Abstract
Camel milk is traditionally considered to have medicinal characteristics that it has potential health benefits and could help to treat several illnesses. Particularly, it is closest to human breast milk and has high levels of nutrients and bioactive components. The aim of this study was to explore the antioxidant peptides derived from protein fractions of camel milk. Camel milk proteins (CMP) were fractionated into camel casein protein (CCP) and camel whey protein (CWP), which were hydrolyzed with pepsin to produce peptic digests P-CCP and P-CWP, respectively. RP-HPLC was used for fractionation of the peptides from the P-CCP and P-CWP. The antioxidant activities were evaluated using superoxide anion generating system of xanthine oxidase (XOD) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay. Active peptides were analyzed using matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) whereas a number of antioxidant peptides, with masses ranging from 913 to 2,951 Da, derived mainly from alpha-casein, lactophorin and lactoferrin, were identified. When yeast cells are used as a system for modeling mitochondrial disease, the peptides in caseins and whey fractions significantly enhanced the tolerance of yeast cells against peroxide-induced oxidative stress. The results show that both caseins and whey proteins of camel milk possess bioactive peptides with significant radical-scavenging activities and thus herald a fascinating opportunity for their potential as nutraceuticals or therapeutic peptides for prevention and treatment of oxidative stress-associated diseases.
Keywords: Camel milk proteins, Antioxidant, Bioactive peptides, Superoxide scavenging, Yeast tolerance
1. Introduction
Milk proteins received increasing attention as they are often precursors of various biologically active peptides within the sequence of proteins which are released by enzymatic actions. They exert multifunctional activities, such as immune-modulatory, anti-inflammatory, antimicrobial and anti-cancer properties (Chen et al., 2014, Zimecki and Kruzel, 2007). In contrast to the milk of other dairy animals, camel milk has been reported to cure severe food allergies in children and diabetes (Abdulrahman et al., 2016, Ehlayel et al., 2011a, Ehlayel et al., 2011b, Shori, 2015, Zibaee et al., 2015). Furthermore, camel milk is suggested to exert a number of therapeutic activities (Hailu et al., 2016, Mihic et al., 2016). Numerous studies suggest that camel milk has distinct therapeutic benefits, such as anti-diabetic (Korish, 2014, Korish et al., 2015, Mirmiran et al., 2017), anti-toxic (Al-Asmari et al., 2017, Khan, 2017), anti-viral (el Agamy et al., 1992, El-Fakharany et al., 2017, Lee et al., 2016), antibacterial (Cardoso et al., 2013, Rahimi and Kheirabadi, 2012, Soliman et al., 2015), anti-rheumatoid arthritis (Arab et al., 2017), anticancer (Ayyash et al., 2017, Chen et al., 2014, Korashy et al., 2012), and wound healing (Ebaid et al., 2015, Ebaid et al., 2017) activities. In addition, camel milk has been used for centuries in the Middle East, Asian and North African cultures as a natural remedy for many common health problems (Agrawal et al., 2011a, Agrawal et al., 2011b, Al-Ayadhi and Elamin, 2013, Ehlayel et al., 2011a, Ehlayel et al., 2011b, Mohamad et al., 2009, Mohamed et al., 2015, Shori, 2015).
Reactive oxygen species is an aspect of oxidative stress which are associated with many diseases such as rheumatoid arthritis, diabetes, inflammation, atherosclerosis and cancers (Collins, 2005, Halliwell, 1994). Thus, the need for naturally occurring antioxidants is of crucial importance. Some biopeptides derived from natural proteins have significant therapeutic activities, including anti-oxidative, anti-inflammation, antimicrobial and antihypertensive actions (FitzGerald et al., 2004, Korhonen and Pihlanto, 2007, Lee et al., 2004, Silva and Malcata, 2005). The interesting feature of bioactive peptides as nutraceuticals or therapeutics is that they display little side effects in human. Some studies reported the antioxidant effects of camel milk; however, these studies were performed on fermented milk (Ayyash et al., 2017, Ayyash et al., 2018), whole milk (Al-Ayadhi and Elamin, 2013, Zhu et al., 2016), total protein hydrolysate (Al-Shamsi et al., 2018), or specific protein such as lactoferrin (Habib et al., 2013). There is little information available in the literature about the study on the antioxidant action of the isolated protein fractions of camel milk or their peptides. Thus, the assessment of the peptides from camel milk protein (CMP) fractions for their abilities to scavenge free radicals to prevent oxidative stress would be highly rewarding, has yet to be investigated and is crucially needed. Therefore, this study is to explore the antioxidant activities of the protein fractions, casein and whey, of camel milk as well as their peptides released by peptic digestion.
2. Materials and methods
2.1. Materials
Pepsin (porcine), xanthine, xanthine oxidase (XOD), nitroblue tetrazolium (NBT) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were from Sigma–Aldrich (Tokyo, Japan); TSK gel ODS-120T column was from TOSOH (Tokyo, Japan); α-cyano-4-hydroxy-cinnamic acid (α-HCCA) was from Bruker Daltonik (Bremen, Germany).
2.2. Fractionation and peptic hydrolysis of proteins
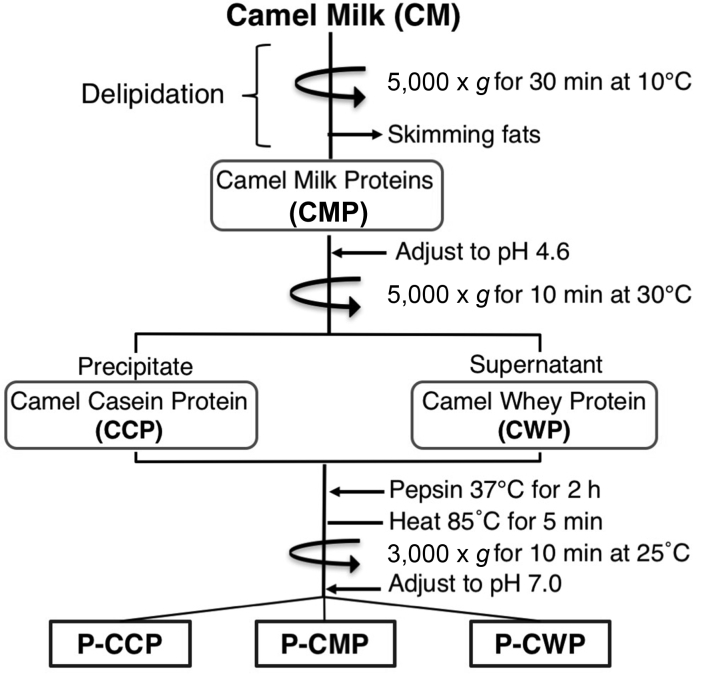
As shown in Fig. 1, fats were removed from raw camel milk by centrifugation at 5,000 × g for 30 min at 10 °C, and the supernatant was passed through 3 layers of gauze and referred to as CMP. A portion of CMP was lyophilized and the rest was adjusted to pH 4.6 with acetic acid (10%) and centrifuged at 5,000 × g for 10 min at 30 °C. The re-suspended pellet (caseins) and the supernatant (whey proteins) were dialyzed, using 1000 MWCO tubes. These fractions were lyophilized and referred to as camel casein protein (CCP) and camel whey protein (CWP). Protein in HCl solution (pH 3.0) was mixed with pepsin to give 50:1 (wt/wt) protein to pepsin. After incubation at 37 °C for 2 h, pepsin was inactivated by heating at 85 °C for 5 min. The reactions were centrifuged at 3,000 × g for 10 min at room temperature, and the supernatants were adjusted to pH 7.0 and freeze-dried. The degree of hydrolysis in the peptic digests of caseins (P-CCP) and whey (P-CWP) was analyzed on reducing sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to standard protocols (Laemmli, 1970). Protein bands were visualized with Coomassie Brilliant Blue (CBB). The hydrolysates (P-CWP and P-CCP) were purified by RP-HPLC on C18 column (7.5 mm × 25 mm) with linear gradient of 1% to 40% acetonitrile over 180 min. Elution of peptides was monitored at 214 nm. Peptides were collected, vacuum dried and re-suspended at the desired concentration in distilled water.
Fig. 1.
Outline of the fractionation of milk proteins into caseins and whey proteins as well as peptic hydrolysis of the fractions.
2.3. Antioxidant activity assay
Antioxidant activity was evaluated by 2 methods: superoxide (O2•−)-scavenging and DPPH-mediated reduction assays. Superoxide generated during the conversion of xanthine into uric acid by XOD in the presence of nitro-blue tetrazolium as a probe was employed to assess superoxide-scavenging activity (Ahmed et al., 2015, Ibrahim et al., 2017). The scavenging capacity is expressed as the degree of NBT reduction by superoxide and measured spectrophotometrically at 562 nm. A 100 μL reaction mixture containing NBT (40 μmol/L), xanthine (5 mmol/L) and various concentrations of protein or peptides in 10 mmol/L Na-phosphate buffer, pH 8.0, in a 96-well plate. Control (Ctrl) lacked test sample. Upon addition of a 100-μL of 5 mU XOD, the kinetics of the reaction was monitored at 562 nm (37 °C) for 20 min by an Ultrospec Biotrak II microplate reader (Amersham Biosciences, Uppsala, Sweden). Two blanks were prepared without XOD or both sample and XOD. Blank values were subtracted from samples, and the results represented as absorbance change, by subtracting the reading at 0 time from the subsequent readings. Results are representative of 2 experiments with 3 wells per sample.
The DPPH-scavenging activity was measured by spraying DPPH solution over protein spotted on a thin layer chromatography (TLC) sheet (Nia et al., 2004). A 6 μL proteins or peptides were spotted on a 5 cm × 7.5 cm TLC sheet (silica gel 60 F254; Merck, Germany) and air-dried. The sheets were sprayed with ethanol solution of DPPH (1.8 mmol/L). White spots will appear within the purple background when spots contain DPPH-scavenger.
2.4. Yeast model for oxidative stress
Yeast Saccharomyces cerevisiae, which displays a moderate sensitivity to oxidative stress induced by H2O2, was used as a system for modeling mitochondrial disease. Briefly, S. cerevisiae (YNN27) cells, grown in yeast extract peptone dextrose (YPD) broth, were suspended in the same broth at absorbance (600 nm) of 0.1. Peptides (200 μg/mL) were incubated at 28 °C with yeast suspension for 1 h. An oxidizing agent (H2O2) was added (2 mmol/L), and the incubation was extended for 48 h. A 10 μL portion of serially diluted cultures in 2 mmol/L H2O2 was spotted on YPD agar plates containing 2 mmol/L H2O2. Plates were incubated for 72 h at 28 °C and the colony forming units of yeast was estimated after incubation of the agar plates at 28 °C for 72 h. Data was presented in cfu/mL.
2.5. Peptides identification
Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) was employed for the identification of the peptides in the RP-HPLC peaks. Peptide solution (2 μL) was mixed with 2 μL of a saturated solution of α-HCCA matrix, α-cyano-4-hydroxy-cinnamic acid (Bruker Daltonik, Bremen, Germany), onto a target plate and air-dried. The analysis was performed using an Autoflex Speed mass spectrometer (Bruker Daltonik GmbH, Germany) in positive reflector mode with mass range of 1,000 to 3,200 Da. Peptides calibration standard (700 to 4,000 Da) was used for calibration according to instructions of the manufacture (Bruker Daltonik GmbH, Germany). The sequences of peptides were identified by subjecting the major precursor ions in each peak to MS/MS analysis, using a de novo routine and an automated application to MASCOT and SEQUEST database.
3. Results
3.1. Peptic hydrolysis of camel milk proteins
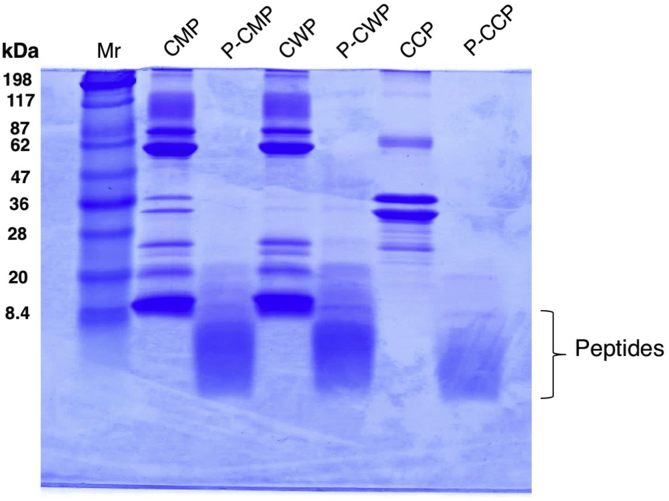
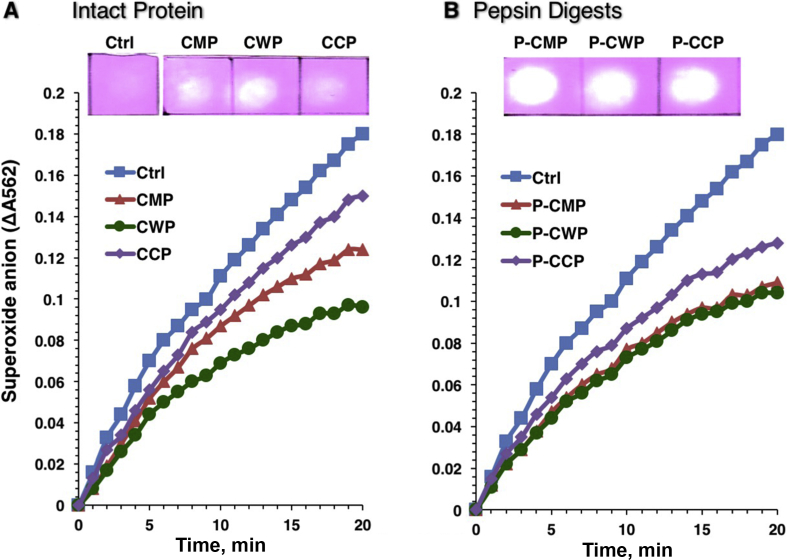
As shown in Fig. 1, total proteins (CMP) caseins (CCP) and whey (CWP) protein fractions of camel milk were separately hydrolyzed with an aspartic acid protease pepsin for 2 h, and then dialyzed before lyophilization. The hydrolysates were analyzed on reducing SDS-PAGE (Fig. 2). It is evident CWP and CCP were adequately separated from each other. All protein fractions (CCP, CWP and CMP) were completely hydrolyzed into peptides with molecular masses less than 8 kDa. The intact proteins as well as the hydrolysates were assessed for their abilities to scavenge superoxide anion and the chemical radical DPPH (Fig. 3). The intact proteins (CMP, CWP and CCP) remarkably reduced the formation of blue diformazan, indicating superoxide-scavenging capacity, with CWP being the strongest superoxide-scavenger (Fig. 3A). The results of DPPH reduction (Fig. 3A upper) paralleled those of the superoxide-scavenging activity (Fig. 3A). However, the hydrolysate of caseins (P-CCP) exhibited greater superoxide-scavenging activity than its intact proteins, while the scavenging capacity of whey protein (P-CWP) paralleled its intact proteins (Fig. 3B). The hydrolysates of the whole proteins (P-CMP) and protein fractions (P-CWP and P-CCP) exhibited significant DPPH reduction, as produced more intense white spots (Fig. 3B, upper) than their undigested proteins (Fig. 3A, upper). The results demonstrate that the peptic hydrolysates of CMP fractions not only can scavenge oxygen superoxide but also have the ability to donate electron to reduce the chemical radical DPPH.
Fig. 2.
Electrophoretic patterns of camel milk proteins (CMP), camel whey proteins (CWP), and camel casein proteins (CCP) before and after peptic digestion on 15% polyacrylamide gels of reducing sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). Mr = molecular weight marker; P-CMP = pepsin digested CMP; P-CWP = pepsin digested CWP; P-CCP = pepsin digested CCP.
Fig. 3.
Superoxide-scavenging activities of (A) camel milk protein (CMP) fractions and (B) their peptic hydrolysates measured in xanthine/XOD/NBT reduction assay at 100 μg/mL protein. The rate of O2•− accumulation measured in real-time kinetics was presented as the rate of absorbance change at 562 nm due to NBT reduction at 37 °C for 20 min. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) reduction of the fractions are shown above the panels. XOD = xanthine oxidase; NBT = nitroblue tetrazolium; CWP = camel whey proteins; CCP = camel casein proteins; P-CMP = pepsin digested CMP; P-CWP = pepsin digested CWP; P-CCP = pepsin digested CCP.
3.2. Fractionation of the antioxidant peptides
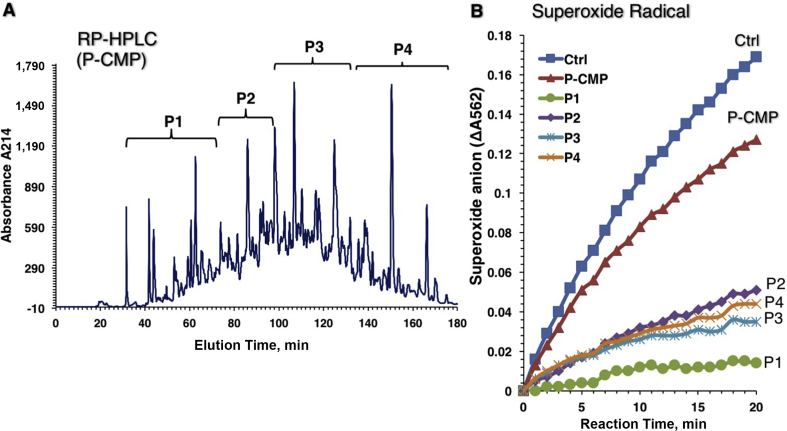
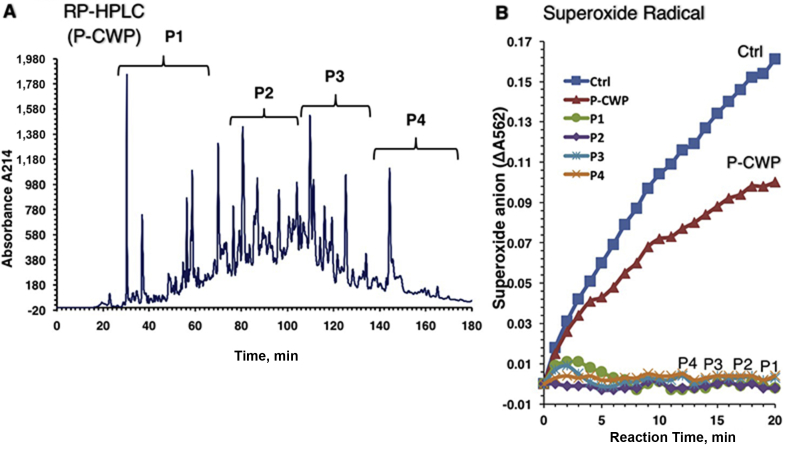
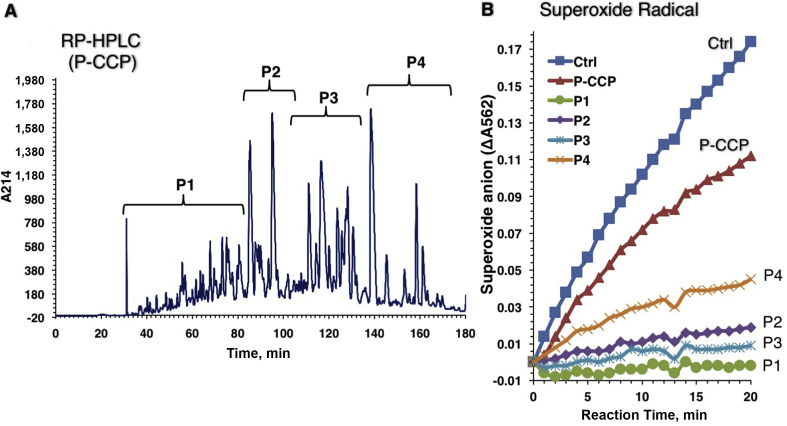
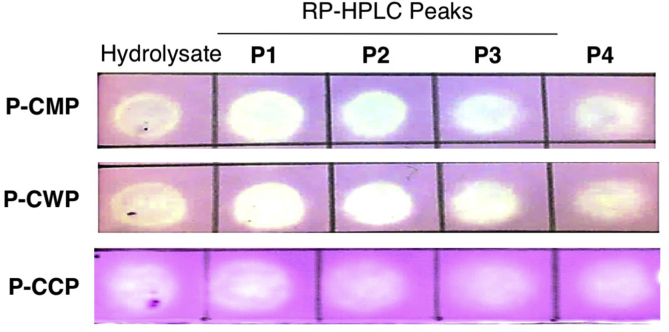
Peptides in P-CMP, P-CWP and P-CCP were separated into 4 peptide sub-fractions, designated P1 to P4, using an RP-HPLC equipped with a C18 column (Figs. 4A, 5A and 6A ). Peaks of sub-fractions P1 and P2 are referred to as fast-eluting peptides (hydrophilic), whereas P3 and P4 are slow-eluting peptides (hydrophobic). The peptide fractions were tested for superoxide scavenging activity at a concentration of 40 μg/mL (Figs. 4B, 5B and 6B). All peptide sub-fractions of P-CMP showed much stronger scavenging activity than their total hydrolysates, indicating that the peptic hydrolysis can produce powerful superoxide-scavenging peptides from CMP without fractionation into caseins and whey proteins (Fig. 4). However, peptides of the whey (P-CWP) showed the strongest scavenging activities regardless of the elution time (Fig. 5), while peptides of total proteins P-CMP (Fig. 4) and P-CCP (Fig. 6) hydrolysates exhibited variable activities ranging from strong (P1 and P3) to moderate (P2 and P4) activities. In all protein types, the fast-eluting peptides (P1 and P2) exhibited more potent DPPH reducing capacities than the slow-eluting peptides (Fig. 7). The peptides fractions P1, P2 and P3 of the whole proteins (P-CMP) and whey protein (P-CWP) exhibited much more significant DPPH reduction than those of P-CCP (Fig. 7). The results demonstrate that the hydrophilic peptides (fast-eluting peptides) are more potent antioxidants than the hydrophobic peptides (slow-eluting peptides).
Fig. 4.
RP-HPLC pattern on a C18 column of (A) the pepsin digested camel milk proteins (P-CMP) hydrolysate and (B) superoxide-scavenging capacities of the peptide peaks. (A) Elution was achieved with a 1% to 40% linear gradient of acetonitrile, and absorbance was monitored at 215 nm. The RP-HPLC patterns represent 4 peptide peaks (P1 to P4). (B) The rate of O2•− accumulation measured in the real-time kinetics of non-stopped xanthine/XOD/NBT reduction assay, and was presented as the rate of absorbance change at 562 nm due to NBT reduction. XOD = xanthine oxidase; NBT = nitroblue tetrazolium.
Fig. 5.
RP-HPLC pattern on a C18 column of (A) the pepsin digested camel whey proteins (P-CWP) hydrolysate and (B) superoxide-scavenging capacities of the peptide peaks. (A) Elution was achieved with a 1% to 40% linear gradient of acetonitrile, and absorbance was monitored at 215 nm. The RP-HPLC patterns represent 4 peptide peaks (P1 to P4). (B) The O2•− scavenging activity is presented.
Fig. 6.
RP-HPLC pattern on C18 column of (A) the pepsin digested camel casein proteins (P-CCP) hydrolysate and (B) superoxide-scavenging capacities of the peptide peaks. (A) Elution was achieved with a 1% to 40% linear gradient of acetonitrile, and absorbance was monitored at 215 nm. The RP-HPLC patterns represent 4 peptide peaks (P1 to P4). (B) The O2•− scavenging activity is presented.
Fig. 7.
DPPH radical-scavenging capacities by pepsin hydrolysates of P-CMP, P-CWP, P-CCP and their RP-HPLC peptide peaks (200 μg/mL). Thin layer chromatography (TLC) blot assay on a silica gel TLC plate stained by spraying with 1.8 mmol/L DPPH solution in ethanol, and visualized for the presence of whitish spots, indicating anti-oxidant activity. DPPH = 2,2-diphenyl-1-picrylhydrazyl; P-CMP = pepsin digested camel milk proteins; P-CWP = pepsin digested camel whey proteins; P-CCP = pepsin digested camel casein proteins.
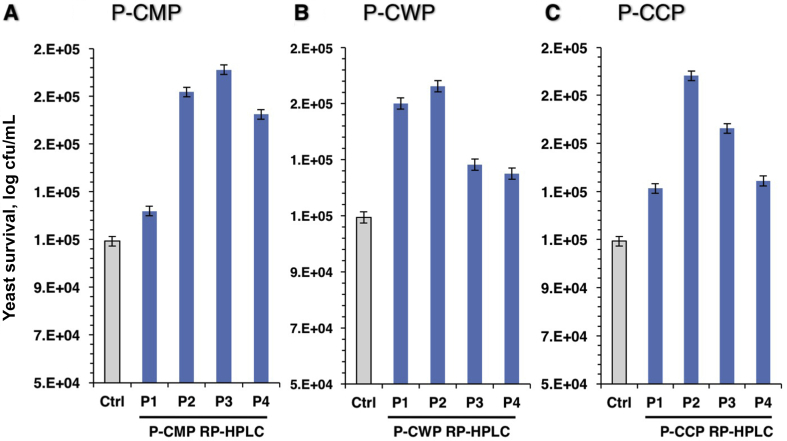
3.3. Peptides impact on the tolerance of yeast cells against oxidative stress
To examine the ability of peptides to induce acquisition of tolerance of yeast cells to oxidative stress, the eukaryote Saccharomyces cervisiae cells were incubated with peptides followed by exposure to the oxidative H2O2 (2 mmol/L). The survival of the treated cells was determined by spotting on agar plates containing 2 mmol/L of H2O2 (Fig. 8). Peptide sub-fractions from all protein types (whole proteins, whey or caseins) showed remarkable increase in the survival (log cfu/mL) compared with the mock-treated cells (Ctrl). However, fast-eluting peptides of whey (P-CWP) were more efficient than the slower ones (Fig. 8B), while slow-eluting peptides of whole proteins (P-CMP) and caseins (P-CCP) were more potent (Fig. 8A and C). The results indicate that pepsin releases different antioxidant peptides from CMP whereas many of them act as intracellular antioxidant peptides. It can be concluded that some antioxidant peptides have the ability to modulate the intracellular redox state and thus impart tolerance against oxidative stress in live cells. Likely, treatments with the peptides can lead to the transient induction of protection against subsequent oxidative conditions.
Fig. 8.
Tolerance of yeast cells to H2O2-induced oxidative stress by RP-HPLC peptide peaks of (A) P-CMP, (B) P-CWP and (C) P-CCP. Cells were pre-cultured to the exponential growth phase in yeast extract peptone dextrose (YPD) broth. Cells treated with peptide peaks then H2O2 was added in YPD to a final concentration of 2 mmol/L and cultured for 48 h at 28 °C. Cultures were serially diluted in YPD containing the same concentration of H2O2, spotted onto YPD agar containing 2 mmol/L H2O2, and then incubated at 28 °C for 48 h before colony count. Data are represented in log cfu/mL. P-CMP = pepsin digested camel milk proteins; P-CWP = pepsin digested camel whey proteins; P-CCP = pepsin digested camel casein proteins.
3.4. Sequences of the active peptides
Peptides in the peaks of RP-HPLC were identified using MALDI-TOF-TOF analysis. Casein hydrolysate (P-CCP) contained 14 peptides in the active peak fractions (P1, P2 and P3) with masses ranging from 913.12 to 2,951.68 m/z (Table 1). The hydrolysate of whey (P-CWP) contained 8 peptides in active peak fractions (P1 and P2) ranging in masses between 1,168.52 and 1,861.14 m/z (Table 2). In caseins, few peptides were from αs1-, αs2- and κ-casein, but β-caseins produced many peptides (Table 1). In the whey, few peptides originated from lactophorin and cysteine-rich protein, but the peptides were mainly generated from lactoferrin (Table 2).
Table 1.
Peptides identified by MALDI-TOF-MS in the RP-HPLC active fractions of camel milk casein hydrolysates. MALDI-TOF-MS = matrix-assisted laser desorption ionization time of flight mass spectrometry
| Fraction | Mass, m/z |
Sequence | Protein identity (fragment) | |
|---|---|---|---|---|
| Signal1 | Predict | |||
| P-CCP P1 | 913.12 | 913.44 | FIPYPNY | κ-casein (55 to 61) |
| 1,091.59 | 1,092.63 | RPKYPLRY | αs1-casein (1 to 8) | |
| 1,168.5 | 1,168.62 | TLTDLENLHL | β-casein (127 to 136) | |
| 1,861.12 | 1,862.93 | QIPQCQALPNIDPPTVE | κ-casein (72 to 88) | |
| 2,001.96 | 2,000.89 | MDQGSSSEESINVSQQKF | αs2-casein (4 to 21) | |
| P-CCP P2 | 1,059.76 | 1,059.51 | FFQLGDYVA | αs1-casein (159 to 167) |
| 1,092.70 | 1,092.63 | RPKYPLRY | αs1-casein (1 to 8) | |
| 1,140.48 | 1,139.57 | QDKIYTFPQ | β-casein (47 to 55) | |
| 1,321.06 | 1,322.66 | LHQGQIVMNPW | αs2-casein (78 to 88) | |
| 1,416.94 | 1,416.71 | MVPYPQRAMPVQ | β-casein (178 to 189) | |
| 2,006.91 | 2,009.06 | PFQEPVPDPVRGLHPVPQ | β-casein (193 to 210) | |
| P-CCP P3 | 2,169.84 | 2,170.19 | VRNIKEVESAEVPTENKISQ | αs2-casein (45 to 64) |
| 2,304.72 | 2,305.2 | SISSSEESITHINKQKIEKF | β-casein (15 to 34) | |
| 2,951.68 | 2,947.68 | QPKVMDVPKTKETIIPKRKEMPLLQ | β-casein (90 to 114) | |
P-CCP = pepsin digested camel casein proteins.
De novo peptide sequencing of the major RP-HPLC peaks of camel casein hydrolysates in MALDI-TOF-MS was performed by manual interpretation of the ion series.
Table 2.
Peptides identified by MALDI-TOF-MS in the RP-HPLC active fractions of camel milk whey hydrolysates. MALDI-TOF-MS = matrix-assisted laser desorption ionization time of flight mass spectrometry.
| Fraction | Mass, m/z |
Sequence | Protein identity (fragment) | |
|---|---|---|---|---|
| Signal1 | Predict | |||
| P-CWP P1 | 1,168.52 | 1,167.67 | ATTLEGKLVEL | Lactophorin B (79 to 89) |
| 2,001.99 | 1,999.98 | KCLQDGAGDVAFVKDSTVF | Lactoferrin (197 to 215) | |
| P-CWP P2 | 1,059.77 | 1,059.57 | KADAVTLDGGL | Lactoferrin (53 to 63) |
| 1,321.07 | 1,321.73 | KFGRGKPSGFQL | Lactoferrin (277 to 288) | |
| 1,421.84 | 1,422.69 | TVVSNNGNREYGL | Lactalbumin (40 to 52) | |
| 1,544.61 | 1,543.83 | CGSIVPRREWRAL | PGRP (7 to 19) | |
| 1,758.37 | 1,760.73 | SSCAMRCLDPVTEDSF | Cys to rich Protein (101 to 116) | |
| 1,861.14 | 1,861.88 | ENTMRETMDFLKSLF | Lactophorin A (113 to 127) | |
P-CWP = pepsin digested camel whey proteins; PGRP = peptidoglycan recognition protein.
De novo peptide sequencing of the major RP-HPLC peaks of camel whey hydrolysates in MALDI-TOF-MS was performed by manual interpretation of the ion series.
4. Discussion
Oxidative stress is a state characterized by increased levels of free radicals which cause damages to vital biomolecules, such as lipids, proteins and DNA. Oxidative stress is generally linked to numerous chronic diseases including atherosclerosis, cancer, diabetics, rheumatoid arthritis, cardiovascular diseases, chronic inflammation and other degenerative diseases in human (Fridovich, 1999, Uttara et al., 2009). Thus, antioxidants are vital in scavenging of radicals and prevention of the oxidative stress and the associated diseases. This study demonstrates that the peptic hydrolysates of CMP contain several peptides with antioxidant activities. A large number of peptides were obtained from caseins (Fig. 6B, P1 to P3), but the most active peptides were originated from whey proteins (Fig. 5B). The active peptides characterized by their hydrophilic nature as they eluted faster from C18 column in RP-HPLC. However, antioxidant peptides are characterized with high contents of the hydrophobic residues (Dávalos et al., 2004). The results of this study argue in favor of high contents of hydrophobic residues, such as Tyr, Leu, Phe and Ile, of the identified peptides, particularly peptides from camel caseins (Table 1). Peptides from whey proteins were rather amphiphilic in nature, due to the presence of hydrophilic residues, such as Lys, Asp, Glu and Ser, scattered along the peptides with the hydrophobic residues (Table 2). The presence of certain amino acid sequence makes contribution to the antioxidant activity of a peptide, particularly Glu–Tyr, Glu–Trp, Asn–Pro and Pro–Tyr sequence motifs (Zou et al., 2016). Peptides from camel milk whey proteins found in this study are rich in these motifs, such as Glu–Tyr, Glu–Trp, and Asp–Pro pairs in peptides with masses of 1,421.84, 1,544.61 and 1,758.37 m/z, respectively of P-CWP P2 fraction (Table 2). In caseins derived peptides, the sequences Pro–Try, Asn–Tyr, Asp–Pro and Tyr–Pro pairs were confirmed in peptides with masses of 913.12, 1,091.59, 1,059.76 and 1,092.70 m/z, respectively, of P-CCP P1 and P-CCP P2 (Table 1). The importance of these sequences for antioxidant activity has been documented that the carboxyls of acidic residues (Glu or Asp) possess excess electrons while the phenolic hydroxyls of Tyr can release hydrogen thus display high reducing abilities (Zou et al., 2016). It is noteworthy that the peptides from whey have generally Leu residue or Phe at the C-terminal (Table 2), which has been documented to be essential in many antioxidant peptides whereas hydrophobic residue at the C-termini of the peptide are essential for radical scavenging activity (Saito et al., 2003). In addition, there are evidences that intact peptides are absorbed in the upper part of human digestive tract, in the duodenum and jejunum through the tight junctions between epithelial cells, and the amphiphilic peptides and those containing Pro residues are readily absorbed (Warren et al., 2014). Thus, the use of pepsin as the hydrolyzing enzyme of CMP can be an effective approach to the production of potent antioxidant peptides and likely for the enhancement of their intestinal absorption. This should await further investigation.
In conclusion, our data highlight that variable anti-oxidative peptides could be released through peptic hydrolysis of CMP fractions. Although casein hydrolysates produced a larger number of peptides (Table 1) with variable antioxidant capacities, all the peptides from whey hydrolysate exhibited higher superoxide scavenging potencies (Fig. 5) than those from caseins (Fig. 6). But casein peptides particularly in peak 1 (P1) were more efficient in reducing DPPH than those from the whey (Fig. 7). The amphiphilic characteristics of peptides of CMP seem to be important for the observed antioxidant potency, most likely as they enhance the solubility of the peptides and participate in protons exchange with radical species. Our study found that peptides from both caseins and whey hydrolysates exhibited remarkable protective effects on H2O2-stressed yeast cells (Fig. 8). Although other proteases could be effective, the peptides generated by pepsin from camel milk caseins and whey proteins could be excellent candidates for the development of novel therapeutic peptides for the treatment of oxidative stress and the associated diseases.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdulrahman A.O., Ismael M.A., Al-Hosaini K., Rame C., Al-Senaidy A.M., Dupont J. Differential effects of camel milk on insulin receptor signaling – toward understanding the insulin-like properties of camel milk. Front Endocrinol (Lausanne) 2016;7:4. doi: 10.3389/fendo.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R.P., Jain S., Shah S., Chopra A., Agarwal V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur J Clin Nutr. 2011;65(9):1048–1052. doi: 10.1038/ejcn.2011.98. [DOI] [PubMed] [Google Scholar]
- Agrawal R.P., Sharma P., Gafoorunissa S.J., Ibrahim S.A., Shah B., Shukla D.K. Effect of camel milk on glucose metabolism in adults with normal glucose tolerance and type 2 diabetes in Raica community: a crossover study. Acta Biomed. 2011;82(3):181–186. [PubMed] [Google Scholar]
- Ahmed A.S., El-Bassiony T., Elmalt L.M., Ibrahim H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Res Int. 2015;74:80–88. doi: 10.1016/j.foodres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- Al-Asmari A.K., Abbasmanthiri R., Al-Elawi A.M., Al-Horaib G., Al-Sadoon K., Al-Asmari B.A. Effect of camel milk against renal toxicity in experimental rats. Pak J Pharm Sci. 2017;30(2):561–565. [PubMed] [Google Scholar]
- Al-Ayadhi L.Y., Elamin N.E. Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD) Evid Based Complement Alternat Med. 2013;2013:602834. doi: 10.1155/2013/602834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamsi K.A., Mudgil P., Hassan H.M., Maqsood S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J Dairy Sci. 2018;101(1):47–60. doi: 10.3168/jds.2017-13194. [DOI] [PubMed] [Google Scholar]
- Arab H.H., Salama S.A., Abdelghany T.M., Omar H.A., Arafa E.A., Alrobaian M.M. Camel milk attenuates rheumatoid arthritis via inhibition of mitogen activated protein kinase pathway. Cell Physiol Biochem. 2017;43(2):540–552. doi: 10.1159/000480527. [DOI] [PubMed] [Google Scholar]
- Ayyash M., Al-Dhaheri A.S., Al Mahadin S., Kizhakkayil J., Abushelaibi A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: a comparative study with fermented bovine milk. J Dairy Sci. 2017;101:900–911. doi: 10.3168/jds.2017-13400. [DOI] [PubMed] [Google Scholar]
- Ayyash M., Al-Nuaimi A.K., Al-Mahadin S., Liu S.Q. In vitro investigation of anticancer and ACE-inhibiting activity, alpha-amylase and alpha-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: a comparative study with fermented bovine milk. Food Chem. 2018;239:588–597. doi: 10.1016/j.foodchem.2017.06.149. [DOI] [PubMed] [Google Scholar]
- Cardoso R.R., Ponte M., Leite V. Protective action of camel milk in mice inoculated with Salmonella enterica. Isr Med Assoc J. 2013;15(1):5–8. [PubMed] [Google Scholar]
- Chen H.Y., Mollstedt O., Tsai M.H., Kreider R.B. Potential clinical applications of multi-functional milk proteins and peptides in cancer management. Curr Med Chem. 2014;21(21):2424–2437. doi: 10.2174/0929867321666140205135739. [DOI] [PubMed] [Google Scholar]
- Collins A.R. Antioxidant intervention as a route to cancer prevention. Eur J Cancer. 2005;41(13):1923–1930. doi: 10.1016/j.ejca.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dávalos A., Miguel M., Bartolomé B., López-Fandiño R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Prot. 2004;67:1939–1944. doi: 10.4315/0362-028x-67.9.1939. [DOI] [PubMed] [Google Scholar]
- Ebaid H., Abdel-Salam B., Hassan I., Al-Tamimi J., Metwalli A., Alhazza I. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2015;14:132. doi: 10.1186/s12944-015-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebaid H., Abdel-Salam B., Hassan I., Al-Tamimi J., Metwalli A., Alhazza I. Erratum to: camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2017;16(1):43. doi: 10.1186/s12944-017-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlayel M., Bener A., Abu Hazeima K., Al-Mesaifri F. Camel milk is a safer choice than goat milk for feeding children with cow milk allergy. ISRN Allergy. 2011;2011:391641. doi: 10.5402/2011/391641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlayel M.S., Hazeima K.A., Al-Mesaifri F., Bener A. Camel milk: an alternative for cow's milk allergy in children. Allergy Asthma Proc. 2011;32(3):255–258. doi: 10.2500/aap.2011.32.3429. [DOI] [PubMed] [Google Scholar]
- el Agamy E.I., Ruppanner R., Ismail A., Champagne C.P., Assaf R. Antibacterial and antiviral activity of camel milk protective proteins. J Dairy Res. 1992;59(2):169–175. doi: 10.1017/s0022029900030417. [DOI] [PubMed] [Google Scholar]
- El-Fakharany E.M., El-Baky N.A., Linjawi M.H., Aljaddawi A.A., Saleem T.H., Nassar A.Y. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp Ther Med. 2017;13(4):1313–1320. doi: 10.3892/etm.2017.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald R.J., Murray B.A., Walsh D.J. Hypotensive peptides from milk proteins. J Nutr. 2004;134(4):980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Habib H.M., Ibrahim W.H., Schneider-Stock R., Hassan H.M. Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem. 2013;141(1):148–152. doi: 10.1016/j.foodchem.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Hailu Y., Hansen E.B., Seifu E., Eshetu M., Ipsen R., Kappeler S. Functional and technological properties of camel milk proteins: a review. J Dairy Res. 2016;83(4):422–429. doi: 10.1017/S0022029916000686. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344(8924):721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim H.R., Ahmed A.S., Miyata T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J Adv Res. 2017;8(1):63–71. doi: 10.1016/j.jare.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A. Immune potentiating and antitoxic effects of camel milk against cyclophosphamide-induced toxicity in BALB/C mice. Int J Health Sci (Qassim) 2017;11(4):18–22. [PMC free article] [PubMed] [Google Scholar]
- Korashy H.M., Maayah Z.H., Abd-Allah A.R., El-Kadi A.O., Alhaider A.A. Camel milk triggers apoptotic signaling pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines through transcriptional mechanism. J Biomed Biotechnol. 2012;2012:593195. doi: 10.1155/2012/593195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen H., Pihlanto A. Bioactive peptides from food proteins. In: Hui Y.H., editor. Handbook of food products manufacturing. John Wiley & Sons, Inc; New Jersey: 2007. pp. 5–37. [Google Scholar]
- Korish A.A. The antidiabetic action of camel milk in experimental type 2 diabetes mellitus: an overview on the changes in incretin hormones, insulin resistance, and inflammatory cytokines. Horm Metab Res. 2014;46(6):404–411. doi: 10.1055/s-0034-1368711. [DOI] [PubMed] [Google Scholar]
- Korish A.A., Abdel Gader A.G., Korashy H.M., Al-Drees A.M., Alhaider A.A., Arafah M.M. Camel milk attenuates the biochemical and morphological features of diabetic nephropathy: inhibition of Smad1 and collagen type IV synthesis. Chem Biol Interact. 2015;229:100–108. doi: 10.1016/j.cbi.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G.H., Tan B.H., Teo E.C., Lim S.G., Dan Y.Y., Wee A. Chronic infection with camelid hepatitis e virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150(2):355–357. doi: 10.1053/j.gastro.2015.10.048. e353. [DOI] [PubMed] [Google Scholar]
- Lee J., Koo N., Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp Rev Food Sci Food Safe. 2004;3(1):21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Mihic T., Rainkie D., Wilby K.J., Pawluk S.A. The therapeutic effects of camel milk: a systematic review of animal and human trials. J Evid Based Complem Altern Med. 2016;21(4):NP110–NP126. doi: 10.1177/2156587216658846. [DOI] [PubMed] [Google Scholar]
- Mirmiran P., Ejtahed H.S., Angoorani P., Eslami F., Azizi F. Camel milk has beneficial effects on diabetes mellitus: a systematic review. Int J Endocrinol Metab. 2017;15(2):e42150. doi: 10.5812/ijem.42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad R.H., Zekry Z.K., Al-Mehdar H.A., Salama O., El-Shaieb S.E., El-Basmy A.A. Camel milk as an adjuvant therapy for the treatment of type 1 diabetes: verification of a traditional ethnomedical practice. J Med Food. 2009;12(2):461–465. doi: 10.1089/jmf.2008.0009. [DOI] [PubMed] [Google Scholar]
- Mohamed W.A., Schaalan M.F., El-Abhar H.S. Camel milk: potential utility as an adjunctive therapy to Peg-IFN/RBV in HCV-4 infected patients in Egypt. Nutr Cancer. 2015;67(8):1305–1313. doi: 10.1080/01635581.2015.1087041. [DOI] [PubMed] [Google Scholar]
- Nia R., Paper D.H., Essien E.E., Iyadi K.C., Bassey A.I.L., Antai A.B. Evaluation of the anti-oxidant and anti-angiogenic effects of Sphenocentrum jollyanum pierre. Afr J Biomed Res. 2004;7:129–132. [Google Scholar]
- Rahimi E., Kheirabadi E.K. Detection of Helicobacter pylori in bovine, buffalo, camel, ovine, and caprine milk in Iran. Foodborne Pathog Dis. 2012;9(5):453–456. doi: 10.1089/fpd.2011.1060. [DOI] [PubMed] [Google Scholar]
- Saito K., Jin D.H., Ogawa T., Muramoto K., Hatakeyama E., Yasuhara T. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem. 2003;51:3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Shori A.B. Camel milk as a potential therapy for controlling diabetes and its complications: a review of in vivo studies. J Food Drug Anal. 2015;23(4):609–618. doi: 10.1016/j.jfda.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S.V., Malcata F.X. Caseins as source of bioactive peptides. Int Dairy J. 2005;15(1):1–15. [Google Scholar]
- Soliman M.M., Hassan M.Y., Mostafa S.A., Ali H.A., Saleh O.M. Protective effects of camel milk against pathogenicity induced by Escherichia coli and Staphylococcus aureus in Wistar rats. Mol Med Rep. 2015;12(6):8306–8312. doi: 10.3892/mmr.2015.4486. [DOI] [PubMed] [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M.W., Stevens B.R., Moughan J.P. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev. 2014;27:308–329. doi: 10.1017/S0954422414000225. [DOI] [PubMed] [Google Scholar]
- Zhu W.W., Kong G.Q., Ma M.M., Li Y., Huang X., Wang L.P. Short communication: camel milk ameliorates inflammatory responses and oxidative stress and downregulates mitogen-activated protein kinase signaling pathways in lipopolysaccharide-induced acute respiratory distress syndrome in rats. J Dairy Sci. 2016;99(1):53–56. doi: 10.3168/jds.2015-10005. [DOI] [PubMed] [Google Scholar]
- Zibaee S., Hosseini S.M., Yousefi M., Taghipour A., Kiani M.A., Noras M.R. Nutritional and therapeutic characteristics of camel milk in children: a systematic review. Electron Physician. 2015;7(7):1523–1528. doi: 10.19082/1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimecki M., Kruzel M.L. Milk-derived proteins and peptides of potential therapeutic and nutritive value. J Exp Ther Oncol. 2007;6(2):89–106. [PubMed] [Google Scholar]
- Zou T.B., He T.P., Li H.B., Tang H.W., Xia E.Q. The structure–activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21:72. doi: 10.3390/molecules21010072. [DOI] [PMC free article] [PubMed] [Google Scholar]