Abstract
This study was to identify the effects of prebiotics supplemented in infant formula on enzyme activity and phosphate uptake in the small intestine of Sprague Dawley (SD) rats. Forty-eight healthy SD rats at 15 days old (a week before weaning) with similar weight were randomly divided into 3 groups: A (control group), B, C, with 16 rats per group. Rats in groups A, B, C were fed a standard infant formula, the standard infant formula supplemented with oligosaccharides, and the standard infant formula supplemented with polysaccharides, respectively. The feeding test was conducted for 28 d. Compared with group A, the results showed the following: 1) the activities of sucrose and lactase in the small intestine were significantly increased in SD rats of group C (P < 0.05); 2) the relative expressions of lactase gene in the anterior and posterior segments of the small intestine were significantly increased by 1.68 and 2.26 in SD rats of group C (P < 0.05), and the relative expression of Mgam gene in the posterior segment of the small intestine was significantly increased by 0.99 in SD rats of group C (P < 0.05); 3) the relative expressions of Na/Pi-IIb gene in the anterior and posterior segments of the small intestine were significantly increased by 1.85 and 2.28 in SD rats of group C (P < 0.05). These results indicate that the supplementation of infant formula with prebiotics can promote enzyme activity in the small intestine by increasing the relative expression of enzyme gene or by decreasing intestinal injury, and can increase the relative expression of Na/Pi-IIb gene. The effect of polysaccharides is better than that of oligosaccharides.
Keywords: Infant formula, Prebiotics, Ussing chamber, Intestinal enzyme activities, Phosphate absorption
1. Introduction
Breast milk is the most major food for mammals after birth. It is rich in proteins, fats, carbohydrates and some biological active substances, providing sufficient energy and nutrition for the healthy growth and development of babies (Hsieh et al., 2015). Prebiotics, as the third largest active nutrient in breast milk, are almost absent in standard infant formulas (Kunz et al., 2000). Vandenplas et al., (2014) found that the intestinal microflora of breastfed infants were different from those of infants fed standard infant formula. Adding prebiotics to standard infant formula can increase its nutritional value. Prebiotics, including oligosaccharides, polysaccharides, polyols, plant extracts, and protein hydrolysates, cannot be degraded by the animals themselves, but can selectively stimulate the growth of beneficial bacteria in the intestine. Polydextrose as a polysaccharide has a role of prebiotics (Kunz et al., 2000). At present, prebiotics that are widely used in human food include lactulose, galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), inulin and its hydrolysates, maltose oligosaccharide, and resistant starch (Al-Sheraji et al., 2013). Clinical trials have shown that inulinase-type FOS and GOS have good effects on human digestive and immune health (Sangwan et al., 2011); GOS and polydextrose can improve the nutrition absorption and possibly enhance their memory through non-humoral regulation mechanism of pigs and Sprague Dawley (SD) rats (Fanaro et al., 2009, McVey Neufeld et al., 2017); polydextrose also affects nutrient uptake, immune regulation, and intestinal function of animals.
In 2011, European Society of Infantile Gastroenterology gave scientific evidence that adding prebiotics to formulas generally did not cause safety and growth side effects in healthy infants (Braegger et al., 2011, Skórka et al., 2017). Some studies showed that the addition of prebiotics to infant formula could improve the intestinal microflora of infants by reducing fecal pH and by increasing short chain fatty acid (SCFA), especially the proliferation of beneficial bacteria such as Bifidobacterium and Lactobacillus, and the inhibition of harmful bacteria such as Clostridium (Holscher et al., 2012, Closa-Monasterolo et al., 2013, Giovannini et al., 2014, Vandenplas et al., 2015). On the other hand, other studies showed that prebiotics supplementation in infant formula had no effect on growth, tolerance, gastrointestinal infections, respiratory tract infections, and allergic manifestations (Skórka et al., 2018). Prebiotics or probiotics could increase intestinal disaccharidases activity by: 1) proliferating beneficial bacteria in the intestine such as Lactobacillus; 2) providing energy directly for the intestine to improve intestinal mucosal growth; 3) increasing the relative expression of related genes, and so on (Moran et al., 2010, Goyal et al., 2013, Jang et al., 2014, Steinhoff-Wagner et al., 2014, Inoue et al., 2015). Phosphorus absorption in the small intestine could be related to intestinal pH, the relative expression of related genes, and transporter levels (Khadeer et al., 2003). However, researchers currently have no consensus on the safety and efficacy of the supplementation of infant formula with prebiotics. Therefore, the research on the safety and efficacy of formula supplemented with prebiotics has become a major task for nutrition researchers. The purpose of this experiment was to study the effects of the supplementation of infant formula with prebiotics on enzyme activity and phosphate uptake in the small intestine of SD rats, and to provide a scientific basis that formula milk better adapts to the nutritional needs of Chinese infants for healthy growth and development.
2. Materials and methods
2.1. Materials
This research was performed in accordance with protocols approved by the Central South University Institutional Animal Care and Use Committee, China. A written informed consent was obtained from the university prior to the study.
In the study, infant formulas were produced by Ausnutria Dairy Corporation Ltd. (China). Infant formulas used in this study are as follows: a standard infant formula, the standard infant formula supplemented with 2.13% of oligosaccharides (1.85% of FOS and 0.28% of GOS), and the standard infant formula supplemented with 2.20% of polyglucose.
Feeds used in this study were produced by Hunan Experimental Animal Co., Ltd., China. Each feed was a mixture of corn, wheat bran and the infant formula in the ratio of 7:2:1. Corn, wheat bran and the standard infant formula were mixed in the ratio of 7:2:1 for feed 1. Corn, wheat bran and the standard infant formula supplemented with oligosaccharides were mixed in the ratio of 7:2:1 for feed 2. Corn, wheat bran and the standard infant formula supplemented with polysaccharides were mixed in the ratio of 7:2:1 for feed 3.
2.2. Animals
A total of 48 healthy SD rats, at 15 days old (a week before weaning) and weighed 27.50 to 31.28 g, were obtained from Changsha Biological Technology Co., Ltd., China. The animals were reared in a specific pathogen free animal laboratory (Xiangya School of Public Health of Central South University, China) with controlled temperature (20 to 26 °C), humidity (40% to 70%), and photoperiod (12 h:12 h light–dark cycle).
2.3. Experimental design
All rats were adaptively fed for 3 d and randomly divided into 3 groups: A, B, C, with 16 rats per group. Rats in groups A, B, C were fed the standard infant formula, the standard infant formula supplemented with oligosaccharides, and the standard infant formula supplemented with polysaccharides, respectively. The feeding test was conducted for 28 d. Feeding status and methods are shown in Table 1.
Table 1.
Feeding status and methods of Sprague Dawley (SD) rats.
| Item | Treatments1 |
||
|---|---|---|---|
| Group A | Group B | Group C | |
| 1 to 7 d (phase 1) | Milk 1 | Milk 2 | Milk 3 |
| 8 to 28 d (phase 2) | Milk 1 + Feed 1 | Milk 2 + Feed 2 | Milk 3 + Feed 3 |
Milk 1 was formulated according to the standard infant formula to water ratio at 1:3. Milk 2 was formulated according to the standard infant formula supplemented with oligosaccharides to water ratio at 1:3. Milk 3 was formulated according to the standard infant formula supplemented with polysaccharides to water ratio at 1:3. Corn, wheat bran and the standard infant formula were mixed in the ratio of 7:2:1 for Feed 1. Corn, wheat bran and the standard infant formula supplemented with oligosaccharides were mixed in the ratio of 7:2:1 for Feed 2. Corn, wheat bran and the standard infant formula supplemented with polysaccharides were mixed in the ratio of 7:2:1 for Feed 3. The milk and the feed were mixed in the ratio of 7:3 in phase 2 (Milk: Feed = 7:3).
2.4. Samples collection
The rats were starved overnight and weighed on the 29th day. After the eyeballs were taken, rats were sacrificed by cervical dislocation, and then the abdominal cavity was opened. The stomach, liver, spleen, kidney, pancreas, and small intestine were separated to observe whether there were any lesions. The gastrointestinal tract was quickly removed. Segments of the small intestine were taken immediately and prepared for Ussing chamber studies. The anterior and posterior segments of the small intestine were collected, and intestinal linings were rinsed with normal saline and stored at −80 °C.
2.5. Using ex vivo Ussing chamber to measure phosphate absorption function of the small intestine
The gastrointestinal tract was gently washed with 0.9% physiological saline and rapidly incubated in the Hepes-Tris buffer in an ice bath for 5 min. Then the serosa of intestine was rapidly stripped and removed, and the rest of intestinal mucosa was fixed in the diffusion cell of an Ussing chamber. Four milliliter of Hepes-Tris buffer was added to both sides of the reaction chamber. After the circuit connection was stabilized, 1 mL of 0.21% phosphorus stock solution was added to the diffusion chamber and then 1 mL of mannitol of the same concentration was added to the receiving chamber (both were preheated to 37 °C, and mixed 95% O2 and 5% CO2). Solution samples, 1.5 mL each, were respectively taken from the receiving and diffusion chamber after 45 min to be detected.
2.6. Activities of sucrose and lactase in the small intestine
Each sample (0.2 to 1.0 g) from the anterior and posterior segments of the small intestine was taken, then according to the tissue to physiological saline ratio (1:9), 0.86% cold saline was added, and samples were homogenized under ice cooling. Then the sample were centrifuged at 3,000 × g at 4 °C for 15 min, and then the supernatant was taken to be tested. The activities of sucrose and lactase enzyme in the anterior and posterior segments of the small intestine were determined by the colorimetric method, and the concentration of tissue protein was measured by using the microplate technique.
2.7. mRNA expression analysis by real-time quantitative polymerase chain reaction (PCR)
The Trizol reagent (Invitrogen Life Technologies) was used to extract total RNA from the anterior and posterior segments of the small intestine, and reverse transcription was performed according to the methods and procedures described in the reverse transcription kit. Then the total mRNA was used as a template to reverse transcribe the cDNA. The reaction procedure was as follows: 95 °C, 10 min; 95 °C, 15 s; 60 °C, 60 s (40 cycles were repeated). Actin was selected as the reference gene, and the relative expression of each target gene mRNA was calculated using the 2−ΔΔCt method, and target genes were lactase, Mgam and Na/Pi-IIb. The primer sequences for each gene are shown in Table 2.
Table 2.
Primer sequences used in the real-time PCR.
| Gene | Primer (5′ to 3′) | Accession number | Length, bp |
|---|---|---|---|
| Actin | F-CATCCTGCGTCTGGACCTGG R-TAATGTCACGCACGATTTCC |
NM_031144 | 116 |
| Lactase | F-CTGCCCTCTTTCTTGTCTCAGTATTT R-TGCTTCCTGGTCTGGATTCTTG |
NM_053841.1 | 126 |
| Mgam | F-CATGGTGCTTCTGAGGCAAAC R-GCAGCATTTCGTTCTTGTGATAG |
NM_001171003.1 | 241 |
| Na/Pi-IIb | F-ATTGGCACCACCACGACTG R-GAAGACTGCGAACCAGCGATA |
NM_053380.2 | 207 |
2.8. Statistical analysis
The data were presented as means ± SD and analyzed by Independent-Samples t Test between the control and trial groups by significant difference using SPSS software (SPSS, V.21.0). Differences were considered statistically significant at P < 0.05. Data were used for plotting the figures with the GraphPad Prism 5 software.
3. Results
3.1. Activities of sucrase and lactase in the small intestine
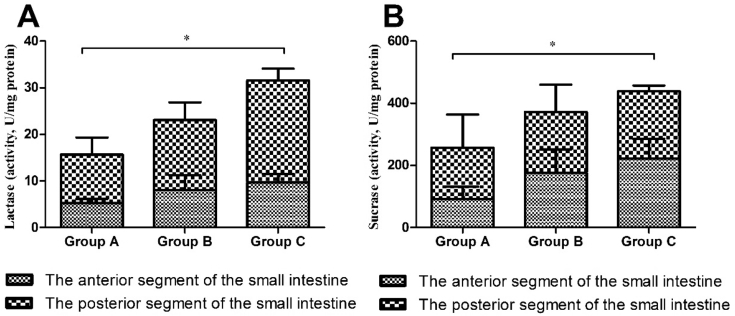
The activities of sucrase and lactase in the small intestine are shown in Fig. 1. Compared with group A, the activities of sucrase and lactase in the small intestine of group B were increased, but not significantly (P > 0.05), and the activities of sucrase and lactase in the small intestine of the group C were significantly increased (P < 0.05).
Fig. 1.
Lactase (A) and sucrase (B) activities in the small intestine of Sprague Dawley (SD) rats in different groups. * means significant difference among 3 groups (P < 0.05).
3.2. Expressions of enzyme genes in the small intestine
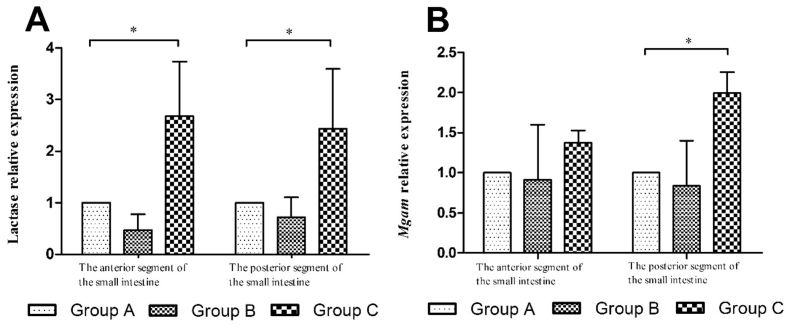
The expressions of enzyme genes in the small intestine are shown in Fig. 2. In comparison with group A, the expression levels of lactase gene in the anterior and posterior segments of the small intestine were significantly increased in group C (P < 0.05); the expression level of Mgam gene in the posterior segment of the small intestine was significantly increased in group C (P < 0.05).
Fig. 2.
Relative expressions of enzyme genes, Lactase (A) and Mgam (B), in the small intestine of Sprague Dawley (SD) rats in different groups. * means significant difference among 3 groups (P < 0.05).
3.3. Absorptive function of phosphate in the small intestine biopsy samples
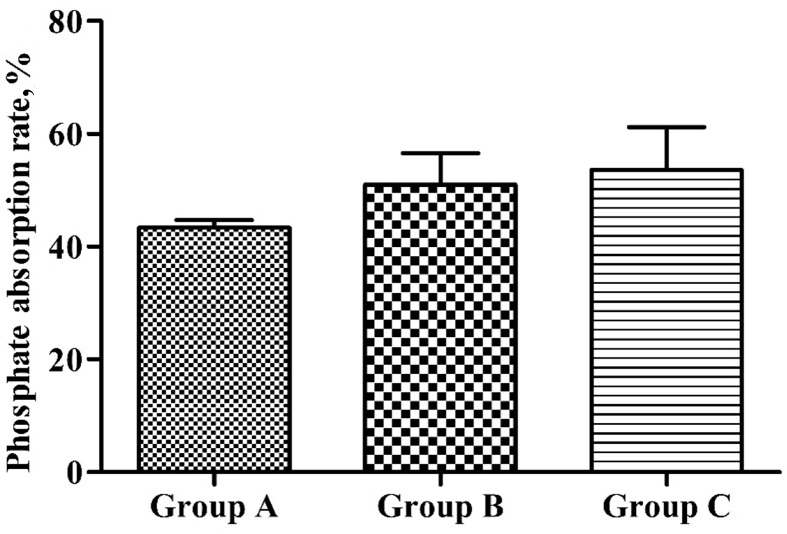
Fig. 3 shows phosphate absorption in the small intestine biopsy specimens of SD rats. In comparison with group A, the phosphate in vitro absorption rate in the small intestine was increased in groups B and C, but not significantly (P > 0.05).
Fig. 3.
Phosphate in vitro absorption rate in the small intestine of Sprague Dawley (SD) rats in different groups.
3.4. Expression of Na/Pi-IIb gene in the small intestine
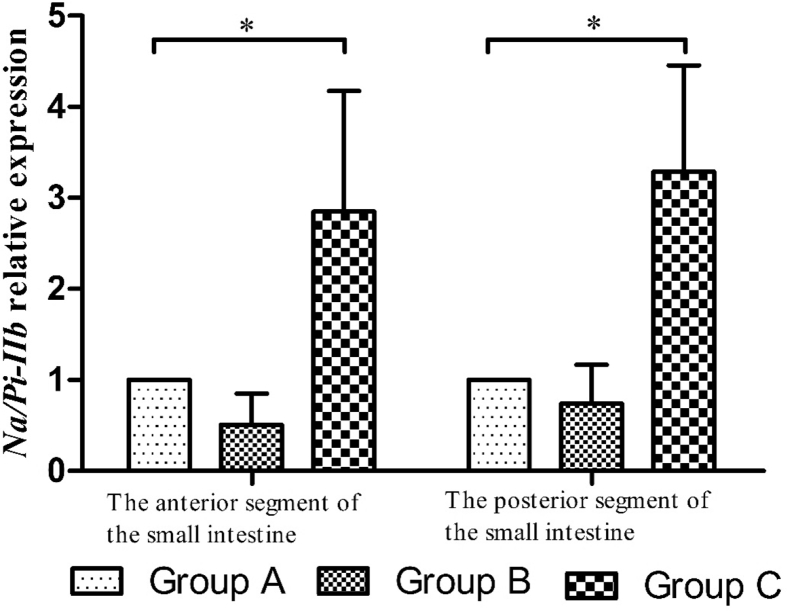
Fig. 4 shows the expression of Na/Pi-IIb gene in the small intestine of SD rats. Compared with group A, the expression level of Na/Pi-IIb gene in the anterior and posterior segments of the small intestine were significantly increased in group C (P < 0.05).
Fig. 4.
The relative expression of Na/Pi-IIb gene in the small intestine of Sprague Dawley (SD) rats in different groups. * means significant difference among 3 groups (P < 0.05).
4. Discussion
4.1. Prebiotics effects on enzyme activity in the small intestine
The present results suggest that the standard infant formula supplemented with polysaccharides can significantly improve enzyme activities in the small intestine of SD rats, but the supplementation of neither polysaccharides nor oligosaccharides has any significant effect on phosphate uptake in the small intestine. A considerable number of studies showed that milk has evolved the most important food source for mammals during infancy, and oligosaccharides are the most prebiotic components in milk (Boehm and Stahl, 2007, Mills et al., 2011, Zhao et al., 2017). But the total and major type of oligosaccharides are more abundant in human milk than in other mammalian milks (Mehra and Kelly, 2006). The structural composition of human milk oligosaccharides (HMO) mostly determines the biological function (Bode, 2015). Studies also have concluded that the HMO can improve the activity of Bifidobacteria and then reduce pathogenic bacteria (Jin et al., 2016, Zhao et al., 2016).
Lactose as the main nutritive carbohydrate abundant in milk can only be absorbed by the intestine when it breaks down into monosaccharides under the action of lactase. In general, the activity of lactase in the intestine of mammals decreased after weaning, but some factors can also affect the small intestinal lactase activity such as the composition of the diet (especially lactose content), the relative expression of lactase gene, and so on (Swallow, 2003, Wang et al., 2006). The mainly carbohydrates in the diet of adult mammalian are starches, and Mgam gene encodes maltase-glucoamylase, which plays an important role in starch digestion and enteral monosaccharide uptake (Ghani, 2015, Joshi et al., 2015, Younkin et al., 2015).
The importance of prebiotics has been recognized due to their fermentative function, nutritional and health benefits that help in promoting the proliferation of Bifidobacteria and Lactobacillus, and the previous studies showed that probiotics can decrease the brush border injury in the intestine by enhancing the activities of lactase and sucrase, and the reverse is also effective (Humen et al., 2005, Southcott et al., 2008, Goyal et al., 2013, Steinhoff-Wagner et al., 2014). However, lactase activity in the small intestine was greater in trial groups than in the control group, especially the supplementation of infant formula with polysaccharides, at the same time, the mRNA expression of lactase gene in each segment of the small intestine was higher in infant formula supplemented with polysaccharides than in the standard infant formula. The reasons of these results are that the standard infant formula supplemented with prebiotics can increase the activities of lactase and sucrase, which maybe by increasing the relative expression of lactase gene in the small intestine or by improving intestinal integrity. On the other hand, the standard infant formula supplemented with polysaccharides can help the neonates better adapt to diets after weaning, which maybe by improving the sucrase activity and the expression of Mgam gene.
4.2. Prebiotics effects on phosphate absorption in the small intestine
Phosphorus, an indispensable element in the growth and development of animals, plays an important role in bone formation, energy metabolism, nucleic acid synthesis, cell signal transduction, and maintenance of blood acid-base balance (Wagner et al., 2014, Miao et al., 2017). Phosphorus reabsorption in the kidney depends on the NaPi-IIa and NaPi-IIc cotransporter, and the absorption in the intestine depends on the NaPi-IIb cotransporter, which are affected by the mRNA expressions of related genes and hyperphosphatemia (Virkki et al., 2007, Biber et al., 2009, Zhang et al., 2016, Sugiura et al., 2018). Prebiotics have been proven to improve the intestinal transport of calcium and protein in rats, however the effect of supplementation of infant formula with prebiotics on phosphorus absorption has rarely been studied. The results showed that the supplementation of infant formula with prebiotics caused significantly higher relative expression of Na/Pi-IIb gene than the standard infant formula. The supplementation of infant formula with prebiotics can promote the absorptive function of phosphate in the small intestine by increasing the relative expression of Na/Pi-IIb gene or by decreasing intestinal injury, but the effect on absorptive function of phosphate in the small intestine has no significant difference among groups, which may due to Na/Pi-IIb transporter of intestinal cells in vitro going down, and the specific mechanism of the research needs further exploration.
The supplementation of infant formula with prebiotics can promote enzyme activity in the small intestine by increasing the mRNA expression of relative gene or by decreasing intestinal injury, and can increase the relative expression of Na/Pi-IIb gene. The effect of polysaccharides is better than that of oligosaccharides.
Acknowledgement
The study was supported by the infant formula of Ausnutria feeding animal experiment study in Central South University (No. H201610130690001).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Jianwu Wang, Email: jianwu_wang@csu.edu.cn.
Rejun Fang, Email: fangrj63@126.com.
References
- Al-Sheraji S.H., Ismail A., Manap M.Y., Mustafa S., Yusof R.M., Hassan F.A. Prebiotics as functional foods: a review. Journal of Functional Foods. 2013;5(4):1542–1553. [Google Scholar]
- Biber J., Hernando N., Forster I., Murer H. Regulation of phosphate transport in proximal tubules. Pflueg Arch Eur J Physiol. 2009;458(1):39–52. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91(11):619–622. doi: 10.1016/j.earlhumdev.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Boehm G., Stahl B. Oligosaccharides from milk. J Nutr. 2007;137(2):847S–849S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- Braegger C., Chmielewska A., Decsi T., Kolacek S., Mihatsch W., Moreno L. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011;52(2):238–250. doi: 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- Closa-Monasterolo R., Gispert-Llaurado M., Luque V., Ferre N., Rubio-Torrents C., Zaragoza-Jordana M. Safety and efficacy of inulin and oligofructose supplementation in infant formula: results from a randomized clinical trial. Clin Nutr. 2013;32(6):918–927. doi: 10.1016/j.clnu.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Fanaro S., Marten B., Bagna R., Viqi V., Fabris C., Pena-Quintana L. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2009;48(1):82–88. doi: 10.1097/MPG.0b013e31817b6dd2. [DOI] [PubMed] [Google Scholar]
- Ghani U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur J Med Chem. 2015;103(48):133–162. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Giovannini M., Verduci E., Gregori D., Ballali S., Soldi S., Ghisleni D. Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: randomized multicenter trial. J Am Coll Nutr. 2014;33(5):385–393. doi: 10.1080/07315724.2013.878232. [DOI] [PubMed] [Google Scholar]
- Goyal N., Rishi P., Shukla G. Lactobacillus rhamnosus GG antagonizes Giardia intestinalis induced oxidative stress and intestinal disaccharidases: an experimental study. World J Microbiol Biotechnol. 2013;29(6):1049–1057. doi: 10.1007/s11274-013-1268-6. [DOI] [PubMed] [Google Scholar]
- Holscher H.D., Faust K.L., Czerkies L.A., Litov R., Ziegler E.E., Lessin H. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN - J Parenter Enter Nutr. 2012;36(1 Suppl):95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- Hsieh C.C., Hernándezledesma B., Fernándeztomé S., Weinborn V., Barile D., de Moura Bell J.M. Milk proteins, peptides, and oligosaccharides: effects against the 21st century disorders. BioMed Res Int. 2015;2015:146840. doi: 10.1155/2015/146840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humen M.A., De Antoni G.L., Benyacoub J., Costas M.E., Cardozo M.I., Kozubsky L. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infect Immun. 2005;73(2):1265–1269. doi: 10.1128/IAI.73.2.1265-1269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Honma K., Mochizuki K., Goda T. Induction of histone H3K4 methylation at the promoter, enhancer, and transcribed regions of the Si and Sglt1 genes in rat jejunum in response to a high-starch/low-fat diet. Nutrition. 2015;31(2):366–372. doi: 10.1016/j.nut.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Jang I., Chang H.K., Ha D.M., Jung D.Y., Sun Y.K., Man J.P. Effects of a lipid-encapsulated zinc oxide supplement on growth performance and intestinal morphology and digestive enzyme activities in weanling pigs. J Anim Sci Technol. 2014;56(1):29–35. doi: 10.1186/2055-0391-56-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.M., Joo W., Ling L., Choi H.S., Han N.S. In vitro, digestion and fermentation of sialyllactoses by infant gut microflora. Journal of Functional Foods. 2016;21:497–506. [Google Scholar]
- Joshi S.R., Standl E., Tong N., Shah P., Kalra S., Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expet Opin Pharmacother. 2015;16(13):1959–1981. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- Khadeer M.A., Tang Z., Tenenhouse H.S., Eiden M.V., Murer H., Hernando N. Na+-dependent phosphate transporters in the murine osteoclast: cellular distribution and protein interactions. Am J Physiol Cell Physiol. 2003;284(6):C1633–C1644. doi: 10.1152/ajpcell.00580.2002. [DOI] [PubMed] [Google Scholar]
- Kunz C., Rudloff S., Baier W., Klein N., Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20(20):699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld K.A., O'Mahony S.M., Hoban A.E., Waworuntu R.V., Berg B.M., Dinan T.G. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2017;4:1–10. doi: 10.1080/1028415X.2017.1397875. [DOI] [PubMed] [Google Scholar]
- Mehra R., Kelly P. Milk oligosaccharides: structural and technological aspects. Int Dairy J. 2006;16(11):1334–1340. [Google Scholar]
- Miao Z., Feng Y., Zhang J., Tian W., Li J., Yang Y. Regulation of phosphate transport and AMPK signal pathway by lower dietary phosphorus of broilers. Oncotarget. 2017;8(64):107825–107832. doi: 10.18632/oncotarget.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S., Ross R.P., Hill C., Fitzgerald G.F., Stanton C. Milk intelligence: mining milk for bioactive substances associated with human health. Int Dairy J. 2011;21(6):377–401. [Google Scholar]
- Moran A.W., Al-Rammahi M.A., Arora D.K., Batchelor D.J., Coulter E.A., Daly K. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010;104(5):637–646. doi: 10.1017/S0007114510000917. [DOI] [PubMed] [Google Scholar]
- Sangwan V., Tomar S.K., Singh R.R., Singh A.K., Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76(4):R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- Skórka A., Pieścik-Lech M., Kołodziej M., Szajewska H. Infant formulae supplemented with prebiotics: are they better than unsupplemented formulae? An updated systematic review. Br J Nutr. 2018;119(7):810–825. doi: 10.1017/S0007114518000120. [DOI] [PubMed] [Google Scholar]
- Skórka A., Pieścik-Lech M., Kołodziej M., Szajewska H. To add or not to add probiotics to infant formulae? An updated systematic review. Benef Microbes. 2017;8(5):717–725. doi: 10.3920/BM2016.0233. [DOI] [PubMed] [Google Scholar]
- Southcott E., Tooley K.L., Howarth G.S., Davidson G.P., Butler R.N. Yoghurts containing probiotics reduce disruption of the small intestinal barrier in methotrexate-treated rats. Dig Dis Sci. 2008;53(7):1837–1841. doi: 10.1007/s10620-008-0275-1. [DOI] [PubMed] [Google Scholar]
- Steinhoff-Wagner J., Zitnan R., Schönhusen U., Pfannkuche H., Hudakova M., Metges C.C. Diet effects on glucose absorption in the small intestine of neonatal calves: importance of intestinal mucosal growth, lactase activity, and glucose transporters. J Dairy Sci. 2014;97(10):6358–6369. doi: 10.3168/jds.2014-8391. [DOI] [PubMed] [Google Scholar]
- Sugiura H., Matsushita A., Futaya M., Teraoka A., Akiyama K.I., Usui N. Fibroblast growth factor 23 is upregulated in the kidney in a chronic kidney disease rat model. PLoS One. 2018;13(3):e0191706. doi: 10.1371/journal.pone.0191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow D.M. Genetics of lactase persistence and lactose intolerance. Annu Rev Genet. 2003;37:197–219. doi: 10.1146/annurev.genet.37.110801.143820. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y., De Greef E., Veereman G. Prebiotics in infant formula. Gut Microb. 2014;5(6):681–687. doi: 10.4161/19490976.2014.972237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas Y., Zakharova I., Dmitrieva Y. Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. Br J Nutr. 2015;113(9):1339–1344. doi: 10.1017/S0007114515000823. [DOI] [PubMed] [Google Scholar]
- Virkki L.V., Biber J., Murer H., Forster I.C. Phosphate transporters: a tale of two solute Carrier families. Am J Physiol. 2007;293(3):F643–F654. doi: 10.1152/ajprenal.00228.2007. [DOI] [PubMed] [Google Scholar]
- Wagner C.A., Hernando N., Forster I.C., Biber J. The SLC34 family of sodium-dependent phosphate transporters. Pflueg Arch Eur J Physiol. 2014;466(1):139–153. doi: 10.1007/s00424-013-1418-6. [DOI] [PubMed] [Google Scholar]
- Wang Z., Maravelias C., Sibley E. Lactase gene promoter fragments mediate differential spatial and temporal expression patterns in transgenic mice. DNA Cell Biol. 2006;25(4):215–222. doi: 10.1089/dna.2006.25.215. [DOI] [PubMed] [Google Scholar]
- Younkin S.G., Scharpf R.B., Schwender H., Parker M.M., Scott A.F., Marazita M.L. A genome-wide study of inherited deletions identified two regions associated with nonsyndromic isolated oral clefts. Birth Defects Res Part A Clin Mol Teratol. 2015;103(4):276–283. doi: 10.1002/bdra.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shi L.M., Li Y., Zhu Q.G., Jin C.L., Wang H.P. Effect of hyperphosphatemia on gene expression of the Na-Pi cotransporter in rats. Genet Mol Res. 2016;14(4):19404–19410. doi: 10.4238/2015.December.30.1. [DOI] [PubMed] [Google Scholar]
- Zhao C., Wu Y., Liu X., Liu B., Cao H., Yu H. Functional properties, structural studies and chemo-enzymatic synthesis of oligosaccharides. Trends Food Sci Technol. 2017;66:135–145. [Google Scholar]
- Zhao C., Wu Y., Yu H., Shah I.M., Li Y., Zeng J. The one-pot multienzyme (OPME) synthesis of human blood group H antigens and a human milk oligosaccharide (HMOS) with highly active Thermosynechococcus elongatus α1-2-fucosyltransferase. Chem Commun. 2016;52(20):3899–3902. doi: 10.1039/c5cc10646j. [DOI] [PMC free article] [PubMed] [Google Scholar]