Abstract
Ruminant production, especially in the tropics and developing countries suffers a setback when compared with the temperate and developed countries, which is attributable to the kinds of available feed resources in the region of production. In the tropics, ruminants are restricted to grazing on low-quality forages, crop residues and agro-industrial by-products with very little or no concentrate diets, which adversely affect the animals in exhibiting their full production potential. Considering this fact, there is an increasing interest in improving the digestibility of these feed resources. In recent years, researchers have explored several methods to enhance the functions of rumen microflora, improve digestion and fermentation processes, as well as increase bioavailability and utilization of nutrients through feed supplementation. This review aims to explore the positive effects of supplementation of ruminant diets with probiotics or botanical extracts and their metabolites on the productivity of the animals. Moreover, the functions of these non-pathogenic and non-toxic live microorganisms (probiotics) and plant biologically active compounds (botanical extract) are explored because of the ban on non-therapeutic use of antibiotics as growth promoters coupled with the critical preference of consumers to high quality and safe animal products. It has been reported that these alternative supplemental products have a beneficial impact on both animal health and productivity, which is affecting stabilization of rumen environment, inhibition of pathogenic bacteria proliferation in gastro-intestinal tract, modulation of immune response, increase in fibre degradation and fermentation, nutrients availability and utilization, animal growth performance and milk production, among others. However, long-term in vivo studies are still required to determine the synergetic effects of these 2 safe supplemental products.
Keywords: Ruminant, Supplementation, Probiotics, Botanical extracts, Tropics
1. Introduction
As opined by Sansoucy (1999), the importance of animal breeding and husbandry is not only the production of high-quality proteins but also sustaining rural livelihoods and possibly contributing to food security. FAO (2010) reported that ruminant animals contributed about 75.7% to the global livestock biomass. However, between 1980 and 2010, the total dietary proteins supplied by animal sources in Africa, which are predominantly in the tropics, and other least developed countries are 25% less than that in developed countries (FAO, 2013). Ruminant feeding and production in the tropics are restricted to grazing on forages, crop residues and agro-industrial by-products with very low allowances of concentrates (Adegoke and Abioye, 2016). These roughage are rich in neutral detergent fibre (NDF) and deficient in nitrogen, and during their ruminal fermentation, fewer amounts of volatile fatty acids (VFA) and microbial biomass (microbial protein) are synthesized (Santra and Karim, 2003), which always results in productivity loss. Hence, manipulation of the digestion process by feed supplementation is imperative to improve the utilization of these available feed resources and increase the productivity of ruminants in the tropics.
Ruminant nutritionists have developed many methods of feed supplementation such as the use of antibiotic growth promoters to enhance production by limiting the effects of pathogenic infection on ruminant productivity (Reti et al., 2013, Valero et al., 2014). However, their usages have been significantly reduced on the basis of its health and environmental implications (Gaggia et al., 2010). Moreover, the non-therapeutic use of antibiotics as growth promoter in livestock diets and for disease prevention has been banned by the European Union in 2006 because of the emergence of resistant pathogenic bacteria and the possible contamination of animal products that may pose health challenges to the consumers (Russell and Houlihan, 2003, Jouany and Morgavi, 2007), and many countries have been following suit. Thus, a need for searching more natural feed additives alternative to antibiotics arises from consumer preferences for more natural animal products (Khan et al., 2016).
In the light of this, many studies have reported the positive effects of probiotics (Khan et al., 2016) and plant extracts (Cruz et al., 2014) as good alternative feed additives to prophylactic use of antibiotics by decreasing the load of pathogenic bacteria, improving dry matter intake and feed conversion efficiency, enhancing nutrient utilization efficiency and production performance, stimulating and activating immune cells, reducing methane production thereby minimizing energy loss, and generally promoting growth and health performance as well as meat and milk production in ruminants.
2. Digestive physiology of rumen
The ruminant gastrointestinal tract hosts a diverse array of microbial species, many of which are directly or indirectly important for the overall well-being of the animals (Stover et al., 2016). Rumen, the main fermentative vat, is a complex biological system where degradation, fermentation and transformation of feed materials into products by microorganisms occur (McSweeney and Mackie, 2012, Albrao et al., 2014). This fermentative vat provides an anaerobic environment, a constant temperature of 38 to 41 °C and pH of 5.5 to 6.9 (Dehority, 2003) for the microbes. Rumen microbes are classified into 3 domains: bacteria, archaea (methanogens), and eucarya (protozoa and fungi). There are more than 200 species of rumen bacteria and their population range is 1010 to 1011 per g. Anaerobic fungi in the rumen are classified into 6 genera with the range population of 103 to 106 per g, rumen methanogen population is up to 109 per g, while bacteriophage and ciliate protozoa having population ranges of 107 to 109 per g and 104 to 106 per g, respectively (Choudhury et al., 2015, Kumar et al., 2009, Wanapat, 2000). Bacteria population are most actively involved in the plant fibre degradation, as revealed by the fact that bacteria associated with feed particles account for nearly 50% to 75% of the total microbial population (Minato et al., 1966). Anaerobic fungi degrade lignocellulosic components of the feed particles. They constitute the smallest proportion (only about 20%) of the rumen microbial biomass (Rezaeian et al., 2004). Rumen protozoa play an important role in fibre digestion and modulation of the fermentation profiles by slowing down the production of acids that lower rumen pH (Vibhute et al., 2011), benefiting the rumen.
3. Probiotics
Ezema (2013) defined probiotics as non-pathogenic and non-toxic live microorganisms that are capable of exerting a beneficial effect on the host animals at the appropriate dosage. Probiotics specific for ruminants include direct-fed microbes such as yeast (Saccharomyces cerevisiae) and bacterial species including Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, Propionibacterium, Megasphaeraelsdenii and Prevotellabryantii (Seo et al., 2010).
3.1. Live yeast (S. cerevisiae)
Live yeast (S. cerevisiae) is one of the most common and efficient probiotics used in ruminant nutrition because of its varieties of function in stabilizing the rumen environment for adequate functioning of the microbial flora, especially the fibrolytic bacteria. The yeast cells are said to be able to maintain viability throughout the digestive tract. Yeast (S. cerevisiae) as feed additives for ruminants provides organic acids and vitamins to stimulate the growth of the lactic acid bacteria (LAB) (Khan et al., 2016), which improves rumen metabolism through stability of the rumen pH, increasing the population of cellulolytic bacteria, competing with lactate producing bacteria for substrate and improving anaerobiosis by scavenging oxygen available in the rumen (Khan et al., 2016, Vibhute et al., 2011, Chiquette, 2009, Marden et al., 2008). Table 1 shows different studies on the effects of yeast strains on rumen functions.
Table 1.
Observations from different articles reporting effects of yeast on rumen microorganisms and functions.
| Title of research | Treatment/Dosage/System | Observations and summary | Authors |
|---|---|---|---|
| Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds | Varying doses of yeast (S. cerevisiae) incubated with corn stover, oat straw, sugarcane bagasse and sorghum straw (in vitro) |
|
Elghandour et al. (2014) |
| Effects of Saccharomyces cerevisiae fermentation product on in vitro fermentation and microbial communities of low-quality forages and mixed diets | S. cerevisiae fermentation/concentrations of 0, 1, 2, 3 g/L (in vitro) |
|
Mao et al. (2014) |
| Effect of yeast culture (Saccharomyces cerevisiae) on the ruminal microbial population in buffalo bulls | Yeast culture (in vivo) |
|
Kumar et al. (2013) |
DM = dry matter; NDF = neutral detergent fibre; RS = rice straw; CS = corn silage; CSNG = corn silage without grain; CSG = corn silage with grains; VFA = volatile fatty acid.
3.2. Bacteria (direct-fed microbes)
Bacterial probiotics have been observed to enhance rumen conditions, improve dry matter intake (DMI), feed efficiency (FE) and weight gain (WG) in ruminants (Elghandour et al., 2015). It may also block the growth of pathogenic organisms, stimulate immune system through secretion of bacteriocin and modulate microbial balance in the gastrointestinal tract (Khan et al., 2016). Increased milk yield, fat-corrected milk yield and milk fat content were also reported with bacterial supplementation (Elghandour et al., 2015, Khan et al., 2016). The summary report of the analysis of literature data on the effects of supplementation of ruminant diets with probiotic bacteria is shown in Table 2.
Table 2.
Observations from different articles reporting effects of bacterial probiotics on rumen microorganisms and functions.
| Title of research | Treatment/Dosage/System | Observations and summary | Authors |
|---|---|---|---|
| Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows | Live Bacillus culture (Bacillus licheniformis), 100 g/d (in vivo) |
|
Qiao et al. (2009) |
| Repeated ruminal dosing of Ruminococcus flavefaciens NJ along with a probiotic mixture in forage or concentrate-fed dairy cows: Effect on ruminal fermentation, cellulolytic populations | Ruminococcus flavefaciens NJ (in vivo) |
|
Chiquette et al. (2007) |
| Effect of L. plantarum, Pediococcusacidilactici, Enterococcus faecium and L. lactis microbial supplementation of grass silage on the fermentation characteristics in the rumen of dairy cows | A mixture of L. plantarum, Pediococcus acidilactici, Enterococcus faecium and L. lactis/5 × 105 colony forming unit/g of fresh herbage (in vivo) | A significant increase in lactic acid concentration and markedly decreased the concentration of acetic acid in silage.
|
Jatkauskas and Vrotniakien (2007) |
VFA = volatile fatty acid; NDF = neutral detergent fibre; ADF = acid detergent fiber; OM = organic matter.
3.3. Effects of probiotics (mostly, yeast) on ruminant performance
3.3.1. Improvement of fibre degradation
Fermentation of plant cell walls (comprising of cellulose, hemicellulose and lignin) in the rumen is of unique importance to the production of good quality and valuable products (i.e., meat and milk) from plants through the utilization of available energy that is not easily accessible by other classes of animals. Interestingly, this process relies on the symbiotic relationship between the rumen microorganisms that produce fibrolytic enzymes and the host animals that provide a conducive anaerobic environment for an efficient fermentation. However, Krause et al. (2003) stated that fibre digestion in the rumen is not efficient enough despite the presence of fibrolytic microorganisms as it is evidenced by the fact that fiber recovered from the faeces can still be further fermented and this has necessitated research in order to increase efficiency of fibre degradation in the rumen.
Probiotics have been reported to improve the digestion of fibre and make structural carbohydrates such as cellulose and hemicellulose more available as an energy source for the host animals. A report by Chaucheyras-Durand et al. (2012) showed that supplementation of SC I-1077 live yeast stimulates the fungi responsible for solubilisation of lignin tissues and also strengthens the activities of cellulolytic bacteria. In another study, Mosoni et al. (2007) observed an increase in the rumen population of Fibrobacter succinogens and Ruminococcus flaveafaciens, which are fibre degrading bacteria, by 45% and 85%, respectively when cattle fed diets supplemented with live yeast. In addition, Guedes et al. (2008) found that low fiber degradation silages were digested 24% higher with live yeast supplementation and concluded that specific live yeast may increase metabolizable energy available from low-quality maize silages, and the glucogenic potential of the diet, both of which would increase the efficiency of cattle production. Chaucheyras-Durand and Fonty (2001) also discussed early establishment and maintenance of inoculated cellulolytic bacteria in the rumen of gnotobiotically-reared lambs in the presence of S. cerevisiae. The authors further explained that there was a decrease in ruminal ammonia concentration and a higher volatile fatty acid (VFA) concentration when lambs were 20 to 50 days old and such activity could be beneficial in preventing microbial imbalance and a reduction of rumen function efficiency in the case of nutritional transitions. In addition, Cömert et al. (2015) evaluated the effect of dietary supplementation of yearling lambs fed untreated wheat straw and ammonia-treated wheat straw supplemented with S. cerevisiae. They observed a higher degradability (18.7%) of water-insoluble fraction of fibre in wheat straw diets supplemented with yeast compared with the unsupplemented group and an additional 8.3% increase in the ammonia-treated wheat straw group. Also, there was a 12% improvement in potential degradability of wheat straw diets supplemented with yeast and an increased average daily total VFA production by 14% compared to the control and not significantly different from the ammonia-treated wheat straw with yeast. The study could explain the fact that yeast supplementation affects the water-insoluble material in the rumen by promoting the development of rumen fauna. Also, Miranda et al. (1996) observed improved in situ degradability of alfalfa NDF and starch (at both levels of 27% and 37% NDF in diets) at 48 h in Holstein heifers diets supplemented with S. cerevisiae and Aspergillus oryzae. The degradability of 27% NDF diets supplemented with A. oryzae was 11.4% higher than control while 37% NDF diets supplemented with S. cerevisiae by 4.6%. Improved ruminal pH stability, TVFA and propionate concentrations and reduced NH3-N concentration were also reported.
3.3.2. Improvement of feed intake and nutrient digestibility
Available feed resources in the tropics have low digestibility (poorly fermented) usually coupled with imbalanced essential nutrients such as protein, leading to low feed intake, as a result of slow passage rate through the rumen, and poor performance of the animal (Wanapat, 1990). Thus, supplementation to enhance the digestibility of a low-quality forage can improve ruminant productivity. In other words, probiotic (i.e., yeast) supplementation has been observed to reduce inter meal interval in lactating cows, which could assist in stabilizing the rumen pH thereby stimulating forage feed intake and nutrient digestibility and consequently increasing the rate of fiber degradation in the rumen (Bach et al., 2007, Chaucheyras-Durand et al., 2012). Bach et al. (2007) observed a significant change in the feeding behaviour of cows whose diets were supplemented with live yeast through increased eating frequency (i.e., shorter inter-meal interval of 3.32 h as against 4.32 h in unsupplemented cows). In addition, Bitencourt et al. (2011) showed that DMI and organic matter intake (OMI) respectively increased by 3.2% (i.e., 21.4 vs. 20.7 kg/d in control) and 4% (20.2% vs. 19.4% in control) in dairy cows whose diets were supplemented with live yeast. The authors also found that NDF digestibility increased by 10% while dry matter digestibility (DMD) and organic matter digestibility (OMD) increased by 2.7% and 2.3%, respectively, and concluded that the positive performance response of supplemented animals was most likely dictated by improved digestibility of fibre in the total digestive tract. Moreover, Gaafar et al. (2009) supplemented the diets of buffaloes with baker's yeast, and they observed that when compared to the unsupplemented group, digestibility of DM and OM increased by 2.7% and 3.2%, and crude protein (CP) and crude fiber (CF) increased by 2.8% and 4.9%, as well as ether extract (EE) and nitrogen free extract (NFE) increased by 4.1% and 3.2%. Using goats as experimental animals, Azzaz et al. (2015) observed improved total tract digestibility of DM, OM, CP, CF and NFE when the animals were fed A. awamori and Lactobacillus acidophilus supplemented diets.
3.3.3. Enhancement of feed conversion ratio (FCR) and growth rate
Probiotics have been reported as an alternative to antibiotics (Callaway et al., 2004) to improve live weight gain in ruminants by enhancing the efficiency of nutrient utilization, improving retention of nitrogen and decreasing the excretion of essential nutrients. De Ondarza et al. (2010) reported that 14 different types of research that live yeast supplementation improved feed efficiency in cows by about 3% (i.e., 1.75 vs. 1.70 for supplemented groups and control, respectively). The improved feed efficiency depicts better utilization of available nutrients in the given diets (Khalid et al., 2011). This is also in line with the findings of Robinson (2002) who reported that probiotics can improve FCR in small ruminants. In addition, Saleem et al. (2017) reported improved performance including final body weight (+3.16 kg), average daily gain (+25.2 g/lamb), total gain (+2.11 kg), and FCR (−1.18) of the lambs fed diets supplemented with Pediococcus acidilactici and Pediococcus pentosaceus probiotics during post weaning phase when compared with the control group. Also, Adams et al. (2008) examined the effect of calves' diets supplemented with Propionibacterium jensenii 702 (PJ702). The probiotic was administered at a dosage of 1.1 × 108 cfu/kg per day. They found that weaning and total live weights increased by 8.2 kg (i.e., 24.4 vs. 16.2 kg in control) and 16.4 kg (i.e., 81.9 vs. 65.5 kg), respectively. Lesmeister et al. (2004) reported a positive influence of yeast culture on the growth of Holstein calves through increased average daily weight gain (ADWG), pre-weaning and weaning weights by 13.5% (i.e., 505 vs. 437 g in calves on the control diet), 11.03% and 20.94%, respectively. By supplementing the diet of growing lambs with a combination of yeast and probiotic bacteria (i.e., S. cerevisiae, Lactobacillus, Streptococcus, Aspergillus) in the diet of growing lambs, Hillal et al. (2011) found a 7.2% increase in ADWG (i.e., 194 vs. 180 g in the control). Similarly, Mudgal and Baghel (2010) found a 31.4% increase in the ADWG of pre-ruminant buffalo calves fed diets supplemented with L. acidophilus at the first month (i.e., 207 vs. 142 g in the unsupplemented group).
3.3.4. Increase in milk production
Song et al. (2012) stated that probiotics are incorporated in livestock feeding in order to improve the health of the animal and also to ensure food safety. Furthermore, yeast is said to optimize rumen function resulting into more nutrient bioavailability, which consequently improve the milk production performance while ensuring digestive comfort of the animal (Maamouri et al., 2014, Ayad et al., 2013). Maamouri et al. (2014) observed an increase in milk yield production of Tunisian Holstein Friesian cows by 1.1 kg/d, which is about 8% higher when compared to unsupplemented group. The author also reported higher milk fat (53 g/cow per day) and protein (41.7 g/cow per day) yields in cows supplemented with yeast as against 47 and 38.7 g/cow per day, respectively in the control group. Moreover, Moallem et al. (2008) supplemented live yeast (Biosaf at 1 g/4 kg of feed consumed) and found that daily average milk yield of dairy cows increased by 4.1% (i.e., 37.8 vs. 36.3 kg in the control). The differences in the response of dairy cows to yeast probiotics among various studies may be as a result of types of offered diets, types and doses of used yeast, and the physiological and nutritional state of animals (Williams et al., 1991). In addition, supplementation of S. cerevisiae (at 5 g/cow per day) improved milk production at 100 days in milk (DIM) by 22.5% (i.e., 2,760.4 vs. 2,253 kg in the control), 13.1% at 200 DIM (i.e., 4,679.9 vs. 4,136.4 kg in the control) and 14.9% (i.e., 5,927.6 vs. 5,161.1 kg/cow in the control) at 305 DIM (Majdoub-Mathlouthi and Kraiem, 2009). Likewise, Ayad et al. (2013) reported that supplementation with yeast probiotic (S. cerevisiae) improved the production of milk at 42 DIM by 23% in cows, and the lactation peak of the cows was stretched longer by 1 week than that of control cows (4 vs. 3 weeks, respectively). According to Majdoub-Mathlouthi and Kraiem, 2009, the extent of milk production improvement with yeast supplementation depends on the stage of lactation, which might be higher in early or late lactation. Furthermore, increased daily milk yield by 3%, and improved milk parameters of milk protein and lactose by 3.3% and 4%, respectively in Holstein dairy cows fed diets supplemented with S. cerevisiae CNCM I-1077 at 1 × 1010 cfu/cow per day was reported by Bitencourt et al. (2011) when compared to the control cows fed diets without yeast additive. However, Bitencourt et al. (2011) found that there was no significant difference in milk fat content of the yeast supplemented cows and the control.
4. Botanical extracts
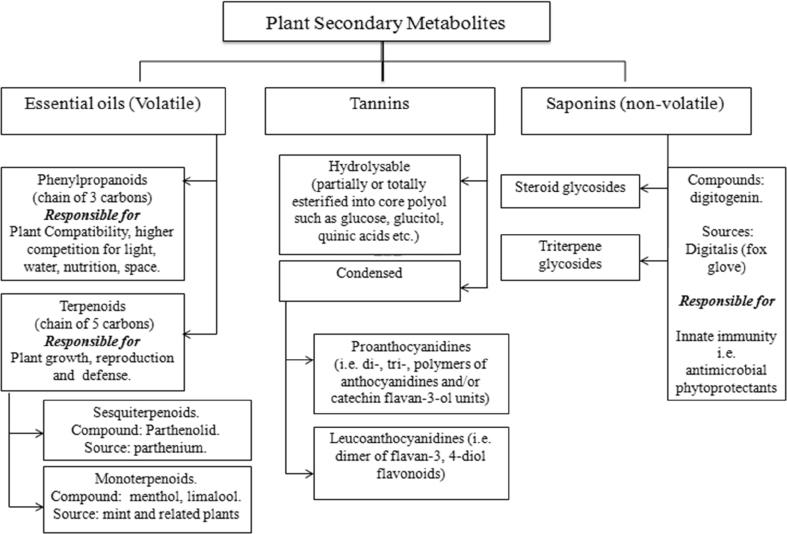
Herbal plants produce secondary metabolites, which are biologically active by providing protection against attack from predators (Iason, 2005) and these metabolites are referred to as phytochemical feed additives, phytobiotics, or herbal and botanical compounds (Kumar et al., 2014), have been researched upon to be used as an alternative to antibiotics and growth promoters in ruminant nutrition. Different plant metabolites are classified in Fig. 1. Valenzuela-Grijalva et al. (2017) outlined the biological activities of these phytochemical feed additives as supporting and improving rumen fermentation processes, modulating of microbiota, improving nutrient digestion and absorption through increased activities of digestive enzymes, decreased oxidative processes and growth of pathogenic bacteria and lastly, improving energy utilization in livestock.
Fig. 1.
Classification of plant secondary metabolites.
These compounds have been observed to exert specific anti-microbial effects against some pathogenic organisms. Itelima et al. (2017) examined the effects of some selected plant species against Escherichia coli 0157:H7 and it was observed that the extracts of Psidium guajava (P. guajava L.), which possesses appreciable quantities of alkaloids, flavonoids, tannins, resins and trace amount of saponins, terpenes steroids and phenols had higher diameter of inhibition zone (29.9 mm) than ampicillin (22.3 mm) at the concentration of 500 mg/mL. This study revealed that the extracts of some of the plant species can be as effective as modern medicine in combating pathogenic microorganisms. Furthermore, Yildiz et al. (2015) reported that some plant metabolites especially essential oils have a unique effect of reducing carbohydrate and protein degradation in the rumen by selectively inhibiting the function of some microorganisms. In addition, many researchers have reported various positive influences of plant secondary metabolites on the reduction of methane gas emission produced by ruminants (Patra and Yu, 2012, Oskoueian et al., 2013, Kim et al., 2014). Apart from being a global phenomenon, Beauchemin et al. (2014) stated that a 25% reduction in methane emissions could also increase body weight gain of growing cattle by approximately 75 g/d or milk production of dairy cows by 1 L/d, due to more effective energy metabolism and lower energy losses in gases produced during rumen fermentation that can contribute up to 10% of gross energy.
4.1. Tannins
Tannins are a complex water-soluble group of polyphenolic compounds found in a wide range of plant species commonly consumed by ruminants (Frutos et al., 2004; Westendarp, 2006). They are reported to be a heterogeneous group of high molecular weight phenolic compounds with the ability to form complexes with proteins (Schofield et al., 2001). It has been demonstrated that tannin can possibly be used to prevent protein degradation and form protein by-pass in the rumen based on its properties, as well increase protein supply and utilization in the small intestine (Westendarp, 2006) thereby, improving ruminant performance. Though, it has been proven that high tannin consumption can affect feed intake and digestibility, which may likely have consequences on the productivity of the animals fed tannins-rich diets (Frutos et al., 2004). In addition, Brogna et al. (2013) reported that addition of tannin extracted from quebracho to lambs' diets at the rate of 80 g/kg feed for parasitic control had no detrimental effect on the sheep meat quality but rather increase ribose, fructose, glucose and sorbitol concentrations in meat. More literature on the effects of tannin on rumen microorganisms and their functions are summarized in Table 3.
Table 3.
Literatures on the effects of tannins on rumen function.
| Type | Plant source | Dosage | System/Host | Reports | Reference |
|---|---|---|---|---|---|
| Condensed tannin (CT) | Quebracho | 0, 1% and 2% CT/kg DMI | in vivo (Steers), in vitro |
|
Min et al. (2016) |
| Condensed tannin | Spray-dried quebracho tannin | 0.1, 0.2 and 0.4 mg/mL of medium | in vitro |
|
Min et al. (2016) |
| Condensed tannin | Quebracho, Silvateam, Ontario, CA | 0, 0.2%, 0.4%, and 0.6% of dry matter basis | in vivo (Holstein steers) |
|
Rivera-Méndez et al. (2017) |
| Condensed and hydrolysable tannin | Quebracho, Silvateam, Ontario, CA Chestnut, Silvateam, Ontario, CA |
1) 0, 0.6% condensed tannin 2) 0.6% hydrolysable tannin 3) 0.3% condensed and 0.3% hydrolysable tannin combined. |
in vivo (Holstein steers) |
|
Rivera-Méndez et al. (2017) |
| Blend of condensed and hydrolysable tannin | – | 0, 2, 4 or 6 g tannin extract/kg dietary dry matter |
in vivo (lambs) |
|
Rojas-Roman et al. (2017) |
DMI = dry matter intake; ADWG = average daily weight gain; SCFA = short-chain fatty acids; NH3 = ammonia; DMD = dry matter digestibility; ADG = average daily gain; NE = net energy.
4.2. Saponins
Saponins are naturally occurring surface-active glycosides produced primarily by plants and the name was derived from their ability to form stable soap-like foams in aqueous solutions (Das et al., 2012). Potter et al. (1993) reported that saponin forms a complex with proteins thereby reducing protein digestibility. This phenomenon could aid protein nutrient utilization in ruminant by preventing ruminal degradation by the microbes. In addition, many studies have reported significant effects of saponin on reducing rumen protozoa population (Hristov et al., 1999, Goel et al., 2008), which consequently increases nitrogen utilization and directly leads to improved ruminant performance (Wina et al., 2005, Wanapat et al., 2013). This naturally occurring compound, saponin, has also been observed to have a substantial effect on the microbial population in the rumen by selectively enhancing or inhibiting the growth of some bacteria species (Wanapat et al., 2013). In the study of Patra et al. (2012), it was observed that saponin supplementation altered rumen bacteria community by selectively and significantly increasing the populations of Ruminococcus flavefaciens, Prevotella and F. succinogenes, thus, improving digestibility of feeds. Table 4 highlights the effects of different types of saponin extracted from plant sources at stipulated doses on the functions of rumen microorganisms.
Table 4.
Literatures on the effects of Saponin on rumen function.
| Type | Plant source | Dosage | System/Host | Reports | References |
|---|---|---|---|---|---|
| Triterpenoid Saponi |
Alfalfa | 2% and 4% of DMI | in vivo (Sheep) |
|
Lu et al. (1987) |
| 10% Saponin (wt/wt) | Quillaja saponara | 0.4% inclusin rate | in vitro |
|
Hristov et al. (2003) |
| Tea saponin (oleane-type triterpene) |
Seeds of tea plant |
8 mg in 30 mL of gas production medium |
in vitro |
|
Hu et al. (2005) |
|
Quillaja saponaria (extract) | 60 g/head per day | in vivo (Cattle) |
|
Baah et al. (2007) |
| – | 1) Fenugreek | 1) 11.54 mg/40 mL | in vitro |
|
Goel et al. (2008) |
| 2) Sesbania | 2) 21.8 mg/40 mL | ||||
| 3) Knautia | 3) 7.76 mg/40 mL | ||||
| Methanol extract saponin | Sapindus rarak | 1) 0.25 mg/mL | in vitro |
|
Wina et al. (2005) |
| 2) 0.5 mg/mL | |||||
| 3) 1.0 mg/mL | |||||
| 4) 2.0 mg/mL | |||||
| 5) 4.0 mg/mL |
DMI = dry matter intake; SCFA = short-chain fatty acids.
5. Area of future research
A comprehensive in vivo research on ruminants to evaluate the sustainability of various plant extracts in improving ruminant production without adverse effects on the animal is needed. In addition, a research that will provide insights on the potential benefits of the synergetic relationship between plant extracts and probiotics to improve production of ruminant animals in the tropics and developing countries where there are limited and low nutrient feed resources is also needed.
6. Summary of findings
Supplementation of probiotics and plant extracts in nutrition of ruminants has been observed to improve productivity through increased stability of rumen environment, inhibition of growth of pathogenic bacteria in gastro-intestinal tract, modulation of immune cells, improved fibre degradation and fermentation, increased nutrients availability and utilization and finally, increased animal products' yield such as meat, milk and wool.
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adams M.C., Luo J., Rayward D., King S., Gibson R., Moghaddam G.H. Selection of a novel direct-fed microbial to enhance weight gain in intensively reared calves. Anim Feed Sci Technol. 2008;145:41–52. [Google Scholar]
- Adegoke T.A., Abioye A.A. Assessment of existing and potential feed resources for improving livestock productivity in Niger. Int J Agric Res. 2016;11:40–55. [Google Scholar]
- Albrao F.O., Duarte E.R., Freitas C.E.S., Vieira E.A., Geraseev L.C., Silva-Hughes A.C. Characterization of fungi from the ruminal fluid of beef cattle with different ages and raised in tropical lignified pastures. Curr Microbiol. 2014;69:649–659. doi: 10.1007/s00284-014-0633-5. [DOI] [PubMed] [Google Scholar]
- Ayad M.A., Benallou B., Saim M.S., Samadi M.A., Meziane T. Impact of feeding yeast culture on milk yield, milk components, and blood components in Algerian dairy herds. J Vet Sci Technol. 2013;4:135–140. [Google Scholar]
- Azzaz H.H., Aziz H.A., Farahat E.S.A., Murad H.A. Impact of microbial feed supplements on the productive performance of lactating Nubian goats. Global Vet. 2015;14:567–575. [Google Scholar]
- Baah J., Ivan M., Hristov A.N. Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim Feed Sci Technol. 2007;137:126–137. [Google Scholar]
- Bach A., Iglesias C., Devant M. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim Feed Sci Technol. 2007;136:156–163. [Google Scholar]
- Beauchemin K.A., McGinn S.M., Martinez T.F., McAllister T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J Anim Sci Ruminant Nutrition. 2014;85(8):1990–1996. doi: 10.2527/jas.2006-686. [DOI] [PubMed] [Google Scholar]
- Bitencourt L.L., Silva J.R.M., De Oliveira B.M.L., Júnior S.S., De Zacaroni O.F., Pereira M.N. Diet digestibility and performance of dairy cows supplemented with live yeast. SciAgric. 2011;68:301–307. [Google Scholar]
- Brogna D.M.R., Tansawat R., Cornforth D., Ward R., Bella M., Luciano G. The quality of meat from sheep treated with tannin- and saponin-based remedies as a natural strategy for parasite control. Meat Sci. 2013;96:744–749. doi: 10.1016/j.meatsci.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Callaway T.R., Anderson R.C., Edrington T.S., Genovese K.J., Bischoff K.M. What are we doing about Escherichia coli O157: H7in cattle? J Anim Sci. 2004;82:93–99. doi: 10.2527/2004.8213_supplE93x. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Chevaux E., Martin C., Forano E. Probiotic in animals. 2012. Use of yeast probiotics in ruminants: effects and mechanisms of action on rumen pH, fibre degradation, and microbiota according to the diet. [Chapter 7] [Google Scholar]
- Chaucheyras-Durand F., Fonty G. Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077. Reprod Nutr Dev. 2001;41:57–68. doi: 10.1051/rnd:2001112. [DOI] [PubMed] [Google Scholar]
- Chiquette J., Talbot G., Markwell F., Nili N., Forster R.J. Repeated ruminal dosing of Ruminococcus flavefaciens NJ along with a probiotic mixture in forage or concentrate-fed dairy cows: effect on ruminal fermentation, cellulolytic populations and in sacco digestibility. Can J Anim Sci. 2007;87:237–249. [Google Scholar]
- Chiquette J. The role of probiotics in promoting dairy production. WCDS Adv Dairy Technol. 2009;21:143–157. [Google Scholar]
- Choudhury P.K., Salem A.Z.M., Jena R., Kumar S., Singh R., Puniya A.K. Rumen microbiology: an overview. In: Puniya K.A., Singh R., Kamra N.D., editors. Rumen microbiology: from evolution to revolution. Springer; New Delhi: 2015. pp. 3–16. [Google Scholar]
- Cömert M., Yılmaz Ş., Hülya Ö., Yeğenoğlu G.B. Effects of Saccharomyces cerevisiae supplementation and anhydrous ammonia treatment of wheat straw on in situ degradability and, rumen fermentation and growth performance of yearling lambs. Asian Australas J Anim Sci. 2015;28(5):639–646. doi: 10.5713/ajas.14.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz O.T.B., Valero M.V., Zawadzki F., Eiras C.E., Rivaroli D.C., Prado R.M. Effect of glycerine and essential oils (Anacardium occidentale and ricinus Communis) on animal performance, feed efficiency and carcass characteristics of crossbred bulls finished in a feedlot system. Ital J Anim Sci. 2014;13(4):3492. [Google Scholar]
- Das T.K., Banerjee D., Chakraborty D., Pakhira M.C., Shrivastava B., Kuhad R.C. Saponin: role in the animal system. Vet World. 2012;5(4):248–254. [Google Scholar]
- De Ondarza M.B., Sniffen C.J., Dussert L., Chevaux E., Sullivan J., PAS Walker N. CASE STUDY: multiple-study analysis of the effect of live yeast on milk yield, milk component content and yield, and feed efficiency. Prof Anim Sci. 2010;26:661–666. [Google Scholar]
- Dehority B.A. Nottingham University Press; Nottingham: 2003. Rumen microbiology. [Google Scholar]
- Elghandour M.M.Y., Chagoyan J.C.V., Salem A.Z.M., Kholif A.E., Castaneda J.S.M., Camacho L.M. Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds. Ital J Anim Sci. 2014;13:295–301. [Google Scholar]
- Elghandour M.M.Y., Salem A.Z.M., Castaneda J.S.M., Camacho L.M., Kholif A.E., Chagoya J.C.V. Direct-fed microbes: a tool for improving the utilization of low-quality roughages in ruminants. J Integr Agric. 2015;14:526–533. [Google Scholar]
- Ezema C. Probiotics in animal production: a review. J Vet Med and Anim Health. 2013;5(11):308–316. [Google Scholar]
- FAO . 2010. Food and Agriculture Organization of the United Nations.http://www.faostat.fao.org/ [Accessed 2017] [PubMed] [Google Scholar]
- FAO . 2013. Food and Agriculture Organization of the United Nations.http://faostat3.fao.org/home/index.html FAOSTAT. [Accessed 2017] [PubMed] [Google Scholar]
- Frutos P., Hervas G., Giráldez F.J., Mantecón A. Review. Tannins and ruminant nutrition. Spanish J Agric Res. 2004;2:191–202. [Google Scholar]
- Gaafar H.M.A., Mohi El-Din A.M.A., Basiuoni M.I., El-riedy K.F.A. Effect of concentrate to roughage ratio and baker's yeast supplementation during the hot season on the performance of lactating buffaloes. Slovak J Anim Sci. 2009;42(4):188–195. [Google Scholar]
- Gaggia F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Goel G., Makkar H.P.S., Becker K. Effect of Sesbania sesban and Carduuspycnocephalus leaves and fenugreek (Trigonellafoenum-graecum L.) seeds and their extracts on the partitioning of nutrient from roughage and concentrate based feeds to methane. Anim Feed Sci Technol. 2008;147:72–89. [Google Scholar]
- Guedes C.M., Gonc D.A., Rodrigues M.A.M., Dias-da-Silva A. Effects of a Saccharomyces cerevisiae yeast on ruminal fermentation and fibre degradation of maize silages in cows. Anim Feed Sci Technol. 2008;145:27–40. [Google Scholar]
- Hillal H., El-Sayaad G., Abdella M. Effect of growth promoters (probiotics) supplementation on performance, rumen activity and some blood constituents in growing lambs. ArchivTierzucht. 2011;54(6):607–617. [Google Scholar]
- Hristov A.N., Ivan M., Neill L., McAllister T.A. Evaluation of several potential bioactive agents for reducing protozoal activity in vitro. Anim Feed Sci Technol. 2003;105:163–184. [Google Scholar]
- Hristov N.A., McAllister T.A., Van Herk F.H., Cheng K.J., Newbold C.J., Cheeke P.R. Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. J Anim Sci. 1999;77:2554–2563. doi: 10.2527/1999.7792554x. [DOI] [PubMed] [Google Scholar]
- Hu W., Wu Y., Liu J., Guo Y., Ye J. Tea saponins affect in vitro fermentation and methanogenesis in faunated and defaunated rumen fluid. J Zhejiang Univ Sci B. 2005;6(8):787–792. doi: 10.1631/jzus.2005.B0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iason G. The role of plant secondary metabolites in mammalian herbivory: ecological perspectives. Proc Nutr Soc. 2005;64:123–131. doi: 10.1079/pns2004415. [DOI] [PubMed] [Google Scholar]
- Itelima J.U., Agina S.E., Pandukur S.G. Antimicrobial activity of selected plant species and anti-biotic drugs against Escherichia coli 0157: H7. Afr J Microbiol Res. 2017;11(20):792–803. [Google Scholar]
- Jatkauskas J., Vrotniakien V. Effect of L-plantarum, Pediococcusacidilactici, Enterococcus faecium and L-lactis microbial supplementation of grass silage on the fermentation characteristics in the rumen of dairy cows. Vet Zootec. 2007;40:29–34. [Google Scholar]
- Jouany J.P., Morgavi D.P. Use of ‘natural’ products as alternatives to antibiotic feed additives in ruminant production. Animal. 2007;10:1443–1466. doi: 10.1017/S1751731107000742. [DOI] [PubMed] [Google Scholar]
- Khalid M.F., Shahzad M.A., Sarwar M., Rehman A.U., Sharif M., Mukhtar N. Probiotics and lamb performance: a review. Afr J Agric Res. 2011;6(23):5198–5203. [Google Scholar]
- Khan R.U., Shabana N., Kuldeep D., Karthik K., Ruchi T., Mutassim M.A. Direct-fed microbial: beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. Int J Pharm. 2016;12:220–231. [Google Scholar]
- Kim E., Park C., Lim D., Kwon E., Ki K., Kim S., Moon Y. Effects of coconut materials on in vitro ruminal methanogenesis and fermentation characteristics. Asian-Australas J Anim Sci. 2014;27:1721. doi: 10.5713/ajas.2014.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D.O., Denman S.E., Mackie R.I., Morrison M., Rae A.L., Attwood G.T. Opportunities to improve fibre degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiol Rev. 2003;27(5):663–693. doi: 10.1016/S0168-6445(03)00072-X. [DOI] [PubMed] [Google Scholar]
- Kumar M., Kumar V., Roy D., Kushwaha R., Vaiswani S. Application of herbal feed additives in animal nutrition-a review. Int J Lives Res. 2014;4:1–8. [Google Scholar]
- Kumar S., Chigurupati D., Prasad S., Prasad R.M.V. Effect of yeast culture (Saccharomyces cerevisiae) on the ruminal microbial population in buffalo bulls. Buff Bull. 2013;32:116–119. [Google Scholar]
- Kumar S., Puniya A.K., Puniya M., Dagar S.S., Sirohi S.K., Singh K. Factors affecting rumen methanogens and methane mitigation strategies. World J Microbiol Biotechnol. 2009;25:1557–1566. [Google Scholar]
- Lesmeister K.E., Heinrichs A.J., Gabler M.T. Effects of supplemental yeast (Saccharomyces cerevisiae) culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J Dairy Sci. 2004;87:1832–1839. doi: 10.3168/jds.S0022-0302(04)73340-8. [DOI] [PubMed] [Google Scholar]
- Lu C.D., Jorgensen N.A. Alfalfa saponins affect site and extent of nutrient digestion in ruminants. J Nutr. 1987;117(5):919–927. doi: 10.1093/jn/117.5.919. [DOI] [PubMed] [Google Scholar]
- Maamouri O., Selmi H., M'Hamd N. Effects of yeast (Saccharomyces cerevisiae) feed supplement on milk production and its composition in Tunisian Holstein Friesian cows. Sci Agric Bohem. 2014;45:170–174. [Google Scholar]
- Majdoub-Mathlouthi L., Kraiem K.L. Effects of feeding Saccharomyces cerevisiae Sc 47 to dairy cows on milk yield and milk components, in Tunisian conditions. Livest Res Rural Dev. 2009;21:271–284. [Google Scholar]
- Mao H.L., Mao Hua-long, Wang J.K., Liu J.X., Yoon I. Effects of Saccharomyces cerevisiae fermentation product on in vitro fermentation and microbial communities of low-quality forages and mixed diets. J Anim Sci. 2014;91:3291–3298. doi: 10.2527/jas.2012-5851. [DOI] [PubMed] [Google Scholar]
- Marden J.P., Julien C., Monteils V., Auclair E., Moncoulon R., Bayourthe C. How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high yielding dairy cows? J Dairy Sci. 2008;91:3528–3535. doi: 10.3168/jds.2007-0889. [DOI] [PubMed] [Google Scholar]
- McSweeney C., Mackie R. 2012. Microorganisms and ruminant digestion: state of knowledge, trends and future prospects. Background Study Paper. Roma. [Google Scholar]
- Min B.R., Pinchak W.E., Anderson R.C., Hume M.E. In vitro bacterial growth and in vivo rumen microbiota populations associated with potential bloat dynamics in winter wheat. J Anim Sci. 2016;84:2546–2554. doi: 10.2527/jas.2005-399. [DOI] [PubMed] [Google Scholar]
- Minato H., Endo A., Higuchi M. Ecological treatise on the rumen fermentation. I. The fractionation of bacteria attached to the rumen digesta solids. J Gen Appl Microbiol. 1966;12:39–52. [Google Scholar]
- Miranda R.L.A., Mendoza M.G.D., Bfircena-Gama J.R., Gonziilez M.S., Ferrara R., Ortega C.M.E. Effect of Saccharomyces cerevisiae or Aspergillus oryzaecultures and NDF level on parameters of ruminal fermentation. Anim Feed Sci Technol. 1996;63:289–296. [Google Scholar]
- Moallem U., Lehrer H., Livshitz L., Zachut M., Yakoby S. The effects of live yeast supplementation to dairy cows during the hot season on production, feed efficiency, and digestibility. J Dairy Sci. 2008;92:343–351. doi: 10.3168/jds.2007-0839. [DOI] [PubMed] [Google Scholar]
- Mosoni P., Chaucheyras-Durand F., Bérat-Maillet C., Forano E. Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates. Effect of a yeast additive. J Appl Microbiol. 2007;103:2676–2685. doi: 10.1111/j.1365-2672.2007.03517.x. [DOI] [PubMed] [Google Scholar]
- Mudgal V., Baghel R.P.S. Effect of probiotic supplementation on growth performance of pre-ruminant Buffalo (Bubalus bubalis) calves. Buffalo Bull. 2010;29(3) [Google Scholar]
- Oskoueian E., Abdullah N., Oskoueian A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res Int. 2013:1–8. doi: 10.1155/2013/349129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A.K., Stiverson J., Yu Z. Effects of quillaja and yucca saponins on communities and select populations of rumen bacteria and archaea, and fermentation in vitro. J Appl Microbiol. 2012;113(6):1329–1340. doi: 10.1111/j.1365-2672.2012.05440.x. [DOI] [PubMed] [Google Scholar]
- Patra A.K., Yu Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl Environ Microbiol. 2012;78:4271–4280. doi: 10.1128/AEM.00309-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S.M., Jimenez-Flores R., Pollack J., Lone, Berber-Jimenez M.D. Protein saponin interaction and its influence on blood lipids. J Agric Food Chem. 1993;41:1287–1291. [Google Scholar]
- Qiao G.H., Shan A.S., Ma N., Ma Q.Q., Sun Z.W. Effect of supplemental bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J Anim Physiol Anim Nutr. 2009;94:429–436. doi: 10.1111/j.1439-0396.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- Reti K.L., Thomas M.C., Yanke L.J., Selinger L.B., Inglis G.D. Effect of antimicrobial growth promoter administration on the intestinal microbiota of beef cattle. Gut Pathog. 2013;5(8):1–16. doi: 10.1186/1757-4749-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeian M., Beakes G.W., Parker D.S. Distribution and estimation of anaerobic zoosporic fungi along the digestive tracts of sheep. Mycol Res. 2004;108:1227–1233. doi: 10.1017/s0953756204000929. [DOI] [PubMed] [Google Scholar]
- Rivera-Méndez C., Plascencia A., Torrentera N., Zinn R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J Appl Anim Res. 2017;45(1):199–203. [Google Scholar]
- Robinson P.H. Yeast products for growing and lactating dairy cattle: impact on rumen fermentation and performance. Dairy Rev. 2002;9:1–4. [Google Scholar]
- Rojas-Roman L.A., Castro-Perez B.I., Estrada-Angulo A., Angulo-Montoya C., Yocupicio-Rocha J.A. Influence of long-term supplementation of tannins on growth performance, dietary net energy and carcass characteristics: finishing lambs. Small Rumin Res. 2017;06:010. [Google Scholar]
- Russell J.B., Houlihan A.J. Ionophoreresistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol Rev. 2003;27:65–74. doi: 10.1016/S0168-6445(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Saleem A.M., Zanouny A.I., Singer A.M. Growth performance, nutrients digestibility, and blood metabolites of lambs fed diets supplemented with probiotics during pre- and post-weaning period. Asian Australas J Anim Sci. 2017;30(4):523–530. doi: 10.5713/ajas.16.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansoucy R. Livestock- a driving force for food security and sustainable development. Rev Mund Zootec. 1999;84/85:5–17. [Google Scholar]
- Santra A., Karim S.A. Rumen manipulation to improve animal productivity. Asian Australas J Anim Sci. 2003;16(5):748–763. [Google Scholar]
- Schofield P., Mbugua D.M., Pell A.N. Analysis of condensed tannins: a review. Anim Feed Sci Technol. 2001;91:21–40. [Google Scholar]
- Seo J.K., Kim S.W., Kim M.H., Upadhaya S.D., Kam D.K., Ha J.K. Direct-fed microbials for ruminant animals. Asian Australas J Anim Sci. 2010;23:1657–1667. [Google Scholar]
- Song D., Ibrahim S., Hayek S. INTECH; 2012. Recent application of probiotics in food and agricultural science. Probiotics; pp. 3–36. [Google Scholar]
- Stover M.G., Watson R.R., Collier R. 2016. Pre- and probiotic supplementation in ruminant livestock production; pp. 25–36. [Google Scholar]
- Valenzuela-Grijalva N.V., Pinelli-Saavedra A., Muhlia-Almazan A., Domínguez-Díaz D., González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol. 2017;59:8. doi: 10.1186/s40781-017-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M.V., Prado R.M., Zawadzki F., Eiras C.E., Madrona G.S., Prado I.N. Propolis and essential oils additives in the diets improved animal performance and feed efficiency of bulls finished in feedlot. Acta Scientiarum Anim Sci. 2014;36:419–426. [Google Scholar]
- Vibhute V.M., Shelke R.R., Chavan S.D., Nage S.P. Effect of probiotics supplementation on the performance of lactating crossbred cows. Vet World. 2011;4:557–561. [Google Scholar]
- Wanapat M. Thailand by Funny Press; Bangkok: 1990. Nutritional aspects of ruminant production in Southeast Asia with special reference to Thailand. 549/1 Senanikom 1, Phaholyothin 32. [Google Scholar]
- Wanapat M., Kang S., Polyorach S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J Anim Sci Biotechnol. 2013;4:32. doi: 10.1186/2049-1891-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanapat M. Rumen manipulation to increase the efficient use of local feed resources and productivity of ruminants in the tropics. Asian-Aust. J Anim Sci. 2000;13:59–67. [Google Scholar]
- Westendarp H. Effects of tannins in animal nutrition. DtschTierarztlWochenschr. 2006;113(7):264–268. [PubMed] [Google Scholar]
- Williams P.E.V., Tait C.A.G., Innes G.M., Newbold C.J. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J Anim Sci. 1991;69:3016–3026. doi: 10.2527/1991.6973016x. [DOI] [PubMed] [Google Scholar]
- Wina E., Muetzel S., Becker K. The impact of saponins or saponin-containing plant on ruminant production - a Review. J Agric Food Chem. 2005;53:8093–8105. doi: 10.1021/jf048053d. [DOI] [PubMed] [Google Scholar]
- Yildiz G., Tekeli A., Drochner W., Steingass H. Determination of the effects of some plant extracts on rumen fermentation and protozoal counts by “in vitro” gas production technique. Int J Anim Vet Adv. 2015;7:18–26. [Google Scholar]