert.2005.05.019. [DOI] [PubMed] [Google Scholar]
- D'Aniello S., Somorjai I., Garcia-Fernandez J., Topo E., D'Aniello A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB journal. Official Publ Fed Am Soc Exp Biol. 2011;25(3):1014–1027. doi: 10.1096/fj.10-168492. [DOI] [PubMed] [Google Scholar]
- D’Aniello S., Garcia-Fernàndez J. D-Aspartic acid and L-amino acids in the neural system of the amphioxus Branchiostoma lanceolatum. Amino Acids. 2006;32(1):21–26. doi: 10.1007/s00726-006-0347-5. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A., Errico F., Aceto G., Tomasetti C., Usiello A., Iasevoli F. D-aspartate dysregulation in Ddo(-/-) mice modulates phencyclidine-induced gene expression changes of postsynaptic density molecules in cortex and striatum. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;62:35–43. doi: 10.1016/j.pnpbp.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Di Fiore M.M., Santillo A., Chieffi Baccari G. Current knowledge of D-aspartate in glandular tissues. Amino Acids. 2014;46(8):1805–1818. doi: 10.1007/s00726-014-1759-2. [DOI] [PubMed] [Google Scholar]
- Di Fiore M.M., Santillo A., Falvo S., Longobardi S., Chieffi Baccari G. Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia. Int J Mol Sci. 2016;17(7) doi: 10.3390/ijms17071127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico F., Mothet J.P., Usiello A. D-Aspartate: An endogenous NMDA receptor agonist enriched in the developing brain with potential involvement in schizophrenia. J Pharmaceut Biomed Anal. 2015;116:7–17. doi: 10.1016/j.jpba.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Errico F., Napolitano F., Squillace M., Vitucci D., Blasi G., de Bartolomeis A. Decreased levels of D-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J Psychiatr Res. 2013;47(10):1432–1437. doi: 10.1016/j.jpsychires.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Errico F., Nistico R., Di Giorgio A., Squillace M., Vitucci D., Galbusera A. Free D-aspartate regulates neuronal dendritic morphology, synaptic plasticity, gray matter volume and brain activity in mammals. Transl Psychiatry. 2014;4 doi: 10.1038/tp.2014.59. e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico F., Nistico R., Napolitano F., Mazzola C., Astone D., Pisapia T. Increased D-aspartate brain content rescues hippocampal age-related synaptic plasticity deterioration of mice. Neurobiol Aging. 2011;32(12):2229–2243. doi: 10.1016/j.neurobiolaging.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Errico F., Nistico R., Napolitano F., Oliva A.B., Romano R., Barbieri F. Persistent increase of D-aspartate in D-aspartate oxidase mutant mice induces a precocious hippocampal age-dependent synaptic plasticity and spatial memory decay. Neurobiol Aging. 2011;32(11):2061–2074. doi: 10.1016/j.neurobiolaging.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Erwan E., Chowdhury V.S., Nagasawa M., Goda R., Otsuka T., Yasuo S. Central injection of L- and D-aspartate attenuates isolation-induced stress behavior in chicks possibly through different mechanisms. Eur J Pharmacol. 2014;736:138–142. doi: 10.1016/j.ejphar.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Erwan E., Tomonaga S., Ohmori T., Mutaguchi Y. Oral Administration of D-aspartate, but not of L-aspartate, reduces food intake in Chicks. J Poultry Sci. 2013;50(2):164–171. [Google Scholar]
- Fiore M.M.D., Santillo A., Falvo S., Baccari G.C., Venditti M., Venditti M., Russo F.D.G. Sex hormone levels in the brain of d -aspartate-treated rats. Comptes Rendus Biologies. 2017;341(1) doi: 10.1016/j.crvi.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Fisher G., Lopez S., Peterson K., Goff T., Philip I., Gaviria R. Is there a correlation between age and D-aspartic acid in human knee cartilage? Amino Acids. 2006;32(1):27–30. doi: 10.1007/s00726-006-0353-7. [DOI] [PubMed] [Google Scholar]
- Fujii N., Kaji Y., Fujii N. D-Amino acids in aged proteins: analysis and biological relevance. J Chromatogr B Anal Tech Biomed Life Sci. 2011;879(29):3141–3147. doi: 10.1016/j.jchromb.2011.05.051. [DOI] [PubMed] [Google Scholar]
- Giacone F., Condorelli R.A., Mongioi L.M., Bullara V., La Vignera S., Calogero A.E. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine. 2017;56(2):408–415. doi: 10.1007/s12020-016-1013-7. [DOI] [PubMed] [Google Scholar]
- Han H., Miyoshi Y., Koga R., Mita M., Konno R., Hamase K. Changes in D-aspartic acid and D-glutamic acid levels in the tissues and physiological fluids of mice with various D-aspartate oxidase activities. J Pharmaceut Biomed Anal. 2015;116:47–52. doi: 10.1016/j.jpba.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Katane M., Homma H. D-Aspartate–an important bioactive substance in mammals: a review from an analytical and biological point of view. J Chromatogr B Anal Tech Biomed Life Sci. 2011;879(29):3108–3121. doi: 10.1016/j.jchromb.2011.03.062. [DOI] [PubMed] [Google Scholar]
- Kim P.M., Duan X., Huang A.S., Liu C.Y., Ming G.L., Song H. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci USA. 2010;107(7):3175–3179. doi: 10.1073/pnas.0914706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H., Sekine M., Furuchi T., Katane M., Nimura N., Shimamoto K. A novel L-glutamate transporter inhibitor reveals endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Life Sci. 2005;76(25):2933–2944. doi: 10.1016/j.lfs.2004.10.057. [DOI] [PubMed] [Google Scholar]
- Krashia P., Ledonne A., Nobili A., Cordella A., Errico F., Usiello A. Persistent elevation of D-Aspartate enhances NMDA receptor-mediated responses in mouse substantia nigra pars compacta dopamine neurons. Neuropharmacology. 2016;103:69–78. doi: 10.1016/j.neuropharm.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Lamanna C., Assisi L., Botte V., Di Fiore M.M. Involvement of D-Asp in P450 aromatase activity and estrogen receptors in boar testis. Amino Acids. 2006;32(1):45–51. doi: 10.1007/s00726-006-0351-9. [DOI] [PubMed] [Google Scholar]
- Lamanna C., Assisi L., Vittoria A., Botte V., Di Fiore M.M. D-Aspartic acid and nitric oxide as regulators of androgen production in boar testis. Theriogenology. 2007;67(2):249–254. doi: 10.1016/j.theriogenology.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Masuda W., Nouso C., Kitamura C., Terashita M., Noguchi T. Free d-aspartic acid in rat salivary glands. Archives Biochem Biophys. 2003;420(1):46–54. doi: 10.1016/j.abb.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Katane M., Maeda K., Kaneko Y., Saitoh Y., Miyamoto T. Biosynthesis of D-aspartate in mammals: the rat and human homologs of mouse aspartate racemase are not responsible for the biosynthesis of D-aspartate. Amino Acids. 2015;47(5):975–985. doi: 10.1007/s00726-015-1926-0. [DOI] [PubMed] [Google Scholar]
- Molinaro G., Pietracupa S., Di Menna L., Pescatori L., Usiello A., Battaglia G. D-aspartate activates mGlu receptors coupled to polyphosphoinositide hydrolysis in neonate rat brain slices. Neurosci Lett. 2010;478(3):128–130. doi: 10.1016/j.neulet.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Motoie R., Fujii N., Tsunoda S., Nagata K., Shimo-oka T., Kinouchi T. Localization of D-beta-aspartyl residue-containing proteins in various tissues. Int J Mol Sciences. 2009;10(5):1999–2009. doi: 10.3390/ijms10051999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzolini A., Bregola G., Bianchi C., Beani L., Simonato M. Characterization of glutamate and [3H]D-Aspartate outflow from various in vitro, preparations of the rat hippocampus. Neurochem Int. 1997;31(1):113–124. doi: 10.1016/s0197-0186(96)00129-5. [DOI] [PubMed] [Google Scholar]
- Opere C.A., Monjok E.M., Kulkarni K.H., Njie Y.F., Ohia S.E. Regulation of [3H] D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochemical Research. 2009;34(11):1962–1968. doi: 10.1007/s11064-009-9984-x. [DOI] [PubMed] [Google Scholar]
- Ota N., Shi T., Sweedler J.V. D-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids. 2012;43(5):1873–1886. doi: 10.1007/s00726-012-1364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E., Luongo L., Guida F., Marabese I., Romano R., Iannotta M. D-Aspartate drinking solution alleviates pain and cognitive impairment in neuropathic mice. Amino Acids. 2016;48(7):1553–1567. doi: 10.1007/s00726-016-2205-4. [DOI] [PubMed] [Google Scholar]
- Punzo D., Errico F., Cristino L., Sacchi S., Keller S., Belardo C. Age-related changes in D-aspartate oxidase promoter methylation control extracellular D-aspartate levels and prevent precocious cell death during brain aging. J Neurosci. 2016;36(10):3064–3078. doi: 10.1523/JNEUROSCI.3881-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci F., D'Aniello A., Di Fiore M.M. Stimulation of androgen production by D-aspartate through the enhancement of StAR, P450scc and 3beta-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells. Steroids. 2014;84:103–110. doi: 10.1016/j.steroids.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Santillo A., Falvo S., Chieffi P., Burrone L., Chieffi Baccari G., Longobardi S. D-aspartate affects NMDA receptor-extracellular signal-regulated kinase pathway and upregulates androgen receptor expression in the rat testis. Theriogenology. 2014;81(5):744–751. doi: 10.1016/j.theriogenology.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Santillo A., Falvo S., Chieffi P., Di Fiore M.M., Senese R., Chieffi Baccari G. D-Aspartate Induces Proliferative Pathways in Spermatogonial GC-1 Cells. J Cell Physiol. 2016;231(2):490–495. doi: 10.1002/jcp.25095. [DOI] [PubMed] [Google Scholar]
- Savage D.D.G.R., Queen S.A., Paxton L.L., Allan A.M. Characterization of electrically evoked [3H]-D-aspartate release from hippocampal slices. Neurochem Int. 2001;38(3):255–267. doi: 10.1016/s0197-0186(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Tanaka-Hayashi A., Hayashi S., Inoue R., Ito T., Konno K., Yoshida T. Is D-aspartate produced by glutamic-oxaloacetic transaminase-1 like 1 (Got1l1): a putative aspartate racemase? Amino Acids. 2015;47(1):79–86. doi: 10.1007/s00726-014-1847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P.P.B.C., Mannaerts G.P. D-aspartate oxidase, a peroxisomal enzyme in liver of rat and man. Biochim Biophys Acta. 1991;1073(1):203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yuan L., Liu Q., Cai B., Li X., Zhao H. Biosafety assessment of genetically modified foods based on the toxicology of the chiral D-amino acid. Scientia Sinica Chimica. 2015;45(1):98. [Google Scholar]