Abstract
Objective:
The associations among socioeconomic disadvantage,amygdala volume,and internalizing symptoms in children and adolescentsare unclear and under-studied in the extant literature. In this study, we examined associations between socioeconomic status (SES) and amygdala volume by age across childhood and adolescence to test whether socioeconomic disadvantage would be associated with larger amygdala volume at younger ages, but with smaller amygdala volume at older ages. We then examined whetherSES and amygdala volume were associated with children’s levels of anxiety and depression.
Method:
Participants were3- to 21-year-oldsfrom the Pediatric Imaging, Neurocognition, and Genetics (PING) study (N = 1196),whichincludedstructural magnetic resonance imaging (MRI). A subsample (N = 327; 7 – 21 years of age) completed self-report measures of anxiety and depression.
Results:
Lower family income and parental educationwere significantly associated with smaller amygdala volume in adolescence (13–21 years), but not significantly associated with amygdala volume at younger ages (3–12 years). Lower parental education, but not family income, was significantly associated with higher levels of anxiety and depression, even after accounting for family history of anxiety/depression. Smaller amygdala volume was significantly associated with higher levels of depression, even after accounting for parental education and family history of anxiety/depression.
Conclusions:
These findings suggest that associations between SES and amygdala structure may vary by age.In addition,smaller amygdala volumemay be linked with an increased risk for depression in children and adolescents.
Keywords: socioeconomic status, amygdala, anxiety, depression
Current estimates indicate that more than 1 in 5 children in the United States grow up in poverty (U.S. Census Bureau, 2015). Although socioeconomic disadvantage is known toincreasechildren’s risk for internalizing problems(Wadsworth, Evans, Grant, Carter, & Duffy, 2016), the neural mechanisms underlying this associationare not well-understood. The amygdala plays a central role in emotion processing, particularly threat detection and fear learning(Adolphs, Tranel, Damasio, & Damasio, 1995; Sergerie, Chochol, & Armony, 2008),and is vulnerable to the effects of early life stress(Tottenham & Sheridan, 2009). Although studies have demonstrated associations between socioeconomic status (SES) and amygdala volume in children, the findings have been inconclusive(Hanson et al., 2015; Luby et al., 2013; Noble, Houston, Kan, & Sowell, 2012), possibly due to differences in age or timing of exposure. Thus, the goal of the present research was to examinewhether associations between SES and amygdala volumevary across development, and how SES and amygdala volume relate to internalizing symptoms in children and adolescents.
SES and Internalizing Problems in Children and Adolescents
Anxiety and depression are among the most common types of psychopathology during childhood and adolescence (Merikangas et al., 2010). In general, children’s risk for these internalizing problems varies based on differences in temperament and environmental exposures, including poverty(Hostinar, Nusslock, & Miller, 2017; McLaughlin, 2016). Indeed, socioeconomic disadvantage,including low family income and/or parental educational attainment, has consistently been found to increase children’s risk for internalizing problems(Wadsworth et al., 2016).For instance, in longitudinal studies, early povertyhas been found to predict elevated later risk for internalizing problems, even after accounting for initial levels of symptoms(Ackerman, Brown, & Izard, 2004; Najman et al., 2010; Spence, Najman, Bor, O’Callaghan, & Williams, 2002). In addition, a longer duration of poverty has been linked with greater risk for internalizing problems(Slopen, Fitzmaurice, Williams, & Gilman, 2010). These findings raise questions about theways in which socioeconomic disadvantage may influence brain development and in turn increase children’s risk for internalizing problems.
SES and Amygdala Volume in Children and Adolescents
SES tends to reflect the quality of multiple aspects of children’s environments, including their level of exposure to chronic stress. Children in lower-SES families are more likely to face numerous stressors,including neighborhood violence, chaotic households, and family turmoil (Evans & Kim, 2013). It has been theorized that poverty-related stressors impact the hypothalamic-pituitary-adrenal (HPA) axis stress response and alter development of brain structures with high concentrations of glucocorticoid receptors, such as the amygdala (Noble et al., 2012).Several structural neuroimaging studies have shown associations betweensocioeconomic factorsand amygdala volume in children, but the directionality of the associations has been inconsistent. Two studies found socioeconomic disadvantage to be associated with smaller amygdala volume (Hanson et al., 2015; Luby et al., 2013) whereas another one found it to be associated with larger amygdala volume (Noble et al., 2012).Other studies have failed to find significant SES-related differences in amygdala structure in children (Hanson, Chandra, Wolfe, & Pollak, 2011; Noble et al., 2015). In particular, in a prior study using the same large sample as the one used here, there were no significant associations between family income or parental education and amygdala volume (Noble et al., 2015). Similardiscrepancies in directionality can be found in the literature linking child maltreatment and early institutionalization with altered amygdala volume (Hanson et al., 2015).
Although there are multiple possible explanations for these discrepancies, including differences in sample characteristics or other methodological factors, one intriguing possibility is that such discrepancies arosedue to differences in the length of time between when the stressor was experienced and when amygdala volume was measured. One possibility, which we term the “acceleration-deceleration hypothesis,”is that initial exposure to chronic stress causes accelerated growth of the amygdala, leading to increased amygdala volume relative to children of the same age. However, over time, stress-relatedheightened amygdala reactivity andvolumetric increasesmay lead to cell death and slower amygdala growth, resulting in smaller amygdala volumes relative to age-matched peers (Tottenham & Sheridan, 2009). This hypothesis has been supported in animal models. For example, rodent studies have revealed that chronic stress, especially early in life, induces neuronal hypertrophyand volumetric increases in the amygdala (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2009). Similarly, functional MRI studies of children exposed to early life stress have shown increased amygdala reactivity to negative emotional stimuli (Tottenham et al., 2011). Thus, long-term exposure to socioeconomic disadvantagein childhood may be ultimately linked withrelatively smaller amygdala volume.
Amygdala Volume and Internalizing Symptoms in Children and Adolescents
Associations between SES and amygdala volume may be relevant for understanding socioeconomic differences in children’s risk for internalizing problems.Indeed, the amygdala has long been a target of investigation for researchers seeking to understand the biological basis of internalizing disorders. However, associations between amygdala volume and internalizing problems in children and adolescents are inconsistent. Children and adolescents with anxiety disorders or major depressive disorder tend to differ in their amygdala structure compared to typically-developing control children, but the directionality of these associationshas been inconsistent. Some studies indicate that children and adolescents with mixed anxiety disorders (Milham et al., 2005; Mueller et al., 2013; Strawn et al., 2015) or major depressive disorder (Rosso et al., 2005)have smaller amygdala volumes.However,other studies indicated that youth with generalized anxiety disorder (De Bellis et al., 2000)and children with higher parent-reported anxiety (Qin et al., 2014)and fearfulness (van der Plas, Boes, Wemmie, Tranel, & Nopoulos, 2010)have larger amygdala volumes. In addition, one longitudinal study found that there was increased growth of the amygdaladuring adolescence associated with depression in girls anddecreased growth in boys (Whittle et al., 2014). Finally, other studieshave not observed structural differences in the amygdala in youth with major depressive disorder (Caetano et al., 2007; MacMaster et al., 2008; Pannekoek et al., 2014)or subthreshold depression (Vulser et al., 2015), or in typically-developing youth varying in parent-reported internalizing symptoms (Koolschijn, van IJzendoorn, Bakermans-Kranenburg, & Crone, 2013).
It is not clear what explains these discrepancies, although multiple suppositions have been made, including arguments about timing. For instance, it has been noted that initial episodes of major depression may lead to increased amygdala volume and reactivity, but after repeated depressive episodes amygdala volume may start to decrease (McEwen, 2003). This pattern has been observed in the literature on major depressive disorder in adults(Campbell & MacQueen, 2006; Frodl, Koutsouleris, Bottlender, Born, Jäger, Mörgenthaler, et al., 2008; Frodl, Koutsouleris, Bottlender, Born, Jäger, Scupin, et al., 2008; Schmaal et al., 2016; Sheline, 2000).
Most of this research has used a categorical approach, comparing diagnostic groups with control groups. Studies that employ a dimensional approach, examining associations of amygdala volume with continuous variability in levels of anxiety and depression in children may complement the existing literature and help to clarify these discrepancies(Fox et al., 2015; Garvey, Avenevoli, & Anderson, 2016). The dimensional approach has been advocated in light of increasing recognition of heterogeneity and comorbidity within diagnostic categories, and the notion that a dimensional understanding may address these issues and ultimately improve treatments. In addition, recent work indicates that dimensional scaling produces measures that evidence greater estimates of reliability and validity compared to categorical scaling (Markon, Chmielewski, & Miller, 2011). In sum, there is evidence to suggest that differences in amygdala structure may be linked with continuous variability in internalizing symptoms in children, but it is difficult to specify directionality. By middle childhood through late adolescence,smaller amygdala volumemay be linked with higher internalizing symptoms.
Current Study
In the current study, we investigated(a) associations between SES and amygdala volume by age and (b) associations of SES andamygdala volume with continuous variability in internalizing symptoms. The sample consisted of children and adolescents from the Pediatric Imaging, Neurocognition, and Genetics (PING) study, which includedstructural MRI and self-report measures ofanxiety and depression (Jernigan et al., 2016). First, using the entire PING sample of 3- to 21-year-olds (N = 1196), we examined whether associations between SES indices (family income, parental education) and amygdala volume varied by age. Family income and parental education were examined separately because they represent distinct aspects of children’s environments that have different roles in their development (Duncan & Magnuson, 2012).Based on a review of previous work (Tottenham & Sheridan, 2009), we hypothesized that lower SES would be associated with larger amygdala volume at younger ages (early childhood) but withsmaller amygdala volume at older ages (adolescence). This study builds on previous work on SES and amygdala volume, including a studyconducted on the PING sample (Noble et al., 2015), by focusingon the role of age as a moderator.
Then, using a PING subsample of 7- to 21-year-olds who completed anxiety and depression measures (N = 327), we examined associations of SES and amygdala volume with levels of anxiety and depression. We hypothesized that lower SES and smaller amygdala volume would be associated with higher levels of anxiety and depression. All analyses utilized a comprehensive set of covariates, including family history of anxiety/depression.Genetic factors have been found to contribute to risk for anxiety and depression and to amygdala structure (Chai et al., 2015), and thus represent a potential confound when examining associations of SES with internalizing symptoms and amygdala volume. Higher levels of internalizing problems or differences in amygdala volume may be due to a genetic predisposition rather than poverty-related factors, such as chronic stress. Yet, previous studies have not controlled for familial contributions to these outcomes. To address this, we controlled for family history of anxiety/depression in all analyses.In addition, includinggenetic ancestry as a covariate in analyses allowed us a precise means of controlling for the confounding of race and SES in the United States(Akshoomoff et al., 2014; Chen, Martin, & Matthews, 2006).
Method
Participants
As described previously(Akshoomoff et al., 2014; Jernigan et al., 2016; Noble et al., 2015), the PING study recruited 3- to 21-year-old participants through a combination of web-basedand community advertising at nine university-based data collection sites in and around the cities of Los Angeles, San Diego, New Haven, Sacramento, Boston, Baltimore, Honolulu, and New York (http://ping.chd.ucsd.edu). Exclusionary criteriaincludedneurological disorder; history of head trauma; preterm birth; autism spectrum disorder, bipolar disorder, schizophrenia, or intellectual disability; and contraindications for MRI (e.g., braces). Written informed consent was provided by parents for all participants < 18 years of age and by the participants themselves if they were ≥ 18 years of age. In addition, child assent was obtained for 7- to 17-year-old participants. Each site’s Institutional Review Board approved the study.
Analyses of SES by age effects utilized the whole PING sample with data on the relevant variables (N = 1196 for parental education models and N = 1185 for family income models). However, other analysesin this study focused on the subsampleof participants who completed web-based, self-report assessments of depression and anxiety from the PhenX Toolkit (https://www.phenxtoolkit.org) (Jernigan et al., 2016; McCarty et al., 2014) (N = 327). Six of the nine PING sites chose to administer the PhenX assessments, and only participants ≥ 8 years of age completed the depression and anxiety measures.The PhenX questionnaires were often completed at a later date, after completion of neuroimaging(McCarty et al., 2014).The time between when participants completed neuroimaging to when they completed the PhenX battery (M = 1.17 years; SD = .67) was not a significant covariate in any of the analyses.Multilevel models (accounting for the nesting of participants within site) indicated that there were no significant differences in age, sex, genetic ancestry, or parental education between this subsample and the sample of participants who were eligible but did not complete the PhenX questionnaires. However, those who completed the PhenX battery came from higher income families compared to those who did not, β = .21, p = .01.
Full sample characteristicsare provided in Table 1. The sample (52% male) ranged in age from 7 to 21 years at neuroimaging and 8 to 22 years at PhenX completion. Note that one older 20-year-old had turned 21 by the completionof neuroimaging (Jernigan et al., 2016).
Table 1.
Descriptive statistics for study variables
| N | M(SD) or n (%) | Range | |
|---|---|---|---|
| Age at neuroimaging (years) | 327 | 13.65(3.62) | 7.08–21.00 |
| Age at PhenX completion (years) | 323 | 14.80(3.91) | 8.25–22.67 |
| Sex (male) | 327 | 171(52%) | -- |
| Parental education (years) | 308 | 15.05(2.26) | 6.00–18.00 |
| Family income (U.S. dollars) | 313 | 104,287.54(76,810.78) | 4,500.00–325,000.00 |
| Genetic ancestry factor (GAF) | 312 | ||
| African | .11(.24) | 0–1.00 | |
| American Indian | .06(.14) | 0-.80 | |
| Central Asian | .03(.13) | 0–1.00 | |
| East Asian | .20(.34) | 0–1.00 | |
| European | .59(.37) | 0–1.00 | |
| Oceanic | .01(.03) | 0-.23 | |
| Family history of anxiety/depression | 326 | .74(1.30) | 0–6.00 |
| Scanner model | 296 | ||
| Philips Achieva | 24(8%) | -- | |
| GE Discovery | 36(12%) | -- | |
| GE Signa | 47(16%) | -- | |
| Siemens TrioTim | 189(64%) | -- | |
| Total amygdala volume (mm3) | 296 | 3354.89 (531.71) | 2112.00–6045.00 |
| Anxiety symptoms | 327 | 28.85(17.38) | 0–100.32 |
| Depression symptoms | 327 | 13.55(10.07) | 0–59.00 |
Note.GAF data show mean, standard deviation, and range across all subjects of the estimated proportion of genetic ancestry for each reference population. For anxiety and depression, SCARED-R and CES-DC total scoresare presented.U.S., United States.
Image Acquisition and Processing
Each site administered a standardized high-resolution structural MRI protocol, using 3T scanners (see Table S1 for scanner models and parameters). Details of the image acquisition and processing protocols have been provided previously(Brown et al., 2012). At the University of California, San Diego (UCSD), the protocol included a sagittal 3D inversion recovery spoiled gradient echo T1-weighted volume optimized for maximum gray/white matter contrast (see parameters in Table S1). Acquisition protocols with pulse sequence parameters identical or near identical to those used at UCSD were installed on scanners at the other eight sites. T1-weighted imaging data included in this study passed standardized quality control procedures, including visual inspection ratings by trained imagingtechnicians and automated quality control algorithms. Morphometric analysis of structural MRI datawas performed using a specialized processing stream and FreeSurfer(http://surfer.nmr.mgh.harvard.edu/). Subcortical structures were labeled using an automated, atlas-based, volumetric segmentation procedure (Fischl et al., 2002). Volumes in mm3 were calculated for each structure.
Measures
Socioeconomic status.
The PING Study Demographics and Child Health History Questionnaire was completed by parents for participants < 18 years and by the participants themselves if they were≥ 18 years of age. Via this questionnaire, the level of educational attainment was reported for parents in the home. The average parental educational attainment was used in all analyses. Total yearly family income was also reported. Both education and income data were originally collected in bins, which were recoded as the means of the bins for analysis (see TableS2). Family income was log-transformed for all analyses due to the typically observed positive skew. As expected, parental education and family income were highly correlated (r = .56, p< .001).
Genetic ancestry factor (GAF).
Details of genetic ancestry assessment have been provided previously (Akshoomoff et al., 2014; Jernigan et al., 2016). In brief, genome-wide genotypingwas performed on the extracted DNA using the IlluminaHuman660W-Quad BeadChip. Replication and quality control filters (i.e., sample call rate>99%, call rates >95%, minor allele frequency>5%) were performed (Bakken et al., 2012). To assess geneticancestry and admixture proportions in the PING participants, a supervisedclusteringapproach implemented in the ADMIXTURE software was used (Alexander & Lange, 2011). Using this approach, GAFs were estimated for each participant, representing the proportion of ancestral descent foreach of six major continental populations: African, Central Asian, East Asian, European,Native American and Oceanic. Implementation of ancestry and admixture proportions in thePING subjects is described in detail elsewhere(Fjell et al., 2012). Previous PINGstudies have shown strong correlations between genetically-determined and self-reported ancestry (Akshoomoff et al., 2014).
Family history of anxiety/depression.
On the Demographics and Child Health History Questionnaire,respondent sindicated whether the following family members had a history of anxiety or depression: maternal and paternal grandmother/grandfather, biological mother/father, maternal and paternal aunt/uncle, male/female sibling. These responses were summed to create a measure of the total number of family positions with a known history of anxiety or depression. This variable was log-transformed to correct for a positively skewed distribution.
Depression.
Participants completed the Center for Epidemiologic Studies Depression Scale for Children (CES-DC) (Fendrich, Weissman, & Warner, 1990), a 20-item self-report measure.Participants rate each item in terms of frequency during the last week using a 4-point scale, ranging from0 (not at all) to 3 (a lot). Higher scores indicatehigher levels of depressive symptoms.The CES-DChasstrong psychometric properties(Myers & Winters, 2002).
Anxiety.
Participants completed the Screen for Child Anxiety Related Emotional Disorders-Revised (SCARED-R) (Muris, Dreessen, Bögels, Weckx, & van Melick, 2004), a 66-item self-report measureofanxiety disorder symptoms (e.g., generalized anxiety disorder). Participants rated how frequently they experience each item on a 3-point scale, ranging from 0 (almost never)to 2 (often). Higher scores reflect higher levels of anxiety symptoms. The SCARED-R has demonstrated high reliability and validity (Muris et al., 2004).
Analysis Plan
Analyses were conducted using SAS software (version 9.3). In the total PING sample ranging in age from 3 to 21 years, we examined associations between SES indices (family income, parental education) and amygdala volume by age. We used multiple linear regression to examine interactions between SES indices and age for amygdala volume. In addition, regression models were employed to examine associations between SES indices and amygdala volume separately in early childhood, middle childhood, and adolescence (3–6, 7–12, and 13–21 years, respectively), to test the hypothesis of an inverse association in early childhood and a positive association in adolescence. Using the PING subsample with anxiety and depression data (7–21 years), regression was used to examine the associations of SES indices with children’s levels of anxiety and depression. Using this same subsample, associationsofamygdala volume with levels of anxiety and depression were examined using regression.
Covariates.
All regression analyses controlled for age, sex, and family history of anxiety/depression. In addition, all regression analyses involving amygdala volume adjusted for whole brain volume and scanner model. GAFsand site were not significant in any of the models and thus were not included in final models.
Past work has suggested that in certainbrain regions, a quadratic term for age may be moreappropriate than a linear term alone (Ostby et al., 2009).Thus,age2was included in analyses predicting amygdala volume. Note that age2 did not account for significant variance in anxiety or depression and thus was not included inthosemodels.
Results
Descriptive statistics are provided in Table 1, andzero-order correlationsare provided in Table 2. Lower family income and parental education were significantly associated with smaller amygdala volume. Lower parental education, but not family income, was significantly associated with higherlevels of depression symptoms. Smaller amygdala volume was significantly associated with higherlevels of anxiety symptoms.
Table 2.
Zero-order correlations for SES indices, family history of anxiety/depression, amygdala volume, and internalizing symptoms
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| 1. | Family income | -- | |||||
| 2. | Parental education | .56*** | -- | ||||
| 3. | Family history of anxiety/depression | .01 | –.01 | -- | |||
| 4. | Amygdala volume | .14*** | .10*** | .03 | -- | ||
| 5. | Anxiety | –.08 | –.10 | .03 | –.16** | -- | |
| 6. | Depression | –.07 | –.12* | .14* | –.10 | .56*** | -- |
p< 05;
p< .01;
p< .001
Associations between SES and Amygdala Volume by Age
Regression analyses using the entire sample (age range: 3–21 years) indicated that interactions between SES indices and age were not significant for amygdala volume.Results for associations between SES and amygdala volume within each age group (early childhood, middle childhood, and adolescence) are shown in Table 3 and Figure 1. As shown in Figure 1, for all participants, amygdala volume tended to increase with ageuntil adolescence when it began to plateau, consistent with previous reports(Ostby et al., 2009). Thepattern of results for associations between SES and amygdala volume was partiallyconsistent with our expectations. During early childhood (3–6 years), associations between SES indices and amygdala volume were not significant.During adolescence(13–21 years), lower family income (p< .05) and parental education (p< .01) were significantly associated with smaller amygdala volume.
Table 3.
Associations of family income and parental education with amygdala volume in early childhood, middle childhood, and adolescence
|
Model 1: Amygdala volume |
||||||
|
3–6 years |
7–12 years |
13–21 years |
||||
|
β |
p |
β |
p |
β |
p |
|
| Whole brain volume | .46 | <.0001 | .47 | <.0001 | .45 | <.0001 |
| Age | −.86 | .6363 | .11 | .4661 | .01 | .9834 |
| Age2 | −.59 | .3887 | −.17 | .3998 | .01 | .9257 |
| Sex | −.16 | .0272 | −.08 | .1163 | −.17 | .0013 |
| Family history of anxiety/depression | −.03 | .3025 | −.02 | .3979 | .02 | .3002 |
| Family income | −.05 | .1706 | −.004 | .8758 | .04 | .0444 |
|
Model 2: Amygdala volume |
||||||
|
3–6 years |
7–12 years |
13–21 years |
||||
|
β |
p |
β |
p |
β |
p |
|
| Whole brain volume | .43 | <.0001 | .48 | <.0001 | .44 | <.0001 |
| Age | −.55 | .7633 | .12 | .4425 | .01 | .9579 |
| Age2 | −.49 | .4728 | −.12 | .5669 | −.01 | .9630 |
| Sex | −.19 | .0107 | −.09 | .1162 | −.18 | .0007 |
| Family history of anxiety/depression | −.02 | .4580 | −.01 | .5167 | .03 | .2010 |
| Parental education | .03 | .4627 | −.01 | .6823 | .06 | .0060 |
Note. These regression analysesalso adjusted for scanner model. Sample sizes for family income models were 218, 466, and 501, respectively. Sample sizes for parental education models were 220, 463, and 513, respectively.
Figure 1.
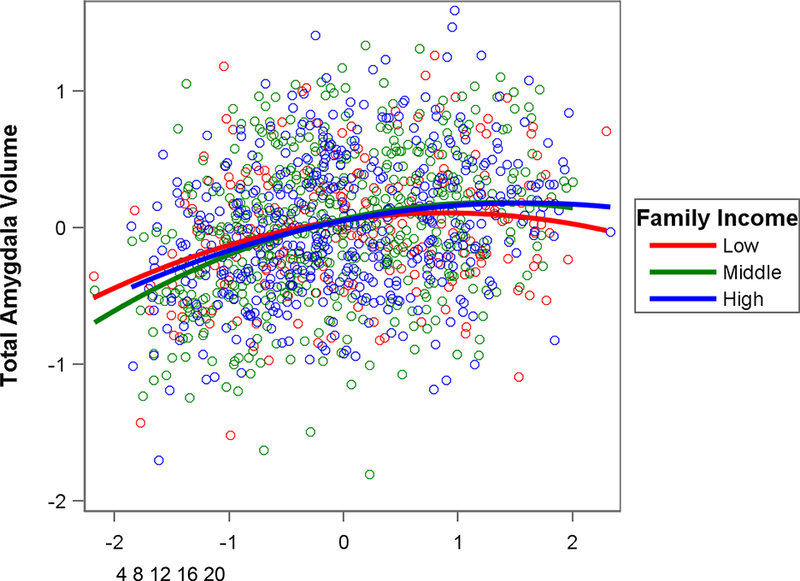
Partial regression plot showing association between age and total amygdala volume by family income, controlling for whole brain volume, sex, and scanner model. Lower family income was significantly associated with smaller amygdala volume during adolescence (see Table 3).For interpretation purposes, the tick marks for age display ages in years corresponding to the standardized residuals for age. Family income was a continuous variable in analyses but is displayed in ecologically-valid family income groups (Low = $4,500 – $25,000; Middle = $35,000 – $75,000; High = $125,000 – $325,000).
For the family income models, the regression coefficients for early childhood (β = −.05) vs. adolescence (β = .04) differed significantly, t(715) = 2.50, p = .01.For the parental education models, these regression coefficients (β = .03 and .06, respectively) did not differ significantly, t(729) = .67, p = .50.
Associations between SES and Internalizing Symptoms
Lower parental education (but not family income) was significantly associated with higher anxiety (p = .03) and depression(p = .03) in children and adolescents (7–21 years) after accounting for age, sex, and family history of anxiety/depression (see Table 4). Thus, we controlled for parental education in the following analyses examining associations of amygdala volume with internalizing symptoms.
Table 4.
Associations of family income and parental educationwith internalizing symptoms
|
Model 1 |
||||||
|
Anxiety |
Depression |
|||||
|
β |
t |
p |
β |
t |
p |
|
| Age | −.08 | −1.30 | .1932 | .07 | 1.06 | .2892 |
| Sex | .37 | 3.35 | .0009 | .17 | 1.54 | .1241 |
| Family history of anxiety/depression | .04 | .74 | .4575 | .16 | 2.85 | .0046 |
| Family income | −.09 | −1.47 | .1428 | −.07 | −1.11 | .2660 |
|
Model 2 |
||||||
|
Anxiety |
Depression |
|||||
|
β |
t |
p |
β |
t |
p |
|
| Age | −.09 | −1.33 | .1842 | .07 | 1.14 | .2549 |
| Sex | .40 | 3.59 | .0004 | .18 | 1.58 | .1159 |
| Family history of anxiety/depression | .05 | .88 | .3804 | .15 | 2.73 | .0067 |
| Parental education | −.13 | −2.24 | .0258 | −.13 | −2.18 | .0298 |
Note. Sex: 1 = female, 0 = male.
Associations between Amygdala Volume and Internalizing Symptoms
Smalleramygdala volume was significantly associated with higher levels ofdepressive symptoms (p = .03),but not anxiety symptoms,after adjusting for parental education,whole brain volume, age, sex, family history of anxiety/depression, and scanner model(see Table 5). Parental education was no longer significant in either regression model after amygdala volume was added.These results remained unchanged when analyses were run without controlling for family history of anxiety/depression or parental education.
Table 5.
Associations of amygdala volume with internalizing symptoms
| Anxiety |
Depression |
|||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| Whole brain volume | .19 | 2.01 | .0452 | .21 | 2.31 | .0218 |
| Age | −.04 | −.63 | .5261 | .05 | .81 | .4209 |
| Sex | .53 | 3.76 | .0002 | .20 | 1.39 | .1645 |
| Family history of anxiety/depression | .03 | .53 | .5982 | .16 | 2.73 | .0068 |
| Parental education | −.09 | −1.54 | .1237 | −.11 | −1.91 | .0576 |
| Amygdala volume | −.15 | −1.39 | .1670 | −.23 | −2.12 | .0347 |
Note. These regression analyses accounted for scanner model.
Sex: 1 = female, 0 = male.
We ran parallel analyses with the hippocampus as a check for specificity. Unlike in the amygdala, we found no differences in SES associations with hippocampal volume by age. Further, while both lower parental education (β = −.12, p = .04) and lower hippocampal volume (β = −.16, p = .04) were associated with increased symptoms of depression, both of these variables remained significant in the model, consistent with the possibility that hippocampal differences were not fully accounting for socioeconomic differences in depressive symptoms.
Discussion
The goals of this study of children and adolescents wereto examine (1) associations between SES and amygdala volume by age and (2) associations of SES and amygdala volume withinternalizing symptoms. To our knowledge, this is the first study to address this set ofquestions. Some previous studies have linked lower SES with smaller amygdala volume (Hanson et al., 2015; Luby et al., 2013)whereasothers have linked lower SES with larger amygdala volume(Noble et al., 2012).In this study, we testedwhether these discrepancies may be due to age differences, as previouslytheorized(Tottenham & Sheridan, 2009).Specifically, we tested the a priori hypothesis of an inverse association between SES and amygdala volume in early childhood and a positive association between SES and amygdala volume in adolescence. Interactions between SES indices (family income, parental education) and age were not significant, possibly becauseof the truncated age range of the sample (i.e., not including children younger than 3 years) or to a threshold effect (e.g., step function) at a certain age.We then conducted separate regression analyses by age group.Results indicated non-significant associations between SES indices and amygdala volumein early and middle childhood (3–12 years) and significantpositive associationsin adolescence (13–21 years). Theseresultssuggest thatadolescents from disadvantaged familiesmay have smaller amygdala volumes than their peers from more advantaged families.
Disadvantaged environments are often characterized by chronic stressors, including crowding/noise, dangerous neighborhoods, household chaos and unpredictability, and lower parental responsiveness (Evans & Kim, 2013). Stressors such as these may have a particularly strong impact on the amygdala, which is closely involved in fear processing, detecting perceived threats, and triggering the HPA axis stress response (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2009). In the context of chronic stress, the amygdala may initially increase and show greater reactivity but then begin to diminish in size with continued exposure over time(Tottenham & Sheridan, 2009). Thus, amygdala hyperactivity may lead to this pattern of “acceleration” followed by “deceleration”. Initial exposure to chronic stress may lead to amygdala hyperactivity and neuronal hypertrophy (e.g., increased dendritic arborization), but after prolonged exposure (by adolescence), chronic hyperactivity might lead to excitotoxic changes and amygdala atrophy(Nacewicz et al., 2006). Adolescentsfrom disadvantaged environments may have accrued histories of long-term exposure to chronic stress, which explains their smaller amygdala volume, relative to their more advantaged peers.Longitudinal studies are needed to formally test this hypothesis and to elucidate the way in which SES may modify trajectories of amygdala development during childhood and adolescence.
In a subsample of 7- to 21-year-olds for whom anxiety and depression data were available, lower parental education, but not family income, was associated with higher levels of anxiety and depression. These associations remained significant even after accounting for family history of anxiety/depression, lending some support to the notion that SES-related factors, such as chronic stress, may be driving these results. Family income and parental education may reflect distinct aspects of children’s environments. Family incomemay more directlyreflect the physical resources available to the family,whereasparental educationmay more directly reflectparenting style and the quality of parent-child interactions(Duncan & Magnuson, 2012).
Findings also indicated that smaller amygdala volumewas significantly associated with higher levels of depression in children and adolescents, even after accounting for parental education and family history of anxiety/depression. The association of smaller amygdala volume with elevateddepressive symptoms was consistent with some prior studies (Milham et al., 2005; Mueller et al., 2013; Rosso et al., 2005; Strawn et al., 2015)but not others(De Bellis et al., 2000; Qin et al., 2014). It is possible that due to the older age of the sample (7–21 years), participants had experienced these symptoms for a prolonged period of time, leading to relatively reduced rather than increased amygdala volume (McEwen, 2003). Most of the previous studies have focus on diagnostic groups. This study provides insight into these associations for dimensionally-scaled anxiety and depression.
Taken together, these findings are consistent with the hypothesis that differences in amygdala development may partially explain the higher levels of depressive symptoms often found among children from disadvantaged families,consistent with a recent functional neuroimaging study (Barch et al., 2016).However, longitudinal studies are needed to formally test such a mediation model(Cole & Maxwell, 2003; Maxwell & Cole, 2007). In terms of clinical implications, it is possible that early preventative interventions targetingemotion management techniques may lead to a reduced risk of internalizing problems among children from disadvantaged families. Since socioeconomic disadvantage may alter children’s brain development prior to the onset of observable internalizing problems, prevention efforts provided early in childhood and potentially targeting reductions in exposure to poverty-related stressors and training in emotion regulation may be very important.
Results from this study should in no way be interpreted to mean that structural differences in the amygdala represent a full explanation for the link between socioeconomic disadvantage andincreased risk for internalizing problems in children. We did not test for regional specificity and thus cannot say whether differences in the amygdala would emerge above and beyond all other brain regions. The amygdala is likely part of a larger neural network (e.g., hippocampus, medial prefrontal cortex) that is impacted by early exposure to socioeconomic disadvantage and also implicated as a mechanistic pathway in internalizing problems. Research is needed to further identifythe ways in which structural and functional connectivity with the amygdala may be altered by socioeconomic disadvantageand linked with higherinternalizing symptoms during childhood. For instance, less efficient prefrontal cortex regulation of the amygdala has been found in adults who grew up in poverty(Kim et al., 2013).
This study had a number of strengths including a large sample size and a comprehensive statistical approach, which included a broad set of covariates. In addition, the use of the PING sample increases the generalizability of these findings. There were also limitations to this study that should be taken into account when interpreting the findings. This was a cross-sectional, correlational study, and such research designs do not allowinferences about developmental trajectories or causality. Also, the anxiety and depression data were only collected for a subsample. In addition, data were not available on variability in exposure to specific poverty-related stressors (e.g., neighborhood violence, family turmoil), and thus we cannot specify which SES-related risk factors might be driving these associations. Given that anxiety and depression were measured solely via self-report, we were unable to examine consistency in reporting across multiple informants(De Los Reyes et al., 2015).Although it is possible that the non-significant associations between SES and amygdala volume for younger children reflect age-related differences in technical artifacts (e.g., differences in the quality of FreeSurfer segmentation, greater registration error in younger children), it is important to note that a rigorous quality control protocol was employed in the PING study(Jernigan et al., 2016).Also, although we took care to limit the number of comparisons and tested a priori hypotheses, it should be noted that results were not strictly corrected for multiple comparisons. Finally, although this was not a clinical sample, it is possible that medication use (e.g., antidepressants) was ongoing in some of the participants in this sample.
In this study, socioeconomic disadvantagewas linked with smaller amygdala volumes in adolescentsbut not childrenbetween 3–12 years, consistent with the notion that these associations may differ by timing or duration of exposure. Lower parental education, but not family income, was associated with higher levels of anxiety and depression, even after accounting for family history of anxiety/depression. Smaller amygdala volumes were significantly associated with higher levels of depressive symptoms, even after accounting for parental education and family history of anxiety/depression. Prevention efforts that combat economic inequality and reduce children’s exposure to poverty-related stressors may reduce their risk for internalizing problems and improve their chances of healthy emotional and behavioral development.
Supplementary Material
Acknowledgments
Data used in preparation of this article were obtained from the Pediatric Imaging, Neurocognition, and Genetics Study (PING) database (http://ping.chd.ucsd.edu). As such, the investigators within PING contributed to the design and implementation of PING and/or provided data but did not participate in analysis or writing of this report. A complete listing of PING investigators can be found at https://pingdataportal.ucsd.edu/sharing/Authors10222012.pdf.
Funding
Data collection and sharing for this projectwas funded by the Pediatric Imaging, Neurocognition,and Genetics Study (PING) (National Institutes ofHealth Grant RC2DA029475). PING is funded by theNational Institute on Drug Abuse and the EuniceKennedy Shriver National Institute of Child Health &Human Development. PING data are disseminatedby the PING Coordinating Center at the Center forHuman Development, University of California, SanDiego.This work was also made possible by fundingfrom the Annie E. Casey Foundation; TeachersCollege, Columbia University; and a National Institute ofMental Health (NIMH) training grant (T32MH13043).
References
- Ackerman BP, Brown ED, & Izard CE (2004). The relations between contextual risk, earned income, and the school adjustment of children from economically disadvantaged families. Developmental Psychology, 40(2), 204–216. 10.1037/0012-1649.40.2.204 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, & Damasio AR (1995). Fear and the human amygdala. The Journal of Neuroscience, 15(9), 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, … Jernigan TL (2014). The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology, 28(1), 1–10. 10.1037/neu0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DH, & Lange K (2011). Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics, 12, 246 10.1186/1471-2105-12-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Roddey JC, Djurovic S, Akshoomoff N, Amaral DG, Bloss CS, … Dale AM (2012). Association of common genetic variants in GPCPD1 with scaling of visual cortical surface area in humans. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3985–3990. 10.1073/pnas.1105829109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, … Luby J (2016). Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School-Age Depression. The American Journal of Psychiatry, 173(6), 625–634. 10.1176/appi.ajp.2015.15081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, … Dale AM (2012). Neuroanatomical assessment of biological maturity. Current Biology: CB, 22(18), 1693–1698. 10.1016/j.cub.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano SC, Fonseca M, Hatch JP, Olvera RL, Nicoletti M, Hunter K, … Soares JC (2007). Medial temporal lobe abnormalities in pediatric unipolar depression. Neuroscience Letters, 427(3), 142–147. 10.1016/j.neulet.2007.06.014 [DOI] [PubMed] [Google Scholar]
- Campbell S, & MacQueen G (2006). An update on regional brain volume differences associated with mood disorders. Current Opinion in Psychiatry, 19(1), 25–33. 10.1097/01.yco.0000194371.47685.f2 [DOI] [PubMed] [Google Scholar]
- Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, … Gabrieli JDE (2015). Functional and structural brain correlates of risk for major depression in children with familial depression. NeuroImage: Clinical, 8, 398–407. 10.1016/j.nicl.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Martin AD, & Matthews KA (2006). Understanding Health Disparities: The Role of Race and Socioeconomic Status in Children’s Health. American Journal of Public Health, 96(4), 702–708. 10.2105/AJPH.2004.048124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, & Maxwell SE (2003). Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology, 112(4), 558–577. 10.1037/0021-843X.112.4.558 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. 10.1016/S0006-3223(00)00835-0 [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DAG, Burgers DE, & Rabinowitz J (2015). The Validity of the Multi-Informant Approach to Assessing Child and Adolescent Mental Health. Psychological Bulletin, 141(4), 858–900. 10.1037/a0038498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, & Magnuson K (2012). Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science, 3(3), 377–386. 10.1002/wcs.1176 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2013). Childhood Poverty, Chronic Stress, Self-Regulation, and Coping. Child Development Perspectives, 7(1), 43–48. 10.1111/cdep.12013 [DOI] [Google Scholar]
- Fendrich M, Weissman MM, & Warner V (1990). Screening for Depressive Disorder in Children and Adolescents: Validating the Center for Epidemiologic Studies Depression Scale for Children. American Journal of Epidemiology, 131(3), 538–551. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, … Gruen J (2012). Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences of the United States of America, 109(48), 19620–19625. 10.1073/pnas.1208243109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, … Kalin NH (2015). Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences of the United States of America, 112(29), 9118–9122. 10.1073/pnas.1508593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, … Meisenzahl EM (2008). Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry, 13(12), 1093–1101. 10.1038/mp.2008.62 [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, … Meisenzahl EM (2008). Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry, 65(10), 1156–1165. 10.1001/archpsyc.65.10.1156 [DOI] [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, & Anderson K (2016). The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. Journal of the American Academy of Child & Adolescent Psychiatry, 55(2), 93–98. 10.1016/j.jaac.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between Income and the Hippocampus. PLOS ONE, 6(5), e18712 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ (2015). Behavior Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Nusslock R, & Miller GE (2017). Future Directions in the Study of Early-Life Stress and Physical and Emotional Health: Implications of the Neuroimmune Network Hypothesis. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 1–15. 10.1080/15374416.2016.1266647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ Jr., Akshoomoff N, Bartsch H, Newman E, … Dale AM (2016). The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. NeuroImage, 124, Part B, 1149–1154. 10.1016/j.neuroimage.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, van IJzendoorn MH, Bakermans-Kranenburg MJ, & Crone EA (2013). Hippocampal volume and internalizing behavior problems in adolescence. European Neuropsychopharmacology, 23(7), 622–628. 10.1016/j.euroneuro.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, … Rosenberg DR (2008). Amygdala and Hippocampal Volumes in Familial Early Onset Major Depressive Disorder. Biological Psychiatry, 63(4), 385–390. 10.1016/j.biopsych.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, & Miller CJ (2011). The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychological Bulletin, 137(5), 856–879. 10.1037/a0023678 [DOI] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12(1), 23–44. 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- McCarty CA, Huggins W, Aiello AE, Bilder RM, Hariri A, Jernigan TL, … Junkins HA (2014). PhenX RISING: real world implementation and sharing of PhenX measures. BMC Medical Genomics, 7, 16 10.1186/1755-8794-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. 10.1016/S0006-3223(03)00177-X [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future Directions in Childhood Adversity and Youth Psychopathology. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 45(3), 361–382. 10.1080/15374416.2015.1110823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, … Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biological Psychiatry, 57(9), 961–966. 10.1016/j.biopsych.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, & Ernst M (2013). Grey Matter Volume in Adolescent Anxiety: An Impact of the Brain-Derived Neurotropic Factor Val66Met Polymorphism? Journal of the American Academy of Child and Adolescent Psychiatry, 52(2), 184–195. 10.1016/j.jaac.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Dreessen L, Bögels S, Weckx M, & van Melick M (2004). A questionnaire for screening a broad range of DSM-defined anxiety disorder symptoms in clinically referred children and adolescents. Journal of Child Psychology and Psychiatry, 45(4), 813–820. 10.1111/j.1469-7610.2004.00274.x [DOI] [PubMed] [Google Scholar]
- Myers K, & Winters NC (2002). Ten-Year Review of Rating Scales. II: Scales for Internalizing Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 41(6), 634–659. 10.1097/00004583-200206000-00004 [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, … Davidson RJ (2006). Amygdala Volume and Nonverbal Social Impairment in Adolescent and Adult Males With Autism. Archives of General Psychiatry, 63(12), 1417–1428. 10.1001/archpsyc.63.12.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najman JM, Hayatbakhsh MR, Clavarino A, Bor W, O’Callaghan MJ, & Williams GM (2010). Family Poverty Over the Early Life Course and Recurrent Adolescent and Young Adult Anxiety and Depression: A Longitudinal Study. American Journal of Public Health, 100(9), 1719–1723. 10.2105/AJPH.2009.180943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family Income, Parental Education and Brain Structure in Children and Adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, & Walhovd KB (2009). Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(38), 11772–11782. 10.1523/JNEUROSCI.1242-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJA, van den Bulk BG, van Lang NDJ, Rombouts SARB, van Buchem MA, … van der Wee NJA (2014). Reduced anterior cingulate gray matter volume in treatment-naïve clinically depressed adolescents. NeuroImage: Clinical, 4, 336–342. 10.1016/j.nicl.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, & Menon V (2014). Amygdala Subregional Structure and Intrinsic Functional Connectivity Predicts Individual Differences in Anxiety During Early Childhood. Biological Psychiatry, 75(11), 892–900. 10.1016/j.biopsych.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, & Yurgelun-Todd DA (2005). Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry, 57(1), 21–26. 10.1016/j.biopsych.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, … Hibar DP (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry, 21(6), 806–812. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, & Armony JL (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32(4), 811–830. 10.1016/j.neubiorev.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Sheline YI (2000). 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry, 48(8), 791–800. [DOI] [PubMed] [Google Scholar]
- Slopen N, Fitzmaurice G, Williams DR, & Gilman SE (2010). Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 49(5), 444–452. [DOI] [PubMed] [Google Scholar]
- Spence SH, Najman JM, Bor W, O’Callaghan MJ, & Williams GM (2002). Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 43(4), 457–469. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, & Phan KL (2015). Neurostructural Abnormalities in Pediatric Anxiety Disorders. Journal of Anxiety Disorders, 32, 81–88. 10.1016/j.janxdis.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14(2), 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2009). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EAA, Boes AD, Wemmie JA, Tranel D, & Nopoulos P (2010). Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience, 5(4), 424–431. 10.1093/scan/nsq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulser H, Lemaitre H, Artiges E, Miranda R, Penttilä J, Struve M, … Stephens D (2015). Subthreshold Depression and Regional Brain Volumes in Young Community Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 54(10), 832–840. 10.1016/j.jaac.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Evans GW, Grant K, Carter JS, & Duffy S (2016). Poverty and the Development of Psychopathology In Developmental Psychopathology (Vol. 4, pp. 1–44). John Wiley & Sons, Inc. [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, … Allen NB (2014). Structural Brain Development and Depression Onset During Adolescence: A Prospective Longitudinal Study. American Journal of Psychiatry, 171(5), 564–571. 10.1176/appi.ajp.2013.13070920 [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2015). http://datacenter.kidscount.org/data/tables/8447-children-in-poverty-100-by-age-group-and-race-and-ethnicity?loc=1&loct=2#detailed/1/any/false/869,36/2757,4087,3654,3301,2322,3307,2664/17079,17080
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


