Abstract
Background
BK virus (BKV), Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivations are common after kidney transplantation and associated with increased morbidity and mortality. Although CMV might be a risk factor for BKV and EBV, the effects of combined reactivations remain unknown. The purpose of this study is to ascertain the interaction and effects on graft function of these reactivations.
Methods
3715 serum samples from 540 kidney transplant recipients were analysed for viral load by qPCR. Measurements were performed throughout eight visits during the first post-transplantation year. Clinical characteristics, including graft function (GFR), were collected in parallel.
Findings
BKV had the highest prevalence and viral loads. BKV or CMV viral loads over 10,000 copies·mL−1 led to significant GFR impairment. 57 patients had BKV-CMV combined reactivation, both reactivations were significantly associated (p = 0.005). Combined reactivation was associated with a significant GFR reduction one year post-transplantation of 11.7 mL·min−1·1.73 m−2 (p = 0.02) at relatively low thresholds (BKV > 1000 and CMV > 4000 copies·mL−1). For EBV, a significant association was found with CMV reactivation (p = 0.02), but no GFR reduction was found. Long cold ischaemia times were a further risk factor for high CMV load.
Interpretation
BKV-CMV combined reactivation has a deep impact on renal function one year post-transplantation and therefore most likely on long-term allograft function, even at low viral loads. Frequent viral monitoring and subsequent interventions for low BKV and/or CMV viraemia levels and/or long cold ischaemia time are recommended.
Fund
Investigator Initiated Trial; financial support by German Federal Ministry of Education and Research (BMBF).
Keywords: BK virus, Cytomegalovirus, Epstein-Barr virus, Kidney transplantation, Graft function, Combined reactivation
Research in context.
Evidence Before this Study
Viral reactivations of BK virus (BKV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) are common complications in recipients of renal transplantation. Combined reactivations of these viruses have been observed repeatedly in the past and interplay between BKV and CMV has been shown in vitro. Different interaction mechanisms have been proposed. However, it is currently unclear whether there are associations in viral reactivations in vivo. Moreover, it is not clear so far what is the cause of such combined reactivations and whether combined reactivations have more serious impact on graft function than the corresponding mono-reactivations. To obtain information on the state-of-art, we searched MEDLINE, PubMed, and Google Scholar for papers published after January 2003, using the terms “renal transplantation BKV”, “renal transplantation CMV”, “renal transplantation EBV”, “coinfection BKV CMV”, “coinfection BKV EBV”, “coinfection CMV EBV”. No language restrictions were employed. The quality of evidence was assessed prioritizing epidemiological studies over case reports and in vitro studies.
Added Value of this Study
This is the first large, prospective multi-centre study to systematically analyse the clinical course of BKV, CMV, and EBV reactivations at eight pre-defined time points during the first post-transplantation year. Almost ten thousand viral load measurements were performed. It is the first study to provide clinical evidence of the relevance of BKV-CMV combined reactivations, showing, already at moderate viral loads (BKV > 1000 and CMV > 4000 copies·mL−1), an impact on renal function one year post-transplantation with a median drop in renal function of 11.7 mL·min−1·1.73 m−2. This observation is reinforced by the fact that a significant association was found between BKV and CMV during the first post-transplantation year. Moreover, it is the first large study to find an association between cold ischaemia time and high level CMV viral load: High-level CMV (>10,000 copies·mL−1) was associated with significantly longer cold ischaemia time for cadaveric graft (median difference: 284 min), compared to patients without CMV or CMV below the threshold. Furthermore, this study shows BKV as the most relevant viral adverse event in kidney transplantation, as it had the highest prevalence, the highest viral loads and lowest clearing rate. Our results have revealed a prevalence of presumptive BKV nephropathy of 10.9% (over the 1–10% prevalence in the literature), in spite of the patients belonging to an immunological low-risk cohort. In conclusion, it is a confirmation that BKV is an emergent pathogen that must be tackled in order to improve the efficacy of current transplantation protocols.
Implications of All the Available Evidence
We have provided the most systematic analysis so far of BKV, CMV, and EBV virus reactivations in renal transplantation, as part of a large, prospective multi-centre study. Their viral loads were analysed at eight time points during the first transplantation year. With our results, we showed a clinical impact of BKV-CMV combined reactivation, even at low viral load levels. In addition, we performed in-depth analyses of the impact of different modifiable and non-modifiable risk factors on virus reactivation. Therefore, we consider our work as crucial for the management of viral reactivations after kidney transplantation, leading to a better monitoring and treatment for kidney transplantation patients with BKV and/or CMV low viral loads, as well as patients with long cold ischaemia times and additional CMV risk factors.
Alt-text: Unlabelled Box
1. Introduction
Viral reactivations are a major cause of morbidity and mortality for recipients of solid organ transplantation [1]. In kidney transplantation, BK virus (BKV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) are major pathogens. These viruses are very common in healthy population, with an approximate prevalence of 80%, 60%, and 90%, respectively [[2], [3], [4]]. Primary infection usually occurs during childhood, but the virus stays latent and asymptomatic under normal conditions [5,6]. Individuals with compromised immune systems, i.e. after a solid organ transplantation, are prone to both primary infection and reactivations with clinically relevant symptoms [7,8].
BKV is an emerging pathogen and the cause of BKV-associated nephropathy (BKVAN), a major complication in renal transplantation [6]. It is linked to kidney malfunction and rejection, leading to graft loss in up to 60% of affected patients [6,8,9]. The incidence of BKVAN is 1–10% in renal transplantation [10]. BKVAN is usually encountered in a context of over-immunosuppression, even though it is not associated with a specific immunosuppressive drug [9,11,12]. Early diagnosis is vital for a successful treatment, but BKVAN progression occurs without clinical signs except for increasing serum creatinine concentrations and diagnosis relies on renal biopsy [9,11]. However, BKV serum load over 10,000 copies·mL−1 is a generally accepted surrogate marker defining “presumptive BKVAN” [11].
CMV is a major viral pathogen after kidney transplantation, linked among others to retinitis, pneumonitis, colitis, encephalitis and importantly, allograft damage, allograft loss and death [5,8,13,14]. CMV proliferation may occur through reactivation of a latent infection, a new donor-transmitted infection or acquired from the general population due to the immunosuppression [13]. However, the highest risk is encountered by CMV seronegative patients receiving a transplant from a seropositive donor (D+R−) [13]. EBV in kidney transplantation is mainly associated with post-transplant lymphoproliferative disorders (PTLD) [5,7]. PTLD is a severe complication in solid organ transplantation, occurring in around 1% of patients mostly after the first post-transplant year [7,15,16]. It comprises a very broad spectrum of disorders, from spontaneously regressing to lethal B cell proliferations [4,7].
In this work, we assess the impact and relevance of BKV, CMV, and EBV reactivations in a large, prospective multi-centre study, analysing renal transplant in clinical follow-up during the first year after transplantation. Our work focuses on potential interactions between viruses and their combined impact on graft function, as well as the risk factors associated with each virus, including the role of immunosuppressive therapy.
2. Patients and Methods
2.1. Patient Population
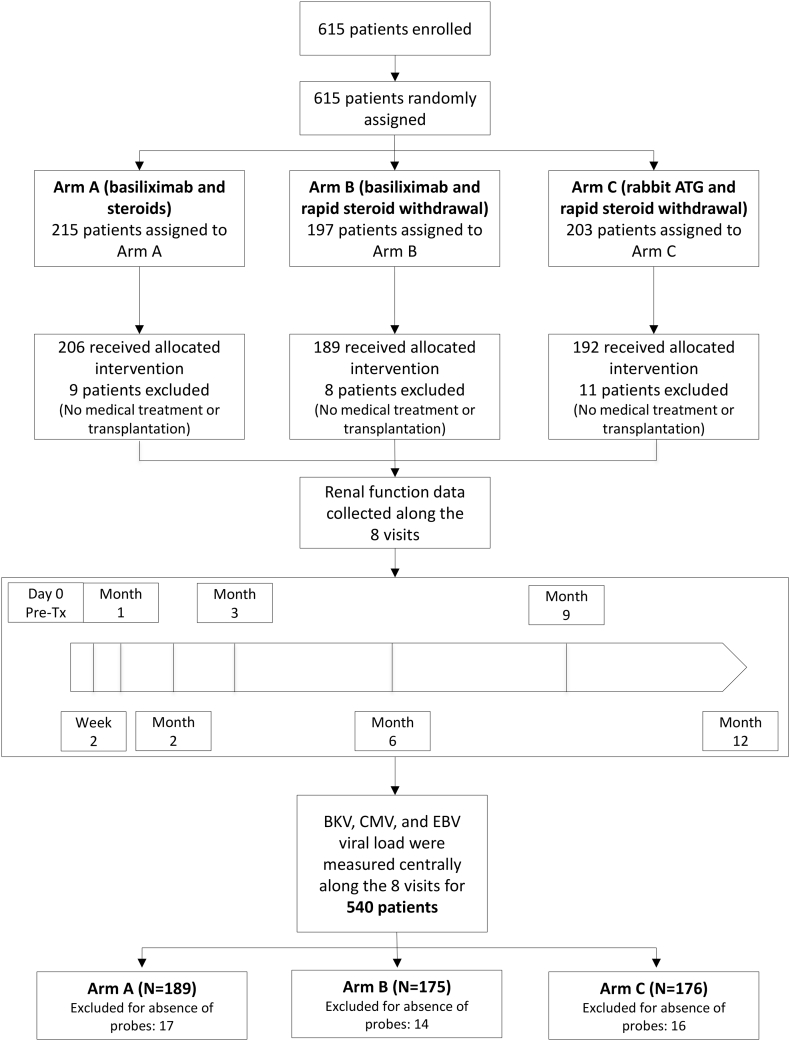
We conducted a sub-study within the randomized, multi-centre, investigator-initiated Harmony trial (NCT 00724022) [17] to prospectively monitor viral load of BKV, CMV, and EBV at predetermined eight study visits and correlate it with clinical outcome parameters. Following the KDIGO clinical guideline, BKV viral load monitoring was performed in serum rather than urine, as the former has a higher BKVAN diagnostic value [18,19]. Viral monitoring was non-interventional and centrally performed. The study was carried out in compliance with the Declaration of Helsinki and Good Clinical Practice. A total of 540 patients undergoing kidney transplantation between 08/2008 and 11/2012 were analysed (Fig. 1).
Fig. 1.
Trial profile.
2.2. Patient Medication
Patients were randomized to one of three therapeutic groups, as described before [17]. The immunosuppressive therapy included induction with either monoclonal IL-2R antibody basiliximab (arms A and B) (Simulect®, Novartis) or rabbit ATG (arm C) (Thymoglobulin®, Sanofi). Maintenance immunosuppression consisted of tacrolimus (Advagraf®, Astellas) and mycophenolate mofetil (MMF) with (arm A) or without steroids (arms B and C). Patients with mismatch-based risk (seronegative recipient and seropositive donor) for CMV or EBV as well as patients from arm C received at least a 3 months prophylaxis with valganciclovir.
2.3. Patient Monitoring
Patients were monitored for creatinine along eight visits, scheduled at day 0 (pre-transplantation), 2nd week, 1st month, 2nd month, 3rd month, 6th month, 9th month, and 12th month. Glomerular filtration rate was calculated using the CKD-EPI formula; values are given in mL·min−1·1.73 m−2 [20]. Tacrolimus blood trough levels were measured independently of the eight visits described above, according to the internal study centre standards. Suspected episodes of acute rejection had to be confirmed through biopsy; histologic characteristics were described according to the Banff criteria of 2005 [21]. Routine surveillance biopsies were allowed but not mandatory. Borderline rejections were disregarded in the analysis.
2.4. Screening of BKV, CMV, and EBV Viraemia
Peripheral blood samples from the eight visits were centrally monitored for BKV, CMV, and EBV by TaqMan quantitative polymerase chain reaction (qPCR), as described previously [19]. Briefly, DNA was isolated from serum (BKV) or whole blood (CMV and EBV) using a QIAamp DNA Mini Kit (Qiagen Corp, Hilden, Germany) according to the manufacturer's instructions. PCR was based on the TaqMan platform and used the Prism 7700 Sequence Detector (ABI). In the case of BKV, PCR amplifications were set up in a reaction volume of 25 μL using primer and probe at final concentrations of 900 nM and 5 μM [19]. Primers and probe were designed to amplify the VP1 gene [19]. CMV and EBV were amplified using the same protocol; primers and probe sequences, as well as reagent concentrations are shown in Table 1. The detection level was the lowest viral load measured within the range of linearity.
Table 1.
CMV and EBV qPCR reagent characteristics.
| Reagent | Sequence | Concentration |
|---|---|---|
| CMV Forward primer | 5′-CTG CGT GAT ATG AAC GTG AAG G-3′ | 300 nM |
| CMV Reverse primer | 5′-GCT GTT GGC GAA ATT AAA GAT GA-3′ | 900 nM |
| CMV Probe | 5′-CGC CAG GAC GCT GCT ACT CAC GA-3′ | 5 μM |
| EBV Forward primer | 5′-TCC CGG GTA CAA GTC CCG-3′ | 900 nM |
| EBV Reverse primer | 5′-TGA CCG AAG ACG GCA GAA AG-3′ | 900 nM |
| EBV Probe | 5′-TGG TGA GGA CGG TGT CTG TGG TTG TCT T-3′ | 5 μM |
2.5. Clinical Management of BKV, CMV, and EBV
BKV, CMV, and EBV reactivations and disease were monitored according to intern centre standards. qPCR (and/or pp65 CMV antigenemia tests) and symptom monitoring were performed. Viral loads over 10,000 copies·mL−1 for BKV and over 1000 copies·mL−1 for CMV and EBV were considered clinically relevant. Reactivations were treated based on centre internal standards. According to the study protocol, suggested treatment included a reduction of the total immunosuppression e.g. reduction of tacrolimus and MMF dose. For CMV, patients would receive additionally a (val)ganciclovir treatment for three weeks according to local standards, followed by (val)ganciclovir prophylaxis for, at least, four weeks. After reactivation, patients were regularly monitored for viral load, first weekly, than monthly and then three-monthly until the end of the study.
2.6. Viraemia-Based Patient Classification
Patients were classified based on their peak viral load values for BKV, CMV, and EBV during follow-up (Table 2). Patients with viral loads over detection level were classified as BKV+, CMV+, or EBV+. Patients of the former group with, at least, one measurement over 2000 copies·mL−1 were classified as elevated viraemia (eBKV, eCMV, and eEBV); patients with viral load over 10,000 copies·mL−1 were classified as high-level viraemia (hBKV, hCMV, and hEBV). Altogether, patients were classified into up to nine overlapping groups.
Table 2.
Summary of viraemia-based patient classification sub-groups.
| Abbreviation | Definition | Threshold |
|---|---|---|
| BKV+ | Detectable BKV viral load for at least one visit | >DL (250 copies·mL−1) |
| CMV+ | Detectable CMV viral load for at least one visit | >DL (250 copies·mL−1) |
| EBV+ | Detectable EBV viral load for at least one visit | >DL (250 copies·mL−1) |
| eBKV | Elevated BKV viral load for at least one visit | >2000 copies·mL−1 |
| eCMV | Elevated CMV viral load for at least one visit | >2000 copies·mL−1 |
| eEBV | Elevated EBV viral load for at least one visit | >2000 copies·mL−1 |
| hBKV | High-level BKV viral load for at least one visit | >10,000 copies·mL−1 |
| hCMV | High-level CMV viral load for at least one visit | >10,000 copies·mL−1 |
| hEBV | High-level EBV viral load for at least one visit | >10,000 copies·mL−1 |
2.7. Statistical Analysis
Qualitative variables were described using counts and frequencies and compared using Pearson's chi-square test with continuity correction (unless otherwise stated), odds ratio (OR) and 95% confidence intervals (95%CI) are provided. Quantitative variables are described as median and interquartile range (IQR). The differences between continuous variables are analysed using the Mann-Whitney test. Three-dimensional contingency tables are reduced to two dimensions (flattened) for chi-square test analysis, iteratively controlling for each one of the three variables; average of the three obtained p values is given. A cut-off of 0.05 for the p value was used on all tests to discard or confirm significant associations. Analyses were performed with R (Version 3.1.1).
2.8. Statistical Analysis of Immunosuppressant Usage
The relation between immunosuppressant usage (MMF daily dose and tacrolimus trough levels) and viral reactivations was analysed by comparing the usage between patients with reactivation (sample) and patients with no viral reactivation (control).
In detail, the analysis was performed as follows: The sample group was defined as the patients with viral load for the virus v over a threshold th at any visit, while the control group were all patients with no reactivation for virus v. Monitoring of drug usage was performed for the sample group at the first visit with reactivation over th, and for the control group for randomly selected visits so that the analysed visits have the same frequencies as in the sample group and that each patient is taken into account only once. For MMF daily drug dose, the dose at viral load monitoring was compared, for tacrolimus trough levels the last measurement before monitoring visit was considered. Only viral reactivations occurring after transplantation were considered.
Mann-Whitney test with 100 replicates was employed for the comparison, with the null hypothesis that drug usage in the sample group was not higher than in the control group. A difference was considered significant if the null hypothesis was rejected (p < 0.05) for at least 80% of the replicates. Statistics of drug usage are given as the median over all replicates of the median and IQR of the sample and control groups, as well as median p value.
2.9. Role of the Funding Source
The trial was designed and run by NB, who received financial support from the German Federal Ministry of Education and Research (BMBF). The funders had no role in data collection, data analysis, data interpretation, or writing of the manuscript. ABN, CH, MO, and NB had full access to all study data and had final responsibility for the decision to submit for publication.
3. Results
3.1. BKV Is the most Relevant Viral Reactivation in Renal Transplantation Recipients
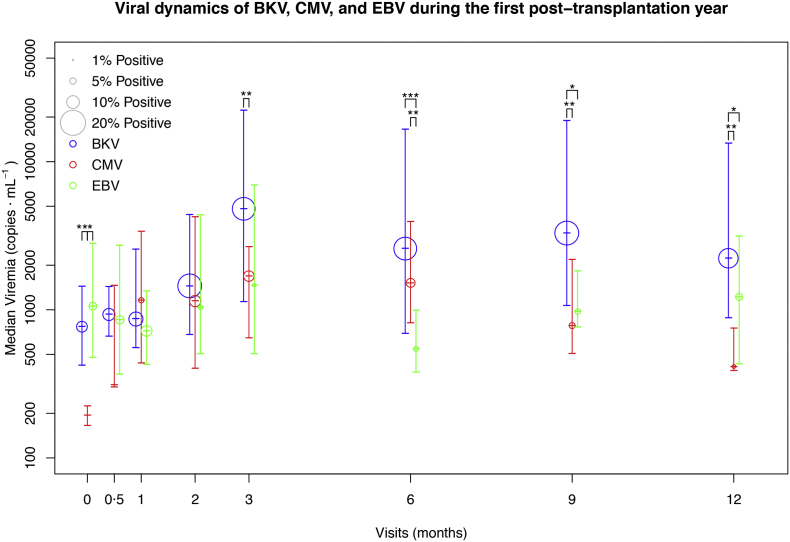
A total of 3715 blood samples from 540 patients (18 centres) were analysed for BKV, CMV, and EBV. Detection limit (DL) was 250 copies·mL−1. Demographic and clinical characteristics of the patients are shown in Table 3. Prevalence, viral load, temporal sequence and recurrence are presented in Table 4 and Fig. 2. Overall, BKV was the most relevant reactivation, with the highest prevalence, viral loads, incidence of prolonged reactivations and the lowest rate of clearing: 260 of the patients (48.1%) were BKV+ (see Viraemia-based patient classification section), 121 (22.4%) were eBKV and 59 (10.9%) were hBKV; 109 (20.2%) patients had prolonged viraemia; median viral load peak value was 1505 [779–8452] copies·mL−1 and rate of clearing was 80.5%.
Table 3.
Patient demographic and clinical characteristics, treatment details and transplantation outcomes. Data are given in number (percentage) or median (interquartile range) and range.
| Variable | Measurement | Total (N = 540) |
|---|---|---|
| Male sex | 346 (64.1%) | |
| Age (years) | Median (IQR) | 56 (45–64) |
| Range | [19, 75] | |
| BMI (kg m−2) | Median (IQR) | 25.8 (23.2–29.0) |
| Range | [16.2, 49.1] | |
| Living donor | 66 (12.2%) | |
| Second transplantation | 22 (4.1%) | |
| Cold ischaemia time: only cadaveric donors (min) | Median (IQR) | 660 (488–880) |
| Range | [35, 1712] | |
| Average MMF daily dose (mg·day-1) | Median (IQR) | 1505 (1058–1990) |
| Range | [0–3994] | |
| Average tacrolimus trough level (ng·mL-1) | Median (IQR) | 9.5 (8.5–10.5) |
| Range | [5.5, 27.0] | |
| Graft loss one year post-transplantation | 22 (4.1%) | |
| Death one year post-transplantation | 16 (3.0%) | |
| Graft survival one year post-transplantation | 504 (93.3%) | |
| GFR one year post-transplantation (mL.min−1·1.73 m−2) | Median (IQR) | 47.6 (35.0–60.8) |
| Range | [7.6, 126.9] | |
Table 4.
Viral reactivation statistics. Data are given in number (percentage) or median (interquartile range) and range. The percentages of the first four categories refer to the total number of patients (N = 540). For the clearing statistics, the percentage corresponds to the ratio of: number of patients with detectable viraemia (at least once between visits 1 and 7) and with no viral load in the eighth visit (cleared patients), and the total number of patients with detectable viraemia; patients who did not have viral load measurements at the last time point (visit 8) were excluded from the analysis.
| BKV | CMV | EBV | ||
|---|---|---|---|---|
| Patients with detectable viraemia (>DL) | 260 (48.1%) | 92 (17.0%) | 109 (20.2%) | |
| Patients with elevated viraemia (>2000 copies·mL−1) | 121 (22.4%) | 39 (7.22%) | 37 (6.85%) | |
| Patients with high-level viraemia (>10,000 copies·mL−1) | 59 (10.9%) | 18 (3.33%) | 11 (2.04%) | |
| Patients with prolonged viraemia (more than one positive measurement) | 109 (20.2%) | 35 (6.48%) | 36 (6.67%) | |
| Viraemia patients with no detectable viraemia one year post-transplantation (clearing) | 128 (80.5%) | 61 (95.3%) | 48 (85.7%) | |
| Time until first detectable viraemia (days) | Median (IQR) | 61 (23–178) | 66 (54–185) | 27 (7–80) |
| Range | [0, 380] | [0, 370] | [0, 386] | |
| Peak viraemia per patient (copies·mL−1) | Median (IQR) | 1505 (779–8452) | 1491 (710–5850) | 926 (550–3075) |
| Range | [DL, 3849694] | [DL, 136722] | [DL, 1369425] | |
Fig. 2.
Viral dynamics of BKV, CMV, and EBV during the first post-transplantation year. Prevalence and viral load levels for BKV (blue), CMV (red), and EBV (green) are plotted for the eight visits of the study. The size of the points is a function of the prevalence of positive measurements (viral load over detection level). The height of the points represents the median viral load (copies·mL−1) of positive measurements; the bars indicate the interquartile range. Asterisks indicate a significant difference calculated with the Mann-Whitney test (* p < 0·05; ** p < 0·01; *** p < 0·001) in viral load (only samples with detectable viral load) for each virus.
3.2. Elevated CMV Is Significantly Associated with Higher Cold Ischaemia Time
Demographic and clinical characteristics were analysed univariately for association with each one of the nine viraemia groups (Table 5). Following characteristics were analysed: sex, age, body mass index, donor type, number of previous transplants, EBV and CMV donor and recipient serostatus and mismatch-associated risk, and cold ischaemia time. CMV donor seropositivity was significantly associated with CMV reactivation for all three thresholds, as was CMV mismatch-associated risk. Interestingly, CMV mismatch-associated risk was similarly associated with eEBV. eEBV was also associated with CMV recipient seronegativity, CMV mismatch-associated risk and EBV mismatch-associated risk. Finally, we found a relation between CMV and cold ischaemia time for patients with cadaveric transplants: this difference was observed for both eCMV and hCMV, with increasing difference for higher viral loads. For BKV, no significant differences were found for any of the three thresholds.
Table 5.
Results of univariate analysis. Demographic and clinical characteristics were analysed for association with each one of the nine pre-defined viraemia sub-groups (Table 2), compared to the rest of population. The effect size is shown according to the employed test: OR (95%CI) for Chi-squared and Fisher's exact test and median of sub-group (IQR) vs. median of rest of cohort (IQR) for Mann-Whitney test. Only significant (P < 0·05) differences are shown.
| Variable | Viraemia Group | P Value | Test | Effect size |
|---|---|---|---|---|
| CMV donor seropositivity | CMV+ | <0.00001 | Chi-squared | 3.75 (2.12–6.64) |
| eCMV | 0.0024 | Chi-squared | 3.89 (1.60–9.45) | |
| hCMV | 0.0237 | Chi-squared | 5.45 (1.24–23.9) | |
| CMV recipient seropositivity | eEBV | 0.0154 | Chi-squared | 0.39 (0.19–0.81) |
| CMV mismatch-based risk (D+R−) | CMV+ | 0.0002 | Chi-squared | 2.46 (1.54–3.93) |
| eCMV | 0.0025 | Chi-squared | 2.87 (1.47–5.60) | |
| hCMV | 0.0254 | Fisher's exact | 3.09 (1.17–8.16) | |
| eEBV | 0.0053 | Chi-squared | 2.70 (1.37–5.31) | |
| EBV mismatch-based risk (D+R−) | eEBV | 0.0236 | Chi-squared | 3.77 (1.31–10.9) |
| Cold ischaemia time (min) (only cadaveric donors) | eCMV | 0.0199 | Mann-Whitney | 819 (539–1078) vs. 660 (484–855) |
| hCMV | 0.0140 | Mann-Whitney | 944 (702–1058) vs. 660 (484–859) |
3.3. CMV Reactivation Is Significantly Associated with BKV and EBV
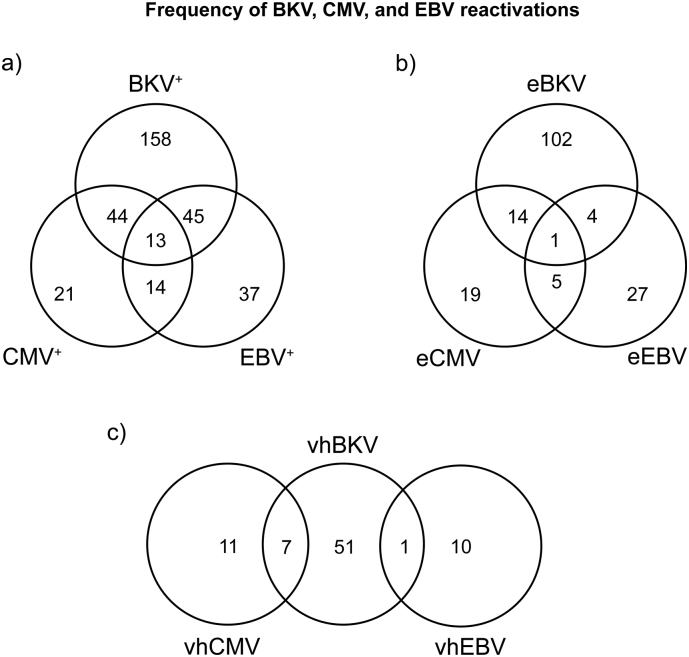
We examined the association between the reactivations, including pair-wise analyses (Fig. 3). 13 patients (2.41%) had viraemia over DL for all three viruses. The association was significant (average p = 0.0021); the number of triple-infected patients was 45% higher than expected for no association.
Fig. 3.
Frequency of triple, combined and mono-reactivations of BKV, CMV, and EBV during the first post-transplantation year. Number of patients with reactivations and all their possible combinations are plotted as a Venn diagram. Fig. 3a depicts the combinations of BKV+, CMV+, and EBV+, i.e. viral load over detection level. Fig. 3b depicts the combinations of elevated viral load sub-groups (eBKV, eCMV, and eEBV, > 2000 copies·mL−1). Fig. 3c depicts the combinations of high-level viral load sub-groups (hBKV, hCMV, and hEBV, > 10,000 copies·mL−1).
There was a highly significant association between BKV and CMV for all three thresholds: BKV+-CMV+(p = 0.0052; OR = 1.97, 95%CI = 1.24–3.11), eBKV and eCMV (p = 0.0216; OR = 2.33, 95%CI = 1.18–4.60) and hBKV and hCMV (Fisher's exact test: p = 0.0016; OR = 5.75, 95%CI = 1.80–17.0). There was a significantly higher number of sera positive for both virus (p = 0.0145; OR = 1.72, 95%CI = 1.13–2.62). There was no clear temporal pattern: 45.6% had detectable BKV before CMV, and 33.3% had CMV before BKV.
CMV and EBV were also significantly associated for CMV+-EBV+ (p = 0.0237; OR = 1.85, 95%CI = 1.11–3.08) and for eCMV-eEBV (Fisher's exact test: p = 0.0416; OR = 2.76, 95%CI = 1.05–7.08) – there were no hCMV-hEBV patients. There was a significantly higher number of sera simultaneously positive for both virus (p = 0.0193; OR = 2.07, 9%CI = 1.17–3.69). EBV preceded CMV in 51.9% of cases and was observed after CMV in 29.6%.
There was no significant association between BKV and EBV.
3.4. CMV Serostatus Is the Only Demographic Characteristic Associated with Combined Reactivations
We analysed the differences of demographic and clinical characteristics for combined reactivations with respect to the rest of patient population. BKV+-CMV+ was associated with CMV seropositivity of donor (p < 0.00001; OR = 5.34, 95%CI = 2.37–12.0) and CMV mismatch-based risk (p = 0.0001; OR = 3.02, 95%CI = 1.72–5.30); eBKV-eCMV was associated with CMV mismatch-based risk (p = 0.0278; OR = 3.64, 95%CI = 1.24–10.7). CMV+-EBV+ was likewise associated with CMV seropositivity of donor (p = 0.0127; OR = 3.97, 95%CI = 1.35–11.6).
3.5. Therapy Arm Was Not Associated with Elevated or High-Level Viral Loads
EBV+ was significantly associated with immunosuppressive regimen (p = 0.0303): Arm C (ATG and rapid steroid withdrawal) had a higher EBV+ prevalence (p = 0.0225; OR = 1.69, 95%CI = 1.10–2.60). Interestingly, the lowest EBV+ prevalence was found in arm B (basiliximab and rapid steroid withdrawal) (p = 0.0432; OR = 0.59, 95%CI = 0.37–0.96). This effect was not found for higher viral load thresholds. There were no significant differences between therapeutic arms for BKV or CMV or their combinations.
3.6. High Tacrolimus Trough Levels Were Associated with Detectable CMV Reactivation
High tacrolimus trough levels were significantly associated with CMV+: With 100 replicates, we obtained a significant p value for 96% of replicates (median p = 0.0142). While the median of the last tacrolimus trough level measured before CMV reactivation was 9.1 [7.1–11.1] ng·mL−1, the trough levels for the control group of patients without CMV reactivation were 8.2 [6.4–10.2] ng·mL−1. On the other hand, we did not find any effect of MMF daily dose on viral reactivation, as there were no significant replicates for any combination of threshold and virus.
To discard the possibility that the lack of detection of an association of MMF daily doses with reactivation is caused by a poor choice of thresholds, the analysis was repeated for both drugs and all thresholds between DL and 20,000 copies·mL−1, with steps of 1000 copies·mL−1. However, the results demonstrated no effect of MMF daily dose levels on viral reactivation, as well as no effect of tacrolimus trough levels on BKV or EBV, with 0% of significant replicates.
3.7. High-Level CMV Viraemia Was Positively Associated with Acute Rejection
hCMV was significantly associated with acute rejection (Fisher's exact test: p = 0.0393; OR = 3.27, 95%CI = 1.08–9.41). Three patients (60.0%) had viral load over DL before acute rejection; only one of the patients had a CMV load >10,000 copies·mL−1 before rejection. No significant association was found between acute rejection and BKV or EBV.
Patients who received an anti-rejection therapy did not have a significantly higher incidence of viral reactivation for any of the pre-defined thresholds. Furthermore, there was no significant association between the use of steroid or ATG anti-rejection therapies and viral reactivation.
3.8. Severe PTLD Was a Rare Event in Conjunction with High EBV Load
There were two cases of PTLD (0.37%) in the cohort, of which one was severe. Even though both PTLD cases affected patients in arm C, there was no significant association between therapy arm and PTLD incidence (Fisher's exact test: p = 0.21). The patient with severe PTLD had EBV viral load over DL for visits 4 and 5, with a peak viral load of 12,271 copies·mL−1; the patient with mild PTLD showed no EBV viral load. None of the patients showed viral load over DL for CMV or BKV.
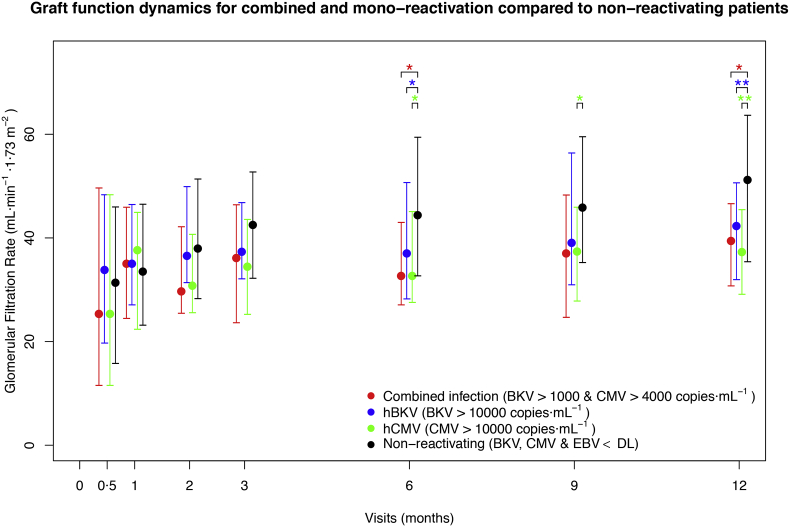
3.9. High-Level BKV and CMV Were Associated with Lower Graft Function One Year Post-Transplantation
Patients with hBKV had a significantly lower GFR (42.3 [31.9–50.6] mL·min−1·1.73 m−2 – p = 0.0096) one year after transplantation compared to patients with no viraemia (BKV, CMV, and EBV below DL; 51.2 [35.4–63.6] mL·min−1·1.73 m−2) (Fig. 4). Median reduction was 8.9 mL·min−1·1.73 m−2. Patients with hCMV had a significant GFR loss from the 6th month onwards, with a median difference of 13.9 mL·min−1·1.73 m−2 one year after transplantation (p = 0.0021–37.3 [29.1–45.4] vs. 51.2 [35.4–63.6] mL·min−1·1.73 m−2) (Fig. 4). The relationship of BKV and CMV with GFR loss was robust for a very wide range of thresholds (Fig. S1, Fig. S2). No significant relationship was observed between EBV and GFR.
Fig. 4.
Graft function dynamics of patients with BKV and CMV mono-reactivations and combined reactivations, in comparison to non-reactivating patients. Median GFR (mL·min−1·1·73 m−2) for patients with BKV-CMV combined reactivation (red; N = 16), hBKV (blue; N = 59), hCMV (green; N = 18) and non-reactivating (black; N = 208) for the last seven visits is plotted. Coloured groups are not mutually exclusive – a patient might belong to more than one sub-group. The bars indicate the interquartile range. Coloured asterisks indicate a significant difference calculated with the Mann-Whitney test (* p < 0·05; ** p < 0·01) in GFR of the corresponding group with respect to the non-reactivating group. Day 0 is not shown, as it is pre-transplantation.
3.10. A Combination of BKV and CMV Viraemia Leads to Lower Graft Function Already at Moderate Viral Loads
To better capture the possible effect of combined viral reactivations on graft function, as well as the effect of the viral load threshold used to classify the patients into viraemia groups, a systematic exploration of viral load thresholds was performed (Fig. S3). Patients with combined BKV and CMV viraemia had lower GFR already for low viral load levels. For example, patients with BKV > 1000 copies·mL−1 and CMV > 4000 copies·mL−1 (N = 16) demonstrated a significant impairment of GFR from the ninth month onwards and a median loss of 11.7 mL·min−1·1.73 m−2 compared to non-reactivating patients at the first post-transplantation year (p = 0.0172; 39.5 [30.7–46.6] vs. 51.2 [35.4–63.6] mL·min−1·1.73 m−2) (Fig. 4). Moreover, these patients had (non-significant) lower GFR than patients with mono-reactivation (N = 166; BKV > 1000 or CMV > 4000 copies·mL−1) from the second week of the study onwards, with a median difference one year post-transplantation of 3.33 mL·min−1·1.73 m−2.
There was no bias in the use of antiviral treatment for combined reactivations (Fisher's exact test: p = 0.70): 68.8% of the patients with BKV > 1000 and CMV > 4000 copies·mL−1 were treated with (val)ganciclovir, while for patients with BKV < 1000 and CMV > 4000 copies·mL−1 the prevalence of treatment was 58.3%.
4. Discussion
In this work, the prevalence of BKV, CMV, and EBV and their impact on patients undergoing kidney transplantation have been analysed for the first time in a large multi-centre study. With the increasing efficacy of immunosuppressive therapies and the subsequent decrease of acute rejection, reactivations are expected to gain clinical importance in renal transplantation. The main findings of our study include:
-
-
Superiority of BKV over CMV and EBV from the epidemiological point of view with the highest incidence and viral load;
-
-
Significant association between CMV with BKV or EBV, but not between EBV and BKV;
-
-
Combined BKV and CMV reactivation significantly associated with lower graft function one year post-transplantation, even at low viral load levels.
Our results show that BKV, with the highest incidence rate and median peak viral load and the lowest clearing rate, is the most relevant viral reactivation of the three from the epidemiological point of view for kidney recipients. Prevalence of presumptive BKV nephropathy (BKV > 10,000 copies·mL−1) [11] was on the higher end of the common estimations for BKV nephropathy (1–10%) [11,22], although the patient cohort consisted of immunological low-risk patients. In contrast to BKV, CMV, and EBV were observed with a lower but still substantial prevalence in around one fifth of the patients. In both cases, viraemia had most frequently an episodic character, with viraemia clearance rates over 85%.
A key finding of our study is the impact of BKV-CMV combined reactivation on GFR one year post-transplantation – an important predictor of transplant survival [23]– even at relatively low viral load levels. Patients with no viral reactivation experimented an increase in the median GFR between the third and the twelfth post-transplantation month. Such positive GFR slopes in patients without transplant complications have been observed before in the literature, e.g. Guba et al. [24]. However, in patients with BKV or CMV viral load over 10,000 copies·mL−1, no such increase was observed, leading to a significantly lower GFR. Remarkably, patients with moderate BKV-CMV combined reactivation (BKV > 1000 copies·mL−1 and CMV > 4000 copies·mL−1) also had a significantly lower GFR (median difference: 11·7 mL·min−1·1.73 m−2). This finding is especially interesting since currently only much higher BKV levels are generally considered of clinical relevance so far [25]. Thus, our data provide evidence regarding BKV-CMV combined reactivation as a relevant complication of the post-transplantation period.
The impact of BKV-CMV combined reactivation on GFR is especially relevant due to the reciprocal effects between the viruses. BKV-CMV combined reactivations have been observed repeatedly in the past and interactions between both are plausible [9,14,[26], [27], [28], [29]]. However, the existence of an epidemiological association is controversial: A previous large retrospective study from our group identified a significant association between CMV and BKV viraemia [9], but a large prospective study by Elfadawy et al. showed a negative association between antecedent CMV and BKV incidence [14]. Moreover, Elfadawy et al. did not find any effect of BKV, CMV or their combination on GFR and only symptomatic CMV was linked with graft survival. A reason for the first difference lies probably on the fact that Elfadawy et al. was an interventional study [14]. As the authors suggest, interventions following CMV diagnosis are a plausible cause for the seemingly protective effect of CMV against BKV [14]. For the contradiction on GFR effects, the most likely cause is the different stratification strategies of viral reactivations: while in our study systematic viral load cut-offs were employed, Elfadawy et al. employed a symptom-based approach for CMV and no stratification strategy for BKV [14]. However, as shown in our results (e.g. Fig. S1, Fig. S2, Fig. S3), the choice of viral load threshold is important for the identification of virus-associated renal function impairments. Our results show that stratification of reactivations according to viral load is key to identifying which patients might develop a lower GFR as a consequence of apparently asymptomatic reactivations.
Our study also showed a significant association between CMV and EBV. This in agreement with a previous study showing an association of these viruses [30]. However, no clinical relevance of this association was found, possibly due to the low number of affected patients. Literature offers likewise an unclear picture on the clinical relevance of this combined reactivation: Even though a previous study showed a link between CMV-EBV combined reactivation and PTLD in liver transplantation recipients [31], we found no case of PTLD in patients with CMV-EBV combined infection. The only case of severe PTLD in the cohort suffered from high-level (>10,000 copies·mL−1) EBV mono-reactivation. Nevertheless, our results cannot exclude a relationship between CMV-EBV combined reactivation and PTLD, as the majority of PTLD cases in adult renal transplantation occur after the first post-transplantation year [7,15,16]. There are to our knowledge no recent studies showing a clinical relevance for CMV-EBV combined reactivation in kidney recipients.
Acute rejection was significantly associated with high-level CMV viral loads (>10,000 copies/mL). A mutual relation of these two phenomena, where CMV boosts rejection and rejection boosts reactivations, seems likely. However, the number of cases was too low to offer an unambiguous cause-effect relation. No relationship with acute rejection was found for BKV or EBV. This is remarkable for BKV, as it is known from the literature to be associated with rejection [9]; the absence of association could be linked to the remarkably low rejection rate found in the patient cohort [17].
Regarding risk factors, therapy arm did not have any significant effect on BKV or CMV incidence. This highlights the safety of both rabbit ATG and steroid withdrawal therapies in renal transplantation with respect to BKV and CMV [17]. On the other hand, EBV reactivations were significantly associated with arm C (ATG and rapid steroid withdrawal). This finding is consistent with a previous study [32]. Likewise, the only two cases of PTLD in the cohort were encountered in arm C, but the association was not statistically significant. Interestingly, arm B (basiliximab and rapid steroid withdrawal) had a significantly lower EBV prevalence than the rest. However, it should be emphasized that there was no association between arm and elevated (>2000 copies·mL−1), high-level EBV (>10,000 copies·mL−1), or PTLD. Finally, no significant relationship was found between MMF daily dose and viraemia. In agreement with Elfadawy et al., we found that higher tacrolimus trough levels were however a risk factor for detectable CMV reactivation [14]. On the other hand, there was no evidence for an effect of tacrolimus on BKV or EBV reactivation. Other clinical risk factors showed no association with BKV. This highlights the current uncertainty on its risk factors, with the literature yielding inconsistent results [9,[33], [34], [35], [36]]. CMV and EBV were, as expected, significantly associated with patient-donor serological mismatch. Interestingly, we have observed a significant association between high-level CMV viraemia (>10,000 copies·mL−1) and longer cold ischaemia time for cadaveric organs, an association first observed in a very recent study with only eight patients [37]. Since such viral loads are associated with lower GFR, our data suggest reinforcing CMV-surveillance after transplantations with a long cold ischaemia time, especially for cases of high CMV mismatch-associated risk.
Our study has some limitations. First, the follow-up period – one year – is too short to observe long-term effects. Our analyses were explorative and are not corrected for multiple testing to maximize the obtained information, on the basis of the precautionary principle. Secondly, other factors affecting renal function (e.g. bacterial infections, use of nephrotoxic drugs) were not analysed. Moreover, transplantation outcomes were assessed on the basis of estimated GFR and not on a histological basis. Nevertheless, the fact that GFR is a recognised predictor for long-term transplantation outcomes supports the relevance of our conclusions [23]. However, these limitations are outweighed by the fact that it is the first large multi-centre study that has examined BKV, CMV, and EBV in kidney transplantation in a systematic and parallel way, as well as the first to offer a detailed analysis of viral associations and their impact on transplantation outcomes. Almost ten thousand viral load measurements were performed along the eight pre-programmed visits – all measurements were performed in the same centre and following the same, standardised protocol, thereby ensuring the comparability of results.
In conclusion, our work offers an extensive outlook on the impact and relevance of BKV, CMV, and EBV and their interactions after kidney transplantation. In our study, BKV emerges as the most relevant viral complication from the epidemiological point of view, with the highest prevalence, highest viral load, and the lowest clearing rates. Long cold ischaemia time is confirmed to be significantly associated with elevated CMV viraemia. An association between CMV and EBV is shown, albeit without any evidence of enhancement of their clinical effects. Finally, we further demonstrate a highly significant association between BKV and CMV reactivations, shown for the first time to be of clinical interest, with an increase of damaging effects by both viruses already at moderate viral loads. The results of our study have the potential to change the BKV and CMV management, appealing for a stricter monitoring and intervention in kidney transplantation patients with BKV or CMV low viral loads as well as long cold ischaemia times.
The following are the supplementary data related to this article.
Influence of BKV threshold on median GFR loss one year post-transplantation. A systematic exploration of the influence of BKV viral load threshold choice on GFR one year post-transplantation was performed. Patients with BKV peak viral load over the threshold were compared with the patients with no viral reactivation (BKV, CMV and EBV below DL, N = 208). All thresholds between 0 and 20,000 copies·mL−1, with steps of 500 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test. The height of the points indicates the median difference in GFR one year post-transplantation between the BKV sub-group and the non-reactivating sub-group. Non-significant differences were plotted as red crosses, significant differences (p < 0.05) as black points whose size indicate the p value. As it can be observed, for all thresholds there was a negative effect of BKV on GFR one year post-transplantation, which was significant for all thresholds between 4000 and 20,000 copies·mL−1.
Influence of CMV threshold on median GFR loss one year post-transplantation. A systematic exploration of the influence of CMV viral load threshold choice on GFR one year post-transplantation was performed. Patients with CMV peak viral load over the threshold were compared with the patients with no viral reactivation (BKV, CMV and EBV below DL, N = 208). All thresholds between 0 and 20,000 copies·mL−1, with steps of 500 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test. The height of the points indicates the median difference in GFR one year post-transplantation between the CMV sub-group and the non-reactivating sub-group. Non-significant differences were plotted as red crosses, significant differences (p < 0.05) as black points whose size indicate the p value. As it can be observed, for all thresholds there was a negative effect of CMV on GFR one year post-transplantation, with increasing GFR loss for higher thresholds. GFR loss was significant for all thresholds between 6000 and 20,000 copies·mL−1.
Exploration of viral load thresholds for BKV and CMV and their influence on GFR one year post-transplantation. A systematic exploration was performed on the influence of viral load threshold choice on GFR one year post-transplantation for patients with BKV-CMV combined reactivation. For this, two sub-groups are generated for the comparison of their GFR at the end of the study: one (combined reactivation) with the patients that had BKV and CMV viral loads over certain thresholds; the second group (non-reactivating) with the patients that did not show any detectable viral load (BKV, CMV and EBV below DL, N = 208). For the BKV and CMV thresholds, all combinations between 0 and 20,000 copies·mL−1, with steps of 100 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test, the colour shows the P value for each combination. Shown is the space BKV ≤ 5000 copies·mL−1, where all significant differences were found. In all cases, the combined reactivation group had a lower median GFR than the non-reactivating group.
Funding Sources
This work was funded by the German Federal Ministry of Education and Research (BMBF)01ZX1312. The funder had no role in data collection, data analysis, data interpretation, writing of the manuscript, or manuscript submission.
Declaration of Interests
The authors declare no competing interests.
Author Contributions
NB, NW, CH, OT, CB, KW, RS, THW, BS, PR, and MO contributed to the study design, sample collection, and sample management. Data were generated by CDH, data interpretation by ABN, NB, and MO, and drafting of the manuscript by ABN, NB, and MO. All authors have contributed to the manuscript and approved the final version of the manuscript for submission.
Acknowledgements
This work was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (01ZX1312).
Contributor Information
Michal Or-Guil, Email: m.orguil@biologie.hu-berlin.de.
Nina Babel, Email: nina.babel@charite.de.
References
- 1.Alangaden G.J., Rama T., Gruber S.A. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20:401–409. doi: 10.1111/j.1399-0012.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 2.Egli A., Infanti L., Dumoulin A. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths P., Baraniak I., Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.-B., Khoury J.D. Epstein-Barr virus in lymphoproliferative processes: an update for the diagnostic pathologist. Adv Anat Pathol. 2009;16:40–55. doi: 10.1097/PAP.0b013e3181916029. [DOI] [PubMed] [Google Scholar]
- 5.Le Page A.K., Mackie F.E., McTaggart S.J., Kennedy S.E. Cytomegalovirus & epstein barr virus serostatus as a predictor of the long-term outcome of kidney transplantation. Nephrology (Carlton) 2013;18:813–819. doi: 10.1111/nep.12149. [DOI] [PubMed] [Google Scholar]
- 6.Babel N., Volk H.-D., Reinke P. BK polyomavirus infection and nephropathy: the virus – immune system interplay. Nat Rev Nephrol. 2011;7:399–406. doi: 10.1038/nrneph.2011.59. [DOI] [PubMed] [Google Scholar]
- 7.Petrara M.R., Giunco S., Serraino D., Dolcetti R., De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37–44. doi: 10.1016/j.canlet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Egli A., Binggeli S., Bodaghi S. Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant. 2007;22:viii72–viii82. doi: 10.1093/ndt/gfm648. [DOI] [PubMed] [Google Scholar]
- 9.Schachtner T., Babel N., Reinke P. Different risk factor profiles distinguish early-onset from late-onset BKV-replication. Transpl Int. 2015;28:1081–1091. doi: 10.1111/tri.12601. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch H.H., Steiger J., Polyomavirus B.K. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch H.H., Brennan D.C., Drachenberg C.B. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 12.Blazquez-Navarro A., Schachtner T., Stervbo U. Differential T cell response against BK virus regulatory and structural antigens: a viral dynamics modelling approach. PLoS Comput Biol. 2018;14:1–20. doi: 10.1371/journal.pcbi.1005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr T., Cippà P.E., Mueller N.J. Cytomegalovirus post kidney transplantation: prophylaxis versus pre-emptive therapy? Transpl Int. 2015;28:1351–1356. doi: 10.1111/tri.12629. [DOI] [PubMed] [Google Scholar]
- 14.Elfadawy N., Flechner S.M., Liu X. CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 2013;96:1097–1103. doi: 10.1097/TP.0b013e3182a6890d. [DOI] [PubMed] [Google Scholar]
- 15.Végso G., Hajdu M., Sebestyén A. Lymphoproliferative disorders after solid organ transplantation-classification, incidence, risk factors, early detection and treatment options. Pathol Oncol Res. 2011;17:443–454. doi: 10.1007/s12253-010-9329-8. [DOI] [PubMed] [Google Scholar]
- 16.Babel N., Vergopoulos A., Trappe R.U. Evidence for genetic susceptibility towards development of posttransplant lymphoproliferative disorder in solid organ recipients. Transplantation. 2007;84:387–391. doi: 10.1097/01.tp.0000269617.60751.c4. [DOI] [PubMed] [Google Scholar]
- 17.Thomusch O., Wiesener M., Opgenoorth M. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (harmony): an open-label, multicentre, randomised controlled trial. Lancet. 2016;388:3006–3016. doi: 10.1016/S0140-6736(16)32187-0. [DOI] [PubMed] [Google Scholar]
- 18.Kasiske B.L., Zeier M.G., Chapman J.R. KDIGO clinical practice guideline for the care of kidney. Rev Nefrol Dial y Traspl. 2011;31:6–21. [Google Scholar]
- 19.Babel N., Fendt J., Karaivanov S. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88:89–95. doi: 10.1097/TP.0b013e3181aa8f62. [DOI] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solez K., Colvin R.B., Racusen L.C. Banff ‘05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (’CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 22.Trofe J., Gordon J., Roy-Chaudhury P. Polyomavirus nephropathy in kidney transplantation. Prog Transplant. 2004;14:130–140. doi: 10.1177/152692480401400207. [DOI] [PubMed] [Google Scholar]
- 23.Kasiske B.L., Israni A.K., Snyder J.J., Skeans M.A. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57:466–475. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 24.Guba M., Pratschke J., Hugo C. Renal function, efficacy, and safety of Sirolimus and mycophenolate Mofetil after short-term Calcineurin inhibitor-based quadruple therapy in De novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplant J. 2010;90:175–183. doi: 10.1097/TP.0b013e3181e11798. [DOI] [PubMed] [Google Scholar]
- 25.Sood P., Senanayake S., Sujeet K. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94:814–821. doi: 10.1097/TP.0b013e31826690c6. [DOI] [PubMed] [Google Scholar]
- 26.Nada R., Sachdeva M.U.S., Sud K., Jha V., Joshi K. Co-infection by cytomegalovirus and BK polyoma virus in renal allograft, mimicking acute rejection. Nephrol Dial Transplant. 2005;20:994–996. doi: 10.1093/ndt/gfh737. [DOI] [PubMed] [Google Scholar]
- 27.Park S.B., Kwak J.H., Lee K.T. Polyoma virus-associated nephropathy and concurrent cytomegalovirus infection in the kidney transplant recipients. Transplant Proc. 2006;38:2059–2061. doi: 10.1016/j.transproceed.2006.06.107. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda M., Puliyanda D.P., Amet N. Co-infection of polyomavirus-BK and cytomegalovirus in renal transplant recipients. Transplantation. 2005;80:198–205. doi: 10.1097/01.tp.0000165110.78397.93. [DOI] [PubMed] [Google Scholar]
- 29.Kristoffersen A.K., Inge J., Morten O. Vol. 52. 1997. The Human Polyomavirus Bk T Antigen Induces Gene Expression in Human Cytomegalovirus; pp. 61–71. [DOI] [PubMed] [Google Scholar]
- 30.Meyer T., Scholz D., Warnecke G. Importance of simultaneous active cytomegalovirus and Epstein-Barr virus infection in renal transplantation. Clin Diagn Virol. 1996;6:79–91. doi: 10.1016/0928-0197(96)00230-9. [DOI] [PubMed] [Google Scholar]
- 31.Mañez R., Breinig M.C., Linden P. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462–1467. doi: 10.1086/514142. [DOI] [PubMed] [Google Scholar]
- 32.Bamoulid J., Courivaud C., Coaquette A. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13:656–662. doi: 10.1111/ajt.12009. [DOI] [PubMed] [Google Scholar]
- 33.Borni-Duval C., Caillard S., Olagne J. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation. 2013;95:1498–1505. doi: 10.1097/TP.0b013e3182921995. [DOI] [PubMed] [Google Scholar]
- 34.Pai D., Mann D.M., Malik A., Hoover D.R., Fyfe B., Mann R.A. Risk factors for the development of BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47:2465–2469. doi: 10.1016/j.transproceed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch H.H., Vincenti F., Friman S. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136–145. doi: 10.1111/j.1600-6143.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonvoisin C., Weekers L., Xhignesse P., Grosch S., Milicevic M., Krzesinski J.-M. Polyomavirus in renal transplantation: a hot problem. Transplantation. 2008;85:S42–S48. doi: 10.1097/TP.0b013e318169c794. [DOI] [PubMed] [Google Scholar]
- 37.Schlott F., Steubl D., Hoffmann D. Primary cytomegalovirus infection in seronegative kidney transplant patients is associated with protracted cold ischemic time of seropositive donor organs. PLoS One. 2017;12:1–10. doi: 10.1371/journal.pone.0171035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence of BKV threshold on median GFR loss one year post-transplantation. A systematic exploration of the influence of BKV viral load threshold choice on GFR one year post-transplantation was performed. Patients with BKV peak viral load over the threshold were compared with the patients with no viral reactivation (BKV, CMV and EBV below DL, N = 208). All thresholds between 0 and 20,000 copies·mL−1, with steps of 500 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test. The height of the points indicates the median difference in GFR one year post-transplantation between the BKV sub-group and the non-reactivating sub-group. Non-significant differences were plotted as red crosses, significant differences (p < 0.05) as black points whose size indicate the p value. As it can be observed, for all thresholds there was a negative effect of BKV on GFR one year post-transplantation, which was significant for all thresholds between 4000 and 20,000 copies·mL−1.
Influence of CMV threshold on median GFR loss one year post-transplantation. A systematic exploration of the influence of CMV viral load threshold choice on GFR one year post-transplantation was performed. Patients with CMV peak viral load over the threshold were compared with the patients with no viral reactivation (BKV, CMV and EBV below DL, N = 208). All thresholds between 0 and 20,000 copies·mL−1, with steps of 500 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test. The height of the points indicates the median difference in GFR one year post-transplantation between the CMV sub-group and the non-reactivating sub-group. Non-significant differences were plotted as red crosses, significant differences (p < 0.05) as black points whose size indicate the p value. As it can be observed, for all thresholds there was a negative effect of CMV on GFR one year post-transplantation, with increasing GFR loss for higher thresholds. GFR loss was significant for all thresholds between 6000 and 20,000 copies·mL−1.
Exploration of viral load thresholds for BKV and CMV and their influence on GFR one year post-transplantation. A systematic exploration was performed on the influence of viral load threshold choice on GFR one year post-transplantation for patients with BKV-CMV combined reactivation. For this, two sub-groups are generated for the comparison of their GFR at the end of the study: one (combined reactivation) with the patients that had BKV and CMV viral loads over certain thresholds; the second group (non-reactivating) with the patients that did not show any detectable viral load (BKV, CMV and EBV below DL, N = 208). For the BKV and CMV thresholds, all combinations between 0 and 20,000 copies·mL−1, with steps of 100 copies·mL−1, were evaluated. Significance was evaluated through the Mann-Whitney test, the colour shows the P value for each combination. Shown is the space BKV ≤ 5000 copies·mL−1, where all significant differences were found. In all cases, the combined reactivation group had a lower median GFR than the non-reactivating group.