Abstract
Background:
Methodologic challenges related to the concomitant use (co-use) of substances and changes in policy and potency of marijuana contribute to ongoing uncertainty about risks to fetal neurodevelopment associated with prenatal marijuana use. In this study, we examined two biomarkers of fetal neurodevelopmental risk—birth weight and length of gestation—associated with prenatal marijuana use, independent of tobacco (TOB), alcohol (ALC), other drug use (OTH), and socioeconomic risk (SES), in a pooled sample (N = 1191) derived from 3 recent developmental cohorts (2003–2015) with state-of-the-art substance use measures. We examined differential associations by infant sex, and multiplicative effects associated with co-use of MJ and TOB.
Methods:
Participants were mother-infant dyads with complete data on all study variables derived from Growing Up Healthy (n = 251), Behavior and Mood in Babies and Mothers (Cohorts 1 and 2; n = 315), and the Early Growth and Development Study (N = 625). We estimated direct effects on birth weight and length of gestation associated with MJ, TOB, and co-use (MJ x TOB), using linear regression analysis in the full sample, and in male (n = 654) and female (n = 537) infants, separately.
Results:
Mean birth weight and length of gestation were 3277 g (SD = 543) and 37.8 weeks (SD = 2.0), respectively. Rates of prenatal use were as follows: any use, n = 748 (62.8%); MJ use, n = 273 (22.9%); TOB use, n = 608 (51.0%); co-use of MJ and TOB, n = 230 (19.3%); ALC use, n = 464 (39.0%); and OTH use n = 115 (9.7%.) For all infants, unique effects on birth weight were observed for any MJ use [B(SE) = − 84.367(38.271), 95% C.I. −159.453 to −9.281, p = .028], any TOB use [B(SE) = −0.99.416(34.418), 95% C.I. −166.942 to − 31.889, p = .004], and each cigarette/day in mean TOB use [B(SE) = −12.233(3.427), 95% C.I. −18.995 to −5.510, p < .001]. Additional effects of co-use on birth weight, beyond these drug-specific effects, were not supported. In analyses stratified by sex, while TOB use was associated with lower birth weight in both sexes, MJ use during pregnancy was associated with lower birth weight of male infants [B(SE) = −153.1 (54.20); 95% C.I. −259.5 to −46.7, p = .005], but not female infants [B(SE) = 8.3(53.1), 95% C.I. −96.024 to 112.551, p = .876]. TOB, MJ, and their co-use were not associated with length of gestation.
Conclusions:
In this sample, intrauterine co-exposure to MJ and TOB was associated with an estimated 18% reduction in birth weight not attributable to earlier delivery, exposure to ALC or ΟΤΉ drugs, nor to maternal SES. We found evidence for greater susceptibility of male fetuses to any prenatal MJ exposure. Examination of dose-dependence in relationships found in this study, using continuous measures of exposure, is an important next step. Finally, we underscore the need to consider (a) the potential moderating influence of fetal sex on exposure-related neurodevelopmental risks; and (b) the importance of quantifying expressions of risk through subtle alterations, rather than dichotomous outcomes.
1. Introduction
Marijuana is the most commonly-reported illicit drug used by pregnant women in the United States (Berg et al., 2015; SAMHSA, 2013). Based on epidemiologic surveys, between 5% and 28% of the approximately 4 million infants born annually in the United States are born prenatally-exposed to marijuana (Ko et al., 2018). As intrauterine exposure to marijuana is not apparent at birth via a recognizable morphologic or physiologic syndrome, as is the case with alcohol (Clarren and Smith, 1978), and late term opioid exposure (Jansson and Velez, 2012), respectively, the nature and magnitude of impact on fetal development is an area of intense inquiry (Volkow et al., 2017).
Marijuana could adversely influence fetal development via several biological mechanisms (Grant et al., 2017; Richardson et al., 2016). A full third of THC consumed during pregnancy reaches the fetal circulation through the placenta (Hurd et al., 2005; Little and VanBeveren, 1996) and is met by cannabinoid receptors present in placental and fetal tissue, including the fetal brain, from early stages of embryonic development (Galve-Roperh et al., 2009; Park et al., 2003). Preclinical studies document alterations in normal patterns of fetal brain development (Jutras-Aswad et al., 2009), intrauterine growth, and early social and cognitive function of offspring prenatally exposed to marijuana, with early evidence for increased vulnerability in males (Bara et al., 2018; Benevenuto et al., 2017). Results from clinical studies are inconclusive, with confounding by concomitant use (or co-use) with tobacco and drugs as a major methodologic limitation of research to date (Huizink, 2014).
Separate from methodologic challenges, are recent changes in public perception, state policy, and apparent potency of marijuana in the United States (ElSohly et al., 2016; Warner et al., 2014). Critically, these changes occurred since the largest and most comprehensive teratologic investigations developed for this purpose were conducted (Day and Richardson, 1991; Jaddoe et al., 2012). While ramifications of these changes are yet to be fully appreciated, concern about greater prevalence and magnitude of prenatal exposures is warranted. Marijuana is perceived by many pregnant women as ‘natural,’ and relatively safe, and even preferable to prescription and over-the-counter remedies for nausea and hyperemesis (Oh et al., 2017). In the context of the resulting urgency to provide contemporary estimates of risk associated marijuana use during pregnancy, we conducted a secondary analysis using existing data from several recent well-described birth cohorts to overcome several critical methodologic barriers to knowledge.
1.1. Co-use of marijuana with tobacco, alcohol and other drugs
Among the most challenging methodologic dilemmas in etiologic research on substance use disorders is the common practice of co-use. In the examination of any particular substance of abuse, failure to detect co-use by women who are categorized as users could inflate estimates of risk, while failure to detect substances used by women categorized as non-users could dilute between-group differences (Conner et al., 2016). Indeed, conflicting findings to date regarding the risk for preterm delivery of low birth weight infants following marijuana during pregnancy derived from the Ottawa Prenatal Prospective Study (Fried et al., 1998), the Maternal Health Practices and Child Development Study (MHPCD) (Day and Richardson, 1991), and the Generation R study (El Marroun et al., 2009) has been attributed to confounding by tobacco and other drugs (Huizink, 2014). Two comprehensive reviews and meta-analyses of these and other studies were similarly limited in their ability to adjust for co-use (Conner et al., 2016; English et al., 1997; Huizink, 2014).
The most recent and well-controlled meta-analysis to our knowledge which included 31 studies of prenatal marijuana exposure published through August of 2015 also concluded a lack of risk for preterm delivery and low birth weight infants associated with prenatal marijuana use once tobacco and maternal socioeconomic factors were controlled (Conner et al., 2016). However, most cohorts included in this metaanalysis were recruited prior to aforementioned policy and potency changes. Moreover, only four of reviewed studies assessed drugs other than tobacco, rendering between-sample harmonization of alcohol or other drug use during pregnancy impossible. Finally, only 5.9% of infants in the meta-analytic sample were prenatally exposed to marijuana; Conner et al. (2016) cautioned that analyses was severely underpowered to detect a statistically-significant effect, were it present. To provide risk estimates that capture recent changes in policy, perception, and potency of marijuana in the U.S., and are adequately powered to detect effects on birth outcome, we examined prenatal cohorts recruited domestically between 2003 and 2015 that were directly or indirectly oversampled for tobacco and other drug exposures.
1.2. Measurement quality
We addressed two additional limitations of research to date that have received less attention, the first of which concerns measurement quality. Measurement can have a robust influence on estimates−of prevalence, extent, and putative effects of−prenatal tobacco exposure (Estabrook et al., 2016; Gunn et al., 2016; Shisler et al., 2017) yet is highly variable. Measurement quality is typically highest in mechanism-focused cohort studies that are usually underpowered to stratify outcomes by substances, and very limited in survey-based reports of use in large epidemiologic cohorts. This dilemma could contribute to mixed findings and inaccurate estimates of effects. To illustrate, in an earlier study of over 1200 women, Zuckerman and colleagues found that prenatal marijuana use was independently associated with fetal growth restriction, but only when urine screens for THC were used, and not when exposure was measured by self-reports alone (Zuckerman et al., 1989).
Indeed, even with widespread legalization and increased social acceptance of marijuana (Berg et al., 2015), when studying pregnant women, non-disclosure of marijuana use (Chang et al., 2017), and also tobacco use (Pickett et al., 2005; Shisler et al., 2017), still constitute potential sources of error. Data used in the current study contained finegrained interview-based multi-substance prenatal exposure measures collected in the context of multiple post-2000 mechanistic studies. These cohorts contained a substantially higher prevalence of exposure (s), (two were specifically oversampled for tobacco exposure) which enhanced our power to quantify substance-specific effects.
1.3. Variations in birth weight and length of gestation versus risk for clinically-defined benchmarks
Separate from measures of independent variables, i.e., co-exposures and other confounders, there has been less attention directed towards the selection of birth outcome measures. Studies to date have overwhelmingly examined dichotomous primary outcomes, namely, low birth weight, defined as < 2500 g, rather than continuously. This provides a clinically-informative risk ratio, but is a coarse measure of neurodevelopmental risk, when compared to the estimation of alteration in birth weight. While reductions in birth weight of normal weight infants may not be clinically significant at the time of birth, there is growing recognition of how the intrauterine environment powerfully shapes trajectories of health and disease across the lifespan (Barker, 1995). Birth weight is a highly-sensitive and well-established biomarker of intrauterine adversity and a strong predictor of neurodevelopmental and cardio-metabolic outcomes in later life (Johnson et al., 2017), including cognitive function 75 years later (Muller et al., 2014). Furthermore, natural variations in birth weight, within the normal range, have been shown to independently predict cortical surface area, total brain volume, and caudate volume, in healthy 6-year-old children, more strongly, in fact, than variations in low or high birth weight ranges (Walhovd et al., 2012). Thus, with a broader focus on estimating of neurodevelopmental risk, rather than simply confirming or refuting short-term morbidity and mortality, in this study we conceptualized birth outcomes as markers of long term risk, and examined them as continuous measures (birth weight, length of gestation).
1.4. Consideration of sex differences
Finally, while mechanisms are not well-understood, sex differences in the developmental impact of maternal smoking during pregnancy (Cross et al., 2017), and many other environmental insults across neonatal medicine (Rosen and Bateman, 2010), are widely observed, yet have not been a central focus of teratologic investigations concerning marijuana. To consider the possibility for sex differences in marijuana’s risk, we analyzed the full sample as a whole, consistent with nearly all studies to date, and then, male and female infants separately.
For clarity, we will use the terms, substance use and drug use, inter changeably, to refer to the use of any of the following: tobacco, alcohol, marijuana, other illicit drugs, and non-medical use of prescribed controlled substances. References made to substances used ‘during pregnancy’ will refer to the period from the estimated date of conception through delivery, consistent with intrauterine exposure.
2. Materials and methods
2.1. Participants and procedures
All study procedures described were approved by respective local Institutional Review Boards (IRB) prior to conduct. A flow chart showing the derivation of the pooled analytic sample of N = 1191 is shown in Fig. 1. Details of individual cohorts are described below.
Fig. 1.
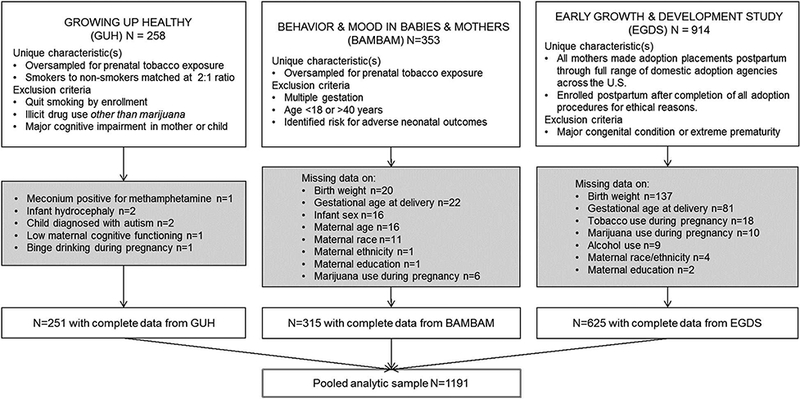
Individual cohorts and derivation of the analytic sample (N = 1191).
2.1.1. The Growing Up Healthy study (GUH)
We included n = 251 mothers with complete data (i.e. all variables described in regression analyses) from the full sample of 258, from GUH, a longitudinal study of prenatal tobacco exposure and child selfregulation. From 2006 to 2009, English-speaking women ≥18 years old with singleton pregnancies at ≤ 20 weeks of gestation were recruited from an urban hospital-based obstetric clinic in the Northeastern United States. Exclusion criteria were: any reported or biomarker (maternal saliva, infant meconium) evidence of illicit drug use other than marijuana during pregnancy, reported consumption of 4 or more alcoholic drinks/drinking occasion after the estimated date conception, or > 1 drink/day following the recognition of the pregnancy. At one-month intervals following enrollment, pregnant smokers were matched on age and education with the closest eligible non-smoking woman from the clinic. Smokers were oversampled to maximize range of smoking frequency, and in anticipation of greater attrition relative to non-smokers. Additional details can be found at (Eiden et al., 2018).
2.1.2. The Mood and Behavior in Mothers and Babies studies (BAM BAM and BAM BAM-2)
Participants in the pooled analytic sample were also derived from two larger studies with complementary methods−the Behavior and Mood in Babies and Mothers (BAM BAM) Study (2006–2010), and the BAM BAM-2 Study (2012–2015), referred to collectively, herein, as BAM BAM. The aim of both studies was to understand the effects of maternal perinatal smoking on fetal and infant outcomes (Stroud et al., 2014; Stroud et al., 2016). For both studies, pregnant women were recruited from obstetric clinics, health centers, and community postings in the Northeastern United States, oversampling for (tobacco) cigarette smokers. Women were excluded from participation if they were <18 or > 40 years of age, had current or prior involvement with child protective services, were pregnant with more than one fetus, or had serious psychiatric conditions (e.g., bipolar or psychotic disorders) or any serious medical condition during pregnancy (e.g., preeclampsia). Both studies utilized prospective interview- and biochemically-based assessments of substance use in the second and/or third trimesters of pregnancy, and at delivery. Additional details may be found in published reports from these data, for example (Massey et al., 2015; Stroud et al., in press).
2.1.3. The Early Growth and Development study (EGDS)
Finally, the pooled sample included 625 biological mothers (from a total of 914) from the EGDS, an ongoing, longitudinal, multi-site study that utilizes the parent-offspring within-family design to examine prenatal and postnatal environmental influences on child health outcomes, separate from genetic influences. Biological mothers were recruited from 2003 to 2010 from 45 adoption agencies in 15 states, across Mid-Atlantic, Southwestern, Midwestern, and Pacific Northwestern regions of the U.S., and represented the spectrum of public, private, religious, and secular agencies, including those favoring both open and closed adoptions. All EGDS biological mothers placed their children with unrelated adoptive parents at or shortly after delivery (M = 6.2 days, SD = 12.5, range = 0–91). Infants born with major congenital conditions, or who were delivered before 28 weeks of gestation (extreme prematurity) were excluded from participation. Additional information about the sample can be found at (Leve et al., 2013; Marceau et al., 2016; Massey et al., 2016).
2.2. Measures
2.2.1. Interview-based maternal reports of substance use during pregnancy
In all cohorts, maternal reports of drug use during pregnancy were assessed using well-validated calendar-based interview methods−the Life History Calendar (LHC) in EGDS (Caspi et al., 1996), and Timeline Follow Back Interviews in GUH and BAM BAM (Sobell and Sobell, 1996). Interviews were conducted prospectively in GUH and BAM BAM, and at 3–6 months postpartum (after finalization of adoption procedures) in EGDS. To minimize social desirability biases in reporting of stigmatizing information, the EGDS utilized computer-assisted personal interviewing (Gnambs and Kaspar, 2015).
2.2.2. Biomarkers of drug use during pregnancy
Biomarker verification of reported use was employed in GUH and BAM BAM, representng nearly half of the full analytic sample (47.5%, n = 566 out of 1191). In GUH, biomarkers of use were quantified from maternal saliva, collected during each trimester of pregnancy, and from meconium at delivery. Infant meconium samples were collected across several days, in one collection bottle, then assayed with a validated LC-MSMS method (Gray et al., 2010a) at 2.5ng/g nicotine, 1 ng/g nicotine, and 5 ng/g OHCOT for tobacco; and with a validated 2-di-mensional GC-MS analytical method for THC, 11-hydroxy-THC; 8,11-dihydroxy-THC; 11-nor-9-carboxy-THC (THC-COOH) and cannabinol (Gray et al., 2010b) for marijuana. Metabolites of cannabis, ethanol, amphetamines, opiates, and cocaine were tested with immunoassay screening (4.0 ng/L cutoff), followed by gas chromatography-mass spectrometry (GC-MS) confirmation (4.0 ng/L cutoff) (Gray et al., 2010). In BAM BAM, mothers provided saliva samples for cotinine assays using ELISA kits at 30 and 35 weeks, and at delivery (Salimetrics, 2006). Following delivery, infant meconium samples were collected for up to three days, then assayed for metabolites of marijuana, cocaine, and other illicit drugs (Gray et al., 2010; Moore et al., 1998).
2.2.3. Infant sex, birth weight, and gestational age at delivery
Data on birth outcomes were derived from medical records for all studies.
2.3. Data analysis
Measures of tobacco use in cigarettes/day across all cohorts enabled harmonization to create a continuous measure of prenatal tobacco exposure. For alcohol, marijuana, and other drugs, GUH and BAM BAM quantified use in units/day, while EGDS assessed severity of use patterns as evidenced by diagnostic criteria for substance use disorders endorsed. Thus, for alcohol and marijuana, we created categorical variables indicating the presence or absence of use at any time during pregnancy. Close to a dozen illicit drugs other than marijuana were assessed across cohorts, including non-medical use of prescription narcotics in EGDS. However, since the frequency of each drug was low, they were collapsed into a fourth dichotomous substance use variable, referred to, herein, as other drugs. As continuous independent variables are methodologically favorable to dichotomous variables in regression models (Royston et al., 2006), mean tobacco exposure in cigarettes/day was selected for use in models. However, we also examined tobacco as a dichotomous variable (any use) to facilitate the comparison of its effect with dichotomous marijuana and other drug variables.
To estimate additional effects on outcomes associated with co-use of tobacco and marijuana (beyond effects of tobacco and marijuana individually), we created the interaction term, mean TOB exposure x MJ, by multiplying standardized mean cigarettes/day by any marijuana use. Among many maternal demographic factors considered for inclusion as covariates (Kramer, 1987), we selected those correlated with either birth weight or length of gestation, in any individual cohort, or in the pooled sample (p < .05). These covariates−maternal age at delivery, minority race (non-White) or ethnicity (Hispanic), less than high school educational attainment, and male sex, were entered in all models (with the exception of male sex, removed for analyses stratified by sex).
After ruling out multi-collinearity among variables (defined a priori, as r > 0.6) using bivariate correlation analysis (Table 2), we estimated specific substance-outcome associations (effects) using linear regression analysis, with birth weight, then length of gestation, as the dependent variable. As independent variables, we entered the 4 exposure variables (mean TOB exposure, any MJ use, any ALC use, and any OTHER drug use) together with all covariates. Next, the interaction term for co-use of MJ and TOB was added to examine any additional effect (Table 3). These same models were then fitted, separately, to male (N = 654) and female (N = 537) infants (Table 4).
3. Results
3.1. Descriptive characteristics (N = 1191)
Characteristics of mothers and infants in pooled and individual cohorts, and between-cohort differences, are shown in Table 1. For the pooled sample, mothers were typically high school-educated women in their mid-20’s (M = 24.4 years, SD = 5.7) who delivered at a mean gestational age of 37.8 weeks (SD = 2.0). About two thirds of mothers (62.8%, n = 748) used at least one substance during pregnancy. Approximately half used tobacco (n = 608, 51.0%); over a third used alcohol (n = 464, 39.0%); almost 1 in 4 used marijuana (n = 273, 22.9%); and about 1 in 10 used a drug other than tobacco, alcohol or marijuana (n = 115, 9.7%). About 1 in 5 women used tobacco and marijuana concomitantly (n = 230, 19.3%). Newborns were 54.9% male, weighing an average of 3277 g (SD = 543), equivalent to 7.2 lbs. Seven percent of infants (n = 85) were low birth weight ( < 2500 g).
Table 1.
Descriptive characteristics of mothers and infants in pooled (N = 1191) and individual cohorts.
| Pooled sample N=1191 | Growing Up Healthy N=251 |
Behavior & Mood in Mothers & Babies N=315 |
Early Growth & Development Study N=625 |
F or χ2 statistic |
|
|---|---|---|---|---|---|
| Mothers, pregnancy | Mean ± SD or n (%) | ||||
| Age at delivery, (years) | 24.4 ± 5.7 | 24.0 ± 5.0 | 25.9 ± 5.1 | 23.8 ± 6.1 | 14.713*** |
| Less than H.S. education | 272 (22.8%) | 74 (29.5%) | 70 (22.2%) | 128 (26.2%) | 8.327* |
| Racial or ethnic minority | 480 (40.3%) | 177 (70.5%) | 139 (44.4%) | 164 (26.2%) | 148.531*** |
| Any use | 748 (62.8%) | 215 (85.7%) | 170 (54.0%) | 363 (58.1%) | 72.615*** |
| Any tobacco (TOB) use | 608 (51.0%) | 178 (70.9%) | 160 (50.8%) | 270 (43.2%) | 55.063*** |
| Mean TOB exposure (cigs/d) | 3.01 ± 4.8 | 3.53 ± 4.5 | 2.71 ± 4.4 | 2.94 ± 5.1 | 2.102 |
| Mean cig/d, TOB users only | 6.15 ± 5.32 | 5.37 ± 4.61 | 5.66 ± 4.96 | 6.92 ± 5.82 | 5.284** |
| Any MJ use | 273 (22.9%) | 101 (40.2%) | 66 (21.0%) | 105 (16.8%) | 56.694*** |
| Mean MJ exposure (joints/d) | 0.12 ± 0.43 | 0.24 ± 0.62 | 0.024 ± 0.12 | Not assessed | 36.330*** |
| Mean joints/d, MJ users only | 0.41 ± 0.73 | 0.57 ± 0.85 | 0.13 ± 0.25 | Not assessed | 15.154*** |
| Co-use of TOB & MJ | 230 (19.3%) | 97 (38.6%) | 56 (17.8%) | 77 (12.3%) | 80.294*** |
| Any alcohol (ALC) use | 464 (39.0%) | 153 (61.0%) | 162 (51.4%) | 149 (23.8%) | 131.743*** |
| Any OTHER drug use includes misuse of prescription drugs | 115 (9.7%) | 5 (2.0%) | 6 (1.9%) | 104 (16.6%) | 73.542*** |
| Infants, at delivery | Mean ± SD or n (%) | ||||
| Male | 654 (54.9%) | 131 (52.2%) | 163 (51.7%) | 360 (57.6%) | 3.850 |
| Birth weight (g) | 3,277 ± 543 | 3,224 ± 582 | 3,335 ± 505.7 | 3,266 ± 543 | 2.762 |
| Low birthweight (<2,500 g) | 85 (7.1%) | 20 (8.0%) | 16 (5.1%) | 49 (7.8%) | 2.740 |
| Gestational age (weeks) | 37.8 ± 2.0 | 38.9 ± 1.8 | 39.4 ± 1.5 | 36.6 ± 1.4 | 440.170*** |
Grey rows shown for descriptive purposes only; all other rows show variables used in subsequent analyses.
p < .05
p < .01
p < .001 for two-tailed tests.
Between-cohort differences in demographic and substance use variables are shown in Table 1. Briefly, GUH participants were least likely to have completed high school, most racially and ethnically diverse, and most likely to use any substance during pregnancy. Mean tobacco use (cig/d) among mothers who smoked at any time during gestation was lowest in GUH, however (M = 5.4 cig/d, SD = 4.6), and highest in EGDS (M = 6.9 cig/d, SD = 5.2; F(all cohorts) = 5.284; df 2, 579; p = .005). GUH mothers were heavier marijuana users, relative to BAM BAM mothers, reporting the equivalent of just over 1 joint consumed every 2 days (M = 0.57 joints/d, SD = 0.85), compared to 1 joint every 8 days in BAM BAM (M = 0.13 joints/d, SD = 0.73, F(GUH, BAM BAM) = 15.154, d/1, 164; p < .001). Mean birth weights among the 3 cohorts were not different (F = 2.762, df 1, 1189, p = NS), while mean lengths of gestation were (F = 440.170, p < .01). EGDS mothers delivered earliest, at a mean of 36.6 weeks (SD = 1.4), followed by GUH mothers (M = 38.9 weeks, SD = 1.8).
3.2. Correlations among maternal and infant characteristics
Bivariate correlations among variables are shown in Table 2. All substances used during pregnancy were moderately inter-correlated with one another, and inversely associated with birth weight, with the exception of alcohol use (no correlation). For length of gestation, maternal alcohol use (r = 0.251, p < .01) was associated with a longer gestation. We interpreted the statistically significant but near zero association between marijuana and length of gestation (r = 0.058, p < .05) as not significant.
Table 2.
Bivariate correlations among), outcomes and variables (N = 1191).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal age | |||||||||
| 2. <H.S. education | −0.131** | ||||||||
| 3. Minority race/eth. | 0.025 | 0.095** | |||||||
| 4. Male infant | 0.023 | −0.009 | −0.030 | ||||||
| 5. TOB (cig/d) | 0.152** | 0.123** | −0.170** | 0.043 | |||||
| 6. Any MJ use | −0.027 | 0.028 | 0.022 | −0.030 | 0.203** | ||||
| 7. Any ALC | 0.023 | −0.115** | 0.018 | −0.020 | 0.088** | 0.218** | |||
| 8. Any OTHER use | 0.094** | 0.012 | −0.130** | 0.005 | 0.216** | 0.134** | −0.034 | ||
| 9. Birth weight | 0.033 | −0.093** | −0.161** | 0.118** | −0.090** | −0.090** | 0.038 | −0.052 | |
| 10. Length of gestation | 0.070* | 0.018 | 0.139** | −0.009 | −0.011 | 0.058* | 0.251** | −0.195** | 0.422** |
p < .05
p < .01 for two-tailed tests.
Highlighted, are correlates of mean tobacco use and any marijuana use during pregnancy.
Use of tobacco and other drugs during pregnancy were unrelated.
3.3. Exposures and birth weight, analysis of full sample (Table 3, top)
Table 3.
Estimated effects on birth weight (top) and length of gestation (bottom) associated with maternal tobacco, marijuana, alcohol, and other drug use during pregnancy from linear regression (N = 1191).
| Birth weight (grams) | B | SE | β | t | P | 95% C.I. for B | |
|---|---|---|---|---|---|---|---|
| Constant | 3232.824 | 73.256 | 44.130 | .000 | 3089.097 | 3376.551 | |
| Maternal age | 4.383 | 2.762 | .046 | 1.587 | .113 | −1.036 | 9.801 |
| < High school | −60.342 | 37.626 | −.047 | −1.604 | .109 | −134.162 | 13.479 |
| Minority race or ethnicity | −196.943 | 32.075 | −.178 | −6.140 | .000 | −259.873 | −134.014 |
| Male infant | 125.525 | 30.667 | .115 | 4.093 | .000 | 65.358 | 185.692 |
| Any alcohol use | 66.332 | 32.415 | .060 | 2.046 | .041 | 2.734 | 129.930 |
| Any other drug use | −83.695 | 53.614 | −.046 | −1.561 | .119 | −188.884 | 21.494 |
| Mean tobacco use (cigs/d) | −12.233 | 3.427 | −.109 | −3.570 | .000 | −18.955 | −5.510 |
| Any marijuana use | −84.367 | 38.271 | −.065 | −2.204 | .028 | −159.453 | −9.281 |
| Length of gestation (weeks) | B | SE | β | t | P | 95% C.I. for B | |
| Constant | 36.549 | .265 | 137.771 | .000 | 36.028 | 37.069 | |
| Maternal age | .031 | .010 | .086 | 3.065 | .002 | .011 | .050 |
| < High school | .231 | .136 | .048 | 1.694 | .090 | −.036 | .498 |
| Minority race or ethnicity | .427 | .116 | .104 | 3.675 | .000 | .199 | .655 |
| Male infant | −.005 | .111 | −.001 | −.046 | .963 | −.223 | .213 |
| Any alcohol use | .992 | .117 | .239 | 8.450 | .000 | .762 | 1.222 |
| Any other drug use | −1.276 | .194 | −.187 | −6.570 | .000 | −1.657 | −.895 |
| Mean tobacco use (cigs/d) | .001 | .012 | .001 | .044 | .965 | −.024 | .025 |
| Any marijuana use | .142 | .139 | .030 | 1.027 | .304 | −.130 | .414 |
Estimates of birth weight effects associated with specific prenatal exposures from linear regression analysis of the full sample all infants are shown in Table 3, top. Any use of tobacco during pregnancy was associated with lower birth weight by approximately 100 g [B (SE) = −0.99.416(34.418), 95% C.I. −166.942 to −31.889,p = .004] (model not shown), with each cigarette/day in mean TOB exposure accounting for an estimated 12.23-gram reduction in birth weight (SE = 3.43, 95% C.I. −18.96 to −5.51, p < .001). Any use of marijuana was also associated with a reduction in birth weight, by 84.367 g (SE = 38.27, 95% C.I. −159.45 to −9.28, p = .028). Maternal minority status was linked to lower birth weight [B (SE) = −196.94 (32.08), 95% C.I. −259.87 to −134.01, p < .001], while male sex and prenatal alcohol use were associated with increased birth weight [B (SE) = 125.53 (30.67), 95% C.I. 65.36 tol85.69, p < .001] and [B (SE) = 66.33 (32.42), 95% C.I. 2.73 to 129.93, p = .041], respectively. Co-use of tobacco and marijuana during pregnancy was not associated with additional effects on birth weight beyond individual drug effects noted above [B (SE) = −55.15 (36.67), 95% C.I. −127.10 to 16.80, p = .133] (model not shown).
3.4. Exposures and length of gestation, analysis of full sample (Table 3, bottom)
As shown in the bottom portion of Table 3, tobacco exposure [B (SE) = 0.001 (0.012), 95% C.I. −0.24 to 0.25, p = .965] and marijuana exposure [B(SE) = 0.142 (0.139), 95% C.I. −0.130 to 0.414, p = .304] were both unrelated to length of gestation. The interaction of tobacco and marijuana also was not significant [B(SE) = −0.082 (0.133), 95% C.I. −0.343 to 0.179 p = .538]. Any alcohol use during pregnancy was associated with a longer length of gestation, by nearly a week [B (SE) = 0.992 (0.117), 95% C.I. 0.762 to 1.222,p < .001], while use of drug(s) other than tobacco, alcohol, and marijuana was associated with a shorter length of gestation by over a week [B(SE) = −1.276 (0.194), 95% C.I. −1.657 to −0.895, p < .001].
3.5. Sex-specific analyses of exposures and birth weight (Table 4, top); and length of gestation (Table 4, bottom)
Table 4.
Estimated effects on birth weight (top) and length of gestation (top) associated with maternal use of tobacco, marijuana, alcohol, and other drug(s) during pregnancy, stratified by infant sex, from linear regression.
| MALE (n=654) | FEMALE (n=537) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight (grams) | 95% C.I. for B | 95% C.I. for B | ||||||||
| B | SE | Lower | Upper | P | B | SE | Lower | Upper | P | |
| Constant | 3441.657 | 99.348 | 3246.573 | 3636.741 | .000 | 3117.925 | 101.110 | 2919.299 | 3316.552 | .000 |
| Maternal age | .126 | 3.828 | −7.391 | 7.644 | .974 | 10.147 | 3.933 | 2.421 | 17.872 | .010 |
| < High school | −103.449 | 53.425 | −208.357 | 1.458 | .053 | −19.821 | 51.971 | −121.917 | 82.274 | .703 |
| Minority race/eth. | −184.974 | 45.744 | −274.800 | −95.149 | .000 | −200.744 | 44.124 | −287.424 | −114.064 | .000 |
| Any alcohol use | 165.613 | 45.413 | 76.439 | 254.787 | .000 | −57.994 | 45.533 | −147.441 | 31.454 | .203 |
| Any other drug use | −90.039 | 74.072 | −235.491 | 55.413 | .225 | −107.041 | 77.042 | −258.386 | 44.305 | .165 |
| Mean TOB use (cigs/d) | −10.995 | 4.736 | −20.295 | −1.695 | .021 | −13.445 | 4.913 | −23.095 | −3.794 | .006 |
| Any MJ use | −153.087 | 54.199 | −259.515 | −46.659 | .005 | 8.263 | 53.087 | −96.024 | 112.551 | .876 |
| Length of gestation (weeks) | 95% C.I. for B | 95% C.I. for B | ||||||||
| B | SE | Lower | Upper | P | B | SE | Lower | Upper | P | |
| Constant | 36.625 | .336 | 35.965 | 37.284 | .000 | 36.420 | .403 | 35.628 | 37.212 | .000 |
| Maternal age | .027 | .013 | .002 | .053 | .035 | .036 | .016 | .006 | .067 | .021 |
| < High school | .320 | .181 | −.035 | .674 | .077 | .099 | .207 | −.308 | .506 | .633 |
| Minority race/eth. | .321 | .155 | .017 | .625 | .039 | .568 | .176 | .223 | .914 | .001 |
| Any alcohol use | 1.240 | .154 | .939 | 1.542 | .000 | .674 | .182 | .318 | 1.031 | .000 |
| Any other drug use | −1.393 | .251 | −1.885 | −.901 | .000 | −1.168 | .307 | −1.771 | −.564 | .000 |
| Mean TOB use (cig/d) | −.007 | .016 | −.039 | .024 | .647 | .009 | .020 | −.030 | .047 | .663 |
| Any MJ use | −.025 | .183 | −.385 | .335 | .890 | .367 | .212 | −.049 | .783 | .084 |
Analyses stratified by infant sex are shown in Table 4. For male infants, each cigarette/day in mean tobacco exposure was associated with a 10.995-gram reduction in birth weight (SE = 4.74, 95% C.I. −20.30 to −1.70, p = .021); any marijuana exposure was associated with a 153.09-gram reduction (SE = 54.20, 95% C.I. −259.52 to −46.66, p = .005). Prenatal tobacco exposure was associated with a similar magnitude of reduction in birth weight in female infants [B (SE) = −13.45 (4.91), 95% C.I. −23.10 to −3.79, p = .006]. However, unlike male infants, no effect of marijuana exposure was observed for females [B (SE) = 8.26, 95% C.I. −96.02 to 112.55, p = .876]. Evidence for effects of co-use (TOB x MJ) above and beyond the effects of each drug alone was not supported, for males [B (SE) = −97.404 (54.358), 95% C.I. [−204.143–9.335], p = .074] nor for females [B (SE) = 3.184 (49.184), 95% C.I. [ −93.437–99.805], p = .948].
4. Discussion
4.1. Unique contributions of the current study
The pooled sample of 1191 mother-infant dyads described in this study represented a rare balance of statistical power, measurement quality, and high rates of drug exposures. Nearly 1 in 4 (22.9%) mothers in the pooled sample used marijuana while pregnant, compared to 2.7% in the Stillbirth Collaborative Research Network (Metz and Stickrath, 2015); 3.2% in the National Birth Defects Prevention Study (van Gelder et al., 2010), 4.6% in the National Survey on Drug Use and Health (Ko et al., 2018), and 5.9% in the most recent and well-con-trolled meta-analysis to date (Conner et al., 2016). Mothers were recruited between 2003 and 2015 concurrent with marijuana’s evolution in policy, public perception, and potency. Third, consistent with the rapidly-expanding body of clinical evidence supporting normal variation in birth weight as a sensitive marker of the intrauterine environment and neurodevelopmental risk, manifested across the life span (Cook and Fletcher, 2015; Muller et al., 2014; Walhovd et al., 2012), we departed from the convention of estimating risks for clinically-defined benchmarks (i.e., low birth weight and preterm birth). Instead, we provided estimates of birth weight effects associated with marijuana and tobacco, independent from one another, and of their co-use, in light of evidence for additive risks of co-use in non-pregnant individuals (Meier and Hatsukami, 2016). Finally, in the context of evidence for sex differences in offspring susceptibility to prenatal tobacco exposure (Cornelius and Day, 2009) and early preclinical evidence for a sex differences in embryonic and fetal vulnerability to THC exposure (Benevenuto et al., 2017), we examined exposure-outcome relationships for male and female infants separately.
4.2. Percentage versus absolute effects and sex differences in mean birth weights
The inverse association between prenatal tobacco exposure (mean cigarettes/day) and birth weight found in this sample mirrors existing evidence in this regard (Ernst et al., 2001). However, while the absolute reduction in birth weight associated with each cigarette/day in mean prenatal tobacco exposure was similar for male (about 11 g) and female infants (13 g), respectively, this estimate represented a greater proportion of total body weight for female infants, who were lighter, when averaged across the pooled sample (3335.4 ± 566 g in males versus 3206.9 ± 504.3 g in females, F = 4.345, df = 1189, p < .001). While this was not an a priori focus of the current investigation, consideration of adjustment for sex differences in sensitive biomarkers of risk such as birth weight may be important. Our recent 3-year follow up of toddlers from GUH showed a stronger association between prenatal tobacco exposure and internalizing, sleep, and attention problems in girls, relative to boys (Eiden et al., 2018). Confirming that female infants were lighter than male infants in the GUH sample, together with these recent findings in toddlers raises the possibility that a more nuanced approach to exposure-outcome associations is needed. Specifically, estimation of relative reduction in birth weight via the integration of sex differences in birth weight (and other biomarkers), may be a more predictive marker for neurodevelopmental risk than estimation of absolute effects, or risk for low birth weight, as has been conventionally done.
4.3. Sex differences in vulnerability may vary by developmental stage
This is the first study to our knowledge to suggest a sex-specific association between prenatal marijuana exposure and birth weight. This observation is consistent with a large body of research supporting sex-specific vulnerability of males to early environmental adversity more broadly (Rosen and Bateman, 2010), and early preclinical evidence for increased morphologic (Benevenuto et al., 2017) and neurodevelopmental (Silva et al., 2012) susceptibility of males to prenatal cannabis exposure. Increased male vulnerability to epigenetic alterations in differentially methylated regions, especially in the Insulin-like Growth Factor 2 regulatory genes (i.e., IGF2 and H19), is a proposed mechanism for sex-specific vulnerability to prenatal tobacco exposure (Murphy et al., 2012). Sex-specific vulnerability could operate even earlier, at the embryonic stage.
For instance, Benevenuto et al. (2017) noted a greater proportion of male pups in litters born to dams exposed to marijuana, relative to litters born to non-exposed dams, coupled with double the rate of postimplantation pregnancy loss of female embryos (though the latter. difference was not statistically significant) (Benevenuto et al., 2017). Regardless, our results emphasize the importance of examining exposure-outcome effects separately by offspring sex, especially since undetected sex differences could dilute true associations. Analysis of pooled data from other recent cohorts well-characterized for exposures, stratified by sex, is recommended to clarify results to date derived from previous studies that have not stratified by sex.
4.4. Estimating subtle alterations in birth weight associated with exposure to elucidate pathways of neurodevelopmental risk
Relatedly, while the majority of prior studies and meta-analyses have not supported an independent risk for low birth weight associated with prenatal marijuana exposure (Conner et al., 2016), the multitude of plausible risk mechanisms (Richardson et al., 2016), combined with preclinical studies suggesting subtle, yet persistent cognitive and social deficits (Silva et al., 2012), together, support a more nuanced approach to quantifying neurodevelopmental risk that involves the estimation of effects on a continuous measure of birth weight rather than a singular focus on risk for low birth weight.
As is the case with prenatal tobacco exposure (Estabrook et al., 2016; Knopik, 2009), association does not imply causality, in light of measured and unmeasured influences, including, but not limited to couse of other substances, maternal stress and its psychopathological manifestations, and associated environmental risks (Borders et al., 2007; Grote et al., 2010). Our results support the presence of risk to fetal growth associated with MJ exposure, particularly in the presence of TOB exposure, combined with the need for more investigation to confirm specificity to male infants. Since our data do not provide the framework for discerning underlying mechanisms of observed sex differences, further investigation using a combination of preclinical and clinical approaches is recommended to establish a dose-dependent relationship, and to elucidate pathways and targets for preventive interventions.
4.5. Lower birth weight associated with marijuana and tobacco exposures was not explained by a shorter length of gestation
Like birth weight, many factors other than cigarette smoking could adversely influence the length of gestation, i.e., maternal infections and other illnesses, pre-pregnancy weight, prior history of spontaneous abortion or preterm delivery, and prenatal exposure to diethyl-stilbestrol (Kramer, 1987). Neither tobacco, nor marijuana, was significantly associated with length of gestation. We considered that the relatively low mean level of prenatal tobacco exposure of approximately 3 cigarettes/day (SD = 4.8) in the full sample, and about 6 cigarettes/day among women who smoked during pregnancy (SD = 5.3), combined with the exclusion criteria typical of developmental cohorts could have contributed to range restriction in length of gestation. However, the significant direct effects on length of gestation observed for drugs other than marijuana do not support this explanation. The observed reduction in birth weight associated with prenatal marijuana and tobacco exposure not accounted for by earlier delivery, then, would be consistent with intrauterine growth restriction. This and other findings must be viewed, however, in the context of limitations of this study.
4.6. Limitations
Despite a much higher prevalence of prenatal exposure to marijuana in our sample compared to epidemiologic cohorts (details above), the prevalence of marijuana use alone, at the exclusion of other substances, especially tobacco, was still quite rare (n = 25; 2.1%). We wish to underscore the impact of this finding alone, that, together with studies across several decades (Coleman-Cowger et al., 2017; Conner et al., 2016; Ko et al., 2018), imply that studying, or evaluating for, one drug, in the scientific or clinical setting, as the case may be, necessitates consideration of the other. Evidence derived from the current study provides an important framework of comparison to be considered with evidence from large epidemiologic studies with much lower rates of exposures, and have not found independent effects (Ko et al., 2018; van Gelder et al., 2010).
Next, the range of exposures was relatively small; frequent and/or heavy use of tobacco, marijuana, and other drugs was rare (Table 1) (Eiden et al., 2018; Massey et al., 2015; Massey et al., 2011). Moreover, we did not utilize a continuous measure of marijuana and other drug exposures, account for timing of exposures in gestation, or assess interindividual and intra-individual fluctuations in use, known to exist for cigarette smoking (Eiden et al., 2013; Pickett et al., 2003).
Third, birth weight and its maternal and child correlates are partially heritable. Quasi-experimental approaches such as genetically-in-formed designs and within-individual designs could provide more robust controls for individual-level confounders (Mosing et al., 2016). Indeed, many unmeasured influences other than marijuana that could have influenced birth weight (Knopik et al., 2016). Notably, socioeconomic status was a robust predictor of birth weight, as expected (Kramer, 1987; Kramer et al., 2000). Being either a racial (non-White) or ethnic (Hispanic) minority showed a consistent inverse association with birth weight−across both sexes (Tables 3 and 4)−and was actually associated with a greater effect on birth weight than any specific exposure [B(SE) = −196.943 (32.075), 95% C.I. [−259.873 to - 134.014], p < .001].
These effects led us to consider the possibility that specific substances used were markers of demographic confounders, especially in the case of alcohol use, which was paradoxically associated with higher birth weight and length of gestation, and inversely associated with having less than a high school education (r = −0.115, p < .01) (Table 2). One plausible explanation for this unexpected finding is selection bias—alcohol users eligible for GUH were women who used alcohol infrequently and/or limited their drinking to 1 drink/day after recognizing the pregnancy (Fig. 1). Relatedly, the apparent protective effect associated with moderate alcohol use could be an artifact, reflecting risks in women who abstained from alcohol completely, mimicking the widely-replicated J-shaped curve phenomenon of moderate drinking and cardiovascular mortality (Di Castelnuovo et al., 2006).
In contrast to alcohol, prenatal marijuana use was neither correlated with education (r = 0.28, p = NS), nor with identification as a racial or ethnic minority (r = 0.022, p = NS), or any other demographic variable. This provided a measure of support for the validity of our results supporting an independent risk of marijuana exposure to fetal growth. Establishing dose dependence of exposure-outcome relationships found in this study are needed, however, prior to translating results to clinical recommendations.
4.7. Conclusion
We reiterate the importance of interpreting these results within the sheer breadth of environmental factors besides substance exposures that could influence birth weight—contextual (i.e., local traumatic events, neighborhood crime), spousal/familial (i.e., intimate partner violence, spousal/household smoking), and individual-level factors (i.e., maternal behavior, mood, and overall health) are well-established determinants of birth weight, all of which are strongly implicated in the transmission of health risks inter-generationally. However, maternal substance abuse is malleable, and arguably, must be addressed to ensure optimal child health.
Maternal behavior may be the most profound determinant of children’s rearing environment, making a focus on maternal well-being paramount in the prevention of child psychopathology (Grant et al., 2018; Massey et al., 2017; Shankaran et al., 2007). To this end, we recommend future research utilizing the approach described in this study—that blends high quality exposure measurement with adequate prevalence and power to understand the extent and nature of risk associated with marijuana use during pregnancy. This knowledge is sorely needed by policymakers, obstetric providers, pregnant women, and sexually-active women of child-bearing age (Mark and Terplan, 2017).
Acknowledgements
We are deeply appreciative to all participating mothers and families, whose contributions made this research possible. We would also like to recognize Sally Guyer at the University of Oregon and Kelcie Kuchenrither at Northwestern University Feinberg School of Medicine for facilitating this collaborative inter-institutional effort.
Role of funding sources
This work was supported by a 2017 Innovation Award (PI Suena Massey) from the Dixon Translational Research Grants Initiative of the Northwestern Memorial Foundation, grants K23DA037913 (PI Suena Massey); R01 DA020585 (PI Jenae Neiderhiser); R01DA019632 (PI Rina Eiden); R01 DA044504, R01 DA031188, and R01DA019558 (PI Laura Stroud); and intramural support (Marilyn Huestis) from the National Institute on Drug Abuse (NIDA); grant R01HD042608 (PI David Reiss Years 1–5; PI Leslie Leve Years 6–10) from the National Institute on Child Health and Human Development; grant R01 MH092118 (Multiple PIs Leslie Leve, Jenae Neiderhiser, and Jody Ganiban) from the National Institute of Mental Health (NIMH); grant R01AG018436 (PI Daniel Mroczek) from the National Institute on Aging (NIA); and grant UG3OD023389 (Multiple PIs Leslie Leve, Jenae Neiderhiser, and Jody Ganiban) from the Office of the Director (OD) of the National Institutes of Health (NIH). Responsibility for this work rests solely with the authors. The NIDA (Extramural and Intramural Divisions), NICHD, NIMH, NIA, OD, and NIH had no role in the study design, data collection, analysis or interpretation of the data, or the decision to submit this manuscript for publication.
Footnotes
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- Bara A, Manduca A, Bernabeu A, Borsoi M, Servado M, Lassalle O, Murphy MN, Wager-Miller J, Mackie K, Pelissier-Alicot A-L, 2018. Sex Specific Endophenotypes of In-utero Cannabinoid Exposure. bioRxiv, pp. 251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, 1995. The fetal and infant origins of disease. Eur. J. Clin. Investig 25 (7), 457–463. [DOI] [PubMed] [Google Scholar]
- Benevenuto SG, Domenico MD, Martins MAG, Costa NS, de Souza ARL, Costa JL, Tavares MFM, Dolhnikoff M, Veras MM, 2017. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: an experimental study in mice. Toxicology 376, 94–101. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Stratton E, Schauer GL, Lewis M, Wang Y, Windle M, Kegler M, 2015. Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Subst. Use Misuse 50 (1), 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders AEB, Grobman WA, Amsden LB, Holl JL, 2007. Chronic stress and low birth weight neonates in a low-income population of women. Obstet. Gynecol 109 (2, Part 1), 331–338. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW,... Harrington H.Silva, 1996. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. Int. J. Methods Psychiatr. Res 6 (2), 101–114. [Google Scholar]
- Chang JC, Holland CL, Tarr JA, Rubio D, Rodriguez KL, Kraemer KL, Day N, Arnold RM, 2017. Perinatal illicit drug and marijuana use: an observational study examining prevalence, screening, and disclosure. Am. J. Health Promot 31 (1), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Smith DW, 1978. The fetal alcohol syndrome. N. Engl. J. Med 298 (19), 1063–1067. [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger VH, Schauer GL, Peters EN, 2017. Marijuana and tobacco co-use among a nationally representative sample of US pregnant and non-pregnant women: 2005–2014 National Survey on Drug Use and Health findings. Drug Alcohol Depend. 177, 130–135. [DOI] [PubMed] [Google Scholar]
- Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG, 2016. Maternal marijuana use and adverse neonatal outcomes. Obstet. Gynecol 128 (4), 713–723. [DOI] [PubMed] [Google Scholar]
- Cook CJ, Fletcher JM, 2015. Understanding heterogeneity in the effects of birth weight on adult cognition and wages. J. Health Econ 41, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Day NL, 2009. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol 22 (2), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Linker KE, Leslie FM, 2017. Sex-dependent effects of nicotine on the developing brain. J. Neurosci. Res 95 (1–2), 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Richardson GA, 1991. Prenatal marijuana use: epidemiology, methodologic issues, and infant outcome. Clin. Perinatol 18 (1), 77–91. [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, De Gaetano G, 2006. Alcohol dosing and total mortality in men and women: an updated metaanalysis of 34 prospective studies. Arch. Intern. Med 166 (22), 2437–2445. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Homish GG, Colder CR, Schuetze P, Gray TR, Huestis MA, 2013. Changes in smoking patterns during pregnancy. Subst. Use Misuse 48 (7), 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Zhao J, Casey M, Shisler S, Schuetze P, Colder CR, 2018. Pre-and postnatal tobacco and cannabis exposure and child behavior problems: bidirectional associations, joint effects, and sex differences. Drug Alcohol Depend. 185, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC, 2009. Intrauterine cannabis exposure affects fetal growth trajectories: the generation R study. J. Am. Acad. Child Adolesc. Psychiatry 48 (12), 1173–1181. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC, 2016. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol. Psychiatry 79 (7), 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D, Hulse G, Milne E, Holman C, Bower C, 1997. Maternal cannabis use and birth weight: a meta-analysis. Addiction 92 (11), 1553–1560. [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML, 2001. Behavioral and neural consequences of prenatal exposure to nicotine. J. Am. Acad. Child Adolesc. Psychiatry 40 (6), 630–641. [DOI] [PubMed] [Google Scholar]
- Estabrook R, Massey SH, Clark CA, Burns JL, Mustanski BS, Cook EH, O’Brien TC, Makowski B, Espy KA, Wakschlag LS, 2016. Separating family-level and direct exposure effects of smoking during pregnancy on offspring externalizing symptoms: bridging the behavior genetic and behavior teratologic divide. Behav. Genet 46 (3), 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R, 1998. Differential effects on cognitive functioning in 9-to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol 20 (3), 293–306. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Palazuelos J, Aguado T, Guzman M, 2009. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur. Arch. Psychiatry Clin. Neurosci 259 (7), 371–382. [DOI] [PubMed] [Google Scholar]
- Gnambs T, Kaspar K, 2015. Disclosure of sensitive behaviors across self-administered survey modes: a meta-analysis. Behav. Res. Methods 47 (4), 1237–1259. [DOI] [PubMed] [Google Scholar]
- Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM, 2018. Cannabis use during pregnancy: pharmacokinetics and effects on child development. J. Stud. Alcohol Drugs 79 (1), 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant TM, Graham JC, Carlini BH, Ernst CC, Brown NN, 2018. Use of marijuana and other substances among pregnant and parenting women with substance use disorders: changes in Washington state after marijuana legalization. J. Stud. Alcohol Drugs 79 (1), 88–95. [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA, 2010. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin. Chem 56 (9), 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ, 2010. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 67 (10), 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J, Rosales C, Center K, Nunez A, Gibson S, Christ C, Ehiri J, 2016. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 6 (4), e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A, 2014. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 52, 45–52. [DOI] [PubMed] [Google Scholar]
- Hurd Y, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D, 2005. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol 27 (2), 221–229. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van IIzendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, 2012. The generation R study: design and cohort update 2012. Eur. J. Epidemiol 27 (9), 739–756. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez M, 2012. Neonatal abstinence syndrome. Curr. Opin. Pediatr 24 (2), 252–258. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Jones S, Paranjothy S, 2017. Reducing low birth weight: prioritizing action to address modifiable risk factors. J. Public Health 39 (1), 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL, 2009. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur. Arch. Psychiatry Clin. Neurosci 259 (7), 395–412. [DOI] [PubMed] [Google Scholar]
- Knopik VS, 2009. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev. Neuropsychol. 34 (1), 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Marceau K, Palmer RH, Smith TF, Heath AC, 2016. Maternal smoking during pregnancy and offspring birth weight: a genetically-informed approach comparing multiple raters. Behav. Genet 46 (3), 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Tong VT, Bombard JM, Hayes DK, Davy J, Perham-Hester KA, 2018. Marijuana use during and after pregnancy and association of prenatal use on birth outcomes: a population-based study. Drug Alcohol Depend. 187, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, 1987. Determinants of low birth weight: methodological assessment and meta-analysis. Bull. World Health Organ 65 (5), 663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Seguin L, Lydon J, Goulet L, 2000. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr. Perinat. Epidemiol 14 (3), 194–210. [DOI] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Shaw DS, Ganiban J, Natsuaki MN, Reiss D, 2013. The early growth and development study: a prospective adoption study from birth through middle childhood. Twin Res. Hum. Genet 16 (1), 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, VanBeveren TT, 1996. Placental Transfer of Selected Substances of Abuse, Seminars in Perinatology. Elsevier, pp. 147–153. [DOI] [PubMed] [Google Scholar]
- Marceau K, De Araujo-Greecher M, Miller ES, Massey SH, Mayes LC, Ganiban JM, Reiss D, Shaw DS, Leve LD, Neiderhiser JM, 2016. The perinatal risk index: early risks experienced by domestic adoptees in the United States. PLoS One 11 (3), e0l50486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark K, Terplan M, 2017. Cannabis and pregnancy: maternal child health implications during a period of drug policy liberalization. Prev. Med 104, 46–49. [DOI] [PubMed] [Google Scholar]
- Massey SH, Lieberman DZ, Reiss D, Leve LD, Shaw DS, Neiderhiser JM, 2011. Association of clinical characteristics and cessation of tobacco, alcohol, and illicit drug use during pregnancy. Am. J. Addict 20 (2), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Bublitz MH, Magee SR, Salisbury A, Niaura RS, Wakschlag LS, Stroud LR, 2015. Maternal-fetal attachment differentiates patterns of prenatal smoking and exposure. Addict. Behav 45, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Reiss D, Neiderhiser JM, Leve LD, Shaw DS, Ganiban JM, 2016. Maternal personality traits associated with patterns of prenatal smoking and exposure: implications for etiologie and prevention research. Neurotoxicol. Teratol 53, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Decety J, Wisner KL, Wakschlag LS, 2017. Specification of change mechanisms in pregnant smokers for malleable target identification: a novel approach to a tenacious public health problem. Front. Public Health 5 (239). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E, Hatsukami DK, 2016. A review of the additive health risk of cannabis and tobacco co-use. Drug Alcohol Depend. 166, 6–12. [DOI] [PubMed] [Google Scholar]
- Metz TD, Stickrath EH, 2015. Marijuana use in pregnancy and lactation: a review of the evidence. Am. J. Obstet. Gynecol 213 (6), 761–778. [DOI] [PubMed] [Google Scholar]
- Moore C, Negrusz A, Lewis D, 1998. Determination of drugs of abuse in meconium. J. Chromatogr. B Biomed. Sci. Appl 713 (1), 137–146. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Cnattingius S, Gatz M, Neiderhiser JM, Pedersen NL, 2016. Associations between fetal growth and self-perceived health throughout adulthood: a co-twin control study. Behav. Genet 46 (3), 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Sigurdsson S, Kjartansson O, Jonsson PV, Garcia M, von Bonsdorff MB, Gunnarsdottir I, Thorsdottir I, Harris TB, van Buchem M, 2014. Birth size and brain function 75 years later. Pediatrics 134 (4), 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C, 2012. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 494 (1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Salas-Wright CP, Vaughn MG, DiNitto DM, 2017. Marijuana use during pregnancy: a comparison of trends and correlates among married and unmarried pregnant women. Drug Alcohol Depend. 181, 229–233. [DOI] [PubMed] [Google Scholar]
- Park B, Gibbons H, Mitchell M, Glass M, 2003. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta 24 (10), 990–995. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wakschlag LS, Dai L, Leventhal BL, 2003. Fluctuations of maternal smoking during pregnancy. Obstet. Gynecol 101 (1), 140–147. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R, 2005. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr. Perinat. Epidemiol 19 (5), 368–376. [DOI] [PubMed] [Google Scholar]
- Richardson KA, Hester AK, McLemore GL, 2016. Prenatal cannabis exposure - the “first hit” to the endocannabinoid system. Neurotoxicol. Teratol 58, 5–14. [DOI] [PubMed] [Google Scholar]
- Rosen TS, Bateman D, 2010. The Effects of Gender in Neonatal Medicine, Principles of Gender-Specific Medicine, Second edition. Elsevier, pp. 3–17. [Google Scholar]
- Royston P, Altman DG, Sauerbrei W, 2006. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat. Med 25 (1), 127–141. [DOI] [PubMed] [Google Scholar]
- Salimetrics, 2006. In: Salimetrics L (Ed.), High Sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay Kit. State College, PA, pp. 3. [Google Scholar]
- SAMHSA, 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Services, D.o.H.a.H., Rockville, MD. [Google Scholar]
- Shankaran S, Lester BM, Das A, Bauer CR, Bada HS, Lagasse L, Higgins R, 2007. Impact of Maternal Substance Use during Pregnancy on Childhood Outcome, Seminars in Fetal and Neonatal Medicine. Elsevier, pp. 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisler S, Eiden RD, Molnar DS, Schuetze P, Huestis M, Homish G, 2017. Smoking in pregnancy and fetal growth: the case for more intensive assessment. Nicotine Tob. Res 19 (5), 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L, Zhao N, Popp S, Dow-Edwards D, 2012. Prenatal tetrahydrocannabinol (THC) alters cognitive function and amphetamine response from weaning to adulthood in the rat. Neurotoxicol. Teratol 34 (1), 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1996. Timeline Followback user’s guide: A calendar method for assessing alcohol and drug use. Addiction Research Foundation, Toronto. [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, Lester B, Huestis MA, Niaura R, Padbury JF, 2014. Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrinology 48, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Salisbury AL, Phipps MG, Huestis MA, Niaura R, Padbury JF, Marsit CJ, Lester BM, 2016. Epigenetic regulation of placental NR3C1: mechanism underlying prenatal programming of infant neurobehavior by maternal smoking? Child Dev. 87 (1), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, McCallum M, Salisbury A, 2018. Impact of Maternal Prenatal Smoking on Fetal to Infant Neurobehavioral Development. Development and Psychopathology. (Special Issue on Developmental Origins of Psychopathology: Mechanisms, Processes, and Pathways Linking the Prenatal Environment and Postnatal Outcomes.), (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N, 2010. Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend. 109 (1), 243–247. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, Blanco C, 2017. MArijuana use during stages of pregnancy in the United States. Ann. Intern. Med 166 (10), 763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Roddey JC, Erhart M, McCabe C, Akshoomoff N, 2012. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc. Natl. Acad. Sci 109 (49), 20089–20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Roussos-Ross D, Behnke M, 2014. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clin. Perinatol 41 (4), 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE, 1989. Effects of maternal marijuana and cocaine use on fetal growth. N. Engl. J. Med 320 (12), 762–768. [DOI] [PubMed] [Google Scholar]


